Abstract
Magnaporthe oryzae-induced rice blast remains a critical threat to sustainable rice farming, causing extensive losses in many rice-producing regions worldwide. Due to increasing concerns about pesticide overuse and its impact on the environment and human health, alternative control methods are being actively explored. Nanotechnology has recently gained attention as a potential tool for sustainable disease management. This review summarises current progress in the use of nanomaterials—including metal and biopolymer nanoparticles, nanoemulsions, targeted delivery systems, and biosensors—for the detection and control of rice blast. Studies have reported that nanomaterials can reduce disease severity by up to 70% and improve rice yield by 10–20% under field or greenhouse conditions. The mode of action, effectiveness under field conditions, and possible integration into integrated pest management (IPM) programs are discussed. The selection of literature followed the PRISMA-P framework to ensure a systematic and transparent review process. Challenges such as biosafety, environmental risks, and regulatory issues are also addressed, with emphasis on green synthesis methods and the need for field validation before practical application.
1. Introduction
Rice (Oryza sativa L.) remains the staple food for over half of the world’s population and plays a particularly crucial role in ensuring food security across Asia and Sub-Saharan Africa [1]. However, rice cultivation faces continual threats from biotic stresses, with rice blast disease—caused by the hemibiotrophic fungus Magnaporthe oryzae (syn. Pyricularia oryzae)—ranking among the most destructive. This pathogen infects all aerial parts of the rice plant, including leaves, stems, nodes, and panicles, often resulting in yield losses exceeding 30% under favorable environmental conditions [2,3,4,5,6]. Although the use of resistant cultivars and fungicides remains central to rice blast management, the rise in new virulent strains and environmental concerns associated with chemical inputs have necessitated alternative strategies [7,8]. The environmental concerns largely arise from the excessive and improper use of synthetic fungicides. These chemicals, when applied intensively, often result in harmful runoff that threatens non-target aquatic species. They may also accumulate in the food chain, alter beneficial microbial populations in soil, and pollute groundwater. In the long term, the persistence of such residues contributes to ecological disruption and raises public health concerns, especially for farm workers and rural communities.
In addition, the growing impact of global climate change further compounds food security challenges, particularly in major rice-producing regions such as China and Southeast Asia. These environmental pressures underscore the need for resilient and sustainable disease management approaches [9]. In this context, nanotechnology has garnered attention for its potential to deliver targeted, efficient, and environmentally responsible solutions. Nanomaterials such as metallic nanoparticles (Ag, ZnO), nanoemulsions, and biopolymer-based carriers (e.g., chitosan, alginate) have demonstrated antifungal activities and elicitor properties that can enhance host resistance [10,11,12,13,14].
Beyond their therapeutic roles, nano-enabled diagnostic platforms—such as biosensors and nucleic acid nanokits—are being explored for the early detection of M. oryzae, enabling timely interventions [15,16,17,18]. These approaches align with the principles of integrated pest management (IPM) and may pave the way for more sustainable crop protection systems. Nevertheless, issues concerning nanoparticle persistence, potential phytotoxicity, and environmental impact necessitate further research, particularly regarding green synthesis and regulation [19,20,21,22,23]. This review provides a critical assessment of recent nanotechnology-based strategies for rice blast control, focusing on mechanisms, efficacy, biosafety, and integration into IPM frameworks.
2. Methods
The review process adhered to PRISMA-P standards, providing a structured and reproducible approach to literature selection and data synthesis. The protocol for this review was prospectively registered on the Open Science Framework (OSF) and is accessible at https://osf.io/z39bq, accessed on 20 August 2025. The PRISMA 2020 checklist [24] was followed to ensure transparency and reproducibility.
A comprehensive literature search was carried out to identify relevant peer-reviewed articles published between 2009 and 2025. The search was performed across multiple electronic databases, including PubMed, ScienceDirect, SpringerLink, Elsevier, and Google Scholar.
The search strategy involved a combination of keywords and Boolean operators: “nanotechnology,” “nanoemulsion,” “nanoparticles,” “nano-formulation,” “Pyricularia oryzae,” “rice blast disease,” “secondary metabolites,” and “nanotechnology for plant disease management.” Only articles published in English and focusing on the application of nanomaterials in the diagnosis or control of rice blast disease were included.
The selection process consisted of four stages: (i) identification of records through database searching; (ii) screening of titles and abstracts for relevance to the review objectives; (iii) eligibility assessment through full-text reading; and (iv) final inclusion based on scientific quality and relevance to nanotechnology-based rice blast control. Scientific quality was assessed based on the journal’s reputation (e.g., impact factor), peer-review status, methodological rigor, and citation metrics when available.
Duplicates, conference abstracts without full texts, non-English publications, and articles unrelated to rice blast or nanotechnology applications were excluded. To enhance the reliability of the selection process, two evaluators reviewed the records separately, and any inconsistencies were reconciled via consensus-based discussion. A flow diagram following the PRISMA format (Figure 1) summarizes the study selection process.
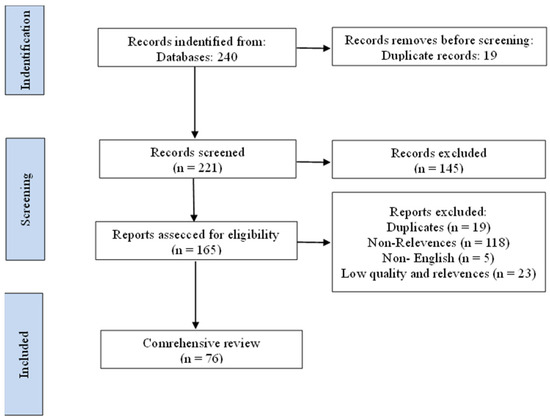
Figure 1.
The overall research framework of this study outlines.
3. Results and Discussions
3.1. Metallic Nanoparticles Against Magnaporthe oryzae
Metal-based nanoparticles such as silver (AgNPs), copper (CuO, CuChNPs), and zinc oxide (ZnO NPs) have shown consistent antifungal efficacy against M. oryzae across laboratory and greenhouse conditions [25,26,27,28,29,30,31,32,33,34,35,36,37,38]. For instance, in a greenhouse study, AgNPs applied at 800 ppm markedly reduced disease severity to 3.23%, with an EC50 of 308.1 ppm under in vitro conditions [25]. This substantial suppression of infection suggests both prophylactic and therapeutic potential. Likewise, 20–30 nm AgNPs used at 100 ppm resulted in a drastic reduction in diseased leaf area (DLA) to 15.3–26.7%, compared to 80% in the control group [26], underscoring their capacity to interfere with early-stage fungal colonization.
Furthermore, combinations of AgNPs with AgNO3 and AgCl showed a synergistic inhibition of conidial formation and hyphal growth, although the absence of precise dose–response data limits direct comparison [27]. Interestingly, copper oxide nanoparticles (CuO NPs) have also been shown to elicit resistance via OsCERK1-mediated signaling, indicating a potential role in priming host immunity [28]. This mechanism, distinct from direct antifungal toxicity, could be particularly valuable under integrated pest management frameworks.
A broader view emerges from review-based work, where AgNPs, CuO NPs, and ZnO NPs were associated with enhanced flavonoid biosynthesis in vitro [29], a response often linked with improved plant defense. Complementing this, AgNPs synthesized using Azadirachta indica extract significantly inhibited fungal growth and spore formation more effectively than AgNO3 alone [30], while biosynthesis via Trichoderma harzianum also yielded particles capable of disrupting fungal hyphae at low doses [31], with supporting SEM evidence.
Zinc oxide nanoparticles (ZnO NPs), particularly those derived from Catharanthus roseus, demonstrated notable improvements over their bulk counterparts in reducing both sporulation and lesion development [32]. The complete suppression of conidia formation at 100 ppm in another ZnO NP study [33] further reinforces their relevance in rice protection strategies.
Among biopolymer-based systems, chitosan nanoparticles (Ch-NPs) have shown encouraging field results. Essential oil-loaded Ch-NPs inhibited spore germination and reduced field-level disease prevalence [34], while chitosan-CuO NP composites provided a dual mode of action through both structural disruption and immune stimulation [35]. Moreover, chitosan-silver nanocomposites have been observed to rupture fungal membranes at relatively low concentrations [36], suggesting strong bioavailability and membrane affinity.
Additional materials such as silicon nanoparticles (SiNPs) and titanium dioxide (TiO2 NPs) offer different modes of action. SiNPs contributed to improved physiological parameters and reduced disease indices under semi-controlled conditions [37], whereas TiO2 NPs acted primarily through oxidative stress generation, disrupting fungal membranes as confirmed via microscopy [38].
Altogether, this body of research illustrates the multifaceted nature of nanoparticle-mediated disease control. Despite the diversity of mechanisms—ranging from membrane damage to immune priming—the variability in experimental designs, formulations, and reporting standards continues to challenge comparative evaluation. Future studies should prioritize standardized protocols and field-scale validation to translate laboratory efficacy into practical applications (Table 1).

Table 1.
Summary of antifungal activities of Nanomaterials against Magnaporthe oryzae.
Notably, ZnO NPs inhibit spore germination and suppress appressorium formation while modulating defense gene expression (e.g., OsNAC4, OsPR1b) and reducing stress-related hormones such as abscisic acid. The transcription factor OsNAC4 plays a key role in plant defense by regulating hypersensitive cell death (HCD), a form of localized programmed cell death that restricts pathogen spread at infection sites [28,39]. In parallel, OsPR1b encodes a pathogenesis-related (PR) protein that functions downstream of the salicylic acid (SA) signaling pathway and is a hallmark of systemic acquired resistance (SAR), a vital mechanism for long-term defense against fungal pathogens [40]. Both genes are widely recognized as molecular markers of plant immune activation under biotic stress. For instance, Dutta et al. The reported that copper oxide nanoparticles (CuO NPs) significantly enhanced OsPR1b expression in rice leaves challenged with M. oryzae, which was accompanied by a reduction in disease symptoms and pathogen load [41].
These particles exert their antifungal effect by disrupting fungal cell membranes, inducing reactive oxygen species (ROS), and releasing metal ions that compromise cellular function. Furthermore, they may trigger systemic acquired resistance (SAR), enhancing the plant’s innate defense. However, their impact on beneficial rhizosphere microbes remains a concern, underlining the need for targeted delivery and ecological assessment. For instance, silver nanoparticles have been shown to influence rhizospheric microbial communities by suppressing pathogenic fungi while also impacting beneficial bacteria such as Bacillus subtilis and Pseudomonas fluorescens [42,43]. In a separate study showed that prolonged exposure to AgNPs in wheat cultivation reduced the activity of key soil enzymes—including dehydrogenase and phosphatase—and altered the overall microbial composition. These findings suggest a potential trade-off between effective pathogen control and the preservation of soil microbial health. Therefore, careful consideration of environmental impacts is essential when designing nanoparticle-based disease management strategies [44,45] (Table 2)

Table 2.
Nanoparticles and Immunity in rice.
3.2. Nanoemulsions of Essential Oils
Nanoemulsions prepared with essential oils like neem, citronella, and eugenol have shown promising antifungal properties, largely due to their enhanced dispersion, stability, and ability to penetrate plant tissues efficiently [46,47,48,49,50,51,52,53,54,55,56]. These nano-formulations not only suppress fungal infections but also promote the plant’s own defense system by upregulating antioxidant enzymes and activating salicylic acid pathways, all without triggering phytotoxic side effects [57].
The nanoscale droplet size (8–20 nm) allows close contact between the nanoemulsion and fungal structures, which enhances antifungal activity. In addition, the formulation’s ability to withstand UV radiation and temperature fluctuations improves its suitability for field applications. Scalable techniques such as phase inversion temperature (PIT) synthesis have also made production more feasible. However, the actual performance of nanoemulsions in field conditions can vary, depending on both environmental factors and the timing of application.
Nanoemulsions are thermodynamically unstable but kinetically stable systems, and their stability can be influenced by temperature, light, pH, and other external factors. For example, high temperatures can lower viscosity and accelerate destabilization mechanisms such as Ostwald ripening—where smaller droplets dissolve and migrate to larger ones—leading to changes in droplet size and reduced availability of active ingredients [58,59].
UV exposure may break down active compounds or weaken the interfacial film around droplets. Changes in pH can affect the charge of surfactants, altering droplet interactions and increasing the risk of aggregation [60,61]. Humid conditions and rainfall may improve droplet spreading on leaves but also increase the chance of wash-off, reducing how long the formulation stays on the plant surface. Other formulation factors—such as the oil-to-water ratio, the concentration of surfactants, and the use of stabilizers—also affect emulsion structure and its ability to remain stable under field conditions [50,59]. Altogether, these factors underline the importance of optimizing both the nanoemulsion formulation and its field application method to achieve consistent and effective results.
3.3. Biopolymer Nanoparticles and Nanochitosan
Natural polymer-based nanoparticles like nanochitosan and nanoalginate are increasingly applied in transporting defense signals and beneficial microbes. These carriers not only possess antifungal effects but also activate plant immune responses, including oxidative bursts and phenolic compound production [13,62,63,64,65,66,67]. Nanochitosan, in particular, has been shown to suppress key rice pathogens by enhancing antioxidant enzyme activity and biosynthesis of flavonoids and phenols [68,69,70].
In recent years, there has been increasing interest in coupling nanocarriers with beneficial microbial agents, particularly nitrogen-fixing cyanobacteria, to enhance soil fertility and promote sustainable crop production. It has been demonstrated that cyanobacteria significantly contribute to biological nitrogen fixation, improving nutrient availability and supporting plant growth under reduced chemical input conditions [71]. Building on this, highlighted the synergistic potential of integrating nanotechnology with cyanobacterial biofertilization, offering a promising strategy for improving crop resilience and system sustainability. By facilitating both pathogen suppression and nutrient delivery, such combined approaches represent a forward-looking model for eco-friendly and integrated rice disease management [72].
Biopolymeric nanoparticles such as Ag–chitosan, Cu–chitosan, Moringa-chitosan nanoparticles (M-CsNPs), and carbon nanospheres (CNs) exhibit strong antifungal activity against Magnaporthe oryzae by inhibiting spore germination and disrupting mycelial growth. They also act as elicitors, triggering ROS production, activating antioxidant enzymes, and upregulating plant defense genes (Figure 2). Additionally, their role as carriers for bioactive compounds or antagonistic microbes enables targeted delivery, supporting their integration into sustainable IPM strategies. Recent molecular studies using transcriptomics and metabolomics have begun to elucidate how nano-formulations regulate rice immune pathways and oxidative balance at the cellular level (e.g., upregulation of OsPR1b, OsWRKY45, and PAL genes Integrative transcriptomic and metabolomic analyses have revealed that moringa–chitosan nanoparticles (M-CsNPs) directly suppress Magnaporthe oryzae, while also modulating host immune pathways at the molecular level. RNA-seq profiling identified significant upregulation of multiple defense-related genes—including those associated with salicylic acid signaling, pathogenesis-related proteins, and antioxidant enzyme systems—supporting the hypothesis that nanochitosan formulations function both as direct antimicrobials and as inducers of endogenous plant defense responses [73]. Similarly, other transcriptomic investigations have observed elevated expression of ROS-scavenging enzymes (e.g., SOD, CAT, POD) and secondary metabolism genes in rice treated with chitosan-based nanoparticles, consistent with enhanced redox homeostasis and phenylpropanoid pathway activation [64,74].
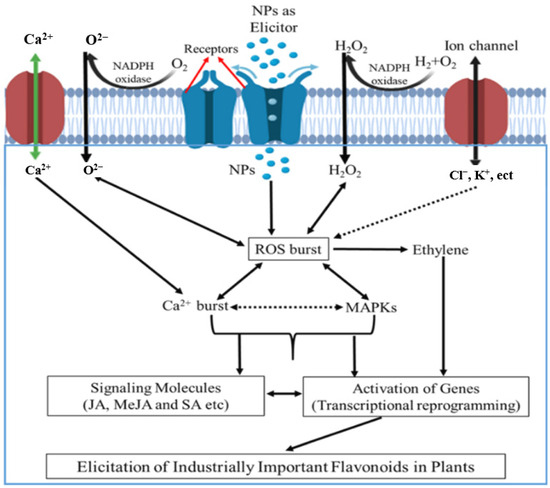
Figure 2.
Illustrates this nano-elicitation pathway in rice.
3.4. Smart Nanocarriers for Controlled Release
Stimuli-responsive nanocarriers—such as Pro@MON@PTA, PYR@FeMOF-pectin, and PYR-HMS-HPC—have been developed to enable site-specific fungicide delivery in response to pH, enzyme activity, or redox signals (e.g., glutathione) [75,76,77,78,79,80].
These nanocarriers are designed to intelligently respond to biochemical triggers present at infection sites, ensuring that fungicide release occurs only where and when it is needed. For example, Pro@MON@PTA, comprising mesoporous organosilica capped with poly (tannic acid), demonstrates dual responsiveness to acidic pH and glutathione (GSH), improving both stability and foliar adhesion [75]. Pro@MSN-Pec, a pectin-gated mesoporous silica nanoparticle, releases active compounds upon exposure to pectinase secreted by Magnaporthe oryzae, enhancing tissue penetration and prolonging fungicidal effect [77].
Likewise, PYR@FeMOF-pectin utilizes a pectin-coated metal–organic framework to trigger enzymatic release of pyraclostrobin, while simultaneously reducing environmental toxicity by up to 8-fold compared to conventional formulations [79]. PYR-HMS-HPC, incorporating hydroxypropyl cellulose and hollow mesoporous silica, offers dual sensitivity to pH and cellulase, significantly enhancing photostability and extending activity in planta [78]. Additionally, virus-like mesoporous silica nanoparticles (VMSNs) have shown immunostimulatory potential by activating systemic acquired resistance (SAR) through PR gene expression in rice [80]. Reviews of nanopesticide technologies have also supported these findings, emphasizing their dual roles in reducing chemical input and improving disease control [76].
Such systems enhance foliar adhesion and prolong efficacy under fluctuating environmental conditions. By optimizing release kinetics, these carriers can reduce fungicide doses by up to 50%, offering a balance between efficacy and environmental safety. Illustrates the mechanism of stimuli-responsive nanocarriers, which release active agents at infection sites in response to pH or enzymatic triggers (Figure 3).
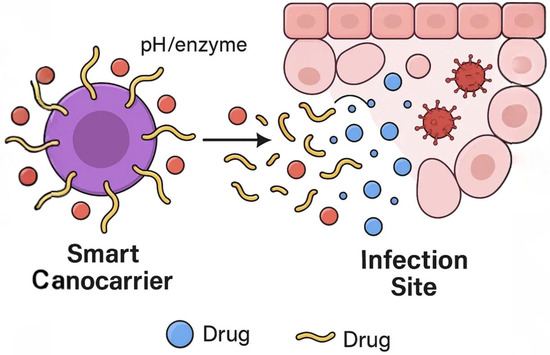
Figure 3.
Schematic representation of a smart nanocarrier releasing fungicide in response to pH or enzymatic conditions at the infection site.
Although promising, most of these systems remain at the laboratory stage. Their synthetic complexity and cost limit their current application in low-resource agricultural settings. Moreover, field performance data are scarce, and their degradation products under soil and plant interactions are poorly characterized. Further studies should focus on upscaling production, biodegradability, and regulatory assessment to ensure safe deployment.
3.5. Nanosensors for Rapid Diagnosis
In recent years, nanosensor-based platforms have received growing attention due to their ability to provide quick and sensitive detection of fungal pathogens, particularly Magnaporthe oryzae—the causal agent of rice blast. These tools are not only useful for detecting the pathogen itself but also for sensing pesticide residues commonly associated with its control. For example, platforms incorporating ZnO-imidoester hybrid nanorods (HINRs) and fluorescein-labeled silver nanoparticles (AgNPs) have shown effectiveness in detecting M. oryzae DNA as well as tricyclazole residues through both optical and electrochemical signal mechanisms [15,18,81,82,83].
Among current technologies, a noteworthy study reported the development of a magnetically controllable electrochemical biosensor specifically designed for early detection of M. oryzae. The system relied on a fungal chitinase (Mgchi) as a marker, with recognition facilitated by a rice-derived lectin (Osmbl). Thanks to the integration of magnetic beads and palladium nanoparticles on a responsive gold electrode, the biosensor was able to detect fungal infection before any visual symptoms were observable. This early detection capability holds significant promise, especially for high-risk regions where blast outbreaks can develop rapidly under favorable weather conditions [18].
What makes these biosensors particularly appealing is their portability and relatively low production cost. Such features make them ideal for deployment in remote or resource-limited agricultural settings, where access to laboratory diagnostics is restricted. Furthermore, their compact form allows for easy integration with portable readers or even smartphone interfaces, which could help farmers or extension workers perform on-site analysis with minimal training.
Other studied show that a variety of nanomaterials including AgNPs, AuNPs, graphene oxide, and quantum dots—have been applied in biosensor construction, enabling detection of not just nucleic acids but also fungal metabolites and enzymatic markers. However, unlike bacterial diagnostics which often rely on well-characterized surface proteins, fungal targets can be more diverse and harder to isolate, partly due to the complexity of fungal cell walls and spore dormancy mechanisms [15].
Looking ahead, integrating nanosensors with microfluidic chips or predictive modeling systems could lead to new diagnostic platforms capable of real-time disease monitoring. Still, issues like environmental stability, cross-reactivity, and the need for fungal-specific probes (e.g., aptamers or monoclonal antibodies) remain challenges that need to be addressed.
Table 3 summarizes selected nanosensor platforms that have been developed or proposed for fungal pathogen detection, particularly targeting Magnaporthe oryzae or related fungal metabolites.

Table 3.
Selected nanosensor platforms relevant to fungal pathogen detection.
3.6. Nanomaterials for Enhanced Host Resistance
Silicon-based nanoparticles (SiNPs), carbon nanospheres (CNS), and rice husk ash (RHA)-derived nanosilica have been investigated for their role in enhancing plant resistance [37,84]. These materials can reinforce cell walls, stimulate lignification, and regulate nutrient uptake through gene modulation (e.g., Lsi1 expression) [46,85,86].
CNS, in particular, modulate microbial dynamics in the rhizosphere, contributing to broader disease suppression. SiNPs have also been linked to improved drought and heat tolerance, supporting overall crop resilience.
This comprehensive overview synthesizes the content from Section 3.1 to Section 3.6 and serves as a reference for selecting appropriate nano-strategies in integrated pest management, including the comparative properties, mechanisms of action, antifungal effectiveness, advantages, and limitations of various nanomaterials applied in managing rice blast disease (Table 4).

Table 4.
Comparative overview of nanomaterials for the management of rice blast disease caused by Magnaporthe oryzae.
3.7. Biosafety and Sustainability Considerations
Environmental and health concerns related to nanoparticle stability, potential toxicity, and their accumulation in ecosystems have driven a shift toward environmentally benign synthesis techniques. Among these, the use of plant-derived compounds or agro-industrial wastes—such as rice straw—has gained attention as sustainable alternatives. Although direct evaluation of rice straw-derived nanoparticles against Magnaporthe oryzae remains limited, rice straw is a rich and sustainable precursor for nanoparticle synthesis. For instance, synthesized silica nanoparticles from rice husk ash with ~47 nm diameter and 99.8% purity, demonstrating good biocompatibility and potential for agricultural integration [87]. Similarly, Other studied show ZnO nanoparticles derived from rice residues inhibited Fusarium oxysporum by 92% and Alternaria alternata by 87%, and also showed >70% antioxidant activity, indicating multifunctionality [88]. Supporting this, results from [89] indicated that ZnO nanoparticles synthesized using rice straw extract showed significant antifungal activity against phytopathogenic fungi, with inhibition zones exceeding 20 mm for Aspergillus niger and Fusarium spp. Moreover, findings from [90] demonstrated that SiO2 nanoparticles obtained from rice husk not only maintained colloidal stability but also reduced disease incidence in tomato plants by 45% under greenhouse trials.
These findings suggest that rice straw–based nanoparticles could serve as eco-friendly alternatives for plant disease control, including rice blast, pending further pathogen-specific validation. Nonetheless, regulatory uncertainties and the lack of harmonized assessment standards remain significant hurdles [19,20,35,91,92,93,94].
A case-by-case classification system for nanoformulations, informed by ecotoxicological studies, is essential. Green-synthesized nanomaterials may improve environmental compatibility and public acceptance.
3.8. Integration with IPM and Comparative Strategies
Several studies have demonstrated that when nanotechnology is used in tandem with biocontrol species like Paecilomyces lilacinus, the outcome in managing plant diseases surpasses that of traditional fungicides including Mancozeb and Carbendazim [7,23,95,96]. This methodology aligns with current trends in IPM, promoting both efficiency and environmental stewardship
When paired with disease forecasting systems and precision delivery, nano-enabled IPM enhances efficacy while minimizing ecological disruption. However, field-scale demonstrations and long-term monitoring are needed to support widespread adoption. Although large-scale validation remains limited, several studies have reported promising results under semi-field or greenhouse conditions. For instance, result demonstrated that formulated a green tea essential oil nanoemulsion using ultrasonication and evaluated its antifungal efficacy against M. oryzae. Their research included both in vitro assays and an in vivo detached leaf assay, where the nanoemulsion significantly inhibited blast lesion development. The mechanism of action was further supported by confocal Raman micro-spectroscopy and SEM imaging, confirming cellular damage in fungal hyphae [97]. Similarly, synthesized chitosan nanoparticles using Camellia sinensis extract and demonstrated complete suppression of both rice blast and bacterial blight using detached leaf bioassays. Although conducted under controlled conditions, such assays closely replicate natural infection dynamics and offer valuable insights into the efficacy of nano-enabled biocontrol agents [98].
In addition, performed pot trials under open-air conditions, applying essential oil-based seed treatments to rice inoculated with M. oryzae. The treatments significantly reduced foliar disease incidence (11.67%) and seedling melt (1.33%), demonstrating the practical potential of botanical formulations within integrated disease management approaches [99].
Taken together, these studies—while not yet conducted at full field scale—provide meaningful experimental evidence supporting the real-world applicability of green nanoformulations in rice blast control. We have revised the manuscript accordingly to incorporate these references and clarify the scope of current field validation.
To synthesize the various strategies discussed, Figure 4 presents an integrated framework that combines nanosensor-based diagnosis, smart nanoformulation delivery, immune elicitation, and IPM feedback mechanisms. This conceptual model illustrates how nanotechnology can be effectively embedded within sustainable crop protection programs.
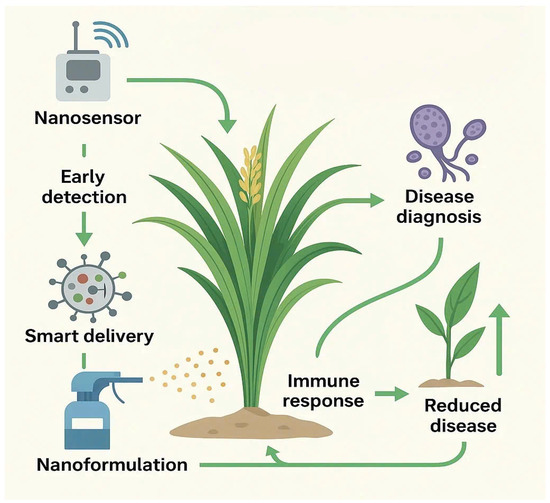
Figure 4.
Nano-IPM integration model.
3.9. Limitations and Future Perspectives
A comparative radar chart is used to visualize relative strengths and limitations of each nanostrategy, serving as a basis for identifying research priorities and optimization pathways (Figure 5).
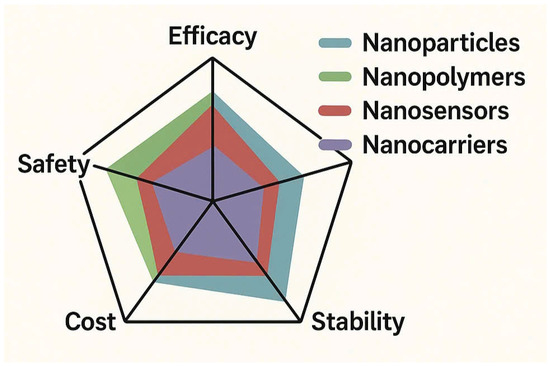
Figure 5.
Comparative radar chart of nanostrategies.
Although nanotechnology shows great promise in controlling rice blast, several limitations remain. Many nanoformulations effective in the lab show inconsistent results in the field due to environmental variability. Biosafety concerns, including nanoparticle persistence and effects on beneficial microbes, are still unresolved. Moreover, the lack of standardized regulations and high production costs limit large-scale application. Additionally, the environmental persistence of nanomaterials varies by type. Silver nanoparticles (AgNPs) can remain active for several weeks to months in soil, gradually transforming into less active forms such as silver sulfide [100] while nanochitosan, being biodegradable, tends to degrade more quickly, typically within 10–30 days depending on humidity and microbial activity [101]. the strategic selection of nanoformulation approaches, Table 5 presents a multi-criteria decision matrix that compares various nanostrategies based on five key performance parameters: efficacy, safety, cost, stability, and IPM compatibility.

Table 5.
Multi-criteria decision matrix comparing five nanostrategies for plant disease control. Each parameter was scored from 1 (low) to 3 (high), based on reported performance in literature.
Recent advances in smart delivery systems offer promising solutions for improving the precision and reliability of nano-based disease management. For example, stimuli-responsive nanocarriers have been designed to release active compounds in response to environmental cues such as pH, temperature, or enzymatic activity, thereby enhancing delivery efficiency and minimizing off-target effects [102,103]. In addition, nano-biocapsules and biodegradable polymer-based carriers have shown improved adhesion, translocation, and sustained release properties, even under semi-field conditions [104]. These innovations represent important steps toward the practical integration of nanotechnology into IPM strategies for managing rice blast and other plant diseases.
Moreover, the compatibility between nanomaterials and microbial biocontrol agents is increasingly recognized as critical in IPM. While metallic nanoparticles like AgNPs may inhibit beneficial microbes at higher concentrations, biopolymer-based carriers such as chitosan nanoparticles are generally considered compatible and may even enhance microbial efficacy [105,106].
Future research should focus on field validation, eco-toxicological studies, and development of green, cost-effective synthesis methods. Interdisciplinary collaboration will be essential to address regulatory gaps and support safe deployment in real-world agriculture. Figure 5 highlights critical knowledge gaps and future development pathways in nanoformulation-based disease control (Figure 6).
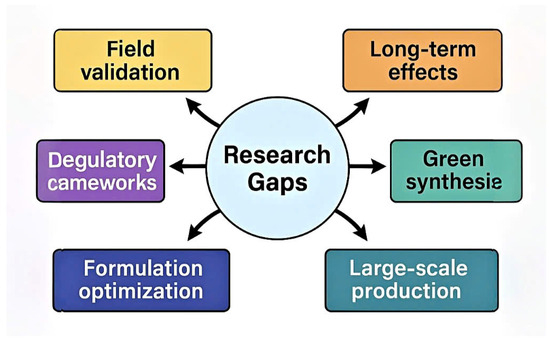
Figure 6.
Research gap map and future directions for nanotechnology applications in plant disease management.
4. Conclusions
Nanotechnology offers significant promise for the sustainable management of rice blast disease. Through improved delivery, diagnostic capabilities, and plant defense modulation, nano-based solutions align well with integrated and eco-friendly pest management strategies. Future efforts should prioritize field validation, biosafety assessments, and development of regulatory frameworks that facilitate responsible adoption. Nanotechnology will be integral to advancing climate-resilient and sustainable agriculture.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Song, J.J.; Soytong, K.; Kanokmedhakul, S. Control of rice blast disease caused by Magnaporthe oryzae by application of antifungal nanomaterials from Emericella nidulans. Plant Prot. Sci. 2021, 58, 40–48. [Google Scholar] [CrossRef]
- Banakar, S.N.; Prasannakumar, M.K.; Parivallal, P.B.; Pramesh, D.; Mahesh, H.B.; Sarangi, A.N.; Puneeth, M.E.; Patil, S.S. Rice-Magnaporthe transcriptomics reveals host defense activation induced by red seaweed-biostimulant in rice plants. Front. Genet. 2023, 14, 1132561. [Google Scholar] [CrossRef]
- Kou, Y.; Shi, H.; Qiu, J.; Tao, Z.; Wang, W. Effectors and environment modulating rice blast disease: From understanding to effective control. Trends Microbiol. 2024, 32, 1007–1020. [Google Scholar] [CrossRef]
- Nalley, L.; Tsiboe, F.; Durand-Morat, A.; Shew, A.; Thoma, G.; Wang, Z. Economic and Environmental Impact of Rice Blast Pathogen (Magnaporthe oryzae) Alleviation in the United States. PLoS ONE 2016, 11, e0167295. [Google Scholar] [CrossRef]
- Skamnioti, P.; Gurr, S.J. Against the grain: Safeguarding rice from rice blast disease. Trends Biotechnol. 2009, 27, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-F.; Islam, T.; Liu, W.-D. Integrated pest management programme for cereal blast fungus Magnaporthe oryzae. J. Integr. Agric. 2022, 21, 3420–3433. [Google Scholar] [CrossRef]
- Sharma, D.; Gupta, A.; Rawat, R.; Sharma, S.; Yadav, J.S.; Saxena, A. Exploring nanoformulation drug delivery of herbal actives for enhanced therapeutic efficacy: A comprehensive review. Intell. Pharm. 2025, 3, 26–34. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Zhang, L.; Zhou, Z.; Zhang, J.; Yang, J.; Gao, X.; Chen, R.; Huang, Z.; Xu, Z.; et al. Isolation of Bacillus siamensis B-612, a Strain That Is Resistant to Rice Blast Disease and an Investigation of the Mechanisms Responsible for Suppressing Rice Blast Fungus. Int. J. Mol. Sci. 2023, 24, 8513. [Google Scholar] [CrossRef]
- Rahman, T.U.; Shah, S.; Hassan, S.; Fahad, S. Food security challenges and adaptation strategies in China amidst global climate change. J. Umm Al-Qura Univ. Appl. Sci. 2025, 1–14. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Osman, A.; El-Saber, M.M.; Camele, I.; Abbas, E. Antifungal Activity of Green and Chemically Synthesized ZnO Nanoparticles against Alternaria citri, the Causal Agent Citrus Black Rot. Plant Pathol. J. 2023, 39, 265–274. [Google Scholar] [CrossRef]
- Jabran, M.; Ali, M.A.; Muzammil, S.; Zahoor, A.; Ali, F.; Hussain, S.; Muhae-Ud-Din, G.; Ijaz, M.; Gao, L. Exploring the potential of nanomaterials (NMs) as diagnostic tools and disease resistance for crop pathogens. Chem. Biol. Technol. Agric. 2024, 11, 75. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z. Chitosan-Based Agronanochemicals as a Sustainable Alternative in Crop Protection. Molecules 2020, 25, 1611. [Google Scholar] [CrossRef] [PubMed]
- Preeti; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R.; et al. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef] [PubMed]
- Vinceković, M.; Jurić, S.; Vlahoviček-Kahlina, K.; Martinko, K.; Šegota, S.; Marijan, M.; Krčelić, A.; Svečnjak, L.; Majdak, M.; Nemet, I.; et al. Novel Zinc/Silver Ions-Loaded Alginate/Chitosan Microparticles Antifungal Activity against Botrytis cinerea. Polymers 2023, 15, 4359. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.; Tripathi, D.; Ranjan, R. Nano-enabled biosensors in early detection of plant diseases. Front. Nanotechnol. 2025, 7, 1545792. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2014, 5, 123–127. [Google Scholar] [CrossRef]
- Le Dang, Q.; Nguyen, C.Q.; Vo, T.K.A.; Nguyen, T.T.T.; Pham, Q.D.; Nguyen, T.X.; Cao, T.H.; De Tran, Q.; Le, T.T.; Do, T.H.; et al. A botanical nanoemulsion against phytopathogenic fungi Colletotrichum sp. and Fusarium oxysporum: Preparation, in vitro and in vivo bioassay. J. Nat. Pestic. Res. 2024, 10, 100099. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, H.; Li, M.; Wang, Z.; Zhou, J.; Wang, S.; Lu, G.; Fu, F. Early diagnosis of blast fungus, Magnaporthe oryzae, in rice plant by using an ultra-sensitive electrically magnetic-controllable electrochemical biosensor. Anal. Chim. Acta 2014, 850, 85–91. [Google Scholar] [CrossRef]
- Bouhadi, M.; Javed, Q.; Jakubus, M.; Elkouali, M.; Fougrach, H.; Ansar, A.; Ban, S.G.; Ban, D.; Heath, D.; Černe, M. Nanoparticles for Sustainable Agriculture: Assessment of Benefits and Risks. Agronomy 2025, 15, 1131. [Google Scholar] [CrossRef]
- Devi, K.A.; Prajapati, D.; Kumar, A.; Pal, A.; Bhagat, D.; Singh, B.R.; Adholeya, A.; Saharan, V. Smart Nano-Chitosan for Fungal Disease Control. In Nanopesticides: From Research and Development to Mechanisms of Action and Sustainable Use in Agriculture; Springer International Publishing: Cham, Switzerland, 2020; pp. 23–47. [Google Scholar] [CrossRef]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef]
- Sidhu, A.K.; Verma, N.; Kaushal, P.; Agrawal, S.B.; Bhatia, S. Green synthesis of polymeric nanoparticles: Agricultural applications and toxicological implications. Discov. Appl. Sci. 2025, 7, 505. [Google Scholar] [CrossRef]
- Zafar, S.; Arshad, M.F.; Khan, H.; Menahil, R.; Iqbal, L.; Prabhavathi, S.J.; Kumar, M.S.; Omar, A.F.; Shaheen, T. Nanoformulations of plant essential oils for managing mycotoxins producing fungi: An overview. Biocatal. Agric. Biotechnol. 2024, 60, 103314. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Akter, R. Efficacy of Silver Nanoparticles Against Rice Blast Disease and Farmers Perception about Its Management in Bangladesh. Master’s Thesis, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2019. [Google Scholar]
- Elamawi, R.M.A.; El-Shafey, R.A.S. Inhibition effects of silver nanoparticles against rice blast disease caused by Magnaporthe grisea. Egypt. J. Agric. Res. 2013, 91, 1271–1283. [Google Scholar] [CrossRef]
- Jo, Y.K.; Kim, B.H.; Jung, G. Antifungal Activity of Silver Ions and Nanoparticles on Phytopathogenic Fungi. Plant Dis. 2009, 93, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Z.; Meng, S.; Shen, Z.; Shi, H.; Qiu, J.; Lin, F.; Zhang, S.; Kou, Y. OsCERK1 Contributes to Cupric Oxide Nanoparticles Induced Phytotoxicity and Basal Resistance against Blast by Regulating the Anti-Oxidant System in Rice. J. Fungi 2022, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Jayabaskaran, C.; Manikandan, A.; Anusuya, S. Synthesis of Nickel-Chitosan Nanoparticles for Controlling Blast Diseases in Asian Rice. Appl. Biochem. Biotechnol. 2022, 195, 2134–2148. [Google Scholar] [CrossRef]
- Liu, H.; Lim, S.M.; Zhang, K.; Shin, J.; Koo, B.; Park, C.O.; Kim, S.-H.; Shin, Y. Efficient handy DNA extraction from fungal spores using modified ZnO nano-rices for rapid pathogen detection. Sens. Actuators B Chem. 2025, 431, 137409. [Google Scholar] [CrossRef]
- Mahmud, Q.; Khan, M.; Akanda, A.; Hossain, M.; Latif, M.; Akter, R. Antifungal potential of commercial silver nanoparticles against rice blast pathogen Magnaporthe oryzae. Ann. Bangladesh Agric. 2024, 27, 17–30. [Google Scholar] [CrossRef]
- Kora, A.J.; Mounika, J.; Jagadeeshwar, R. Rice leaf extract synthesized silver nanoparticles: An in vitro fungicidal evaluation against Rhizoctonia solani, the causative agent of sheath blight disease in rice. Fungal Biol. 2020, 124, 671–681. [Google Scholar] [CrossRef]
- Kanhed, P.; Birla, S.; Gaikwad, S.; Gade, A.; Seabra, A.B.; Rubilar, O.; Duran, N.; Rai, M. In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater. Lett. 2014, 115, 13–17. [Google Scholar] [CrossRef]
- Ngoc, D.T.B.; Du, B.D.; Tuan, L.N.A.; Thach, B.D.; Kien, C.T.; Van Phu, D.; Hien, N.Q. Study on Antifungal Activity and Ability Against Rice Leaf Blast Disease of Nano Cu-Cu2O/Alginate. Indian J. Agric. Res. 2020, 54, 802–806. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Chen, Y.; Liu, Z.; Wen, H.; Jiang, N.; Shi, H.; Kou, Y. The application of zinc oxide nanoparticles: An effective strategy to protect rice from rice blast and abiotic stresses. Environ. Pollut. 2023, 331, 121925. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Application of Copper-Chitosan Nanoparticles Stimulate Growth and Induce Resistance in Finger Millet (Eleusine coracana Gaertn.) Plants against Blast Disease. J. Agric. Food Chem. 2018, 66, 1784–1790. [Google Scholar] [CrossRef]
- Yin, W.; Pang, Z.; Feng, X.; Wang, Y.; Peng, H.; Liang, Y. Comparison of the effects of silicic acid, organosilicon and Nano-silicon on rice cell wall phosphorus. Plant Physiol. Biochem. 2025, 227, 110089. [Google Scholar] [CrossRef]
- Kaneda, T.; Taga, Y.; Takai, R.; Iwano, M.; Matsui, H.; Takayama, S.; Isogai, A.; Che, F.-S. The transcription factor OsNAC4 is a key positive regulator of plant hypersensitive cell death. EMBO J. 2009, 28, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, Y.; Gao, R.; Wu, Z.; Zhang, W.; Zhang, C.; Zhang, P.; Ye, C.; Yao, L.; Jin, Y.; et al. Complete biosynthesis of salicylic acid from phenylalanine in plants. Nature, 2025; online ahead of print. [Google Scholar] [CrossRef]
- Bian, Z.; Gao, H.; Wang, C. NAC Transcription Factors as Positive or Negative Regulators during Ongoing Battle between Pathogens and Our Food Crops. Int. J. Mol. Sci. 2020, 22, 81. [Google Scholar] [CrossRef] [PubMed]
- Júnior, A.H.d.S.; Mulinari, J.; de Oliveira, P.V.; de Oliveira, C.R.S.; Júnior, F.W.R. Impacts of metallic nanoparticles application on the agricultural soils microbiota. J. Hazard. Mater. Adv. 2022, 7, 100103. [Google Scholar] [CrossRef]
- Kumar, N.; Shah, V.; Walker, V.K. Perturbation of an arctic soil microbial community by metal nanoparticles. J. Hazard. Mater. 2011, 190, 816–822. [Google Scholar] [CrossRef]
- Wang, J.; Shu, K.; Zhang, L.; Si, Y. Effects of Silver Nanoparticles on Soil Microbial Communities and Bacterial Nitrification in Suburban Vegetable Soils. Pedosphere 2017, 27, 482–490. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, M.; Zhang, W.; Gardea-Torresdey, J.L.; White, J.C.; Ji, R.; Zhao, L. Silver Nanoparticles Alter Soil Microbial Community Compositions and Metabolite Profiles in Unplanted and Cucumber-Planted Soils. Environ. Sci. Technol. 2020, 54, 3334–3342. [Google Scholar] [CrossRef]
- Choupanian, M.; Omar, D.; Basri, M.; Asib, N. Preparation and characterization of neem oil nanoemulsion formulations against Sitophilus oryzae and Tribolium castaneum adults. J. Pestic. Sci. 2017, 42, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, N.; Rajput, V.D.; Mandzhieva, S.; Minkina, T.; Saharan, B.S.; Kumar, D.; Sadh, P.K.; Duhan, J.S. Advances in Biopolymeric Nanopesticides: A New Eco-Friendly/Eco-Protective Perspective in Precision Agriculture. Nanomaterials 2022, 12, 3964. [Google Scholar] [CrossRef] [PubMed]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108-109, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Kalboush, Z.A.; Mazrou, Y.S.; Hassan, A.A.; El Badeea, O.A.; Nehela, Y. Oil-in-water nano-emulsions boost rice innate immune response against Pyricularia oryzae via the induction of salicylic acid-mediated pathway and the enhancement of antioxidant machinery. Plant Stress 2025, 16, 100889. [Google Scholar] [CrossRef]
- Kumar, R.; Raykar, N.D.; Kumar, H.S.; Dutta, K.; Choudhary, V.; Singh, A.; Kumar, S.; Prajapati, S. Formulation and Characterization of Efavirenz Nanoemulsion Using Grapeseed Oil: A Strategy to Enhance Solubility and Stability. J. Neonatal Surg. 2025, 14, 1473–1485. [Google Scholar]
- Maurya, A.; Singh, V.K.; Das, S.; Prasad, J.; Kedia, A.; Upadhyay, N.; Dubey, N.K.; Dwivedy, A.K. Essential Oil Nanoemulsion as Eco-Friendly and Safe Preservative: Bioefficacy Against Microbial Food Deterioration and Toxin Secretion, Mode of Action, and Future Opportunities. Front. Microbiol. 2021, 12, 751062. [Google Scholar] [CrossRef]
- Ali, E.O.M.; Shakil, N.A.; Rana, V.S.; Sarkar, D.J.; Majumder, S.; Kaushik, P.; Singh, B.B.; Kumar, J. Antifungal activity of nano emulsions of neem and citronella oils against phytopathogenic fungi, Rhizoctonia solani and Sclerotium rolfsii. Ind. Crop. Prod. 2017, 108, 379–387. [Google Scholar] [CrossRef]
- Du, J.; Liu, B.; Zhao, T.; Xu, X.; Lin, H.; Ji, Y.; Li, Y.; Li, Z.; Lu, C.; Li, P.; et al. Silica nanoparticles protect rice against biotic and abiotic stresses. J. Nanobiotechnol. 2022, 20, 197. [Google Scholar] [CrossRef]
- Singh, A.; Das, S.; Chaudhari, A.K.; Deepika; Soni, M.; Yadav, A.; Dwivedy, A.K.; Dubey, N.K. Laurus nobilis essential oil nanoemulsion-infused chitosan: A safe and effective antifungal agent for masticatory preservation. Plant Nano Biol. 2023, 5, 100043. [Google Scholar] [CrossRef]
- Septiyanti, M. Evaluation of Nanoemulsion Concentrate Botanical Fungicide from Neem, Citronella and Eugenol Oil Using Palm Oil Based Surfactant. Am. J. Phys. Appl. 2019, 7, 14. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Solans, C.; Solé, I. Nano-emulsions: Formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.-H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef]
- Raj, S.N.; Anooj, E.; Rajendran, K.; Vallinayagam, S. A comprehensive review on regulatory invention of nano pesticides in Agricultural nano formulation and food system. J. Mol. Struct. 2021, 1239, 130517. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, N.; Devi, L.S.; Kumar, S.; Kamle, M.; Kumar, P.; Mukherjee, A. Neem oil and its nanoemulsion in sustainable food preservation and packaging: Current status and future prospects. J. Agric. Food Res. 2022, 7, 100254. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Manikandan, A. Chitosan nanoparticle induced defense responses in fingermillet plants against blast disease caused by Pyricularia grisea (Cke.) Sacc. Carbohydr. Polym. 2016, 154, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, R.; Guo, J.; Ahmed, T.; Jiang, H.; Raza, M.; Shahid, M.; Ibrahim, E.; Wang, Y.; Wang, J.; Yan, C.; et al. Bio-formulated chitosan nanoparticles enhance disease resistance against rice blast by physiomorphic, transcriptional, and microbiome modulation of rice (Oryza sativa L.). Carbohydr. Polym. 2024, 334, 122023. [Google Scholar] [CrossRef]
- Heuskin, S.; Lorge, S.; Godin, B.; Leroy, P.; Frère, I.; Verheggen, F.J.; Haubruge, E.; Wathelet, J.; Mestdagh, M.; Hance, T.; et al. Optimisation of a semiochemical slow-release alginate formulation attractive towards Aphidius ervi Haliday parasitoids. Pest Manag. Sci. 2011, 68, 127–136. [Google Scholar] [CrossRef]
- Mirara, F.; Dzidzienyo, D.K.; Mwangi, M. Nano-enhanced defense: Titanium-enriched Alginate–Bentonite coating augments Bacillus amyloliquefaciens D203 efficacy against Magnaporthe oryzae in Kenyan rice cultivation. Heliyon 2024, 10, e36141. [Google Scholar] [CrossRef]
- Zheng, F.; Li, Y.; Zhang, Z.; Jia, J.; Hu, P.; Zhang, C.; Xu, H. Novel strategy with an eco-friendly polyurethane system to improve rainfastness of tea saponin for highly efficient rice blast control. J. Clean. Prod. 2020, 264, 121685. [Google Scholar] [CrossRef]
- Divya, K.; Thampi, M.; Vijayan, S.; Varghese, S.; Jisha, M. Induction of defence response in Oryza sativa L. against Rhizoctonia solani (Kuhn) by chitosan nanoparticles. Microb. Pathog. 2020, 149, 104525. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, M.; Muthukumar, S. Chitosan guar nanoparticle preparation and its in vitro antimicrobial activity towards phytopathogens of rice. Int. J. Biol. Macromol. 2020, 153, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, M.; Parthasarathy, R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 2016, 151, 321–325. [Google Scholar] [CrossRef]
- Nawaz, T.; Fahad, S.; Gu, L.; Xu, L.; Zhou, R. Harnessing Nitrogen-Fixing Cyanobacteria for Sustainable Agriculture: Opportunities, Challenges, and Implications for Food Security. Nitrogen 2025, 6, 16. [Google Scholar] [CrossRef]
- Nawaz, T.; Gu, L.; Fahad, S.; Saud, S.; Bleakley, B.; Zhou, R. Exploring Sustainable Agriculture with Nitrogen-Fixing Cyanobacteria and Nanotechnology. Molecules 2024, 29, 2534. [Google Scholar] [CrossRef]
- Hafeez, R.; Guo, J.; Ahmed, T.; Ibrahim, E.; ALI, M.A.; Rizwan, M.; Ijaz, M.; An, Q.; Wang, Y.; Wang, J.; et al. Integrative transcriptomic and metabolomic analyses reveals the toxicity and mechanistic insights of bioformulated chitosan nanoparticles against Magnaporthe oryzae. Chemosphere 2024, 356, 141904. [Google Scholar] [CrossRef]
- Sanches, P.H.G.; de Melo, N.C.; Porcari, A.M.; de Carvalho, L.M. Integrating Molecular Perspectives: Strategies for Comprehensive Multi-Omics Integrative Data Analysis and Machine Learning Applications in Transcriptomics, Proteomics, and Metabolomics. Biology 2024, 13, 848. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Yu, C.; Li, J.; Sun, D.; Wang, J.; Mmby, M.; Li, J.; You, H.; He, S. A smart dual-responsive nanoplatform for delivery of prochloraz for the control of rice blast disease. Adv. Agrochem 2024, 3, 328–336. [Google Scholar] [CrossRef]
- Chaud, M.; Souto, E.B.; Zielinska, A.; Severino, P.; Batain, F.; Oliveira, J., Jr.; Alves, T. Nanopesticides in Agriculture: Benefits and Challenge in Agricultural Productivity, Toxicological Risks to Human Health and Environment. Toxics 2021, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, T.M.; Qin, X.; Li, D.; Senosy, I.A.; Mmby, M.; Wan, H.; Li, J.; He, S. Pectinase-responsive carriers based on mesoporous silica nanoparticles for improving the translocation and fungicidal activity of prochloraz in rice plants. Chem. Eng. J. 2021, 404, 126440. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; Qin, X.; Guo, Z.; Li, D.; Li, C.; Wan, H.; Zhu, F.; Li, J.; Zhang, Z.; et al. Dual stimuli-responsive fungicide carrier based on hollow mesoporous silica/hydroxypropyl cellulose hybrid nanoparticles. J. Hazard. Mater. 2021, 414, 125513. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, S.; Jia, H.; Yao, Y.; Song, J.; Dong, H.; Cao, Y.; Zhu, F.; Huo, Z. Pectin functionalized metal-organic frameworks as dual-stimuli-responsive carriers to improve the pesticide targeting and reduce environmental risks. Colloids Surf. B Biointerfaces 2022, 219, 112796. [Google Scholar] [CrossRef]
- Tan, N.; Yuan, W.; Xu, Y.; Wang, J.; Yuan, B.; Huo, H.; Qiu, W.; Zhou, Y. Migrated silicon dioxide nanoparticles activates the rice immunity for systemic resistance against two pathogens. Adv. Agrochem 2025, 4, 78–89. [Google Scholar] [CrossRef]
- Elmer, W.; White, J.C. The Future of Nanotechnology in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef]
- Liu, T.; Xu, H.; Zheng, S.; Gu, H.; Wen, D.; Shan, Y.; Jiang, G.; Dai, T. Study on the effect of rice husk ash and nano silica on the early hydration kinetic characteristics of oil well cement. Thermochim. Acta 2025, 748, 179995. [Google Scholar] [CrossRef]
- Su, Y.-C.; Lin, A.-Y.; Hu, C.-C.; Chiu, T.-C. Functionalized silver nanoparticles as colorimetric probes for sensing tricyclazole. Food Chem. 2021, 347, 129044. [Google Scholar] [CrossRef]
- Santhoshkumar, R.; Parvathy, A.H.; Soniya, E. Biocompatible silver nanoparticles as nanopriming mediators for improved rice germination and root growth: A transcriptomic perspective. Plant Physiol. Biochem. 2024, 210, 108645. [Google Scholar] [CrossRef]
- Asgari, F.; Majd, A.; Jonoubi, P.; Najafi, F. Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L.). Plant Physiol. Biochem. 2018, 127, 152–160. [Google Scholar] [CrossRef]
- Li, D.; Li, T.; Yang, X.; Wang, H.; Chu, J.; Dong, H.; Lu, P.; Tao, J.; Cao, P.; Jin, J.; et al. Carbon nanosol promotes plant growth and broad-spectrum resistance. Environ. Res. 2024, 251, 118635. [Google Scholar] [CrossRef]
- Yuan, S.; Hou, Y.; Liu, S.; Ma, Y. A Comparative Study on Rice Husk, as Agricultural Waste, in the Production of Silica Nanoparticles via Different Methods. Materials 2024, 17, 1271. [Google Scholar] [CrossRef] [PubMed]
- Baraketi, S.; Khwaldia, K. Nanoparticles from agri-food by-products: Green technology synthesis and application in food packaging. Curr. Opin. Green Sustain. Chem. 2024, 49, 100953. [Google Scholar] [CrossRef]
- Al Jabri, H.; Saleem, M.H.; Rizwan, M.; Hussain, I.; Usman, K.; Alsafran, M. Zinc Oxide Nanoparticles and Their Biosynthesis: Overview. Life 2022, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawy, M.; Alotaibi, M.O.; Li, J.; Mahmoud, E.; Ghoneim, A.M.; Ramadan, M.S.; Shabana, M. Effect of Nano-Zinc Oxide, Rice Straw Compost, and Gypsum on Wheat (Triticum aestivum L.) Yield and Soil Quality in Saline–Sodic Soil. Nanomaterials 2024, 14, 1450. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Singh, R.; Harakeh, S.; Teklemariam, A.D.; Tayeb, H.H.; Deen, P.R.; Srivastava, U.C.; Srivastava, M. Green synthesis of nanostructures from rice straw food waste to improve the antimicrobial efficiency: New insight. Int. J. Food Microbiol. 2022, 386, 110016. [Google Scholar] [CrossRef]
- Liang, L.; Cui, M.; Zhang, M.; Zheng, P.; Deng, Z.; Gao, S.; Wang, X.; Zhang, X.; Wang, C.; Liu, Y.; et al. Nanoparticles’ interference in the evaluation of in vitro toxicity of silver nanoparticles. RSC Adv. 2015, 5, 67327–67334. [Google Scholar] [CrossRef]
- Saritha, G.N.G.; Anju, T.; Kumar, A. Nanotechnology—Big impact: How nanotechnology is changing the future of agriculture? J. Agric. Food Res. 2022, 10, 100457. [Google Scholar] [CrossRef]
- Sonawane, H.; Shelke, D.; Chambhare, M.; Dixit, N.; Math, S.; Sen, S.; Borah, S.N.; Islam, N.F.; Joshi, S.J.; Yousaf, B.; et al. Fungi-derived agriculturally important nanoparticles and their application in crop stress management—Prospects and environmental risks. Environ. Res. 2022, 212, 113543. [Google Scholar] [CrossRef] [PubMed]
- Hajano, J.-U.-D.; Lodhi, A.M.; Pathan, M.A.; Ali, M.; Serwar Shah, G. In-vitro evaluation of fungicides, plant extracts and bio-controlagents against rice blast pathogen magnaporthe oryzae couch. In Pak. J. Bot. 2012, 44, 1775–1778. [Google Scholar]
- Khan, M.A.I.; Ali, M.A.; Kawasaki-Tanaka, A.; Hayashi, N.; Yanagihara, S.; Obara, M.; Mia, M.A.T.; Latif, M.A.; Fukuta, Y. Diversity and Distribution of Rice Blast (Pyricularia oryzae Cavara) Races in Bangladesh. Plant Dis. 2016, 100, 2025–2033. [Google Scholar] [CrossRef]
- Perumal, A.B.; Li, X.; Su, Z.; He, Y. Preparation and characterization of a novel green tea essential oil nanoemulsion and its antifungal mechanism of action against Magnaporthae oryzae. Ultrason. Sonochem. 2021, 76, 105649. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Boomija, R.V.; Muthukumar, S.; Gandhi, M.; Salma, S.; Prinsha, T.K.; Rengasamy, B. Green synthesis of chitosan nanoparticles using tea extract and its antimicrobial activity against economically important phytopathogens of rice. Sci. Rep. 2024, 14, 7381. [Google Scholar] [CrossRef]
- Souleymane, O.; Itolou, K.A.; Sylvain, Z.; Abdoulaye, S.; Kadidia, K. Efficacy of Essential Oil Formulations in Rice Seed Treatment Against Magnaporthe oryzae B.C Couch, a Rice Blast Pathogen. Am. J. Biosci. 2024, 12, 75–79. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, Q.; Rui, Y. The impact of nanomaterials on plant health: A review of exposure, toxicity, and control. Environ. Sci. Nano 2025, 12, 2965–2982. [Google Scholar] [CrossRef]
- Tang, Y.; Gao, C.; Zhang, Y.; Tang, X. The microstructure and physiochemical stability of Pickering emulsions stabilized by chitosan particles coating with sodium alginate: Influence of the ratio between chitosan and sodium alginate. Int. J. Biol. Macromol. 2021, 183, 1402–1409. [Google Scholar] [CrossRef]
- Ma, C.; Li, G.; Xu, W.; Qu, H.; Zhang, H.; Noruzi, E.B.; Li, H. Recent Advances in Stimulus-Responsive Nanocarriers for Pesticide Delivery. J. Agric. Food Chem. 2024, 72, 8906–8927. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Wu, H.; Zhang, Y.; Kang, J.; Dong, A.; Liang, W. Advances in stimuli-responsive systems for pesticides delivery: Recent efforts and future outlook. J. Control. Release 2022, 352, 288–312. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Das, P.; Chunduri, R.; Kumar, D.; Dulta, K.; Kaushal, A.; Gupta, S.; Rj, S.; Yadav, A.N.; Nagraik, R.; et al. Nanocomposite-based agricultural delivery systems: A sustainable approach to enhanced crop productivity and soil health. J. Nanopart. Res. 2025, 27, 110. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Suwanchaikasem, P.; Idnurm, A.; Selby-Pham, J.; Walker, R.; Boughton, B.A. The Impacts of Chitosan on Plant Root Systems and Its Potential to be Used for Controlling Fungal Diseases in Agriculture. J. Plant Growth Regul. 2024, 43, 3424–3445. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).