Current Developments of Iron Oxide Nanomaterials as MRI Theranostic Agents for Pancreatic Cancer
Abstract
1. Introduction
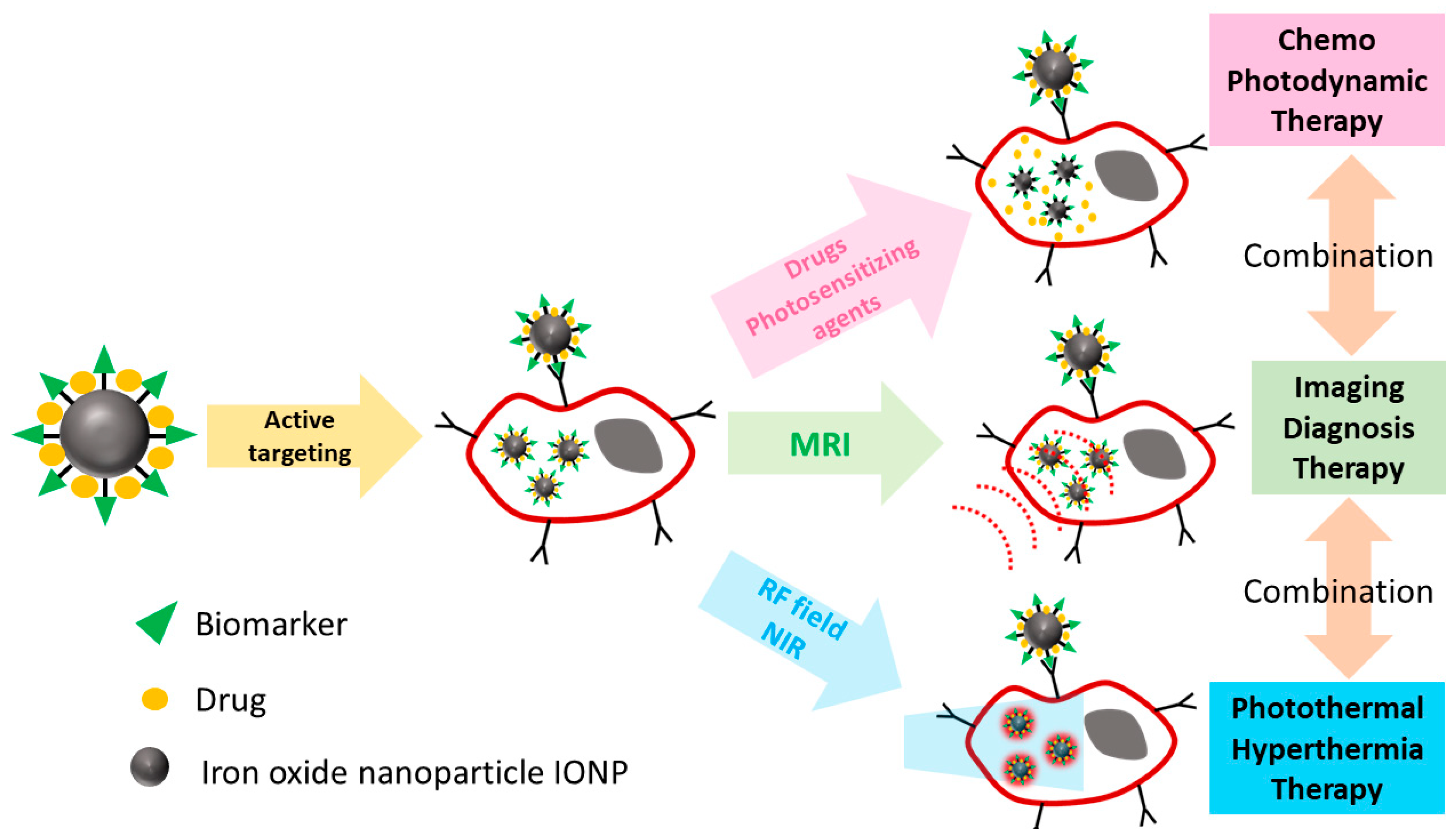
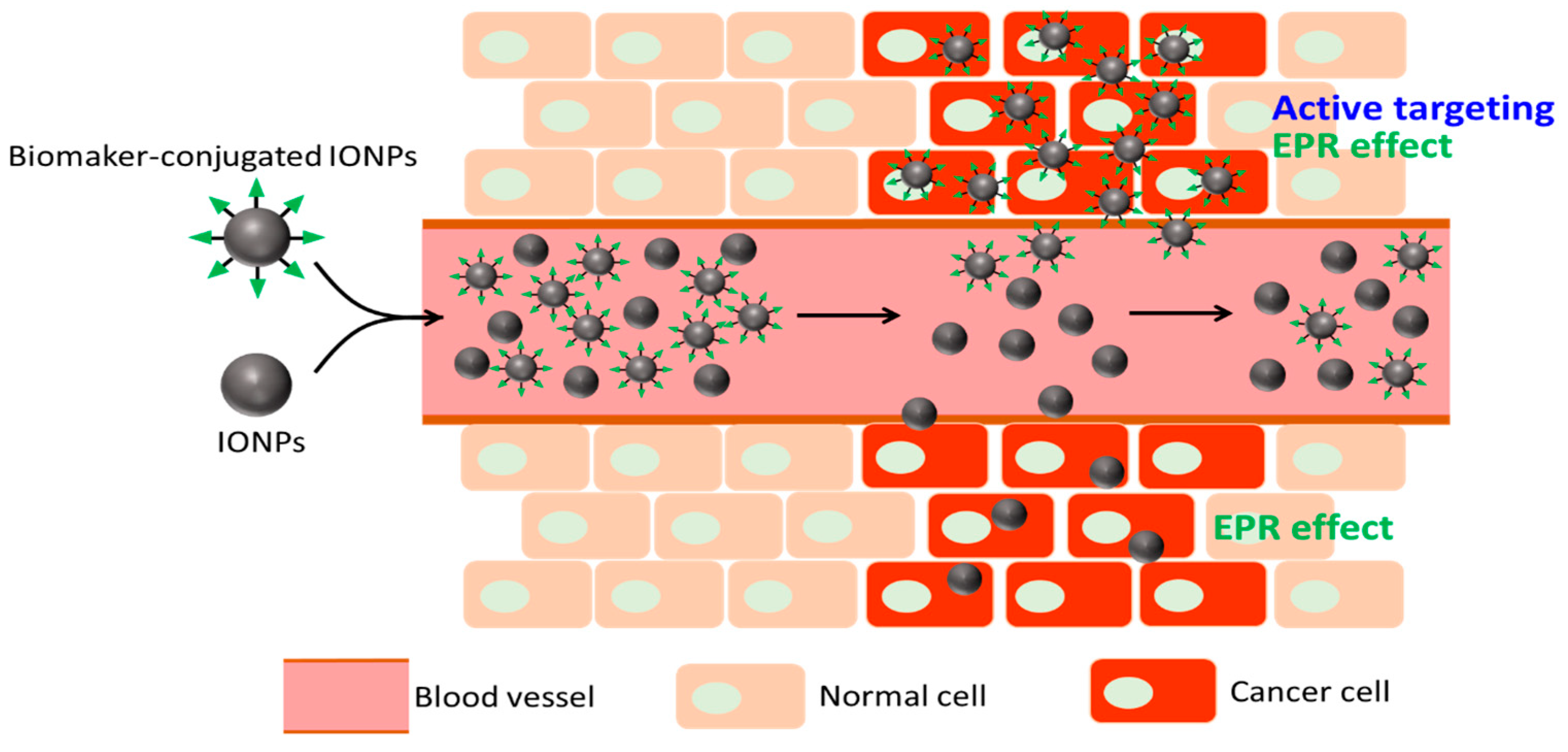
2. Iron Oxide Nanoparticles (NPs) as Theranostic Agents for MR Imaging (MRI) and Pancreatic Ductal Carcinoma (PDAC) Treatment
| Name | Short Name | Size (nm) | Coating | Blood Half Life in Patient | r1 (mmol−1s−1) | r2 (mmol−1s−1) | Type of Contrast | Clinical Dose | Application |
|---|---|---|---|---|---|---|---|---|---|
| Ferumoxide | AMI-258 | 120–180 | Dextran | 10 min | 24 | 100–160 | Negative | 30 μmol Fe/kg | Liver/spleen imaging |
| Ferumoxytol | AMI-7228 | 3 | Carboxymethyl-dextran | 14 h | 15 | 89 | Positive | 50–400 μmol Fe/kg | Angiography IV |
| Ferumoxsil | AMI-121 | 300 | Silica | Not available | 3 | 72 | Negative | 105 mg/patient | GI oral imaging |
| Ferumoxtran | AMI-227 | 30 | Dextran | 24–30 h | 22 | 44–85 | Positive /Negative | 45 μmol Fe/kg | Lymph node bone imaging |
| Feruglose | NC1011 50 | 10–20 | Carboxydrate polyethylene glycol | 2 h | 20 | 35 | Positive | 36 μmol Fe/kg | Perfusion angiography |
| Ferucarbotran SHU-555A | SHU-555A | 60–80 | Carboxydextran | 12 min | 25 | 164–177 | Negative | 8-12 μmol Fe/kg | Liver/spleen IV imaging |
| Ferucarbotran SHU-555C | SHU-555C | 20–50 | Carboxydextran | 6–8 h | 7 | 57 | Positive | 40 μmol Fe/kg | Perfusion lymph node bone marrow IV |
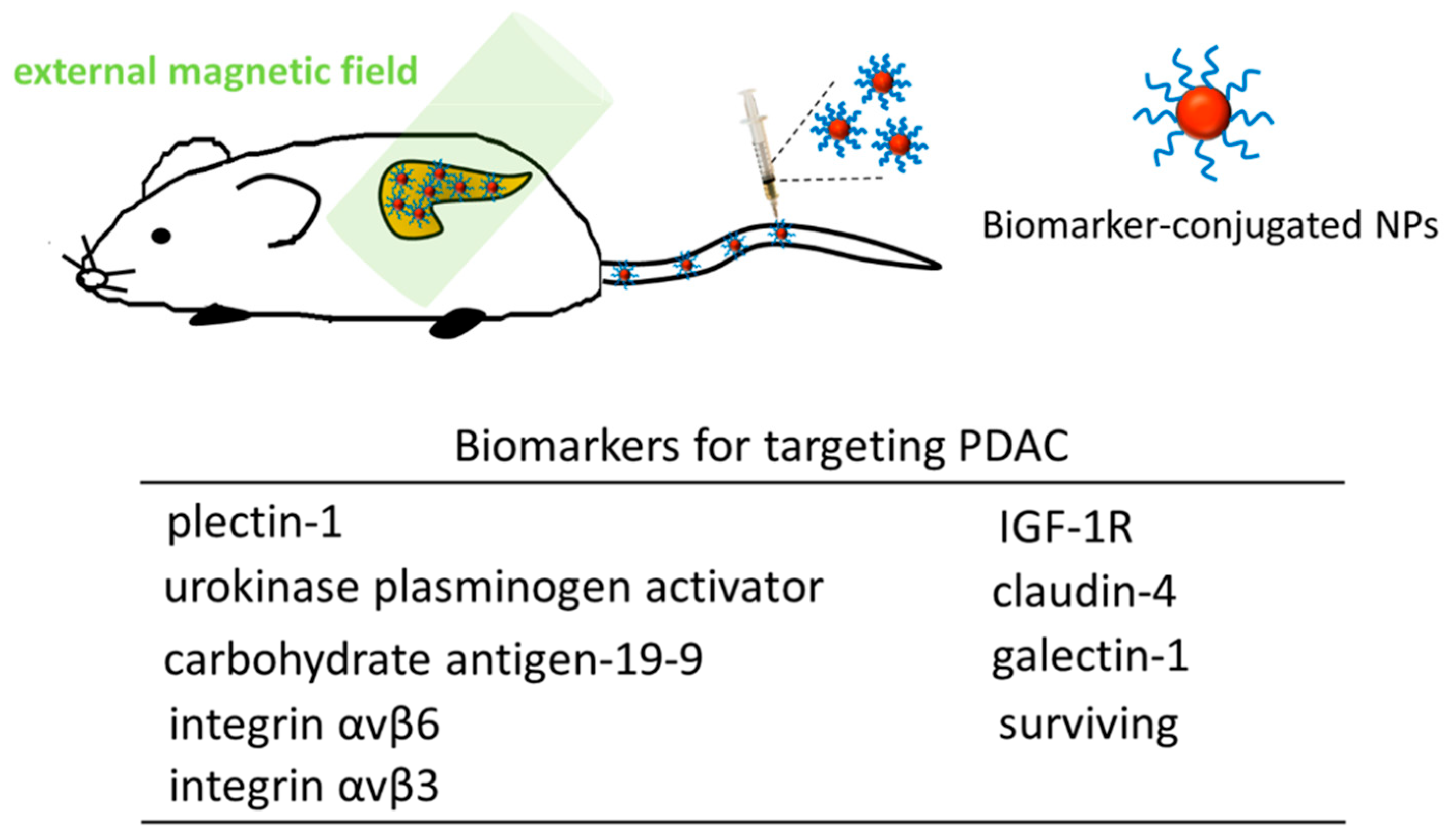
| Type of NPs | Hydrodynamic Size (or Core Size) | Biomarker on NPs | Status and Relevant Findings | Reference |
|---|---|---|---|---|
| MFN | ~39 nm | Plectin-1 targeted peptides (PTP) | About 3.13% of injected dose of MFN presented in the tumors. The MRI sensitivity was 20-fold higher than the detection threshold. | [61] |
| SPION | 29 nm (SPION: 9–15 nm) | Plectin-1 antibody | The accumulation amount of the plectin-1 antibody conjugated SPION was ~10 times greater than that of bare SPION in tumor tissue. | [15] |
| IONP | 41 nm | Bombesin (BN) peptide | The cellular uptake amount of BN-IONPs was ~1.5 times greater than that of IONPs in BxPC-3 cells. | [83] |
| USPIO | 96 nm | pancreatic cancer targeting peptide (CKAAKN) | CKAAKN-USPIO could specifically and highly internalize into CKAAKN-positive BxPC-3 cells. The CKAAKN-USPIO uptake efficiency of positive BxPC-3 cells is ~1.2 times larger than that of negative BxPC-3 cells. | [12] |
| IONP | 24 nm (IONP: 10 nm) | triple single chain antibodies (triple scAbs) | The cellular uptake (Fe amount) of IONPs-PEG-MCC triple scAbs and IONPs were separately ~1.0 pg/cell and ~0.2 pg/cell. | [84] |
| Fe3O4 NPs | 27 nm (Fe3O4 core: 9.9 nm) | Emodin (EMO) | The cumulative EMO amount of EMO-Fe3O4 NPs is ~1.5 times larger than that of EMO alone in BxPC-3 cells. | [13] |
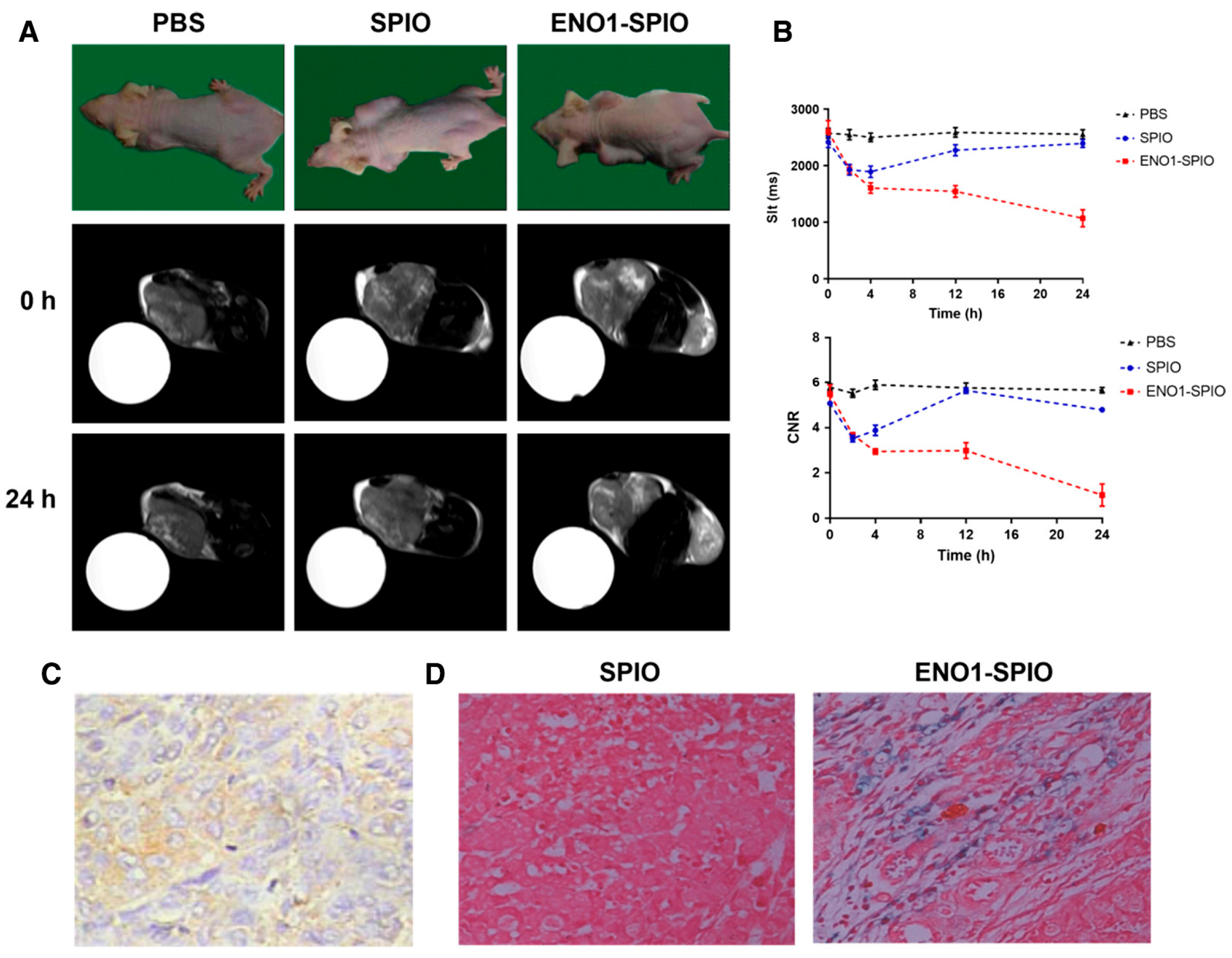
| SPION | 30 nm (SPION: 5–10 nm) | Enolase 1 (ENO1) | ENO1-SPIO nanoparticles using the ENO1 antibody can increase the efficiency of detection of PDAC by in vitro and in vivo MRI (the r1/r2 values of SPIO and ENO1-SPIO were 2.7 and 3.0 in CFPAC-1 cells, respectively) | [14] |
| SPIO | SPIO: 10 nm | Urokinase plasminogen activator receptor (uPAR) | The targeting efficiency of Cy5.5-uPAR-SPIOs was 3- to 4-fold higher than that of the mice that received free Cy5.5-peptides. | [63] |
| IONP | 65.9 nm (IONP: 22 nm) | uPAR | 1. uPAR-IONP-Gem showed approximately 50% tumor growth inhibition, which was significantly different from the free Gem and non-targeted IONP-Gem groups. 2. This work found that there was a 4.8-fold signal decrease in the tumors of mice treated with targeted ATF-IONP-Gem compared to the tumors of mice that received non-targeted IONPs. | [85] |
| IONP | 107 nm (IONP: 12 nm) | anti-CD47 antibody | 1. This work demonstrated that functionalizing the anti-CD47-IONPs greatly improves their cellular uptake by pancreatic cancer cells. 2. The anti-CD47-IONPs and anti-CD47 antibody promoted apoptosis the induction of Panc354 cells were separately ~2.1 and 1.1 (fold change to control). | [78] |
| IONP | 17 nm (IONP: 10 nm) | human insulin-like growth factor1 (IGF1) | 1. The signal intensities of the tumor area in the mice that received IGF1-IONPs were 996 and 1301, as compared to 319 and 371 in the mice that received BSA-IONPs. 2. The ex vivo images of tumors and normal organs showed the presence of high levels of optical signal in tumors injected with IGF1-IONPs (signal intensity: 898) but not BSA-IONPs (signal intensity: 398). | [11] |
3. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Casolino, R.; Braconi, C.; Malleo, G.; Paiella, S.; Bassi, C.; Milella, M.; Dreyer, S.B.; Froeling, F.E.M.; Chang, D.K.; Biankin, A.V.; et al. Reshaping preoperative treatment of pancreatic cancer in the era of precision medicine. Ann. Oncol. 2021, 32, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic Adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Song, L.N.; Zhao, R.; Tian, Y.; Wang, Z.Q. Serum exosomal hsa-let-7f-5p: A potential diagnostic biomarker for metastatic pancreatic cancer detection. World J. Gastroenterol. 2025, 31, 109500. [Google Scholar] [CrossRef] [PubMed]
- Yeo, C.J.; Cameron, J.L.; Maher, M.M.; Sauter, P.K.; Zahurak, M.L.; Talamini, M.A.; Lillemoe, K.D.; Pitt, H.A. A prospective randomized trial of pancreaticogastrostomy versus pancreaticojejunostomy after pancreaticoduodenectomy. Ann. Surg. 1995, 222, 580–592. [Google Scholar] [CrossRef]
- Henriksen, A.; Dyhl-Polk, A.; Chen, I.; Nielsen, D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat. Rev. 2019, 78, 17–30. [Google Scholar] [CrossRef]
- Yang, J.; Xu, R.; Wang, C.; Qiu, J.; Ren, B.; You, L. Early screening and diagnosis strategies of pancreatic cancer: A comprehensive review. Cancer Commun. 2021, 41, 1257–1274. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Borrebaeck, C.A. Precision diagnostics: Moving towards protein biomarker signatures of clinical utility in cancer. Nat. Rev. Cancer 2017, 17, 199–204. [Google Scholar] [CrossRef]
- Hwang, R.F.; Moore, T.; Arumugam, T.; Ramachandran, V.; Amos, K.D.; Rivera, A.; Ji, B.; Evans, D.B.; Logsdon, C.D. Cancer-Associated Stromal Fibroblasts Promote Pancreatic Tumor Progression. Cancer Res. 2008, 68, 918–926. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, W.; Uckun, F.M.; Wang, L.; Wang, Y.A.; Chen, H.; Kooby, D.; Yu, Q.; Lipowska, M.; Staley, C.A.; et al. IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer. ACS Nano 2015, 9, 7976–7991. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, N.; Zhou, Y.; Xuan, S.; Zhang, J.; Giampieri, F.; Zhang, Y.; Yang, F.; Yu, R.; Battino, M.; et al. Targeting pancreatic cancer cells with peptide-functionalized polymeric magnetic nanoparticles. Int. J. Mol. Sci. 2019, 20, 2988. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Song, L.; Tian, Y.; Zhu, L.; Guo, K.; Zhang, H.; Wang, Z. Emodin-Conjugated PEGylation of Fe3O4 Nanoparticles for FI/MRI Dual-Modal Imaging and Therapy in Pancreatic Cancer. Int. J. Nanomed. 2021, 16, 7463–7478. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, H.; Bi, R.; Gao, G.; Li, K.; Liu, H.L. ENO1-targeted superparamagnetic iron oxide nanoparticles for detecting pancreatic cancer by magnetic resonance imaging. J. Cell. Mol. Med. 2020, 24, 5751–5757. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, H.; Li, X.; Duan, N.; Hu, S.; Liu, Y.; Yue, Y.; Song, L.; Zhang, Y.; Li, D.; et al. Plectin-1 targeted dual-modality nanoparticles for pancreatic cancer imaging. EBioMedicine 2018, 30, 129–137. [Google Scholar] [CrossRef]
- Affram, K.; Smith, T.; Helsper, S.; Rosenberg, J.T.; Han, B.; Trevino, J.; Agyare, E. Comparative study on contrast enhancement of Magnevist and Magnevist-loaded nanoparticles in pancreatic cancer PDX model monitored by MRI. Cancer Nanotechnol. 2020, 11, 5. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, Z.; Chen, L.; Pan, C.; Sun, S.; Liu, C.; Li, Z.; Ren, W.; Wu, A.; Huang, P. PCN-Fe(III)-PTX nanoparticles for MRI guided high efficiency chemo-photodynamic therapy in pancreatic cancer through alleviating tumor hypoxia. Nano Res. 2020, 13, 273–281. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Malekigorji, M.; Curtis, A.D.M.; Hoskins, C. The Use of Iron Oxide Nanoparticles for Pancreatic Cancer Therapy. J. Nanomed. Res. 2014, 1, 12. [Google Scholar] [CrossRef]
- Moore, A.; Medarova, Z.; Potthast, A.; Dai, G. In Vivo Targeting of Underglycosylated MUC-1 Tumor Antigen Using a Multimodal Imaging Probe. Cancer Res. 2004, 64, 1821–1827. [Google Scholar] [CrossRef]
- Stirrat, C.G.; Newby, D.E.; Robson, J.M.J.; Jansen, M. The Use of Superparamagnetic Iron Oxide Nanoparticles to Assess Cardiac Inflammation. Curr. Cardiovasc. Imaging Rep. 2014, 7, 9263. [Google Scholar] [CrossRef]
- Barrow, M.; Taylor, A.; Murray, P.; Rosseinsky, M.J.; Adams, D.J. Design considerations for the synthesis of polymer coated iron oxide nanoparticles for stem cell labelling and tracking using MRI. Chem. Soc. Rev. 2015, 44, 6733–6748. [Google Scholar] [CrossRef]
- Saladino, G.M.; Mangarova, D.B.; Nernekli, K.; Wang, J.; Annio, G.; Varniab, Z.S.; Khatoon, Z.; Ribeiro, M.G.; Shi, Y.; Chang, E.; et al. Multimodal imaging approach to track theranostic nanoparticle accumulation in glioblastoma with magnetic resonance imaging and intravital microscopy. Nanoscale 2025, 17, 9986–9995. [Google Scholar] [CrossRef] [PubMed]
- Dehghankhold, M.; Ahmadi, F.; Nezafat, N.; Abedi, M.; Iranpour, P.; Dehghanian, A.; Koohi-Hosseinabadi, O.; Akbarizadeh, A.R.; Sobhani, Z. A versatile theranostic magnetic polydopamine iron oxide NIR laser-responsive nanosystem containing doxorubicin for chemo-photothermal therapy of melanoma. Biomater. Adv. 2024, 159, 213797. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, R.; Abnous, K.; Alibolandi, M.; Mosafer, J.; Dehghani, S.; Taghdisi, S.M.; Ramezani, M. Targeted SPION siderophore conjugate loaded with doxorubicin as a theranostic agent for imaging and treatment of colon carcinoma. Sci. Rep. 2021, 11, 13065. [Google Scholar] [CrossRef] [PubMed]
- Shirangi, A.; Mottaghitalab, F.; Dinarvand, S.; Atyabi, F. Theranostic silk sericin/SPION nanoparticles for targeted delivery of ROR1 siRNA: Synthesis, characterization, diagnosis and anticancer effect on triple-negative breast cancer. Int. J. Biol. Macromol. 2022, 221, 604–612. [Google Scholar] [CrossRef]
- Saesoo, S.; Sathornsumetee, S.; Anekwiang, P.; Treetidnipa, C.; Thuwajit, P.; Bunthot, S.; Maneeprakorn, W.; Maurizi, L.; Hofmann, H.; Rungsardthong, R.U.; et al. Characterization of liposome-containing SPIONs conjugated with anti-CD20 developed as a novel theranostic agent for central nervous system lymphoma. Colloids Surf. B: Biointerfaces 2018, 161, 497–507. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zhu, A.; Yu, N.; Xia, J.; Li, J. Dual-Targeting Biomimetic Semiconducting Polymer Nanocomposites for Amplified Theranostics of Bone Metastasis. Angew. Chem. Int. Ed. Engl. 2024, 63, e202310252. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bruix, J. Molecular targeted therapies in hepatocellular carcinoma†. Hepatology 2008, 48, 1312–1327. [Google Scholar] [CrossRef]
- Motzer, R.J.; Hutson, T.E.; Glen, H.; Michaelson, M.D.; Molina, A.; Eisen, T.; Jassem, J.; Zolnierek, J.; Maroto, J.P.; Mellado, B.; et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015, 16, 1473–1482, Erratum in: Lancet Oncol. 2016, 17, e270. https://doi.org/10.1016/S1470-2045(16)30233-9; Erratum in: Lancet Oncol. 2018, 19, e509. https://doi.org/10.1016/S1470-2045(18)30672-7. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W.; Tang, G.; Wang, H.; Wu, M.; Yu, W.; Zhou, Z.; Mou, Y.; Liu, X. Targeted Codelivery of Docetaxel and Atg7 siRNA for Autophagy Inhibition and Pancreatic Cancer Treatment. ACS Appl. Bio Mater. 2019, 2, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kievit, F.M.; Sham, J.G.; Jeon, M.; Stephen, Z.R.; Bakthavatsalam, A.; Park, J.O.; Zhang, M. Iron-Oxide-Based Nanovector for Tumor Targeted siRNA Delivery in an Orthotopic Hepatocellular Carcinoma Xenograft Mouse Model. Small 2016, 12, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Nejadnik, H.; Daldrup-Link, H.E. Next-generation superparamagnetic iron oxide nanoparticles for cancer theranostics. Drug Discov. Today 2017, 22, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Philip, P.A.; Lacy, J.; Portales, F.; Sobrero, A.; Pazo-Cid, R.; Mozo, J.L.M.; Kim, E.J.; Dowden, S.; Zakari, A.; Borg, C.; et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): A multicentre, open-label phase 2 study. Lancet Gastroenterol. Hepatol. 2020, 5, 285–294. [Google Scholar] [CrossRef]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar] [CrossRef]
- Green, M.R.; Manikhas, G.M.; Orlov, S.; Afanasyev, B.; Makhson, A.M.; Bhar, P.; Hawkins, M.J. Abraxane, a novel Cremophor-free, albumin-bound particle form of paclitaxel for the treatment of advanced non-small-cell lung cancer. Ann. Oncol. 2006, 17, 1263–1268. [Google Scholar] [CrossRef]
- Yang, X.-Y.; Lu, Y.-F.; Xu, J.-X.; Du, Y.-Z.; Yu, R.-S. Recent Advances in Well-Designed Therapeutic Nanosystems for the Pancreatic Ductal Adenocarcinoma Treatment Dilemma. Molecules 2023, 28, 1506. [Google Scholar] [CrossRef]
- Xie, J.; Liu, G.; Eden, H.S.; Ai, H.; Chen, X. Surface-Engineered Magnetic Nanoparticle Platforms for Cancer Imaging and Therapy. Acc. Chem. Res. 2011, 44, 883–892. [Google Scholar] [CrossRef]
- Ahmed, N.; Fessi, H.; Elaissari, A. Theranostic applications of nanoparticles in cancer. Drug Discov. Today 2012, 17, 928–934. [Google Scholar] [CrossRef]
- Hu, X.; Xia, F.; Lee, J.; Li, F.; Lu, X.; Zhuo, X.; Nie, G.; Ling, D. Tailor-Made Nanomaterials for Diagnosis and Therapy of Pancreatic Ductal Adenocarcinoma. Adv. Sci. 2021, 8, 2002545. [Google Scholar] [CrossRef]
- El-Zahaby, S.A.; Elnaggar, Y.S.; Abdallah, O.Y. Reviewing two decades of nanomedicine implementations in targeted treatment and diagnosis of pancreatic cancer: An emphasis on state of art. J. Control. Release 2019, 293, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.F.L.; Hackenberger, C.P.R. Fluorescent labelling in living cells. Curr. Opin. Biotechnol. 2017, 48, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.Y.; Kano, M.R. Stromal barriers to nanomedicine penetration in the pancreatic tumor microenvironment. Cancer Sci. 2018, 109, 2085–2092. [Google Scholar] [CrossRef]
- Adiseshaiah, P.P.; Crist, R.M.; Hook, S.S.; McNeil, S.E. Nanomedicine strategies to overcome the pathophysiological barriers of pancreatic cancer. Nat. Rev. Clin. Oncol. 2016, 13, 750–765. [Google Scholar] [CrossRef]
- Meng, H.; Nel, A.E. Use of nano engineered approaches to overcome the stromal barrier in pancreatic cancer. Adv. Drug Deliv. Rev. 2018, 130, 50–57. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Cuevas, C.; Chang, A.E.; Goel, V.K.; Von Hoff, D.D.; Hingorani, S.R. Enzymatic Targeting of the Stroma Ablates Physical Barriers to Treatment of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2012, 21, 418–429. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Hubner, R.A.; Siveke, J.T.; Von Hoff, D.D.; Belanger, B.; de Jong, F.A.; Mirakhur, B.; Chen, L.-T. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer 2019, 108, 78–87. [Google Scholar] [CrossRef]
- Woo, W.; Carey, E.T.; Choi, M. Spotlight on liposomal irinotecan for metastatic pancreatic cancer: Patient selection and perspectives. Onco Targets Ther. 2019, 12, 1455–1463. [Google Scholar] [CrossRef]
- Dijke, P.T.; Arthur, H.M. Extracellular control of TGFβ signalling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.R.; Bae, Y.; Iwata, C.; Morishita, Y.; Yashiro, M.; Oka, M.; Fujii, T.; Komuro, A.; Kiyono, K.; Kaminishi, M.; et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-β signaling. Proc. Natl. Acad. Sci. USA 2007, 104, 3460–3465. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Teesalu, T.; Prakash Karmali, P.; Ramana Kotamraju, V.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Couvreur, P.; Reddy, L.H.; Mangenot, S.; Poupaert, J.H.; Desmaële, D.; Lepêtre-Mouelhi, S.; Pili, B.; Bourgaux, C.; Amenitsch, H.; Ollivon, M. Discovery of new hexagonal supramolecular nanostructures formed by squalenoylation of an anticancer nucleoside analogue. Small 2008, 4, 247–253. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Wang, M.; Liu, K.; Hoover, A.R.; Li, M.; Towner, R.A.; Mukherjee, P.; Zhou, F.; Qu, J.; et al. Synergistic interventional photothermal therapy and immunotherapy using an iron oxide nanoplatform for the treatment of pancreatic cancer. Acta Biomater. 2022, 138, 453–462. [Google Scholar] [CrossRef]
- Salvanou, E.A.; Kolokithas-Ntoukas, A.; Liolios, C.; Xanthopoulos, S.; Paravatou-Petsotas, M.; Tsoukalas, C.; Avgoustakis, K.; Bouziotis, P. Preliminary Evaluation of Iron Oxide Nanoparticles Radiolabeled with 68Ga and 177Lu as Potential Theranostic Agents. Nanomaterials 2022, 12, 2490. [Google Scholar] [CrossRef]
- Jang, H.M.; Jung, M.H.; Lee, J.S.; Lee, J.S.; Lim, I.-C.; Im, H.; Kim, S.W.; Kang, S.-A.; Cho, W.-J.; Park, J.K. Chelator-Free Copper-64-Incorporated Iron Oxide Nanoparticles for PET/MR Imaging: Improved Radiocopper Stability and Cell Viability. Nanomaterials 2022, 12, 2791. [Google Scholar] [CrossRef]
- Duncan, Z.N.; Summerlin, D.; West, J.T.; Packard, A.T.; Morgan, D.E.; Galgano, S.J. PET/MRI for evaluation of patients with pancreatic cancer. Abdom. Radiol. 2023, 48, 3601–3609. [Google Scholar] [CrossRef]
- Irvine, D.J.; Dane, E.L. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020, 20, 321–334. [Google Scholar] [CrossRef]
- Liu, L.; Kshirsagar, P.G.; Gautam, S.K.; Gulati, M.; Wafa, E.I.; Christiansen, J.C.; White, B.M.; Mallapragada, S.K.; Wannemuehler, M.J.; Kumar, S.; et al. Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies. Theranostics 2022, 12, 1030–1060. [Google Scholar] [CrossRef]
- Kelly, K.A.; Bardeesy, N.; Anbazhagan, R.; Gurumurthy, S.; Berger, J.; Alencar, H.; DePinho, R.A.; Mahmood, U.; Weissleder, R.; Gambhir, S. Targeted Nanoparticles for Imaging Incipient Pancreatic Ductal Adenocarcinoma. PLoS Med. 2008, 5, e85. [Google Scholar] [CrossRef]
- Houghton, J.L.; Zeglis, B.M.; Abdel-Atti, D.; Aggeler, R.; Sawada, R.; Agnew, B.J.; Scholz, W.W.; Lewis, J.S. Site-specifically labeled CA19.9-targeted immunoconjugates for the PET, NIRF, and multimodal PET/NIRF imaging of pancreatic cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 15850–15855. [Google Scholar] [CrossRef]
- Yang, L.; Mao, H.; Cao, Z.; Wang, Y.A.; Peng, X.; Wang, X.; Sajja, H.K.; Wang, L.; Duan, H.; Ni, C.; et al. Molecular imaging of pancreatic cancer in an animal model using targeted multifunctional nanoparticles. Gastroenterology 2009, 136, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Neesse, A.; Hahnenkamp, A.; Griesmann, H.; Buchholz, M.; Hahn, S.A.; Maghnouj, A.; Fendrich, V.; Ring, J.; Sipos, B.; Tuveson, D.A.; et al. Claudin-4-targeted optical imaging detects pancreatic cancer and its precursor lesions. Gut 2013, 62, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- England, C.G.; Kamkaew, A.; Im, H.-J.; Valdovinos, H.F.; Sun, H.; Hernandez, R.; Cho, S.Y.; Dunphy, E.J.; Lee, D.S.; Barnhart, T.E.; et al. ImmunoPET imaging of insulin-like growth factor 1 receptor in a subcutaneous mouse model of pancreatic cancer. Mol. Pharm. 2016, 13, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, I.; Strauss, A.; Dobiasch, S.; Weis, C.; Szanyi, S.; Gil-Iceta, L.; Alonso, E.; Esparza, M.G.; Gómez-Vallejo, V.; Szczupak, B.; et al. Targeted diagnostic magnetic nanoparticles for medical imaging of pancreatic cancer. J. Control. Release 2015, 214, 76–84. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Ma, T.; Sun, X.; Shi, J.; Jia, B.; Sun, Y.; Zhan, J.; Zhang, H.; Zhu, Z.; et al. Integrin αvβ6–targeted SPECT imaging for pancreatic cancer detection. J. Nucl. Med. 2014, 55, 989–994. [Google Scholar] [CrossRef]
- Trajkovic-Arsic, M.; Mohajerani, P.; Sarantopoulos, A.; Kalideris, E.; Steiger, K.; Esposito, I.; Ma, X.; Themelis, G.; Burton, N.; Michalski, C.W.; et al. Multimodal molecular imaging of integrin αvβ3 for in vivo detection of pancreatic cancer. J. Nucl. Med. 2014, 55, 446–451. [Google Scholar] [CrossRef]
- Tong, M.; Xiong, F.; Shi, Y.; Luo, S.; Liu, Z.; Wu, Z.; Wang, Z. In vitrostudy of SPIO-labeled human pancreatic cancer cell line BxPC-3. Contrast Media Mol. Imaging 2012, 8, 101–107. [Google Scholar] [CrossRef]
- Bausch, D.; Thomas, S.; Mino-Kenudson, M.; Fernández-Del, C.C.; Bauer, T.W.; Williams, M.; Warshaw, A.L.; Thayer, S.P.; Kelly, K.A. Plectin-1 as a novel biomarker for pancreatic cancer. Clin. Cancer Res. 2011, 17, 302–309. [Google Scholar] [CrossRef]
- Zhao, S.; Yu, X.; Qian, Y.; Chen, W.; Shen, J. Multifunctional magnetic iron oxide nanoparticles: An advanced platform for cancer theranostics. Theranostics 2020, 10, 6278–6309. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.J. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.J. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J. Gastroenterol. 2015, 21, 13400. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.J.; Hussain, S.M.; Krestin, G.P. Superparamagnetic iron oxide contrast agents: Physicochemical characteristics and applications in MR imaging. Eur. Radiol. 2001, 11, 2319–2331. [Google Scholar] [CrossRef]
- Shen, Z.; Wu, A.; Chen, X. Iron Oxide Nanoparticle Based Contrast Agents for Magnetic Resonance Imaging. Mol. Pharm. 2017, 14, 1352–1364. [Google Scholar] [CrossRef]
- Ma, X.; Gong, A.; Chen, B.; Zheng, J.; Chen, T.; Shen, Z.; Wu, A. Exploring a new SPION-based MRI contrast agent with excellent water-dispersibility, high specificity to cancer cells and strong MR imaging efficacy. Colloids Surf. B: Biointerfaces 2015, 126, 44–49. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, K.; Zhao, R.; Ji, T.; Wang, X.; Yang, X.; Zhang, Y.; Cheng, K.; Liu, S.; Hao, J.; et al. Inducing enhanced immunogenic cell death with nanocarrier-based drug delivery systems for pancreatic cancer therapy. Biomaterials 2016, 102, 187–197. [Google Scholar] [CrossRef]
- Trabulo, S.; Aires, A.; Aicher, A.; Heeschen, C.; Cortajarena, A.L. Multifunctionalized iron oxide nanoparticles for selective targeting of pancreatic cancer cells. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 1597–1605. [Google Scholar] [CrossRef]
- Fernández-Barahona, I.; Muñoz-Hernando, M.; Ruiz-Cabello, J.; Herranz, F.; Pellico, J. Iron Oxide Nanoparticles: An Alternative for Positive Contrast in Magnetic Resonance Imaging. Inorganics 2020, 8, 28. [Google Scholar] [CrossRef]
- Wu, L.; Wen, W.; Wang, X.; Huang, D.; Cao, J.; Qi, X.; Shen, S. Ultrasmall Iron Oxide Nanoparticles Cause Significant Toxicity by Specifically Inducing Acute Oxidative Stress to Multiple Organs. Part. Fibre Toxicol. 2022, 19, 24. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Etxeberria, A.E.; Alhadi, A.A.; Dezfooli, S.M.; Julkapli, N.B.M.; Basirun, W.J.; Seyfoddin, A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants 2019, 9, 24. [Google Scholar] [CrossRef]
- Mesárošová, M.; Kozics, K.; Bábelová, A.; Regendová, E.; Pastorek, M.; Vnuková, D.; Buliaková, B.; Rázga, F.; Gábelová, A. The Role of Reactive Oxygen Species in the Genotoxicity of Surface-Modified Magnetite Nanoparticles. Toxicol. Lett. 2014, 226, 303–313. [Google Scholar] [CrossRef]
- Montet, X.; Weissleder, R.; Josephson, L. Imaging pancreatic cancer with a peptide−nanoparticle conjugate targeted to normal pancreas. Bioconjugate Chem. 2006, 17, 905–911. [Google Scholar] [CrossRef]
- Zou, J.; Chen, S.; Li, Y.; Zeng, L.; Lian, G.; Li, J.; Chen, S.; Huang, K.; Chen, Y. Nanoparticles modified by triple single chain antibodies for MRI examination and targeted therapy in pancreatic cancer. Nanoscale 2020, 12, 4473–4490. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, H.; Chen, J.; Hong, Z.; Liao, Y.; Zhang, Q.; Tong, H. Emodin sensitizes human pancreatic cancer cells to egfr inhibitor through suppressing stat3 signaling pathway. Cancer Manag. Res. 2019, 11, 8463–8473. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Wang, L.; Liu, H.L. ENO1 Overexpression in pancreatic cancer patients and its clinical and diagnostic significance. Gastroenterol. Res. Pract. 2018, 2018, 3842198. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, B.; Robbins, D.H.; Lewin, D.N.; Mikhitarian, K.; Graham, A.; Rumpp, L.; Glenn, T.; Gillanders, W.E.; Cole, D.J.; et al. Accurate Discrimination of Pancreatic Ductal Adenocarcinoma and Chronic Pancreatitis Using Multimarker Expression Data and Samples Obtained by Minimally Invasive Fine Needle Aspiration. Int. J. Cancer 2007, 120, 1511–1517. [Google Scholar] [CrossRef]
- Lee, G.Y.; Qian, W.P.; Wang, L.; Wang, Y.A.; Staley, C.A.; Satpathy, M.; Nie, S.; Mao, H.; Yang, L. Theranostic Nanoparticles with Controlled Release of Gemcitabine for Targeted Therapy and MRI of Pancreatic Cancer. ACS Nano 2013, 7, 2078–2089. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, F.-Y.; Tomanek, B.; Blasiak, B. Current Developments of Iron Oxide Nanomaterials as MRI Theranostic Agents for Pancreatic Cancer. J. Nanotheranostics 2025, 6, 22. https://doi.org/10.3390/jnt6030022
Cheng F-Y, Tomanek B, Blasiak B. Current Developments of Iron Oxide Nanomaterials as MRI Theranostic Agents for Pancreatic Cancer. Journal of Nanotheranostics. 2025; 6(3):22. https://doi.org/10.3390/jnt6030022
Chicago/Turabian StyleCheng, Fong-Yu, Boguslaw Tomanek, and Barbara Blasiak. 2025. "Current Developments of Iron Oxide Nanomaterials as MRI Theranostic Agents for Pancreatic Cancer" Journal of Nanotheranostics 6, no. 3: 22. https://doi.org/10.3390/jnt6030022
APA StyleCheng, F.-Y., Tomanek, B., & Blasiak, B. (2025). Current Developments of Iron Oxide Nanomaterials as MRI Theranostic Agents for Pancreatic Cancer. Journal of Nanotheranostics, 6(3), 22. https://doi.org/10.3390/jnt6030022






