Impacts of Am Aggregation on the Bulk Properties of Mixed Oxides (U, Am)O2 from First Principles
Abstract
1. Introduction
2. Methodology
3. Results and Discussion
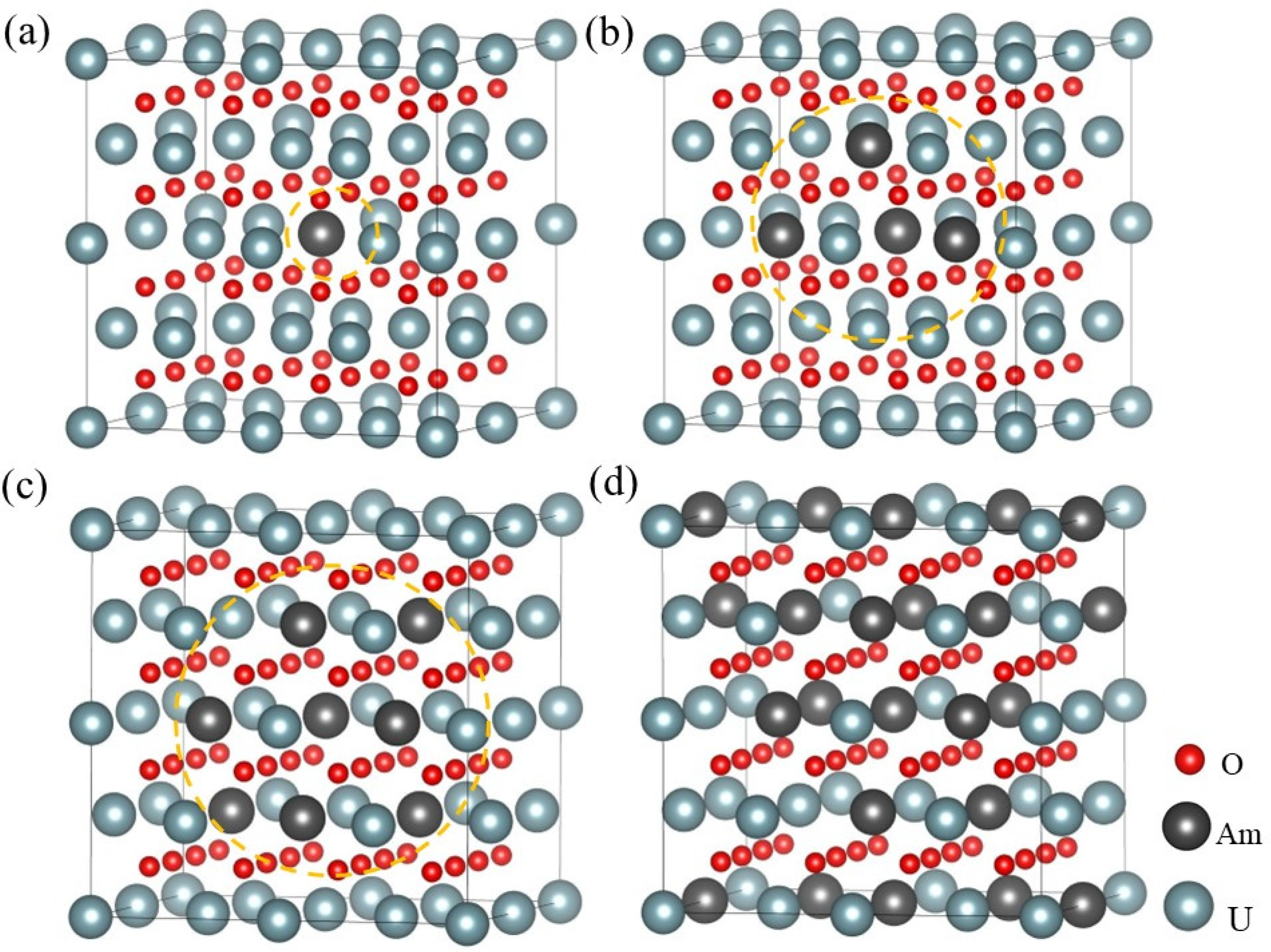
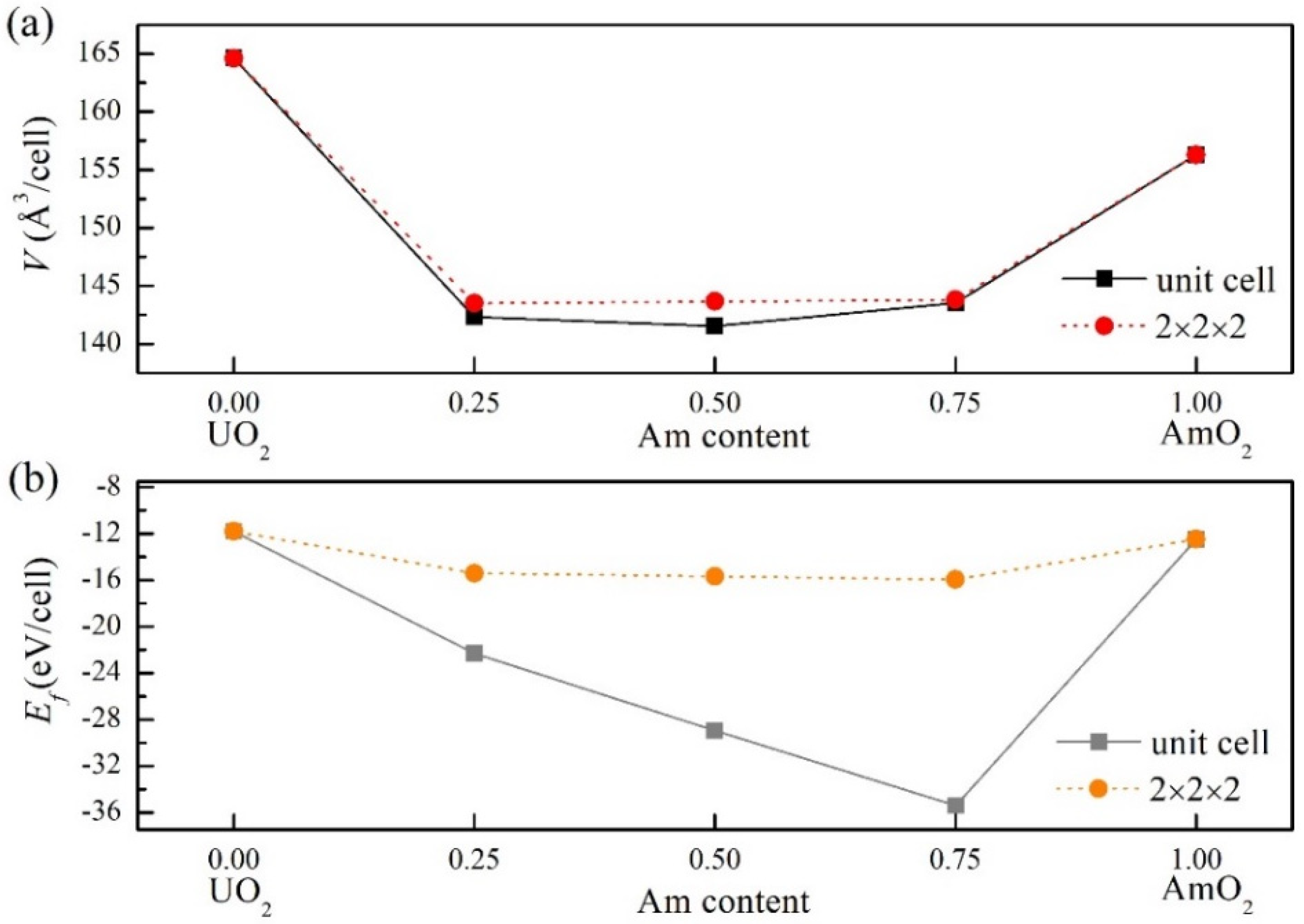
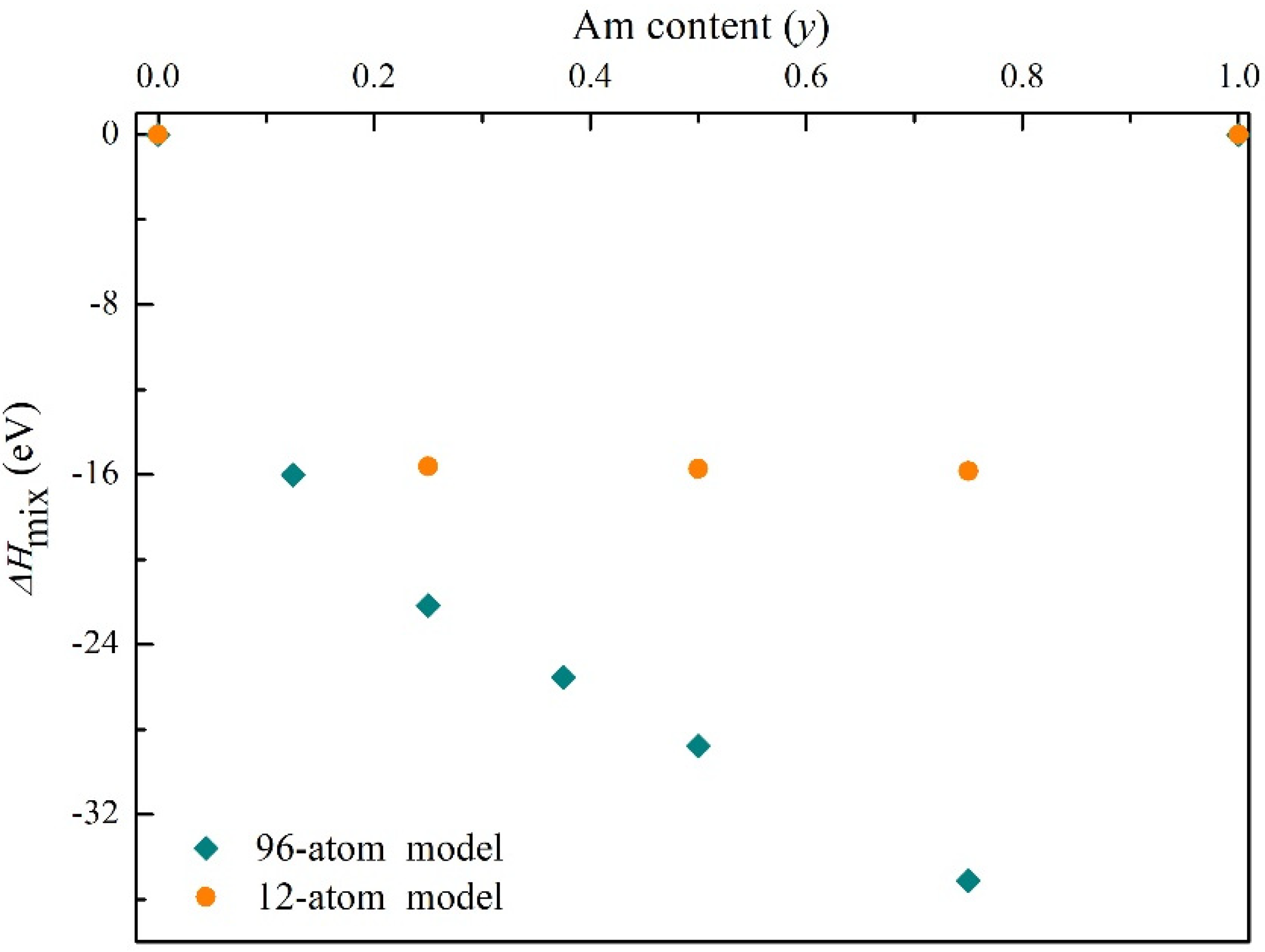
3.1. The Bulk Properties for (U, Am)O2 Using the Supercell (96-Atom) Defect Models
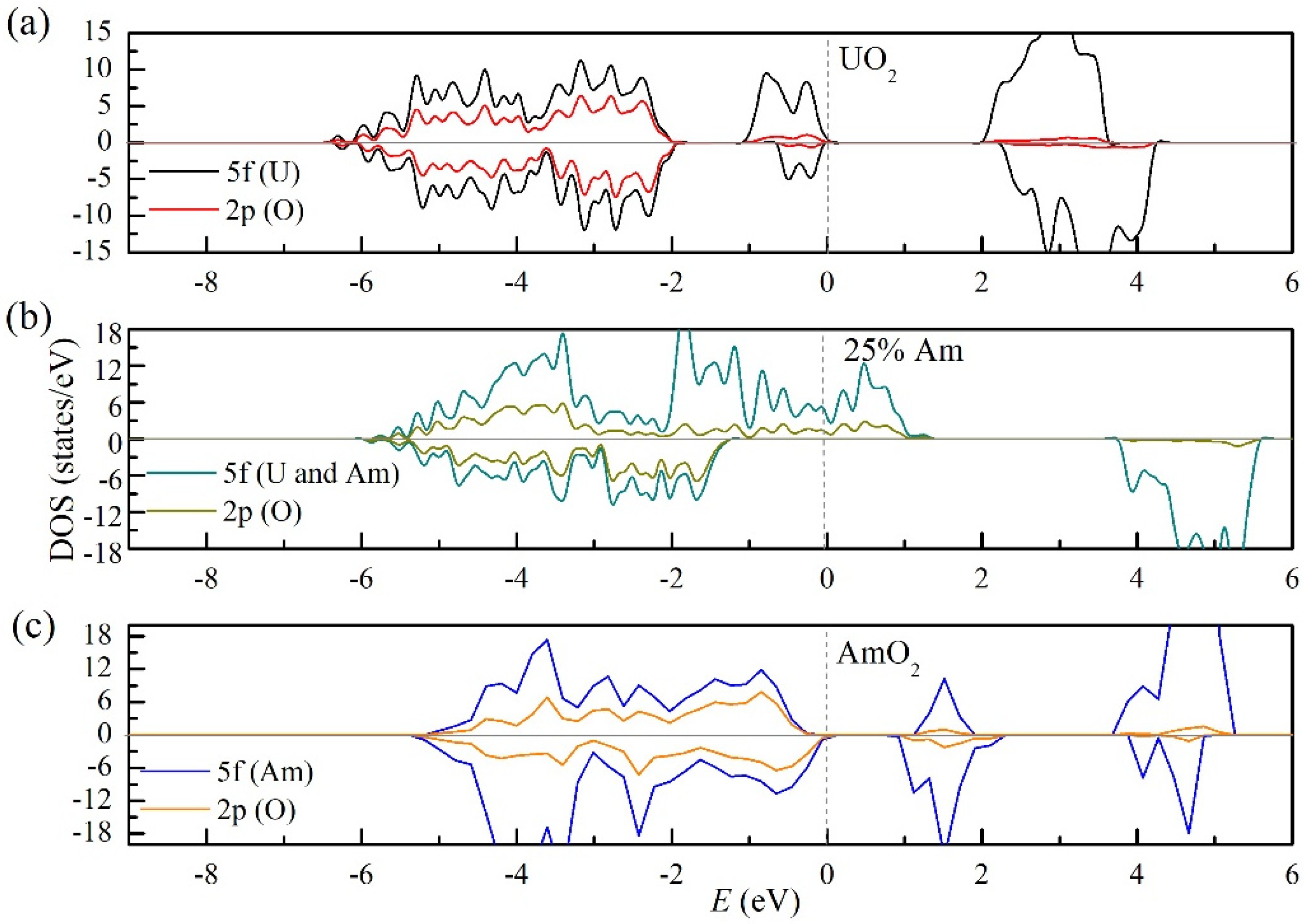
3.2. The Structure and Properties of (U, Am)O2 Using the Supercell (12-Atom) Method
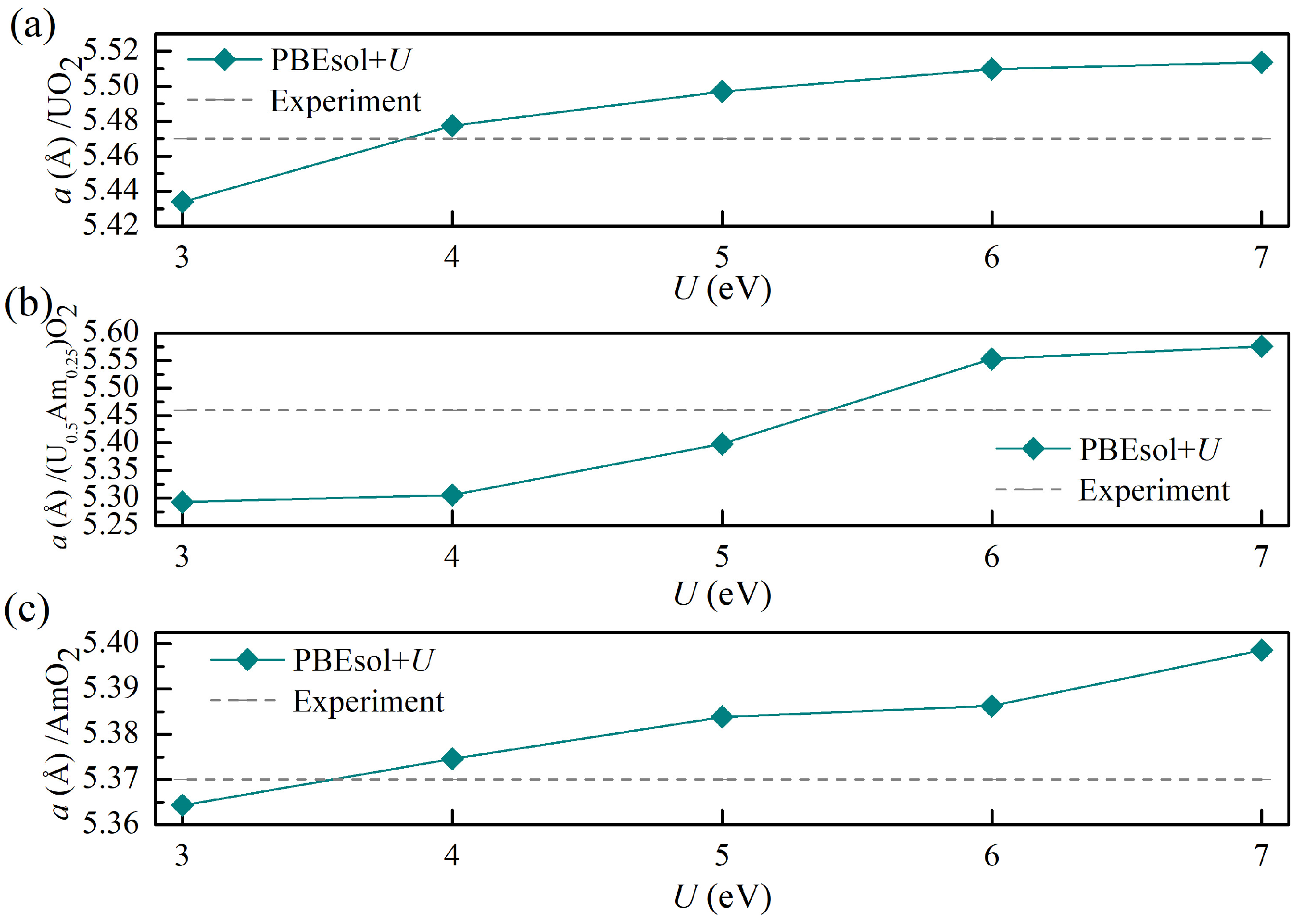
3.3. Energetic Properties for (U, Am)O2 Using PBEsol + U
3.4. Elastic Properties of the (U, Am)O2 Using PBEsol + U
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | antiferromagnetic |
| CALPHAD | computer coupling of phase diagrams and thermochemistry |
| DFT | density functional theory |
| DOS | density of states |
| FM | ferromagnetic |
| GGA | generalized gradient approximation |
| HSE | hybrid density functional |
| LDA | local density approximation |
| MOX | mixed oxides |
| PBE | Perdew–Burke–Erzenhorf |
| PBEsol | PBE for solids |
| SOC | spin–orbit coupling |
| SQS | special quasirandom structures |
| VASP | Vienna Ab initio Simulation Package |
| vdW-opt | van der Waals optimized |
References
- Kelly, J.E. Generation IV international forum: A decade of progress through international cooperation. Prog. Nucl. Energ. 2014, 77, 240–246. [Google Scholar] [CrossRef]
- Crawford, D.C.; Porter, D.L.; Hayes, S.L. Fuels for sodium-cooled fast reactors: US perspective. J. Nucl. Mater. 2007, 371, 202–231. [Google Scholar] [CrossRef]
- Parrish, R.; Aitkaliyeva, A. A review of microstructural features in fast reactor mixed oxides fuels. J. Nucl. Mater. 2018, 510, 644–660. [Google Scholar] [CrossRef]
- Dorado, B.; Garcia, P. First-principles DFT + U modeling of actinide-based alloys: Application to paramagnetic phases of UO2 and (U, Pu) mixed oxides. Phys. Rev. B 2013, 87, 195139. [Google Scholar] [CrossRef]
- Njifon, I.C.; Bertolus, M.; Hayn, R.; Michel, F. Electronic structure investigation of the bulk properties of uranium-plutonium mixed oxides (U, Pu)O2. Inorg. Chem. 2018, 57, 10974–10983. [Google Scholar] [CrossRef]
- Noutack, M.S.T.; Jomard, G.; Freyss, M.; Geneste, G. Structural, electronic and energetic properties of uranium-americium mixed oxides U1-yAmyO2 using DFT + U calculations. J. Phys. Condens. Mat. 2019, 31, 485501. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, S.C.; Gao, T.; Ao, B.Y. Theoretical prediction of some layered Pa2O5 phases: Structure and properties. RSC. Adv. 2019, 9, 31398–31405. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, S.C.; Gao, T.; Ao, B.Y. Density functional investigation of fluorite-based Pa2O5 phases: Structure and properties. Chin. J. Phys. 2020, 66, 115–122. [Google Scholar] [CrossRef]
- James, T.P.; Xavier, A.A.; Mark, S.; Nora, H.L. DFT+U study of the structures and properties of the actinide dioxides. Chin. J. Phys. 2017, 492, 269–278. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Band theory and mott insulators: Hubbard U instead of stoner I. Phys. Rev. B 1991, 44, 943–954. [Google Scholar] [CrossRef]
- Liechtenstein, A.I.; Anisimov, V.I.; Zaanen, J. Density functional theory and strong interactions: Orbital ordering in mott-hubbard insulators. Phys. Rev. B 1995, 52, 5467–5470. [Google Scholar] [CrossRef]
- Vathonne, E.; Wiktor, J.; Freyss, M.; Jomard, G.; Bertolus, M. DFT+U investigation of charged point defects and clusters in UO2. J. Phys. Condens. Matter 2014, 26, 325501. [Google Scholar] [CrossRef]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Szotek, Z.; Temmerman, W.M.; Sutton, A.P. Electronic structure and elastic properties of strongly correlated metal oxides from first principles: LSDA + U, SIC-LSDA and EELS study of UO2 and NiO. Phys. Status Solidi A 1998, 166, 429–443. [Google Scholar] [CrossRef]
- Jomard, G.; Amadon, B.; Bottin, F.; Torrent, M. Structural, thermodynamic, and electronic properties of plutonium oxides from first principles. Phys. Rev. B 2008, 78, 075125. [Google Scholar] [CrossRef]
- Noutack, M.S.T.; Geneste, G.; Jomard, G.; Freyss, M. First-principles investigation of the bulk properties of americium dioxide and sesquioxides. Phys. Rev. Mater 2019, 3, 035001. [Google Scholar] [CrossRef]
- Andersson, D.A.; Lezama, J.; Uberuaga, B.P.; Deo, C.; Conradson, S.D. Cooperativity among defect sites in AO2+x and A4O9 (A=U, Np, Pu): Density functional calculations. Phys. Rev. B 2009, 79, 024110. [Google Scholar] [CrossRef]
- Dorado, B.; Garcia, P.; Carlot, G.; Davoisne, C.; Fraczkiewicz, M.; Pasquet, B.; Freyss, M.; Valot, C.; Baldinozzi, G.; Simëone, D.; et al. First-principles calculation and experimental study of oxygen diffusion in uranium dioxide. Phys. Rev. B 2011, 83, 035126. [Google Scholar] [CrossRef]
- Wang, J.; Ewing, R.C.; Becker, U. Electronic structure and stability of hyperstoichiometric UO2+x under pressure. Phys. Rev. B 2013, 88, 024109. [Google Scholar] [CrossRef]
- Prodan, I.D.; Scuseria, G.E.; Sordo, J.A.; Kudin, K.N.; Martin, R.L. Lattice defects and magnetic ordering in plutonium oxides: A hybrid density-functional-theory study of strongly correlated materials. J. Chem. Phys. 2005, 123, 014703. [Google Scholar] [CrossRef]
- Prodan, I.D.; Scuseria, G.E.; Martin, R.L. Assessment of metageneralized gradient approximation and screened Coulomb hybrid density functionals on bulk actinide oxides. Phys. Rev. B 2006, 73, 045104. [Google Scholar] [CrossRef]
- Santini, P.; Amoretti, S.G.; Caciuffo, R.; Magnani, N.; Lander, G.H. Multipolar interactions in f-electron systems: The paradigm of actinide dioxides. Rev. Mod. Phys. 2009, 81, 807–863. [Google Scholar] [CrossRef]
- Caciuffo, R.; Amoretti, G.; Santini, P.; Lander, G.H.; Kulda, J.; Plessis, P.V.D. Magnetic excitations and dynamical Jahn-Teller distortions in UO2. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 13892–13900. [Google Scholar] [CrossRef]
- Wilkins, S.B.; Caciuffo, R.; Detlefs, C.; Rebizant, J.; Colineau, E.; Wastin, F.; Lander, G.H. Direct observation of electric-quadrupolar order in UO2. Phys. Rev. B 2006, 73, 060406. [Google Scholar] [CrossRef]
- Laskowski, R.; Madsen, G.K.H.; Blaha, P.; Schwarz, K. Magnetic structure and electric-field gradients of uranium dioxide: An Ab initio study. Phys. Rev. B 2004, 69, 140408. [Google Scholar] [CrossRef]
- Martin, P.; Grandjean, S.; Valot, C.; Carlot, G.; Ripert, M.; Blanc, P.; Hennig, C. XAS study of (U1−yPuy)O2 solid solutions. J. Alloys Compd. 2007, 444, 410–414. [Google Scholar] [CrossRef]
- Hurtgen, C.; Fuger, J. Self-irradiation effects in americium oxides. Inorg. Nucl. Chem. Lett. 1977, 13, 179–188. [Google Scholar] [CrossRef]
- Huber, E.J.; Holley, C.E. Enthalpies of formation of triuranium octaoxide and uranium dioxide. J. Chem. Thermodyn. 1969, 1, 267–272. [Google Scholar] [CrossRef]
- Guéneau, C.; Baichi, M.; Labroche, D.; Chatillon, C.; Sundman, B. Thermodynamic assessment of the uranium-oxygen system. J. Nucl. Mater. 2002, 304, 161–175. [Google Scholar] [CrossRef]
- Konings, R.J.M.; Beneš, O.; Kovács, A.; Manara, D.; Sedmidubský, D.; Gorokhov, L.; Iorish, V.S.; Yungman, V.; Shenyavskaya, E.; Osina, E. The thermodynamic properties of the f-elements and their compounds. Part 2. The lanthanide and actinide oxides. J. Phys. Chem. Ref. Data 2014, 43, 013101. [Google Scholar] [CrossRef]
| Am Content | AFM | FM | EFM–EAFM/Atom (eV) | ||||
|---|---|---|---|---|---|---|---|
| a0 (Å) | c/a | Gap (eV) | a0 (Å) | c/a | Gap (eV) | ||
| UO2 | 5.480 | 1.0 | 2.0 | 5.469 | 1.0 | 1.8 | 0.12 |
| 12.5% | 5.221 | 1.0 | Metal | 5.223 | 1.0 | Metal | 0.00 |
| 25% | 5.268 | 0.996 | Metal | 5.269 | 0.996 | Metal | 0.00 |
| 37.5% | 5.368 | 0.995 | Metal | 5.305 | 0.995 | Metal | 0.00 |
| 50% | 5.407 | 0.988 | Metal | 5.420 | 0.990 | Metal | 0.00 |
| 75% | 5.345 | 0.994 | Metal | 5.409 | 0.994 | Metal | 0.00 |
| AmO2 | 5.387 | 1.0 | 1.1 | 5.375 | 1.0 | 1.0 | 0.57 |
| Compounds | Functional | a0 (Å) | Gap (eV) | μmag (μB) | EFM–EAFM/Atom (eV) | |
|---|---|---|---|---|---|---|
| AFM | FM | AFM | AFM | |||
| UO2 | PBEsol + U | 5.480 | 5.469 | 2.1 | 2.0 | 0.12 |
| PBE + U [5] | 5.543 | 5.547 | 2.5 | |||
| Experiment [31] | 5.470 | |||||
| (U0.75Am0.25)O2 | PBEsol + U | 5.221 | 5.223 | Metal | 7.2 | 0.00 |
| (U0.5Am0.5)O2 | PBEsol + U | 5.226 | 5.227 | Metal | 7.0 | 0.00 |
| (U0.25Am0.75)O2 | PBEsol + U | 5.236 | 5.236 | Metal | 7.1 | 0.00 |
| AmO2 | PBEsol + U | 5.387 | 5.375 | 1.1 | 5.3 | 0.57 |
| Experiment [32] | 5.376 | 1.3 | ||||
| Compound | Ef (eV) | ||||
|---|---|---|---|---|---|
| Experiment | CALPHAD | PBE + U | vdW-optPBE + U | PBEsol + U | |
| UO2 | −11.24 [33] | −11.23 [34] | −10.86 [5] | −11.27 [5] | −11.78 |
| (U0.75Am0.25)O2 | −15.39 | ||||
| (U0.5Am0.5)O2 | −15.67 | ||||
| (U0.25Am0.75)O2 | −15.95 | ||||
| AmO2 | −9.51 [35] | −8.29 [21] | −10.46 | ||
| Functional | UO2 | (U0.75Am0.25)O2 | (U0.5Am0.5)O2 | (U0.25Am0.75)O2 | AmO2 | |
|---|---|---|---|---|---|---|
| C11 (GPa) | PBEsol + U | 383 | 358 | 325 | 274 | 321 |
| PBE + U [5] | 364 | 363 | ||||
| LDA + U [4] | 401 | |||||
| experiment [33] | 389 | |||||
| C12 (GPa) | PBEsol + U | 126 | 92 | 80 | 75 | 161 |
| PBE + U [5] | 112 | 102 | ||||
| LDA + U [4] | 132 | |||||
| experiment [33] | 119 | |||||
| C44 (GPa) | PBEsol + U | 72.3 | 25 | 43 | 37 | 58 |
| PBE + U [5] | 58 | 71 | ||||
| LDA + U [4] | 94 | |||||
| experiment [33] | 60 | |||||
| B0 (GPa) | PBEsol + U | 212 | 181 | 162 | 141 | 215 |
| PBE + U [5] | 196 | 189 | ||||
| LDA + U [4] | 222 | |||||
| experiment [33] | 207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Yang, Z.; Yu, X.; Gao, T. Impacts of Am Aggregation on the Bulk Properties of Mixed Oxides (U, Am)O2 from First Principles. Physics 2024, 6, 1240-1250. https://doi.org/10.3390/physics6040076
Liu T, Yang Z, Yu X, Gao T. Impacts of Am Aggregation on the Bulk Properties of Mixed Oxides (U, Am)O2 from First Principles. Physics. 2024; 6(4):1240-1250. https://doi.org/10.3390/physics6040076
Chicago/Turabian StyleLiu, Tao, Ziyi Yang, Xiaoyan Yu, and Tao Gao. 2024. "Impacts of Am Aggregation on the Bulk Properties of Mixed Oxides (U, Am)O2 from First Principles" Physics 6, no. 4: 1240-1250. https://doi.org/10.3390/physics6040076
APA StyleLiu, T., Yang, Z., Yu, X., & Gao, T. (2024). Impacts of Am Aggregation on the Bulk Properties of Mixed Oxides (U, Am)O2 from First Principles. Physics, 6(4), 1240-1250. https://doi.org/10.3390/physics6040076






