Efficient Decolourisation of Astrazon Pink Dye Using Biocarbon Derived from Prosopis juliflora Shells: Kinetics, Isotherms, and RSM-Based Optimization for Sustainable Wastewater Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Biochar
2.2. Batch Study
2.3. Instrumental Analysis
2.4. Isotherm and Kinetics Adsorption Studies
2.5. Thermodynamic Study
2.6. Full Factorial Design of Optimisation of Astrazon Pink (FG) Dye Removal
3. Results
3.1. Instrumental Analysis
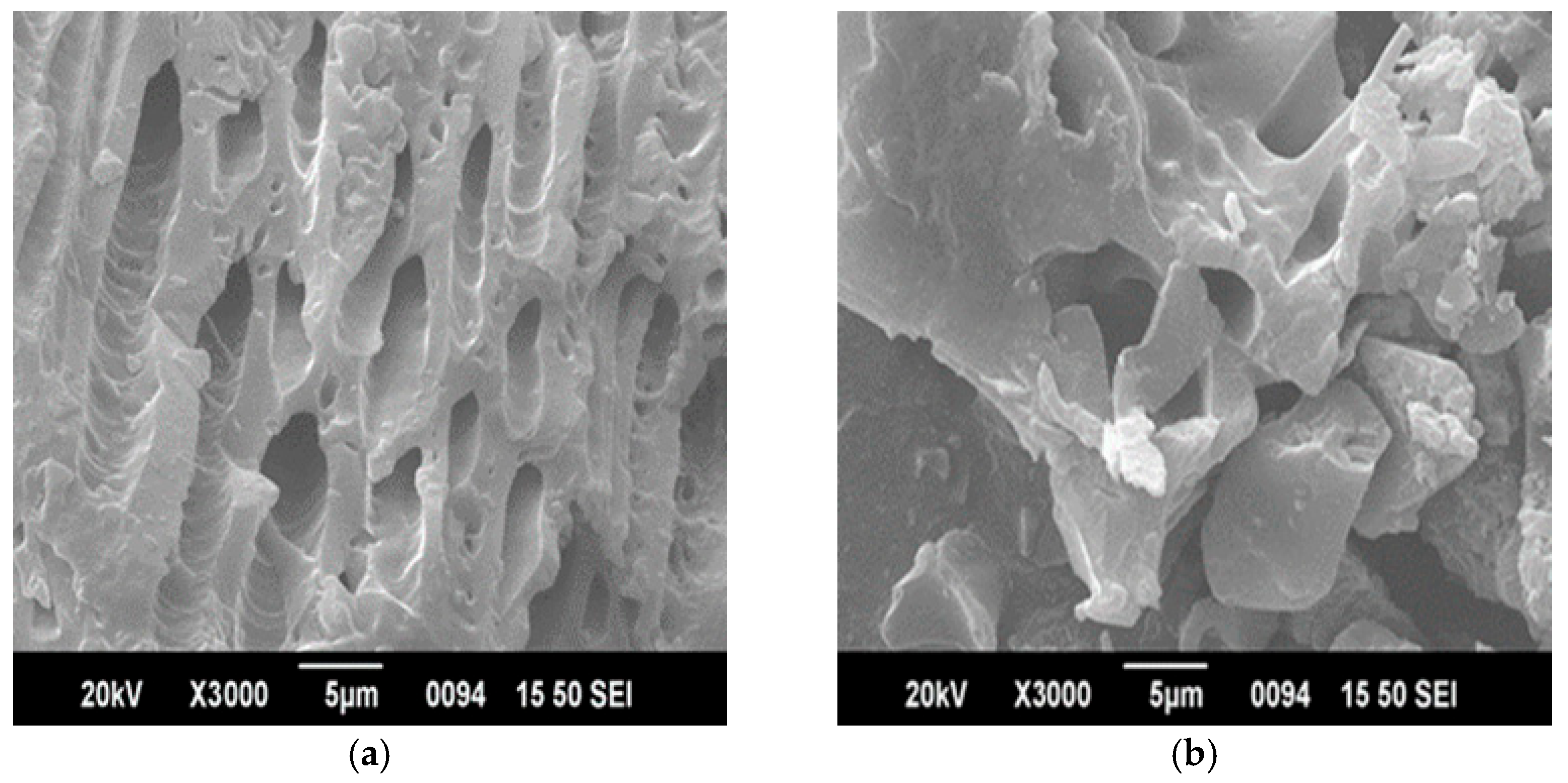
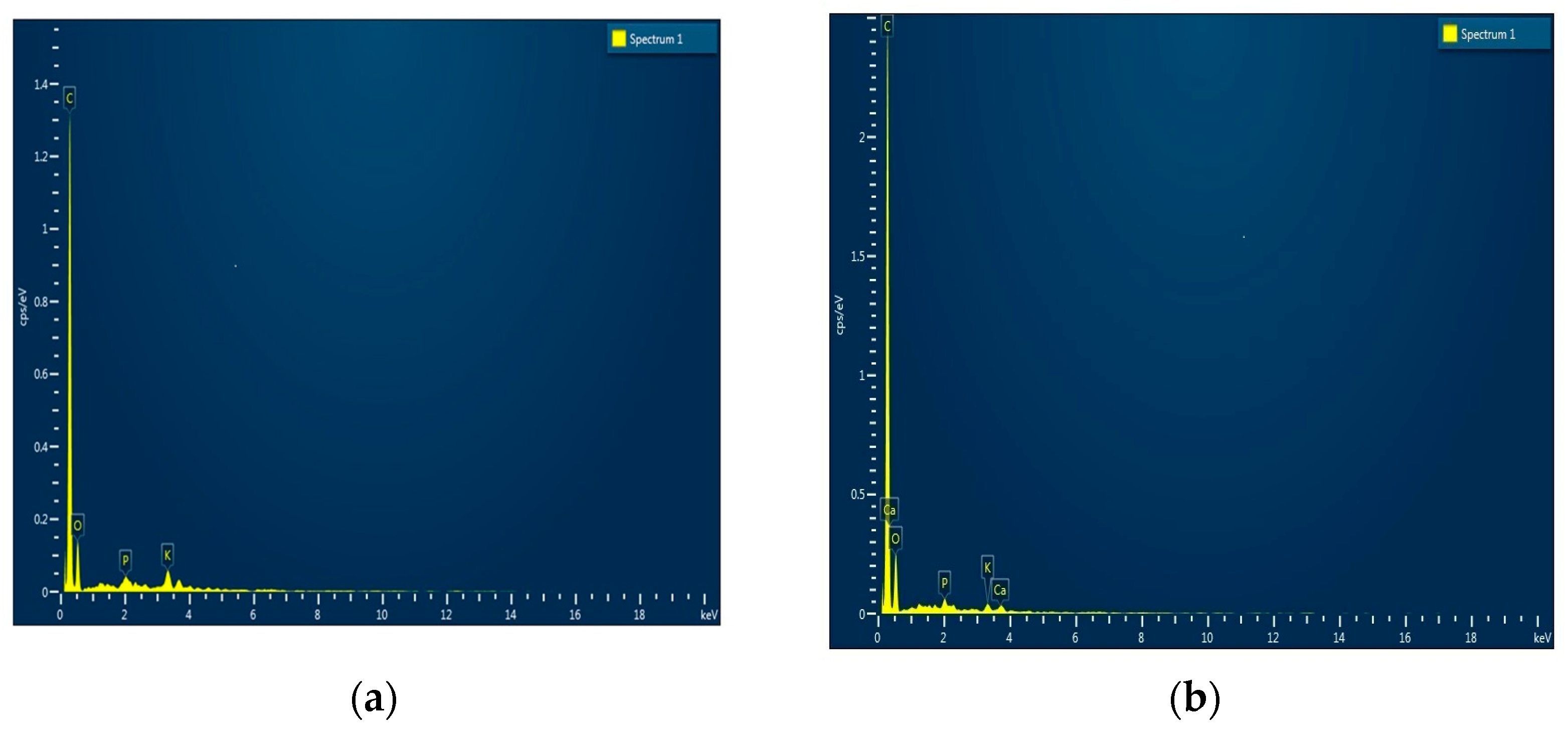
3.1.1. SEM with EDX
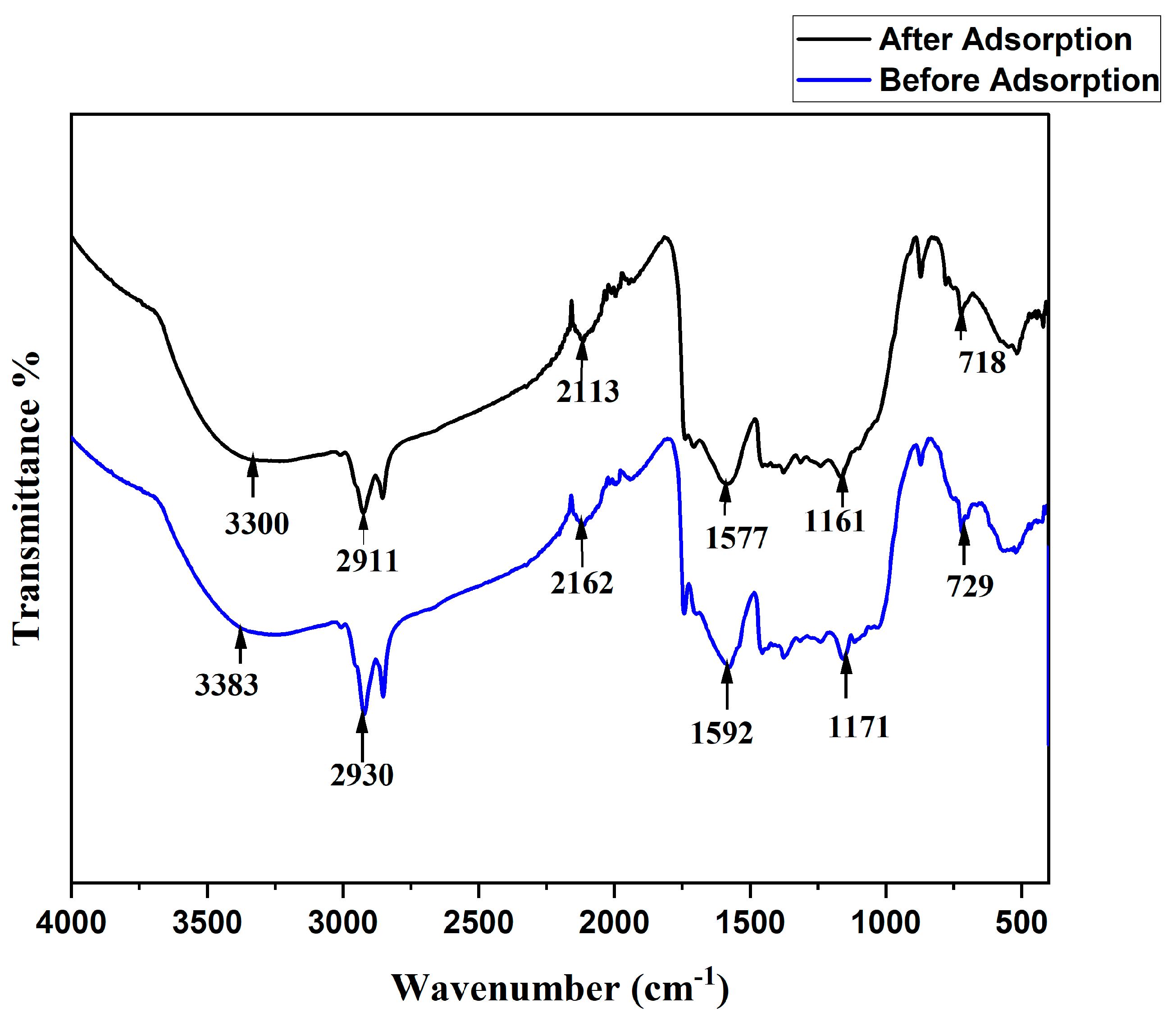
3.1.2. FT- IR Spectrum
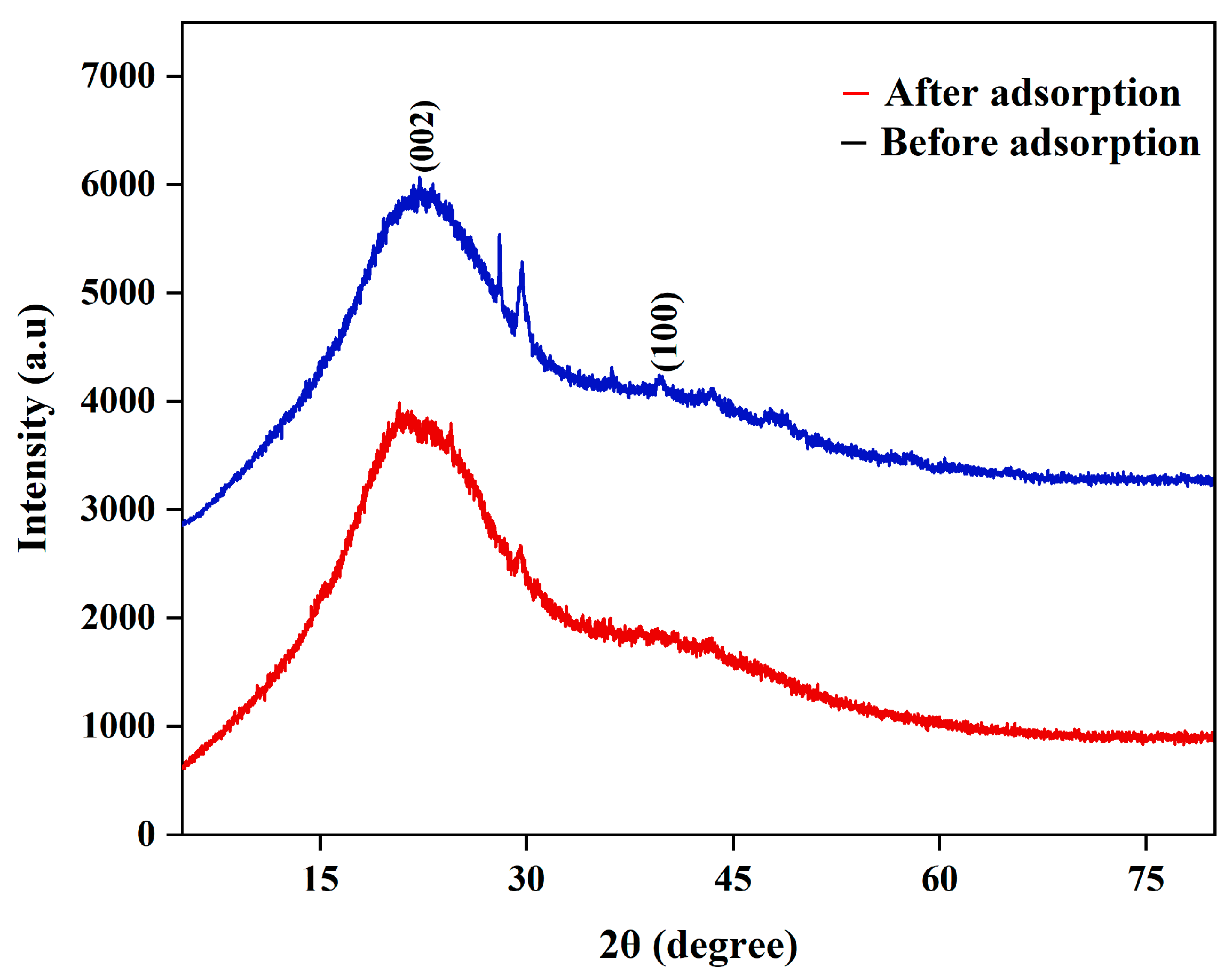
3.1.3. X-Ray Diffraction Analysis
3.2. Batch Adsorption Study
3.2.1. Effect of Dosage of Biochar
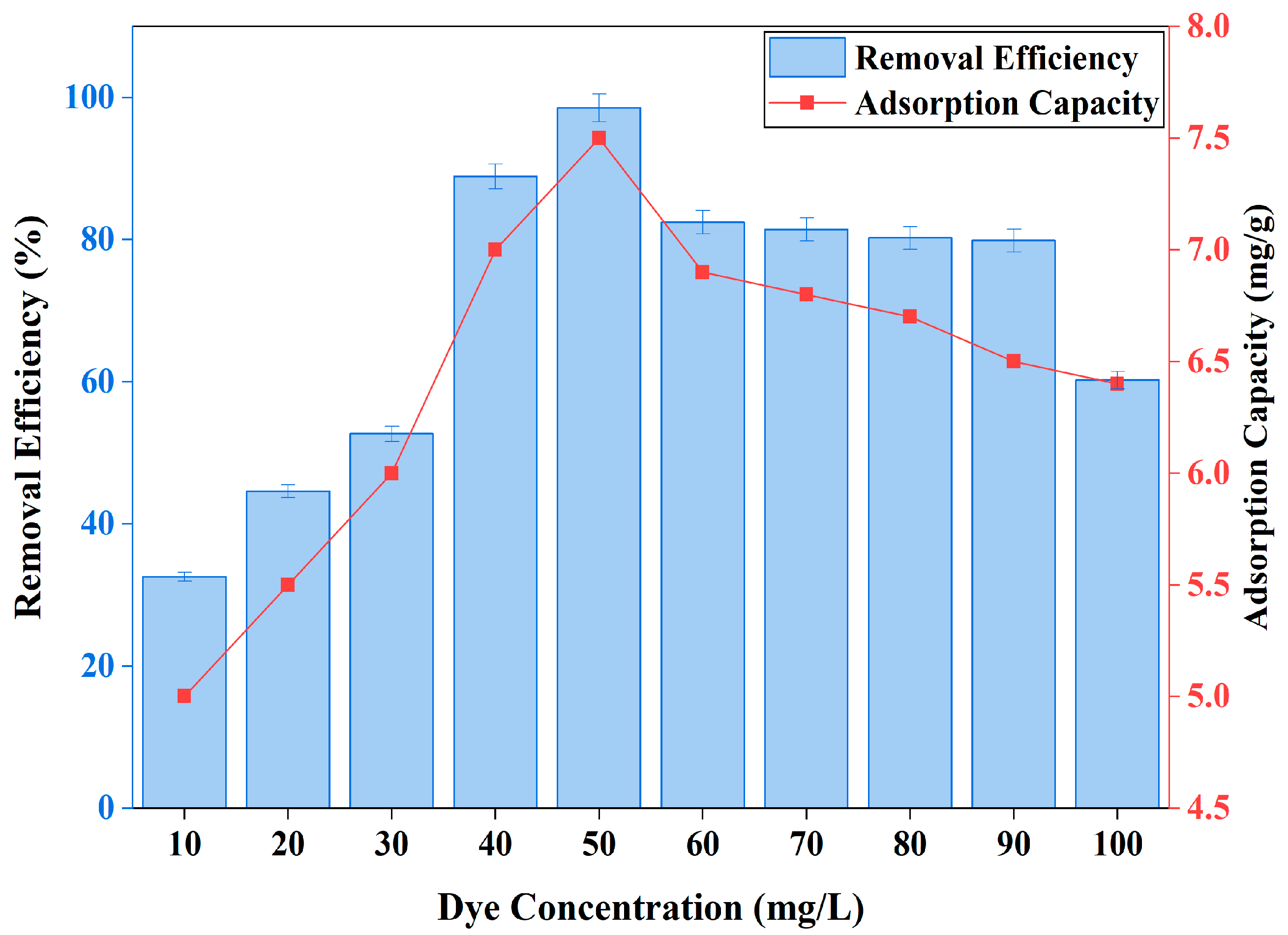
3.2.2. Effect of Concentration of Dye
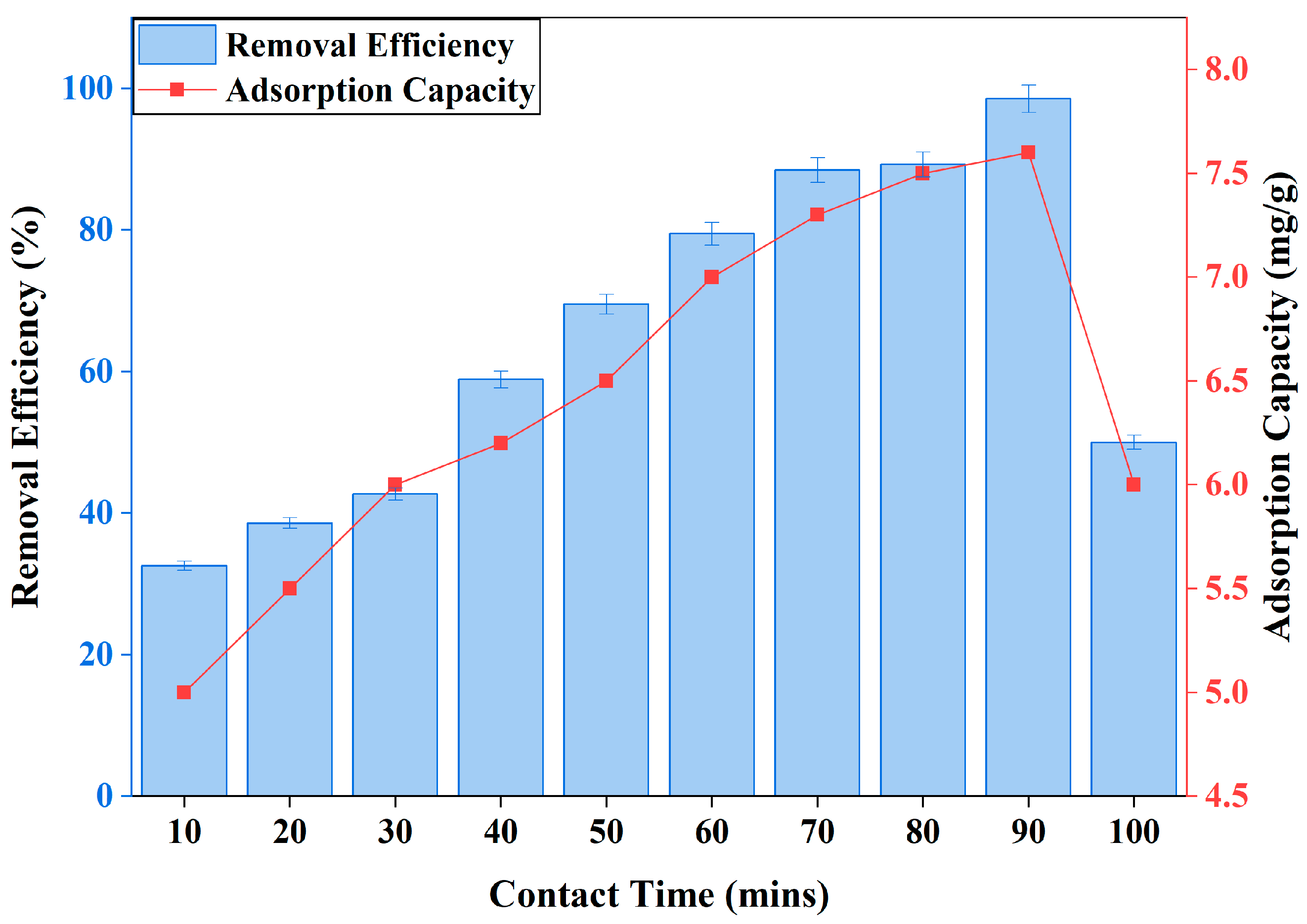
3.2.3. Effect of Time of Contact
3.2.4. Effect of pH
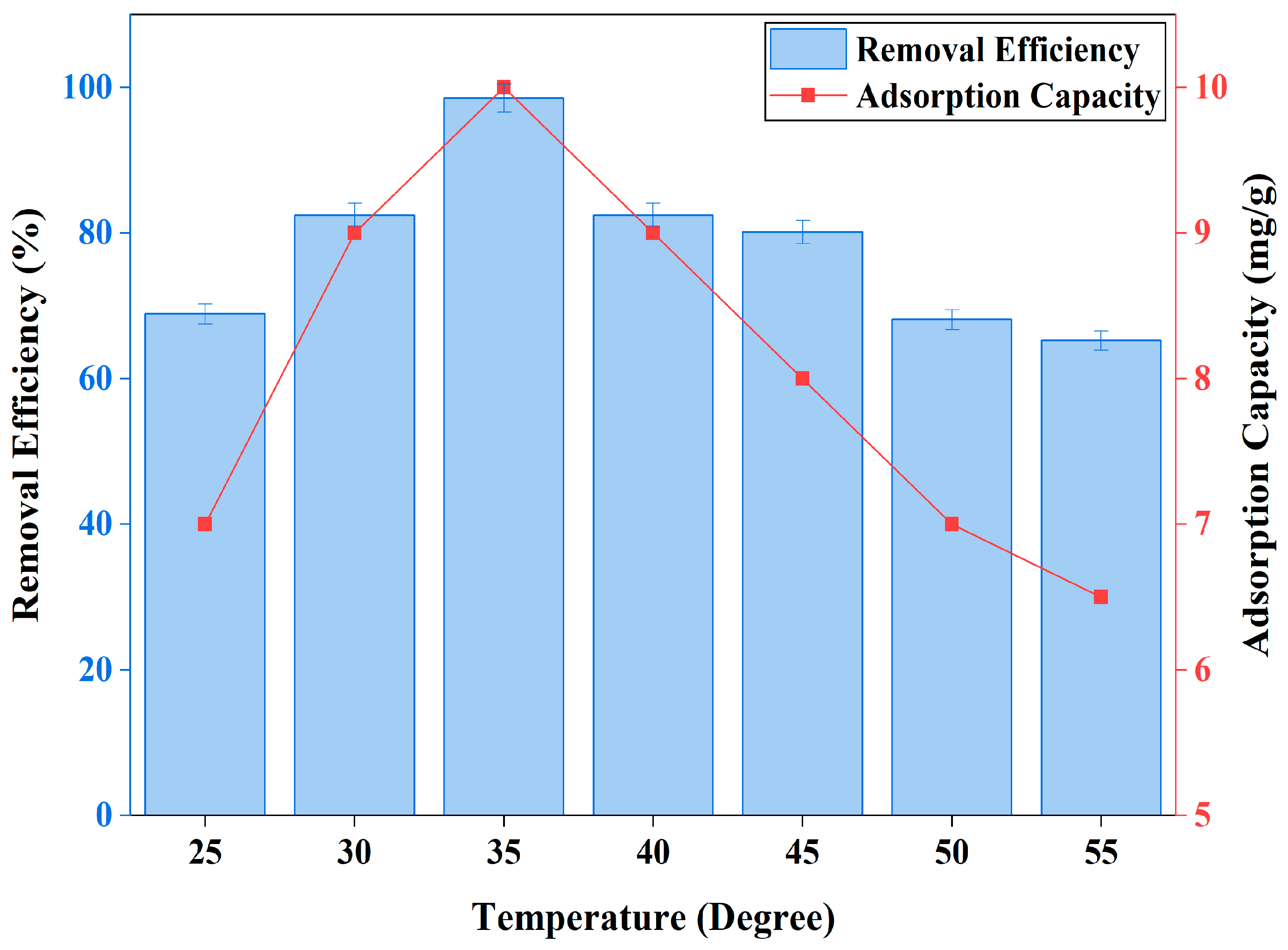
3.2.5. Effect of Temperature
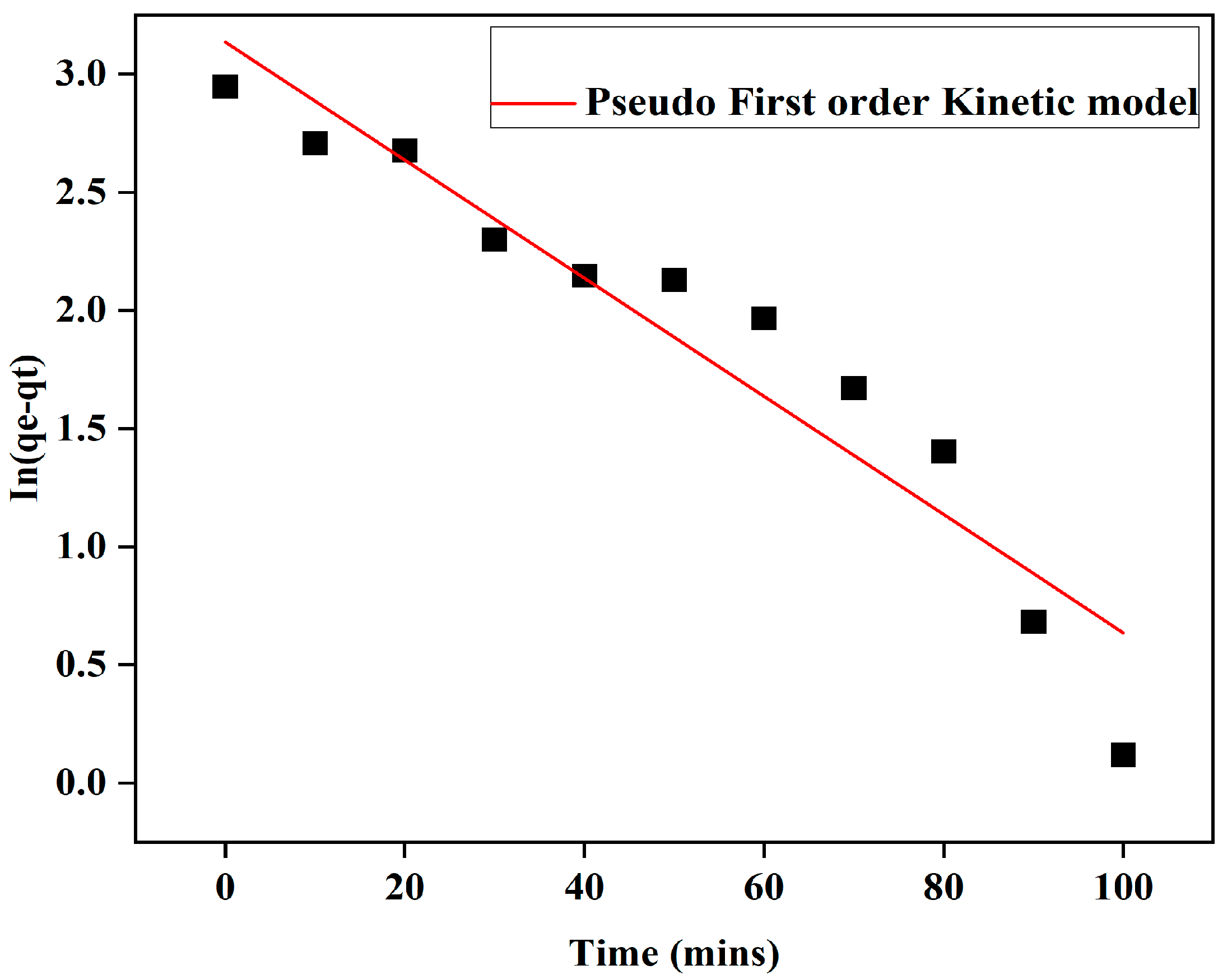
3.3. Adsorption Kinetics
3.4. Isotherm of Adsorption
3.5. Thermodynamics Study
3.6. Optimisation of Astrazon Pink Dye Removal Using Prosopis juliflora Biochar Using Central Composite Design
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdolali, A.; Guo, W.; Ngo, H.; Chen, S.; Nguyen, N.; Tung, K. Typical lignocellulosic wastes and by-products for biosorption process in water and wastewater treatment: A critical review. Bioresour. Technol. 2014, 160, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Yan, F.; Zhu, Z.; Zhao, Y.; Luo, Q.-D.; Fan, Y.-H.; Zhang, X.; Bi, C.-F. Highly selective removal of organic dyes from aqueous solutions with recyclable Fe3O4@PDA-Ag magnetic microspheres. Desalination Water Treat. 2019, 148, 296–311. [Google Scholar] [CrossRef]
- Mohammed, N.; Lian, H.; Islam, M.S.; Strong, M.; Shi, Z.; Berry, R.M.; Yu, H.-Y.; Tam, K.C. Selective adsorption and separation of organic dyes using functionalised cellulose nanocrystals. Chem. Eng. J. 2021, 417, 129237. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Parveen, T.; Umar, K.; Mohamad Ibrahim, M.N. Role of nanomaterials in the treatment of wastewater: A review. Water 2020, 12, 495. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K.; Ang, M.L.; Nishioka, H. Adsorption of methylene blue dye from aqueous solution by novel biomass Eucalyptus sheathiana bark: Equilibrium, kinetics, thermodynamics and mechanism. Desalination Water Treat. 2016, 57, 5858–5878. [Google Scholar] [CrossRef]
- Gokulan, R.; Ganesh Prabhu, G.; Jegan, J. A novel sorbent Ulva lactuca-derived biochar for remediation of Remazol Brilliant Orange 3R in packed column. Water Environ. Res. 2019, 91, 642–649. [Google Scholar] [CrossRef]
- Dutta, P.K. An overview of textile pollution and its remedy. Indian J. Environ. Prot. 1994, 14, 443–446. [Google Scholar]
- Da Silva Alves, D.C.; Healy, B.; Pinto, L.A.d.A.; Cadaval, T.R.S.; Breslin, C.B. Recent developments in Chitosan-based adsorbents for the removal of pollutants from aqueous environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.G.; Shi, K.; Xue, J.L.; Chen, C.; Bai, Y.; Qiao, Y.L.; Liu, Y.X.; Hu, X.M.; Gao, Y.; Yu, H. Systematic adsorption process of petroleum hydrocarbon by immobilised petroleum-degradation bacteria system in degradation pathways. Pet. Sci. 2021, 18, 1543–1550. [Google Scholar] [CrossRef]
- Oladipo, A.C.; Abodunrin, T.O.; Bankole, D.T.; Oladeji, O.S.; Egharevba, G.O.; Bello, O.S. Environmental applications of metal-organic frameworks and derivatives: Recent advances and challenges. In Metal—Organic Frameworks for Environmental Sensing; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; pp. 257–298. [Google Scholar] [CrossRef]
- Morais Da Silva, P.M.; Camparotto, N.G.; Grego Lira, K.T.; Franco Picone, C.S.; Prediger, P. Adsorptive removal of basic dye onto sustainable chitosan beads: Equilibrium, kinetics, stability, continuous-mode adsorption and mechanism. Sustain. Chem. Pharmacy 2020, 18, 100318. [Google Scholar] [CrossRef]
- Ahmed, M.N.; Ram, R.N. Removal of basic dye from wastewater using silica as adsorbent. Environ. Pollut. 1992, 77, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wasti, A.; Ali Awan, M. Adsorption of textile dye onto modified immobilised activated alumina. J. Assoc. Arab. Univ. Basic Appl. Sci. 2016, 20, 26–31. [Google Scholar]
- Hammed, A.K.; Al-Asfoor, I.K.; Al-Dahan, A.K. Comparison between nano zero valent iron supporting onto activated carbon collected via two types of reagents statistically: Carbon for cationic dye removal. J. Phys. Conf. Ser. 2021, 1853, 012016. [Google Scholar] [CrossRef]
- Wang, H.; Cao, X.; Rinklebe, J. Biochar effects on environmental qualities in multiple directions. Chemosphere 2020, 250, 126306. [Google Scholar] [CrossRef]
- Kumar, U.; Vibhute, B.; Parikh, S. Experimental study of adsorption efficiency of methylene blue dye by using banana leaf biochar as an adsorbent. J. Phys. Conf. Ser. 2021, 1979, 012003. [Google Scholar] [CrossRef]
- Reeshma, P.; Balavijayalakhshmi, J. Investigation on structural, spectral, and morphological analysis of polymer functionalised graphene oxide—Metal oxide nanocomposites for removal of dyes from aqueous solution. Mater. Res. Express 2019, 6, 095617. [Google Scholar] [CrossRef]
- Hawal, L.H.; Allah, S.A.; Karbol, M.S. Cadmium ions adsorption from aqueous solutions by Bentonite clay, fixed bed column. J. Phys. Conf. Ser. 2021, 1895, 012040. [Google Scholar] [CrossRef]
- Wang, S.; Boyjoo, Y.; Choueib, A.; Zhu, Z.H. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005, 39, 129–138. [Google Scholar] [CrossRef]
- Bashir, M.; Mohan, C.; Tyagi, S.; Annachhatre, A. Copper removal from aqueous solution using chemical precipitation and adsorption by Himalayan pine forest residue as biochar. Water Sci. Technol. 2022, 86, 530–554. [Google Scholar] [CrossRef]
- Inyinbor, A.A.; Bankole, D.T.; Solomon, P. Adsorptive removal of acetaminophen onto acid-modified Raphia hookeri fruit epicarp. Biomass Convers. Biorefin. 2023, 14, 16219–16230. [Google Scholar] [CrossRef]
- Srivatsav, P.; Bhargav, B.S.; Shanmugasundaram, V.; Arun, J.; Gopinath, K.P.; Bhatnagar, A. Biochar as an eco-friendly and economical adsorbent for the removal of colorants (Dyes) from aqueous environment: A review. Water 2020, 12, 3561. [Google Scholar] [CrossRef]
- Wang, S.; Kwak, J.-H.; Islam, M.S.; Naeth, M.A.; Gamal El-Din, M.; Chang, S.X. Biochar surface complexation and Ni(II), Cu(II), and Cd(II) adsorption in aqueous solutions depend on feedstock type. Sci. Total Environ. 2020, 712, 136538. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Aichour, A.; Zaghouane-Boudiaf, H.; Djafer Khodja, H. Highly removal of anionic dye from aqueous medium using a promising biochar derived from date palm petioles: Characterisation, adsorption properties and reuse studies. Arabian J. Chem. 2022, 15, 103542. [Google Scholar] [CrossRef]
- Dahiru, M.; Zango, Z.U.; Haruna, M.A. Cationic Dyes Removal Using Low-Cost Banana Peel Biosorbent. Am. J. Mater. Sci. 2018, 8, 32–38. [Google Scholar]
- Abdel-Ghani, N.T.; El-Chaghaby, G.A.; Zahran, E.M. Pentachlorophenol (PCP) adsorption from aqueous solution by activated carbons prepared from corn wastes. Int. J. Environ. Sci. Technol. 2015, 12, 211–222. [Google Scholar] [CrossRef]
- Kanojia, A.; Jain, S.K. Performance of coconut shell as coarse aggregate in concrete. Constr. Build. Mater. 2017, 140, 150–156. [Google Scholar] [CrossRef]
- Wang, L. Application of activated carbon derived from ‘waste’bamboo culms for the adsorption of azo disperse dye: Kinetic, equilibrium and thermodynamic studies. J. Environ. Manag. 2012, 102, 79–87. [Google Scholar] [CrossRef]
- Nithyalakshmi, B.; Saraswathi, R.; Praveen, S. Removal of basic fuchsin red dye by turmeric leaf waste biochar: Batch adsorption studies, isotherm kinetics and RSM studies. Glob. NEST J. 2023, 25, 17–27. [Google Scholar]
- Vyavahare, G.; Jadhav, P.; Jadhav, J.; Patil, R.; Aware, C.; Patil, D.; Gophane, A.; Yang, Y.H.; Gurav, R. Strategies for Crystal Violet Dye Sorption on Biochar Derived from Mango Leaves and Evaluation of Residual Dye Toxicity. J. Clean. Prod. 2019, 207, 296–305. [Google Scholar] [CrossRef]
- Vieira, S.S.; Magriotis, Z.M.; Santos, N.A.V.; Cardoso, M.D.; Saczk, A.A. Macauba palm (Acrocomia aculeata) cake from biodiesel processing: An efficient and low-cost substrate for the adsorption of dyes. Chem. Eng. J. 2012, 183, 152–161. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Ngo, H.H.; Guo, W.; Wen, H.; Li, C.; Zhang, Y.; Ma, C. Comparison study on the ammonium adsorption of the biochars derived from different kinds of fruit peel. Sci. Total Environ. 2019, 707, 135544. [Google Scholar] [CrossRef]
- Nirmaladevi, S.; Palanisamy, N. A comparative study of the removal of cationic and anionic dyes from aqueous solutions using biochar as an adsorbent. Desalination Water Treat. 2020, 175, 282–292. [Google Scholar] [CrossRef]
- Liu, S.B.; Tan, X.F.; Liu, Y.G. Production of biochars from Ca impregnated ramie biomass (Boehmeria nivea (L.) Gaud.) and their phosphate removal potential. RSC Adv. 2016, 6, 5871–5880. [Google Scholar] [CrossRef]
- Babaei, A.A.; Alavi, S.N.; Akbarifar, M.; Ahmadi, K.; Esfahani, A.R.; Kakavandi, B. Experimental and modeling study on adsorption of cationic methylene blue dye onto mesoporous biochars prepared from agrowaste. Desalination Water Treat. 2016, 57, 27199–27212. [Google Scholar] [CrossRef]
- Santhy, K.; Selvapathy, P. Removal of reactive dyes from wastewater by adsorption on coir pith activated carbon. Bioresour. Technol. 2006, 97, 1329–1336. [Google Scholar] [CrossRef]
- Garg, S.; Das, P. High-grade activated carbon from pyrolytic biochar of Jatropha and Karanja oil seed cakes—Indian biodiesel industry wastes. Biomass Convers. Biorefinery 2018, 8, 545–561. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Ortiz, J.; Duran, F.; Vallejo, W.; Fals, J. Methyl Orange Adsorption on Biochar Obtained from Prosopis juliflora Waste: Thermodynamic and Kinetic Study. ChemEngineering 2023, 7, 114. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.; Walteros, L.; Duran, F.; Vallejo, W.; Romero Bohórquez, A.R. Prosopis juliflora Seed Waste as Biochar for the Removal of Blue Methylene: A Thermodynamic and Kinetic Study. ACS Omega 2022, 7, 42916–42925. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhamid, A.I.; Emran, M.; El-Sadek, M.H.; El-Shanshory, A.A.; Soliman, H.M.A.; Akl, M.A.; Rashad, M. Enhanced removal of cationic dye by eco-friendly activated biochar derived from rice straw. Appl. Water Sci. 2020, 10, 45. [Google Scholar] [CrossRef]
- Muller, G.C.; Junnila, A.; Traore, M.M.; Traore, S.F.; Doumbia, S.; Sissoko, F.; Dembele, S.M.; Schlein, Y.; Arheart, K.L.; Revay, E.E.; et al. The Invasive Shrub Prosopis Juliflora Enhances the Malaria Parasite Transmission Capacity of Anopheles Mosquitoes: A Habitat Manipulation Experiment. Malar. J. 2017, 16, 237. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Zheng, H.; Baldock, J.A.; Sun, O.J.; Shao, Q. Convergent modelling of past soil organic carbon stocks but divergent projections. Biogeosciences 2015, 12, 4373–4383. [Google Scholar] [CrossRef]
- Mahdi, Z.; El Hanandeh, A.; Yu, Q. Date seed-derived biochar for Ni(II) removal from aqueous solutions. MATEC Web Conf. 2017, 120, 05005. [Google Scholar] [CrossRef]
- Li, H.; Mahyoub, S.A.A.; Liao, W.J.; Xia, S.Q.; Zhao, H.C.; Guo, M.Y.; Ma, P.S. Effect of pyrolysis temperature on characteristics and aromatic contaminants adsorption behavior of magnetic biochar derived from pyrolysis oil distillation residue. Bioresour. Technol. 2017, 223, 20–26. [Google Scholar] [CrossRef]
- Saravanan, P.; Josephraj, J.; Thillainayagam, B.P.; Ravindiran, G. Evaluation of the adsorptive removal of cationic dyes by greening biochar derived from agricultural bio-waste of rice husk. Biomass Convers. Biorefinery 2021, 13, 4047–4060. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent: Equilibrium, thermodynamic, kinetics, mechanism and process design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, kinetics and mechanism of removal of Methylene Blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiata. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Agarwal, S.; Tyagi, I.; Gupta, V.K. Removal of basic dye Auramine-O by ZnS:Cu nanoparticles loaded on activated carbon: Optimisation of parameters using response surface methodology with central composite design. RSC Adv. 2015, 5, 18438–18450. [Google Scholar] [CrossRef]
- Mourabet, M.; Rhilassi, E.I.; Boujaady, A.I.; Bennani-Ziatni, H.; Taitai, M. A Use of response surface methodology for optimisation of fuoride adsorption in an aqueous solution by Brushite. Arab. J. Chem. 2017, 10, S3292–S3302. [Google Scholar] [CrossRef]
- Owolabi, R.U.; Usman, M.A.; Kehinde, A.J. Modelling and optimisation of process variables for the solution polymerisation of styrene using response surface methodology. J. King Saud. Univ. Eng. Sci. 2018, 30, 22–30. [Google Scholar] [CrossRef]
- Bayuo, J.; Pelig-Ba, K.B.; Abukari, M.A. Optimisation of adsorption parameters for efective removal of lead(II) from. Phys. Chem. Indian J. 2019, 14, 123. [Google Scholar]
- Auta, M. Optimization of tea waste activated carbon preparation parameters for removal of cibacron yellow dye from textile waste waters. Int. J. Adv. Eng. Res. 2012, 1, 50–56. [Google Scholar]
- Bagheri, R.; Ghaedi, M.; Asfaram, A.; Dil, E.A.; Javadian, H. RSM-CCD design of malachite green adsorption onto activated carbon with multimodal pore size distribution prepared from Amygdalus scoparia: Kinetic and isotherm studies. Polyhedron 2019, 171, 464–472. [Google Scholar] [CrossRef]
- Bhagavathi Pushpa, T.; Jegan, J.; Praveen, S.; Gokulan, R. Biodecolorization of basic blue 41 using EM based composts: Iso therm and kinetics. Chem. Sel. 2019, 4, 10006–10012. [Google Scholar] [CrossRef]
- Oh, T.K.; Choi, B.; Shinogi, Y.; Chikushi, J. Characterization of biochar derived from three types of biomass. J. Fac. Agric. Kyushu Univ. 2012, 57, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Kamath, S.R.; Proctor, A. Silica gel from rice hull ash: Preparation and characterisation. Cereal Chem. J. 1998, 75, 484–487. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Yao, Y. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass. Bioresour. Technol. 2012, 110, 50–56. [Google Scholar] [CrossRef]
- Torrades, F.; García-Montaño, J. Using central composite experimental design to optimise the degradation of real dye wastewater by Fenton and photo-Fenton reactions. Dye. Pigm. 2014, 100, 184–189. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, M.; Taghizadeh, A. Ultrasound-assisted green synthesis and application of recyclable nanoporous chromium-based metal-organic framework. Korean J. Chem. Eng. 2019, 36, 287–298. [Google Scholar] [CrossRef]
- Kyi, P.P.; Quansah, J.O.; Lee, C.G.; Moon, J.K.; Park, S.J. The Removal of Crystal Violet from Textile Wastewater Using Palm Kernel Shell-Derived Biochar. Appl. Sci. 2020, 10, 2251. [Google Scholar] [CrossRef]
- Tsai, W.T.; Chen, H.R. Adsorption kinetics of herbicide paraquat in aqueous solution onto a low-cost adsorbent, swine-manurederived biochar. Int. J. Environ. Sci. Technol. 2013, 10, 1349–1356. [Google Scholar] [CrossRef]
- Priya, A.K.; Gokulan, R.; Vijayakumar, A.; Praveen, S. Biodecolorization of remazol dyes using biochar derived from ulva reticulata: Isotherm, kinetics, desorption, and thermodynamic studies. Desalination Water Treat. 2020, 200, 286–295. [Google Scholar] [CrossRef]
- Abuabara, A.; Diaz-Uribe, C.; Vallejo, W.; Duran, F.; Mosquera-Vargas, E. Kinetic and Thermodynamic Study of Cationic Dye Removal Using Activated Biochar Synthesised from Prosopis juliflora Waste. ChemEngineering 2025, 9, 64. [Google Scholar] [CrossRef]
- Sutar, S.; Patil, P.; Jadhav, J. Recent advances in biochar technology for textile dyes wastewater remediation: A review. Environ. Res. 2022, 209, 112841. [Google Scholar] [CrossRef]
- N’Dah, F.M.; N’daye, A.D.; Ech-chihbi, E.; Azzaoui, K.; Sabbahi, R.; Siaj, M.; Kankou, M. Kinetics and Equilibrium Study for the Adsorption of Maxilon Blue Dye on Prosopis juliflora Fruit Seeds as a Low-Cost Adsorbent. Moroc. J. Chem. 2024, 12, 1042–1057. [Google Scholar]
- Mahmoud, E.R.I.; Aly, H.M.; Hassan, N.A.; Aljabri, A.; Khan, A.L.; El-Labban, H.F. Biochar from Date Palm Waste via Two-Step Pyrolysis: A Modified Approach for Cu (II) Removal from Aqueous Solutions. Processes 2024, 12, 1189. [Google Scholar] [CrossRef]
- Gokulan, R.; Ganesh Prabhu, G.; Avinash, A.; Jegan, J. Experimental and chemometric analysis of bioremediation of remazol dyes using biochar derived from green seaweeds. Desalination Water Treat. 2020, 184, 340–353. [Google Scholar] [CrossRef]
- Gokulan, R.; Avinash, A.; Prabhu, G.G.; Jegan, J. Remediation of remazol dyes by biochar derived from Caulerpa scalpelliformis—An eco-friendly approach. J. Environ. Chem. Eng. 2019, 7, 103297. [Google Scholar] [CrossRef]
- Gokulan, R.; Prabhu, G.G.; Jegan, J. Remediation of complex remazol effluent using biochar derived from green seaweed biomass. Int. J. Phytoremediation 2019, 21, 1179–1189. [Google Scholar] [CrossRef]
- Mohammad, Y.S.; Shaibu-Imodagbe, E.M.; Igboro, S.B.; Giwa, A.; Okuofu, C.A. Modeling and optimisation for production of rice husk activated carbon and adsorption of phenol. J. Eng. 2014, 2014, 278075. [Google Scholar] [CrossRef]
- Yusuf, A.S. Optimization of adsorption of Cr(VI) from aqueous solution by Leucaena leucocephala seed shell activated carbon using design of experiment. Appl. Water Sci. 2018, 8, 232. [Google Scholar] [CrossRef]
- Ani, I.; Okafor, J.; Olutoye, M.; Akpan, U. Optimization of base oil regeneration from spent engine oil via solvent extraction. Adv. Res. 2015, 4, 403–411. [Google Scholar] [CrossRef]





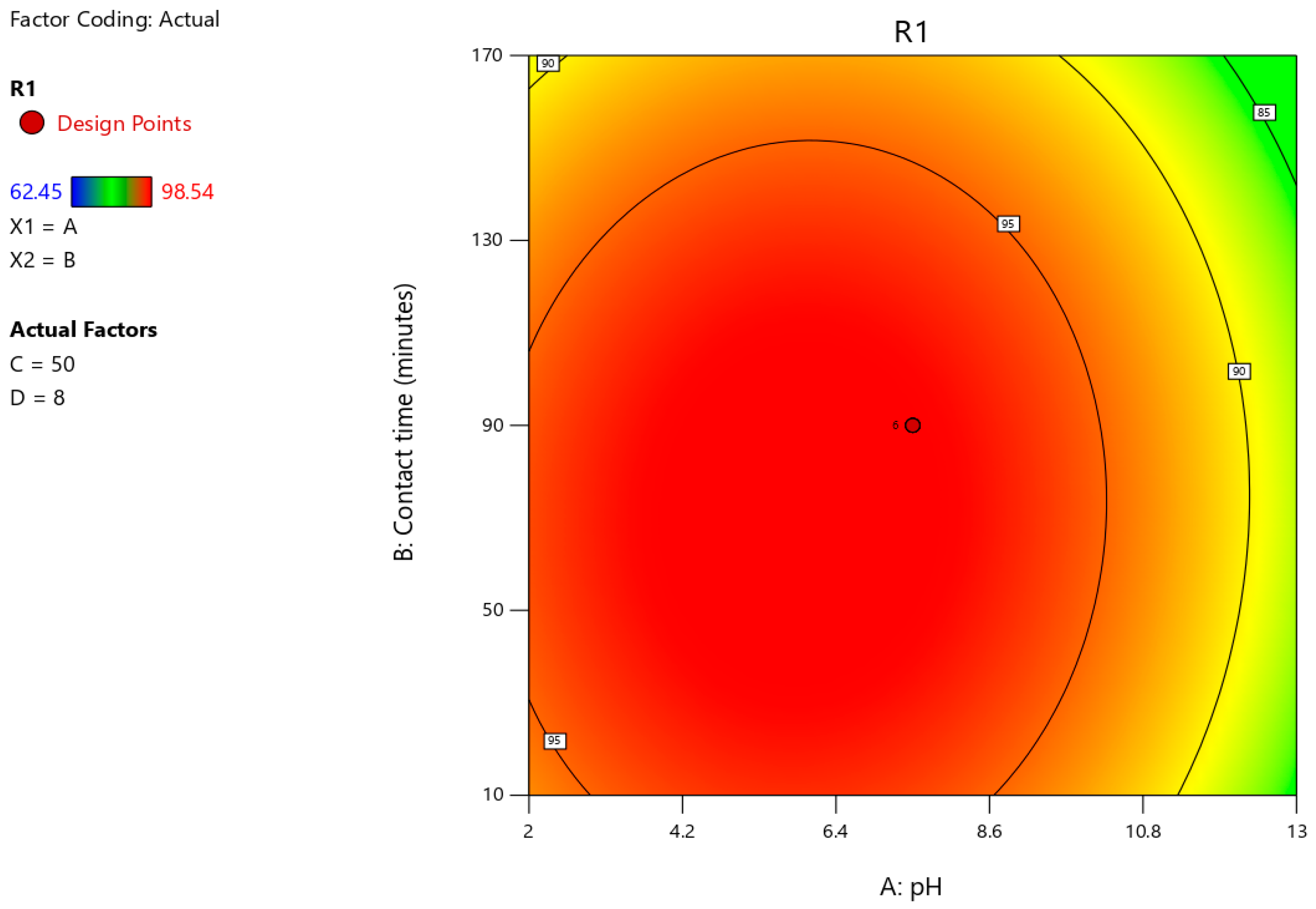
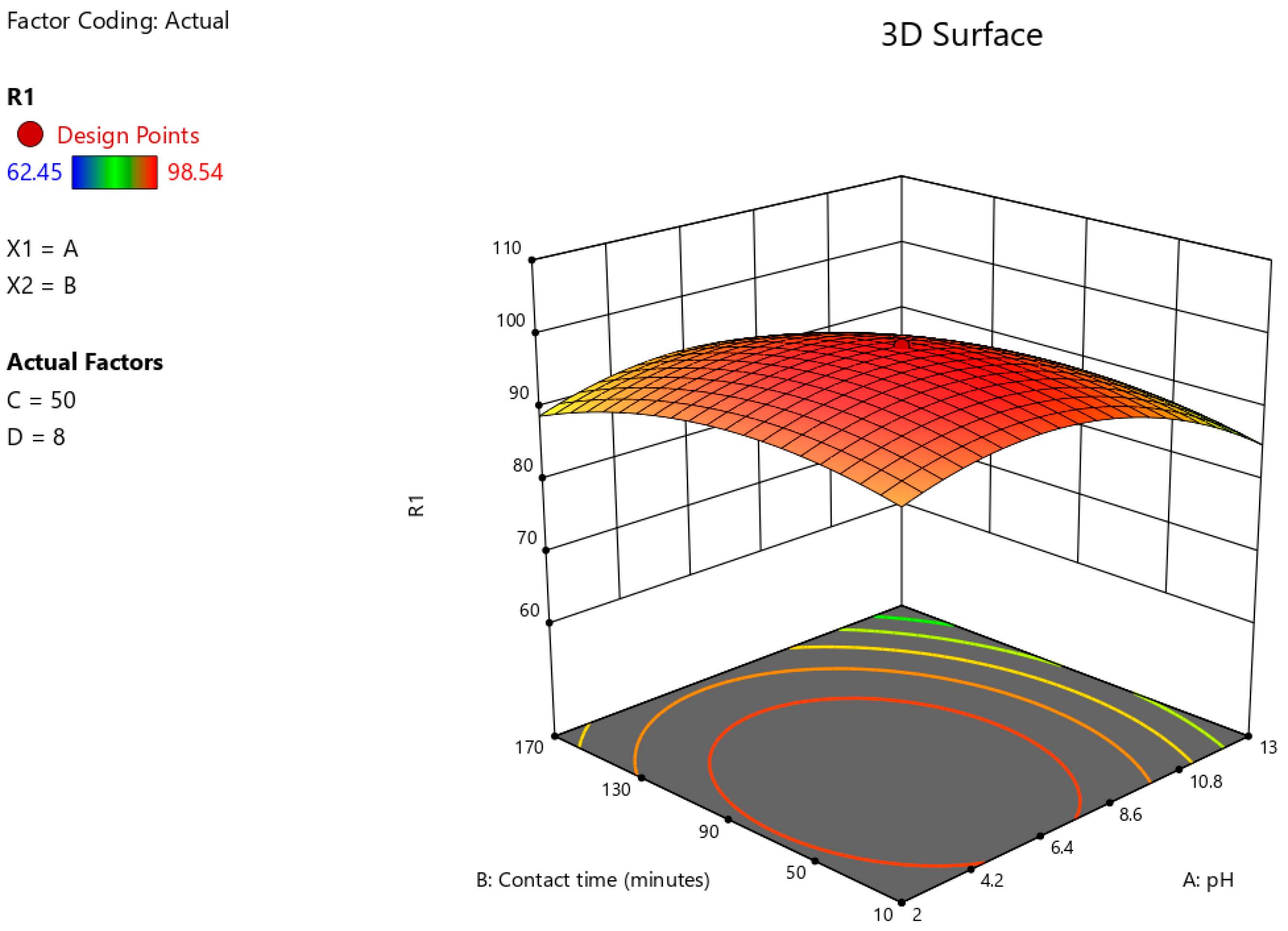
| Parameters | Independent Variables | Units | Levels | ||
|---|---|---|---|---|---|
| Coded Low (−1) | Mean | Coded High (+1) | |||
| pH | A | - | 2 | 7 | 13 |
| Contact time | B | min | 10 | 90 | 170 |
| Dye Concentration | C | mg/L | 10 | 50 | 90 |
| Biochar dose | D | g/L | 1 | 8 | 15 |
| Model | Parameters | |||
|---|---|---|---|---|
| Pseudo-first order | qe (mg/g) | k1 (min−1) | R2 | Standard Errors of Regression |
| 23.0245 | 0.025 | 0.8964 | 0.70685 | |
| Pseudo-second order | qe (mg/g) | k2 (g/mg) min−1 | R2 | |
| 23.7473 | 0.1008 | 0.9999 | 8.05312 × 10−4 | |
| Dye | Adsorbent | Qmax (mg g−1) | References |
|---|---|---|---|
| Astrazon Pink | Prosopis juliflora | 84.175 | This study |
| Methylene blue | Prosopis juliflora activated with citric acid | 37.6 | [65] |
| Methylene orange | Prosopis juliflora | 8.31 | [66] |
| Maxilon Blue dye | Prosopis juliflora fruit seeds | 84.54 | [67] |
| Cu (II) | Date palm leaf waste | 70 | [68] |
| Adsorbent | Temp (k) | KL | Regression of Standard Errors | ∆G° (kjmol−1) | ∆H° (kjmol−1) | ∆S° (jk−1mol−1) | R2 |
|---|---|---|---|---|---|---|---|
| Prosopis juliflora shell biochar | 298 | 2.201 | 2.10869 × 10−5 | −1.9545 | 7.1651 | 30.6142 | 0.999 |
| 308 | 2.422 | −2.2651 | |||||
| 318 | 2.653 | −2.2579 | |||||
| 328 | 2.864 | −2.8693 |
| Source | Std. Dev. | R2 | Adjusted R2 | Predicted R2 | PRESS | |
|---|---|---|---|---|---|---|
| Linear | 10.45 | 0.1701 | 0.0374 | −0.1048 | 3631.70 | |
| 2FI | 11.42 | 0.2468 | −0.1496 | −0.9922 | 6549.10 | |
| Quadratic | 6.50 | 0.8073 | 0.6275 | −0.1099 | 3648.53 | Suggested |
| Cubic | 6.51 | 0.9097 | 0.6257 | −12.0090 | 42,764.94 | Aliased |
| Run | Factor 1: A pH | Factor 2: B Contact Time Minutes | Factor 3: C Dye Concentration mg/L | Factor 4: D Biochar Dose g/L | Response (%) Experimental Values | Response (%) Predicted Values |
|---|---|---|---|---|---|---|
| 1 | 7.5 | 70 | 50 | 8 | 92.78 | 91.78 |
| 2 | 3.5 | 90 | 50 | 8 | 85.54 | 86.54 |
| 3 | 7.5 | 90 | 50 | 8 | 97.44 | 98.54 |
| 4 | 2 | 10 | 90 | 1 | 82.31 | 81.31 |
| 5 | 13 | 170 | 90 | 1 | 72.78 | 71.78 |
| 6 | 2 | 10 | 10 | 1 | 75.45 | 74.45 |
| 7 | 8.5 | 90 | 50 | 8 | 62.454 | 63.75 |
| 8 | 2 | 10 | 90 | 15 | 90.89 | 91.08 |
| 9 | 7.5 | 90 | 50 | 6 | 86.76 | 85.78 |
| 10 | 7.5 | 90 | 50 | 8 | 98.54 | 98.54 |
| 11 | 7.5 | 150 | 50 | 8 | 75.67 | 76.67 |
| 12 | 13 | 170 | 10 | 1 | 82.34 | 83.34 |
| 13 | 2 | 170 | 10 | 15 | 88.78 | 89.78 |
| 14 | 13 | 10 | 90 | 1 | 83.56 | 84.56 |
| 15 | 7.5 | 90 | 50 | 8 | 98.54 | 98.54 |
| 16 | 2 | 170 | 90 | 1 | 80.89 | 80.15 |
| 17 | 2 | 170 | 90 | 15 | 79.89 | 80.89 |
| 18 | 7.5 | 90 | 50 | 8 | 97.54 | 98.54 |
| 19 | 13 | 170 | 10 | 15 | 64.56 | 65.56 |
| 20 | 7.5 | 90 | 50 | 8 | 96.34 | 98.54 |
| 21 | 7.5 | 90 | 50 | 12 | 90.87 | 90.86 |
| 22 | 2 | 10 | 10 | 15 | 83.45 | 83.31 |
| 23 | 13 | 170 | 90 | 15 | 82.45 | 82.45 |
| 24 | 13 | 10 | 10 | 1 | 80.12 | 81.12 |
| 25 | 7.5 | 90 | 30 | 8 | 76.54 | 75.65 |
| 26 | 12 | 170 | 10 | 1 | 70.56 | 70.58 |
| 27 | 13 | 10 | 10 | 15 | 73.45 | 74.54 |
| 28 | 7.5 | 90 | 50 | 8 | 96.44 | 98.54 |
| 29 | 7.5 | 90 | 30 | 8 | 93.45 | 92.45 |
| 30 | 13 | 10 | 90 | 15 | 65.89 | 66.59 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 2653.92 | 14 | 189.57 | 4.49 | 0.0033 | significant |
| A-pH | 367.54 | 1 | 367.54 | 8.70 | 0.0099 | |
| B-Contact time | 89.78 | 1 | 89.78 | 2.132 | 0.1654 | |
| C-Dye Concentration | 98.66 | 1 | 98.66 | 2.34 | 0.1472 | |
| D-Biochar Dose | 3.30 | 1 | 3.30 | 0.0782 | 0.7836 | |
| AB | 2.24 | 1 | 2.24 | 0.0529 | 0.8212 | |
| AC | 7.37 | 1 | 7.37 | 0.1746 | 0.6820 | |
| AD | 233.63 | 1 | 233.63 | 5.53 | 0.0327 | |
| BC | 0.0729 | 1 | 0.0729 | 0.0017 | 0.9674 | |
| BD | 8.64 | 1 | 8.64 | 0.2047 | 0.6574 | |
| CD | 0.1444 | 1 | 0.1444 | 0.0034 | 0.9541 | |
| A2 | 1275.77 | 1 | 1275.77 | 30.21 | <0.0001 | |
| B2 | 498.35 | 1 | 498.35 | 11.80 | 0.0037 | |
| C2 | 454.35 | 1 | 454.35 | 10.76 | 0.0051 | |
| D2 | 266.15 | 1 | 266.15 | 6.30 | 0.0240 | |
| Residual | 633.42 | 15 | 42.23 | |||
| Lack of Fit | 633.42 | 10 | 63.34 | |||
| Pure Error | 0.0000 | 5 | 0.0000 | significant | ||
| Cor Total | 3287.34 | 29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanraj, L.; Jambulingam, R. Efficient Decolourisation of Astrazon Pink Dye Using Biocarbon Derived from Prosopis juliflora Shells: Kinetics, Isotherms, and RSM-Based Optimization for Sustainable Wastewater Treatment. Reactions 2025, 6, 57. https://doi.org/10.3390/reactions6040057
Mohanraj L, Jambulingam R. Efficient Decolourisation of Astrazon Pink Dye Using Biocarbon Derived from Prosopis juliflora Shells: Kinetics, Isotherms, and RSM-Based Optimization for Sustainable Wastewater Treatment. Reactions. 2025; 6(4):57. https://doi.org/10.3390/reactions6040057
Chicago/Turabian StyleMohanraj, Lakshmi, and Ranjitha Jambulingam. 2025. "Efficient Decolourisation of Astrazon Pink Dye Using Biocarbon Derived from Prosopis juliflora Shells: Kinetics, Isotherms, and RSM-Based Optimization for Sustainable Wastewater Treatment" Reactions 6, no. 4: 57. https://doi.org/10.3390/reactions6040057
APA StyleMohanraj, L., & Jambulingam, R. (2025). Efficient Decolourisation of Astrazon Pink Dye Using Biocarbon Derived from Prosopis juliflora Shells: Kinetics, Isotherms, and RSM-Based Optimization for Sustainable Wastewater Treatment. Reactions, 6(4), 57. https://doi.org/10.3390/reactions6040057





