Kinetics and Mechanism of Electrochemical Reactions Occurring during the Chromium Electrodeposition from Electrolytes Based on Cr(III) Compounds: A Literature Review
Abstract
1. Introduction
2. Kinetics and Mechanism of the Reactions during the Electrodeposition of Coatings from Electrolytes Based on Cr(III)
2.1. Water-Based Electrolytes Containing Salts of Trivalent Chromium
2.1.1. Main Features of Reaction Schemes and Mechanisms
2.1.2. Main Influencing Factors and Some Results Published in the Last Few Years
2.2. Electrolytes Based on Ionic Liquids and Deep Eutectic Solvents Containing Trivalent Chromium Salts
2.2.1. Electrolytes Based on “Common” Ionic Liquids
2.2.2. Electrolytes Based on Deep Eutectic Solvents
3. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Okonkwo, B.O.; Jeong, C.; Jang, C. Advances on Cr and Ni electrodeposition for industrial applications—A review. Coatings 2022, 12, 1555. [Google Scholar] [CrossRef]
- Kwon, S.C.; Kim, M.; Park, S.U.; Kim, D.Y.; Kim, D.; Nam, K.S.; Choi, Y. Characterization of intermediate Cr-C layer fabricated by electrodeposition in hexavalent and trivalent chromium baths. Surf. Coat. Technol. 2004, 183, 151–156. [Google Scholar] [CrossRef]
- Dennis, J.K.; Such, T.E. Nickel and Chromium Plating, 3rd ed.; Woodhead Publishing: Cambridge, UK, 1993; 385p. [Google Scholar]
- Hoare, J.P. On the mechanisms of chromium electrodeposition. J. Electrochem. Soc. 1979, 126, 190–199. [Google Scholar] [CrossRef]
- Liebreich, E. Zur Theorie der elektrolytischen Abscheidung des Chroms aus wassrigen Chromsaurelosungen. Z. Elektrochem. Angew. Physik. Chem. 1927, 33, 69–72. [Google Scholar]
- Liebreich, E. Uber die Ursachen der periodischen Erscheinungen bei der Elektrolyse von Chromsaure und uber die Abscheidung metallischen Chroms. Z. Elektrochem. Angew. Physik. Chem. 1921, 27, 94–110. [Google Scholar]
- DesMarias, T.L.; Costa, M. Mechanism of chromium-induced toxicity. Curr. Opin. Toxicol. 2019, 14, 1–7. [Google Scholar] [CrossRef]
- Standeven, A.M.; Wetterhahn, K.E. Chromium (VI) toxicity: Uptake, reduction, and DNA damage. J. Am. Coll. Toxicol. 1989, 8, 1275–1283. [Google Scholar] [CrossRef]
- Yao, H.; Guo, L.; Jiang, B.-H.; Luo, J.; Shi, X. Oxidative stress and chromium (VI) carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2008, 27, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Directive 2002/95/EC of the European Parliament and of the Council of 27 January 2003 on the restriction of the use of certain hazardous substances in electrical and electronic equipment. Off. J. Eur. Union 2003, 37, 19–23.
- Vaiopoulou, E.; Gikas, P. Regulations for chromium emissions to the aquatic environment in Europe and elsewhere. Chemosphere 2020, 254, 126876. [Google Scholar] [CrossRef]
- Jabasingh, S.A.; Lalith, D.; Garre, P. Sorption of chromium(VI) from electroplating effluent onto chitin immobilized Mucor racemosus sorbent (CIMRS) impregnated in rotating disk contactor blades. J. Ind. Eng. Chem. 2015, 23, 79–92. [Google Scholar] [CrossRef]
- Aydin, Y.A.; Aksoy, N.D. Adsorption of chromium on chitosan: Optimization, kinetics and thermodynamics. Chem. Eng. J. 2009, 151, 188–194. [Google Scholar] [CrossRef]
- Fontanesi, C.; Giovanardi, R.; Cannio, M.; Soragni, E. Chromium electrodeposition from Cr(VI) low concentration solutions. J. Appl. Electrochem. 2008, 38, 425–436. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, M.; Kwon, S.C. Characterization of chrome layer formed by pulse plating. Surf. Coat. Technol. 2003, 169–170, 81–84. [Google Scholar] [CrossRef]
- Tsai, R.-Y.; Wu, S.-T. Phase stability of chromium plating from chromic acid electrolyte containing formic acid. J. Electrochem. Soc. 1990, 137, 3057–3060. [Google Scholar] [CrossRef]
- Hoshino, S.; Laitinen, H.A.; Hoflund, G.B. The electrodeposition and properties of amorphous chromium films prepared from chromic acid solutions. J. Electrochem. Soc. 1986, 133, 681–685. [Google Scholar] [CrossRef]
- Danilov, F.I.; Protsenko, V.S. Electroreduction of hexavalent chromium compounds on the gold electrode. Russ. J. Electrochem. 1998, 34, 276–281. [Google Scholar]
- Danilov, F.I.; Protsenko, V.S. Effect of formic acid on the electroreduction of compounds of hexavalent chromium. Russ. J. Electrochem. 1998, 34, 568–571. [Google Scholar]
- Danilov, F.I.; Popov, E.R.; Burykina, V.S.; Protsenko, V.S. Corrosive and electrochemical behavior of deposits from chromic acid electrolytes containing formic acid. Russ. J. Electrochem. 1997, 33, 499–502. [Google Scholar]
- Protsenko, V.S.; Danilov, F.I. Chromium electroplating from trivalent chromium baths as an environmentally friendly alternative to hazardous hexavalent chromium baths: Comparative study on advantages and disadvantages. Clean Technol. Environ. Policy 2014, 16, 1201–1206. [Google Scholar] [CrossRef]
- Yoshida, T.; Yoshida, R. XXXIV. Mechanism of chromium electrodeposition, the modified process for the electrolytic chromium alloy and a summary of the serial papers. J. Soc. Chem. Ind. Jpn. 1955, 58, 89–91. [Google Scholar]
- Ward, J.J.B.; Christie, I.R.A. Electrodeposition of chromium from trivalent salts. Trans. Inst. Met. Finish. 1971, 49, 148–152. [Google Scholar] [CrossRef]
- Smart, D.; Such, T.E.; Wake, S.J. A novel trivalent chromium electroplating bath. Trans. Inst. Met. Finish. 1983, 61, 105–110. [Google Scholar] [CrossRef]
- Ward, J.J.B.; Christie, I.R.A.; Carter, V.E. Pilot scale evaluation of a trivalent chromium plating process. Trans. Inst. Met. Finish. 1971, 49, 97–104. [Google Scholar] [CrossRef]
- Drela, I.; Szynkarczuk, J.; Kubicki, J. Electrodeposition of chromium from Cr (III) electrolytes in the presence of formic acid. J. Appl. Electrochem. 1989, 19, 933–936. [Google Scholar] [CrossRef]
- Datta, J. Chrom(III)–Baeder: Grundlagen fuer die Entwicklung, Eigenschaften, Abscheidungsmechanismus [Trivalent chromium baths: Principles for development, properties, and deposition mechanisms]. Galvanotechnik 1982, 73, 106–112. [Google Scholar]
- Kolthoff, I.M.; Lingane, J.J. Polarography; Interscience Publishers: New York, NY, USA, 1941; 508p. [Google Scholar]
- Fischerova, E.; Fischer, O. Polarographisches Verhalten einiger Chromkomplexe. I. Hexarhodanochromat(III)-Ion. Collect. Czech. Chem. Commun. 1961, 26, 2570–2586. [Google Scholar] [CrossRef]
- Szynkarczuk, J.; Drela, I.; Kubicki, J. Electrochemical behaviour of chromium (III) in the presence of formic acid–I. Electrochim. Acta 1989, 34, 399–403. [Google Scholar] [CrossRef]
- Howarth, J.N.; Pletcher, D. The electrodeposition of chromium from chromium(III) solutions—A study using microelectrodes. J. Appl. Electrochem. 1988, 18, 644–652. [Google Scholar] [CrossRef]
- Del Pianta, D.; Frayret, J.; Gleyzes, C.; Cugnet, C.; Dupin, J.C.; Le Hecho, I. Determination of the chromium(III) reduction mechanism during chromium electroplating. Electrochim. Acta 2018, 284, 234–241. [Google Scholar] [CrossRef]
- Drela, I.; Szynkarczuk, J.; Kubicki, J. Electroreduction of chromium(III)–acetate complex to metallic chromium on the copper electrode. Electrochim. Acta 1988, 33, 589–592. [Google Scholar] [CrossRef]
- Marcus, R.A. On the theory of oxidation-reduction reactions involving electron transfer. I. J. Chem. Phys. 1956, 24, 966–979. [Google Scholar] [CrossRef]
- Marcus, R.A. Chemical and electrochemical electron-transfer theory. Annu. Rev. Phys. Chem. 1964, 15, 155–196. [Google Scholar] [CrossRef]
- Hush, N.S. Adiabatic rate processes at electrodes. I. Energy-charge relationships. J. Chem. Phys. 1958, 28, 962–973. [Google Scholar] [CrossRef]
- Hush, N.S. Electron transfer in retrospect and prospect. 1: Adiabatic electrode processes. J. Electroanal. Chem. 1999, 470, 170–195. [Google Scholar] [CrossRef]
- Koper, M.T.M. Thermodynamic theory of multi-electron transfer reactions: Implications for electrocatalysis. J. Electroanal. Chem. 2011, 660, 254–260. [Google Scholar] [CrossRef]
- Song, Y.B.; Chin, D.-T. Current efficiency and polarization behavior of trivalent chromium electrodeposition process. Electrochim. Acta 2002, 48, 349–356. [Google Scholar] [CrossRef]
- Zeng, Z.; Sun, Y.; Zhang, J. The electrochemical reduction mechanism of trivalent chromium in the presence of formic acid. Electrochem. Commun. 2009, 11, 331–334. [Google Scholar] [CrossRef]
- Vargalyuk, V.F.; Seredyuk, V.O. Features of the process of electroreduction of chromium(III) cations. Nauk. Visnyk Chernivetskogo Universytetu. Seriya Khimiya 2008, 399-400, 119–121. (In Ukrainian) [Google Scholar]
- Seredyuk, V.O.; Vargalyuk, V.F. Effect of the composition of inner sphere of Cr3+ cations on the dynamics of chromium deposition. Vopr. Khimii I Khimicheskoi Tekhnologii 2011, 4, 174–176. (In Ukrainian) [Google Scholar]
- Kuznetsov, V.V.; Vinokurov, E.G.; Kudryavtsev, V.N. Kinetics of electroreduction of Cr3+ ions in sulfate solutions. Russ. J. Electrochem. 2001, 37, 699–703. [Google Scholar] [CrossRef]
- Kuznetsov, V.V.; Vinokurov, E.G.; Kudryavtsev, V.N. Effect of hydrodynamic electrolysis conditions on the kinetics of cathodic processes in chromium(III) sulfate electrolytes. Russ. J. Electrochem. 2000, 36, 756–760. [Google Scholar] [CrossRef]
- Ben-Ali, M.N.; Danilov, F.I.; Mandryka, M.M. Chromium(III) ion electroreduction from a formate bath. Sov. Electrochem. 1991, 27, 484–487. [Google Scholar]
- Danilov, F.I.; Protsenko, V.S. Kinetics and mechanism of chromium electroplating from Cr(III) baths. Prot. Met. 2001, 37, 223–228. [Google Scholar] [CrossRef]
- Danilov, F.I.; Protsenko, V.S.; Butyrina, T.E. Chromium electrodeposition kinetics in solutions of Cr(III) complex ions. Russ. J. Electrochem. 2001, 37, 704–709. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Butyrina, T.E.; Danilov, F.I. Applying a theory of generalized variables to electrochemical kinetics: Interpreting the results of studying chromium deposition from Cr(III) baths. Prot. Met. 2007, 43, 398–406. [Google Scholar] [CrossRef]
- Protsenko, V.; Danilov, F. Kinetics and mechanism of chromium electrodeposition from formate and oxalate solutions of Cr(III) compounds. Electrochim. Acta 2009, 54, 5666–5672. [Google Scholar] [CrossRef]
- Danilov, F.I.; Protsenko, V.S.; Gordiienko, V.O. Electrode processes occurring during the electrodeposition of chromium-carbon coatings from solutions of Cr(III) salts with carbamide and formic acid additions. Russ. J. Electrochem. 2013, 49, 475–482. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised Pourbaix diagrams for chromium at 25–300 °C. Corros. Sci. 1997, 39, 43–57. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Danilov, F.I. Application of dimensional analysis and similarity theory for simulation of electrode kinetics described by the Marcus-Hush-Chidsey formalism. J. Electroanal. Chem. 2012, 669, 50–54. [Google Scholar] [CrossRef]
- Protsenko, V.S. Using the similarity theory and dimensional analysis to quantify the periodic dependence of the properties of chemical elements. RSC Adv. 2023, 13, 8327–8337. [Google Scholar] [CrossRef]
- Protsenko, V.S. Dimensional analysis and similarity theory in electrochemistry. In Advances in Chemistry Research; Taylor, J.C., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2020; Volume 59, Chapter 4, pp. 153–185. [Google Scholar]
- Lira, I. Dimensional analysis made simple. Eur. J. Phys. 2013, 34, 1391–1401. [Google Scholar] [CrossRef]
- Phuong, N.V.; Kwon, S.-C.; Lee, J.-Y.; Shin, J.; Huy, B.T.; Lee, Y.-I. Mechanistic study on the effect of PEG molecules in a trivalent chromium electrodeposition process. Microchem. J. 2011, 99, 7–14. [Google Scholar] [CrossRef]
- Phuong, N.V.; Kwon, S.C.; Lee, J.Y.; Lee, J.H.; Lee, K.H. The effects of pH and polyethylene glycol on the Cr(III) solution chemistry and electrodeposition of chromium. Surf. Coat. Technol. 2012, 206, 4349–4355. [Google Scholar] [CrossRef]
- Li, L.; Wang, Z.; Wang, M.-Y.; Zhang, Y. Modulation of active Cr(III) complexes by bath preparation to adjust Cr(III) electrodeposition. Int. J. Miner. Metall. Mater. 2013, 20, 902–908. [Google Scholar] [CrossRef]
- Lu, C.-E.; Lee, J.-L.; Sheu, H.-H.; Hou, K.-H.; Tseng, C.-C.; Ger, M.-D. Preparation and characterizations of high carbon content Cr-C coatings electroplated from a trivalent chromium-based bath. Int. J. Electrochem. Sci. 2015, 10, 5405–5419. [Google Scholar] [CrossRef]
- Wijenberg, J.H.O.J.; Steegh, M.; Aarnts, M.P.; Lammers, K.R.; Mol, J.M.C. Electrodeposition of mixed chromium metal-carbide-oxide coatings from a trivalent chromium-formate electrolyte without a buffering agent. Electrochim. Acta 2015, 173, 819–826. [Google Scholar] [CrossRef]
- Danilov, F.; Obraztsov, V.; Kapitonov, A. The inhibiting effect of organic substances at polycrystalline and amalgam electrodes. J. Electroanal. Chem. 2003, 552, 69–76. [Google Scholar] [CrossRef]
- Burdykina, R.I. Electrochemistry of Compounds of Chromium (III) and Chromium (VI); Voronezhskii Politekhnicheskii Institute Publishers: Voronezh, Russia, 1991; 166p. [Google Scholar]
- Burdykina, R.I.; Falicheva, A.I. Oscillopolarographic investigation of the reaction of Cr(III) reduction in the presence of aminoacetic acids. Elektrokhimiya 1973, 9, 1272–1274. [Google Scholar]
- Surviliene, S.; Nivinskiene, O.; Cesuniene, A.; Selskis, A. Effect of Cr(III) solution chemistry on electrodeposition of chromium. J. Appl. Electrochem. 2006, 36, 649–654. [Google Scholar] [CrossRef]
- Surviliene, S.; Jasulaitiene, V.; Nivinskiene, O.; Cesuniene, A. Effect of hydrazine and hydroxylaminophosphate on chrome plating from trivalent electrolytes. Appl. Surf. Sci. 2007, 253, 6738–6743. [Google Scholar] [CrossRef]
- Demin, A.A.; Nechaev, E.A.; Danilov, F.I. Influence of organic substances on chromium electrodeposition from Cr(III)-based electrolytes. Sov. Electrochem. 1987, 23, 235–238. [Google Scholar]
- Danilov, F.I.; Protsenko, V.S.; Butyrina, T.E.; Vasil’eva, E.A.; Baskevich, A.S. Electroplating of chromium coatings from Cr(III)-based electrolytes containing water soluble polymer. Prot. Met. 2006, 42, 560–569. [Google Scholar] [CrossRef]
- Protsenko, V.; Gordiienko, V.; Butyrina, T.; Vasil’eva, E.; Danilov, F. Hard chromium electrodeposition from a trivalent chromium bath containing water-soluble polymer. Turk. J. Chem. 2014, 38, 50–55. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, M.; Kwon, S.-C. Effect of polyethylene glycol on electrochemically deposited trivalent chromium layers. Trans. Nonferr. Met. Soc. China 2009, 19, 819–823. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, L.; Niu, D.; Xu, H.; Zhang, X.; Hu, S. Effect of methoxypolyethylene glycol on trivalent chromium electrodeposition. Int. J. Electrochem. Sci. 2019, 14, 6586–6602. [Google Scholar] [CrossRef]
- Kuznetsov, V.V.; Vinokurov, E.G.; Telezhkina, A.V.; Filatova, E.A. Electrodeposition of corrosion-resistant Cr–P and Cr–P–W coatings from solutions based on compounds of trivalent chromium. J. Solid State Electrochem. 2019, 23, 2367–2376. [Google Scholar] [CrossRef]
- Li, B.; Lin, A.; Gan, F. Preparation and characterization of Cr–P coatings by electrodeposition from trivalent chromium electrolytes using malonic acid as complex. Surf. Coat. Technol. 2006, 201, 2578–2586. [Google Scholar] [CrossRef]
- Zeng, Z.; Liang, A.; Zhang, J. Electrochemical corrosion behavior of chromium–phosphorus coatings electrodeposited from trivalent chromium baths. Electrochim. Acta 2008, 53, 7344–7349. [Google Scholar] [CrossRef]
- Ramezani-Varzaneh, H.A.; Allahkaram, S.R.; Isakhani-Zakaria, M. Effects of phosphorus content on corrosion behavior of trivalent chromium coatings in 3.5 wt.% NaCl solution. Surf. Coat. Technol. 2014, 244, 158–165. [Google Scholar] [CrossRef]
- Schröder, N.; Sonntag, B.; Plieth, W. Deposition of amorphous chromium layers. J. Solid State Electrochem. 2012, 16, 3551–3558. [Google Scholar] [CrossRef]
- Baral, A.; Engelken, R. Modeling, optimization, and comparative analysis of trivalent chromium electrodeposition from aqueous glycine and formic acid baths. J. Electrochem. Soc. 2005, 152, C504–C512. [Google Scholar] [CrossRef]
- Edigaryan, A.A.; Safonof, V.A.; Lubnin, E.N.; Vykhodtseva, L.N.; Chusova, G.E.; Polukarov, Y.M. Properties and preparation of amorphous chromium carbide electroplates. Electrochim. Acta 2002, 47, 2775–2786. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, L.; Liang, A.; Chen, L.; Zhang, J. Fabrication of a nanocrystalline Cr-C layer with excellent anti-wear performance. Mater. Lett. 2007, 61, 4107–4109. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, L.; Liang, A.; Zhang, J. Tribological and electrochemical behavior of thick Cr-C alloy coatings electrodeposited in trivalent chromium bath as an alternative to conventional Cr coatings. Electrochim. Acta 2006, 52, 1366–1373. [Google Scholar] [CrossRef]
- Huang, C.A.; Liu, Y.W.; Yu, C.; Yang, C.-C. Role of carbon in the chromium deposit electroplated from a trivalent chromium-based bath. Surf. Coat. Technol. 2011, 205, 3461–3466. [Google Scholar] [CrossRef]
- Sziraki, L.; Kuzmann, E.; Papp, K.; Chisholm, C.U.; El-Sharif, M.R.; Havancsak, K. Electrochemical behaviour of amorphous electrodeposited chromium coatings. Mater. Chem. Phys. 2012, 133, 1092–1100. [Google Scholar] [CrossRef]
- Liang, A.; Zhang, J. Why the decorative chromium coating electrodeposited from trivalent chromium electrolyte containing formic acid is darker. Surf. Coat. Technol. 2012, 206, 3614–3618. [Google Scholar] [CrossRef]
- Chien, C.W.; Liu, C.L.; Chen, F.J.; Lin, K.H.; Lin, C.S. Microstructure and properties of carbon–sulfur-containing chromium deposits electroplated in trivalent chromium baths with thiosalicylic acid. Electrochim. Acta 2012, 72, 74–80. [Google Scholar] [CrossRef]
- Safonova, O.V.; Vykhodtseva, L.N.; Polyakov, N.A.; Swarbrick, J.C.; Sikora, M.; Glatzel, P.; Safonov, V.A. Chemical composition and structural transformations of amorphous chromium coatings electrodeposited from Cr(III) electrolytes. Electrochim. Acta 2010, 56, 145–153. [Google Scholar] [CrossRef]
- Safonov, V.A.; Vykhodtseva, L.N.; Polukarov, Y.M.; Safonova, O.V.; Smolentsev, G.; Sikora, M.; Eeckhout, S.G.; Glatzel, P. Valence-to-core X-ray emission spectroscopy identification of carbide compounds in nanocrystalline Cr coatings deposited from Cr(III) electrolytes containing organic substances. J. Phys. Chem. B 2006, 110, 23192–23196. [Google Scholar] [CrossRef] [PubMed]
- Safonov, V.A.; Habazaki, H.; Glatzel, P.; Fishgoit, L.A.; Drozhzhin, O.A.; Lafuerza, S.; Safonova, O.V. Application of valence-to-core X-ray emission spectroscopy for identification and estimation of amount of carbon covalently bonded to chromium in amorphous Cr-C coatings prepared by magnetron sputtering. Appl. Surf. Sci. 2018, 427, 566–572. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Gordiienko, V.O.; Danilov, F.I. Unusual “chemical” mechanism of carbon co-deposition in Cr-C alloy electrodeposition process from trivalent chromium bath. Electrochem. Commun. 2012, 17, 85–87. [Google Scholar] [CrossRef]
- Song, S.-J.; Ko, S.J.; Lee, J.-R.; Kim, J.-G. Effect of bis-3-sulfopropyl-disulfide on the characteristics of trivalent chromium electrodeposition. Int. J. Electrochem. Sci. 2018, 13, 7489–7504. [Google Scholar] [CrossRef]
- Büker, L.; Dickbreder, R.; Böttcher, R.; Sadowski, S.; Bund, A. Investigation of the reaction kinetics of chromium(III) ions with carboxylic acids in aqueous solutions and the associated effects on chromium deposition. J. Electrochem. Soc. 2020, 167, 162509. [Google Scholar] [CrossRef]
- Xu, L.; Pi, L.; Dou, Y.; Cui, Y.; Mao, X.; Lin, A.; Fernandez, C.; Peng, C. Electroplating of thick hard chromium coating from a trivalent chromium bath containing a ternary complexing agent: A methodological and mechanistic study. ACS Sustain. Chem. Eng. 2020, 8, 15540–15549. [Google Scholar] [CrossRef]
- Danilov, F.I.; Protsenko, V.S. Chromium electrodeposition using electrolytes based on trivalent chromium compounds: A review. Voprosy Khimii i Khimicheskoi Tekhnologii 2020, 2, 4–29. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Liu, F.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Electrodeposition of metals and alloys from ionic liquids. J. Alloys Compd. 2016, 654, 163–170. [Google Scholar] [CrossRef]
- Surviliene, S.; Eugenio, S.; Vilar, R. Chromium electrodeposition from [BMIm][BF4] ionic liquid. J. Appl. Electrochem. 2011, 41, 107–114. [Google Scholar] [CrossRef]
- Eugenio, S.; Rangel, C.M.; Vilar, R.; Botelho do Rego, A.M. Electrodeposition of black chromium spectrally selective coatings from a Cr(III)–ionic liquid solution. Thin Solid Films 2011, 519, 1845–1850. [Google Scholar] [CrossRef]
- Eugenio, S.; Rangel, C.M.; Vilar, R.; Quaresma, S. Electrochemical aspects of black chromium electrodeposition from 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid. Electrochim. Acta 2011, 56, 10347–10352. [Google Scholar] [CrossRef][Green Version]
- He, X.; Hou, B.; Li, C.; Zhu, Q.; Jiang, Y.; Wu, L. Electrochemical mechanism of trivalent chromium reduction in 1-butyl-3-methylimidazolium bromide ionic liquid. Electrochim. Acta 2014, 130, 245–252. [Google Scholar] [CrossRef]
- He, X.; Zhu, Q.; Hou, B.; Cai, Y.; Li, C.; Fu, L.; Wu, L. Electrodeposition of nanocrystalline chromium coatings based on 1-butyl-3-methylimidazolium-bromide ionic liquid. J. Nanosci. Nanotechnol. 2015, 15, 9431–9437. [Google Scholar] [CrossRef]
- He, X.; Li, C.; Jiang, Y.; Zhu, Q.; Wang, W.; Zhang, C.; Wu, L. Electrochemical mechanism of Cr(III) reduction for preparing crystalline chromium coatings based on 1-ethyl-3-methylimidazolium bisulfate ionic liquid. J. Electrochem. Soc. 2015, 162, D435–D443. [Google Scholar] [CrossRef]
- Sun, L.; Brennecke, J.F. Characterization of imidazolium chloride ionic liquids plus trivalent chromium chloride for chromium electroplating. Ind. Eng. Chem. Res. 2015, 54, 4879–4890. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.D.O.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Tome, L.I.N.; Baiao, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Abbott, A.P.; McKenzie, K.J. Application of ionic liquids to the electrodeposition of metals. Phys. Chem. Chem. Phys. 2006, 8, 4265–4279. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ryder, K.S.; König, U. Electrofinishing of metals using eutectic based ionic liquids. Trans. Inst. Met. Finish. 2008, 86, 196–204. [Google Scholar] [CrossRef][Green Version]
- Abbott, A.P.; Frisch, G.; Ryder, K.S. Electroplating using ionic liquids. Annu. Rev. Mater. Res. 2013, 43, 335–358. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K. Ionic liquid analogues formed from hydrated metal salts. Chem. Eur. J. 2004, 10, 3769–3774. [Google Scholar] [CrossRef]
- Abbott, A.P.; Al-Barzinjy, A.A.; Abbott, P.D.; Frish, G.; Harris, R.C.; Hartley, J.; Ryder, K.S. Speciation, physical and electrolytic properties of eutectic mixtures based on CrCl3⋅6H2O and urea. Phys. Chem. Chem. Phys. 2014, 16, 9047–9055. [Google Scholar] [CrossRef]
- Ferreira, E.S.C.; Pereira, C.M.; Silva, A.F. Electrochemical studies of metallic chromium electrodeposition from a Cr(III) bath. J. Electroanal. Chem. 2013, 707, 52–58. [Google Scholar] [CrossRef]
- Mejia-Caballero, I.; Aldana-Gonzalez, J.; Manh, T.L.; Romero-Romo, M.; Arce-Estrada, E.M.; Campos-Silva, I.; Ramirez-Silva, M.T.; Palomar-Pardave, M. Mechanism and kinetics of chromium electrochemical nucleation and growth from a choline chloride/ethylene glycol deep eutectic solvent. J. Electrochem. Soc. 2018, 165, D393–D401. [Google Scholar] [CrossRef]
- Qian, H.; Sun, J.; Li, Q.; Sun, H.; Fu, X. Electrochemical mechanism of trivalent chromium reduction in ChCl-EG deep eutectic solvents containing trivalent chromium. J. Electrochem. Soc. 2020, 167, 102511. [Google Scholar] [CrossRef]
- Mares (Badea), M.L.; Ciocirlan, O.; Cojocaru, A.; Anicai, L. Physico-chemical and electrochemical studies in choline chloride based ionic liquid analogues containing trivalent chromium chloride. Rev. Chim. 2013, 64, 815–824. [Google Scholar]
- Ma, C.; Laaksonen, A.; Liu, C.; Lu, X.; Ji, X. The peculiar effect of water on ionic liquids and deep eutectic solvents. Chem. Soc. Rev. 2018, 47, 8685–8720. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The effect of water upon deep eutectic solvent nanostructure: An unusual transition from ionic mixture to aqueous solution. Angew. Chem. Int. Ed. 2017, 56, 9782–9785. [Google Scholar] [CrossRef]
- Alfurayj, I.; Fraenza, C.C.; Zhang, Y.; Pandian, R.; Spittle, S.; Hansen, B.; Dean, W.; Gurkan, B.; Savinell, R.; Greenbaum, S.; et al. Solvation dynamics of wet ethaline: Water is the magic component. J. Phys. Chem. B 2021, 125, 8888–8901. [Google Scholar] [CrossRef]
- McCalman, D.C.; Sun, L.; Zhang, Y.; Brennecke, J.F.; Maginn, E.J.; Schneider, W.F. Speciation, conductivities, diffusivities, and electrochemical reduction as a function of water content in mixtures of hydrated chromium chloride/choline chloride. J. Phys. Chem. B 2015, 119, 6018–6023. [Google Scholar] [CrossRef]
- Bobrova, L.S.; Danilov, F.I.; Protsenko, V.S. Effects of temperature and water content on physicochemical properties of ionic liquids containing CrCl3⋅xH2O and choline chloride. J. Mol. Liq. 2016, 223, 48–53. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Bobrova, L.S.; Danilov, F.I. Physicochemical properties of ionic liquid mixtures containing choline chloride, chromium (III) chloride and water: Effects of temperature and water content. Ionics 2017, 23, 637–643. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Bobrova, L.S.; Baskevich, A.S.; Korniy, S.A.; Danilov, F.I. Electrodeposition of chromium coatings from a choline chloride based ionic liquid with the addition of water. J. Chem. Technol. Metall. 2018, 53, 906–915. [Google Scholar]
- Protsenko, V.; Bobrova, L.; Danilov, F. Trivalent chromium electrodeposition using a deep eutectic solvent. Anti-Corros. Methods Mater. 2018, 65, 499–505. [Google Scholar] [CrossRef]
- Abbott, A.P.; El Ttaib, K.; Ryder, K.S.; Smith, E.L. Electrodeposition of nickel using eutectic based ionic liquids. Trans. Inst. Met. Finish. 2008, 86, 234–240. [Google Scholar] [CrossRef]
- Protsenko, V.; Bobrova, L.; Kityk, A.; Danilov, F. Kinetics of Cr(III) ions discharge in solutions based on a deep eutectic solvent (ethaline): Effect of water addition. J. Electroanal. Chem. 2020, 864, 114086. [Google Scholar] [CrossRef]
- Mejía-Caballero, I.; Le Manh, T.; Aldana-González, J.; Arce-Estrada, E.M.; Romero-Romo, M.; Campos-Silva, I.; Ramírez-Silva, M.T.; Palomar-Pardavé, M. Electrodeposition of nanostructured chromium conglomerates from Cr(III) dissolved in a deep eutectic solvent: Influence of forced convection. J. Electrochem. Soc. 2021, 168, 112512. [Google Scholar] [CrossRef]
- Barzinjy, A.A.A. Surface roughness reduction of Cr-films deposited from novel deep eutectic solvent: Effect of acetylacetone. Surf. Rev. Lett. 2019, 26, 1850150. [Google Scholar] [CrossRef]
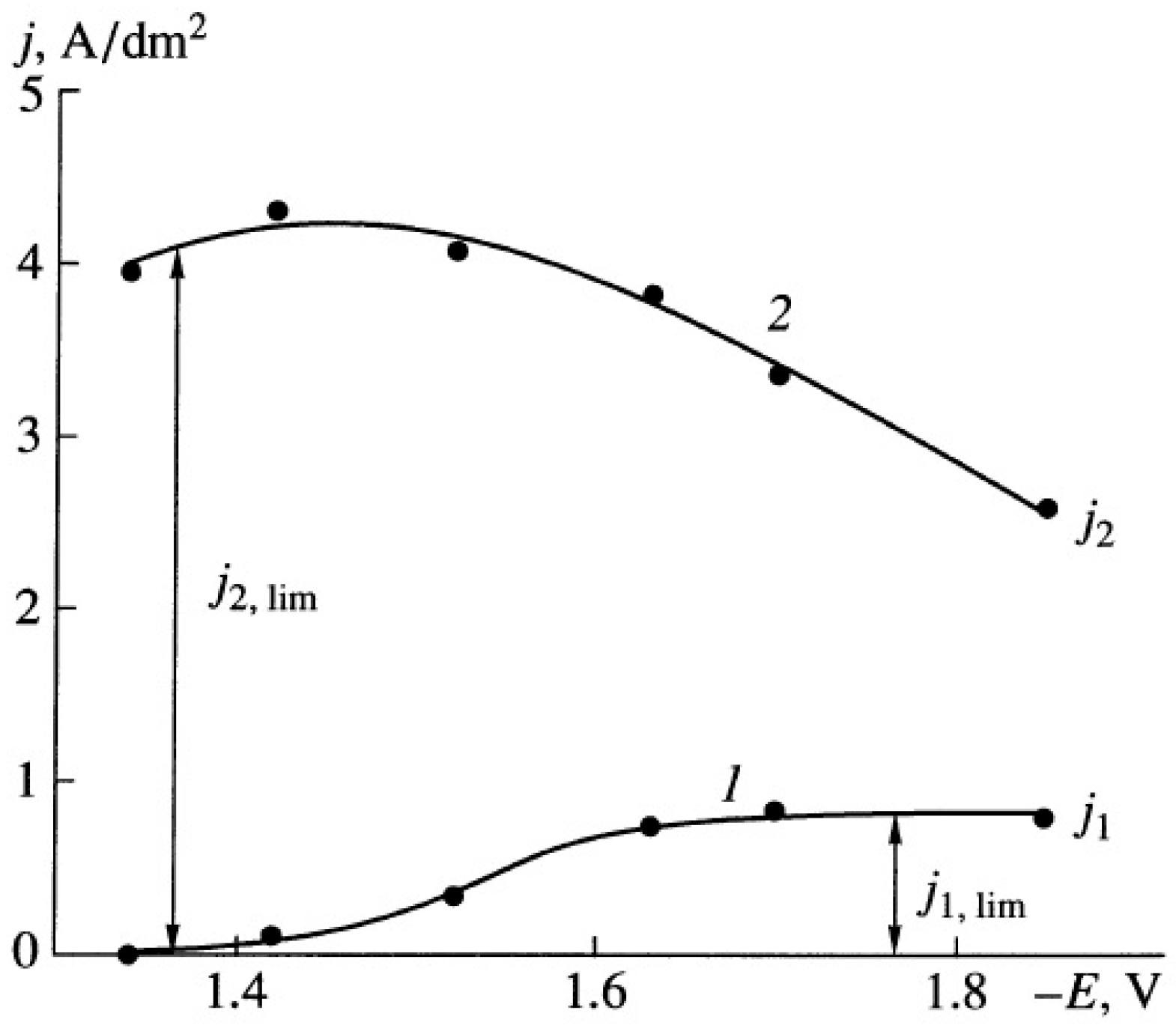
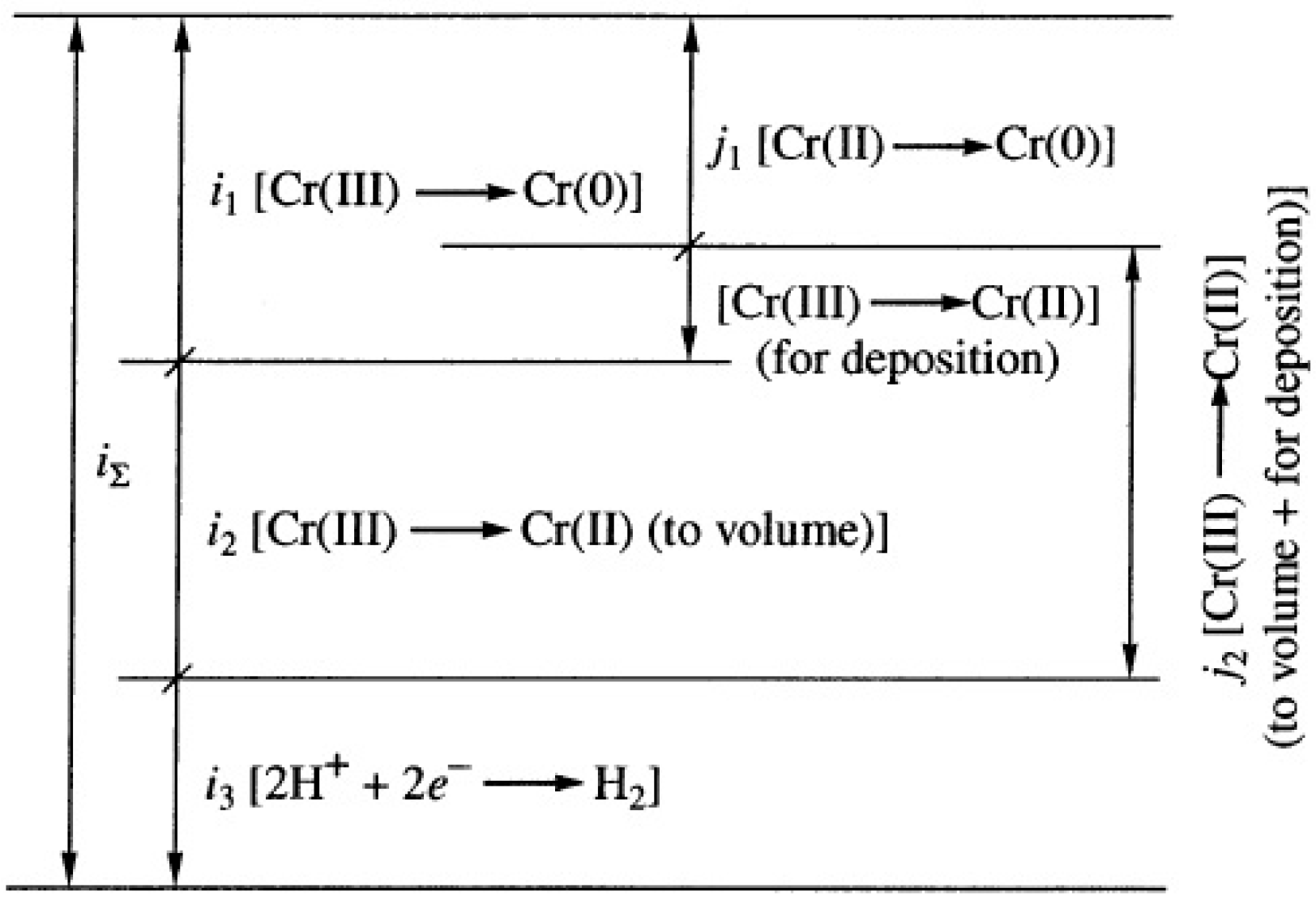
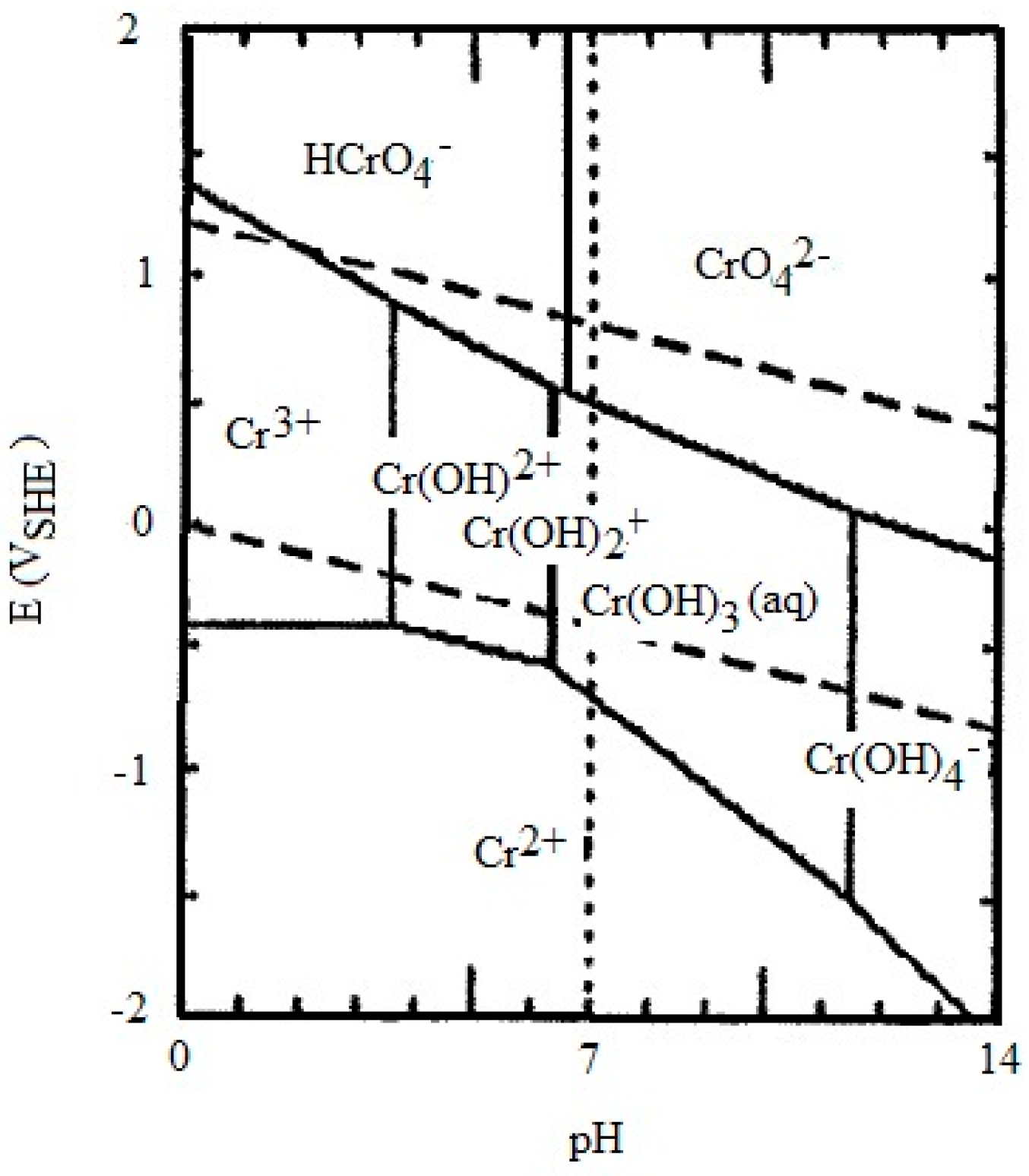
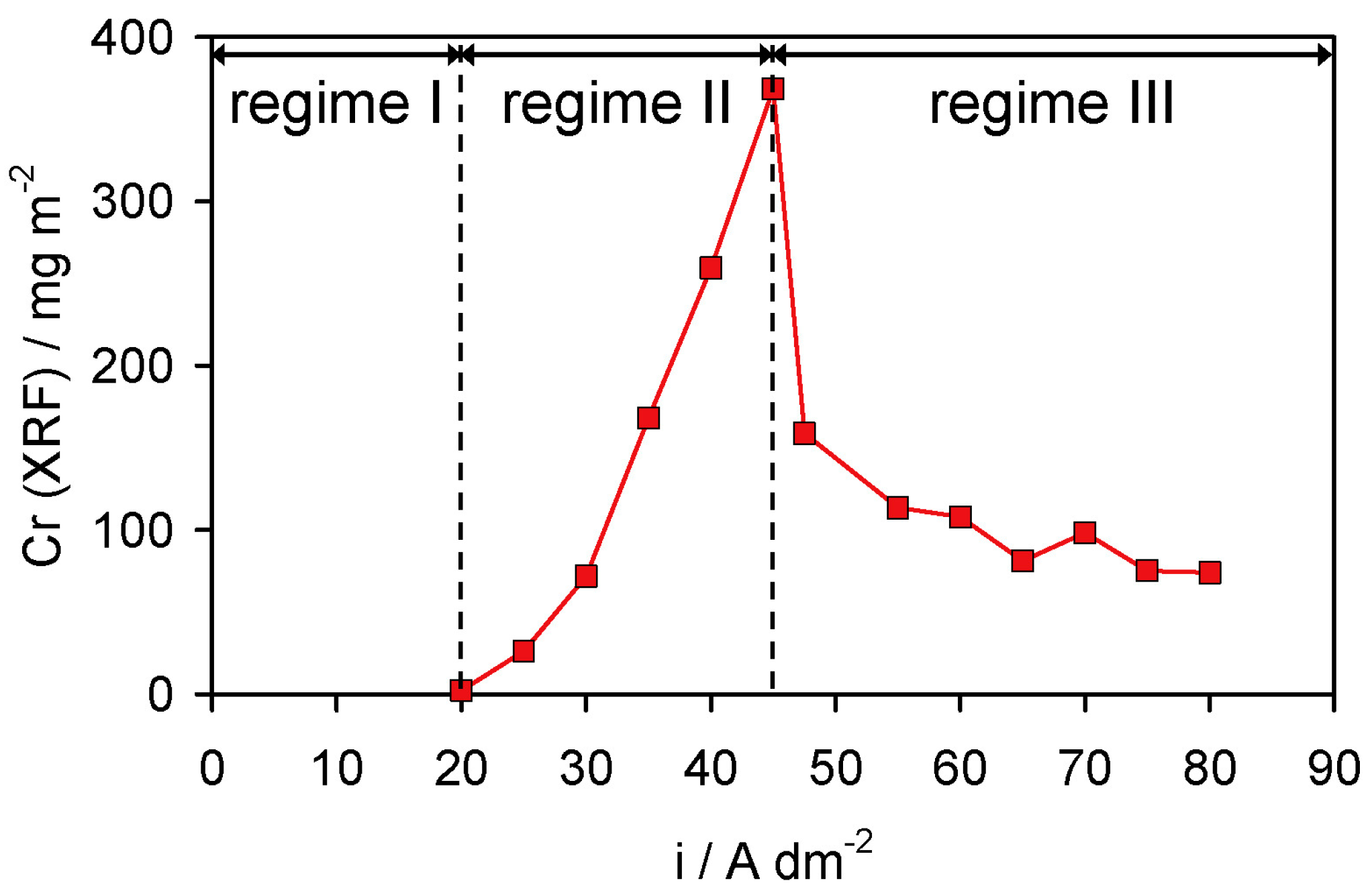
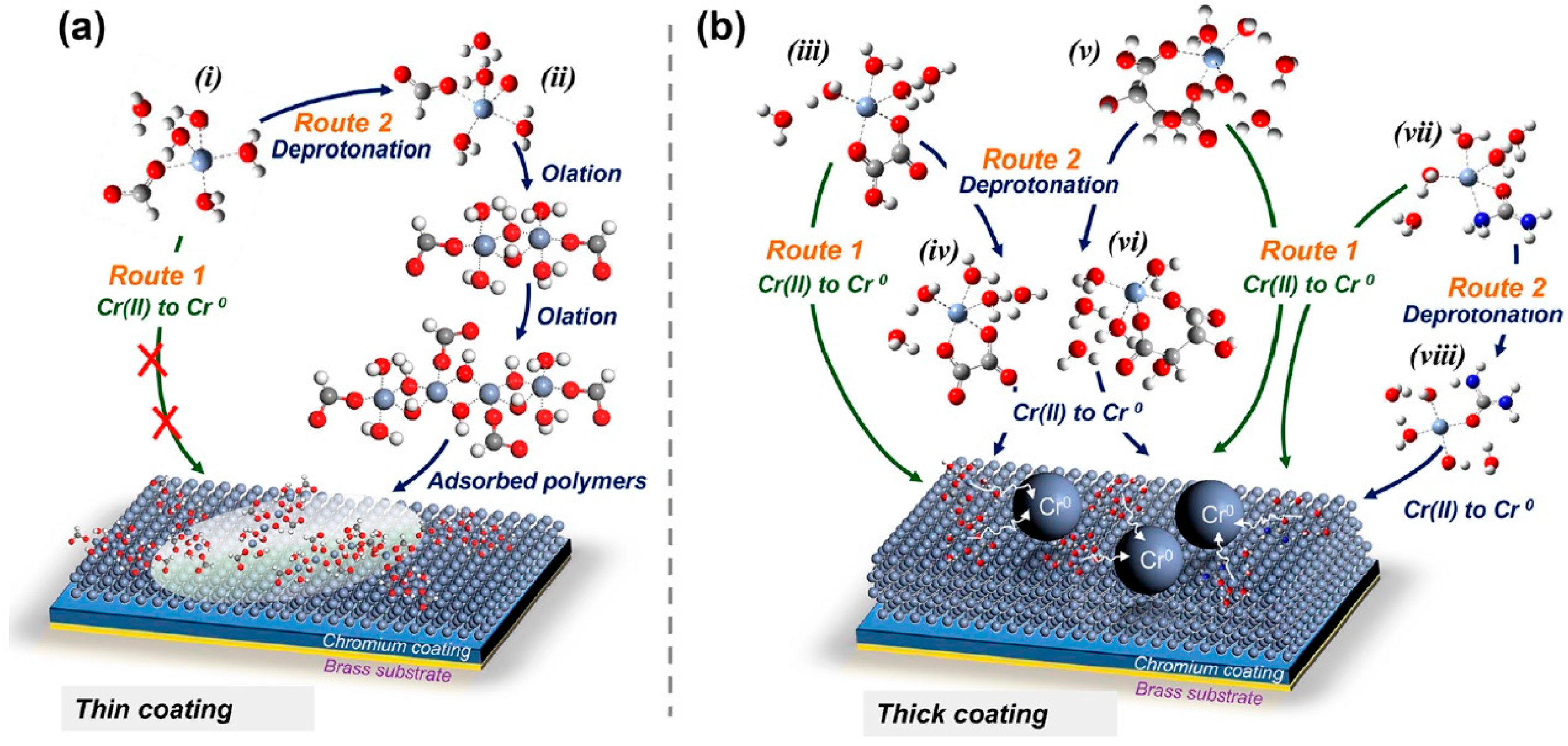
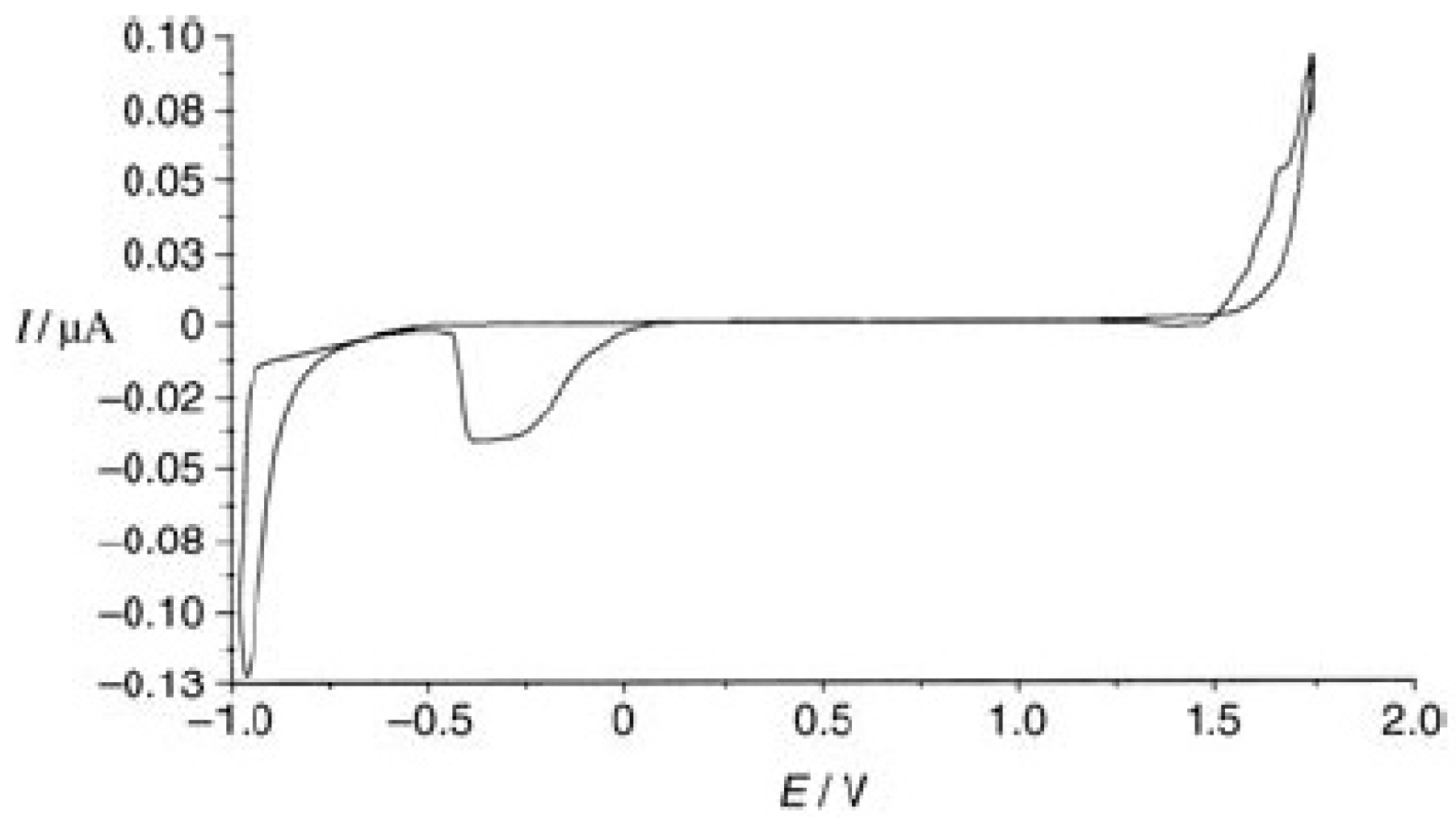
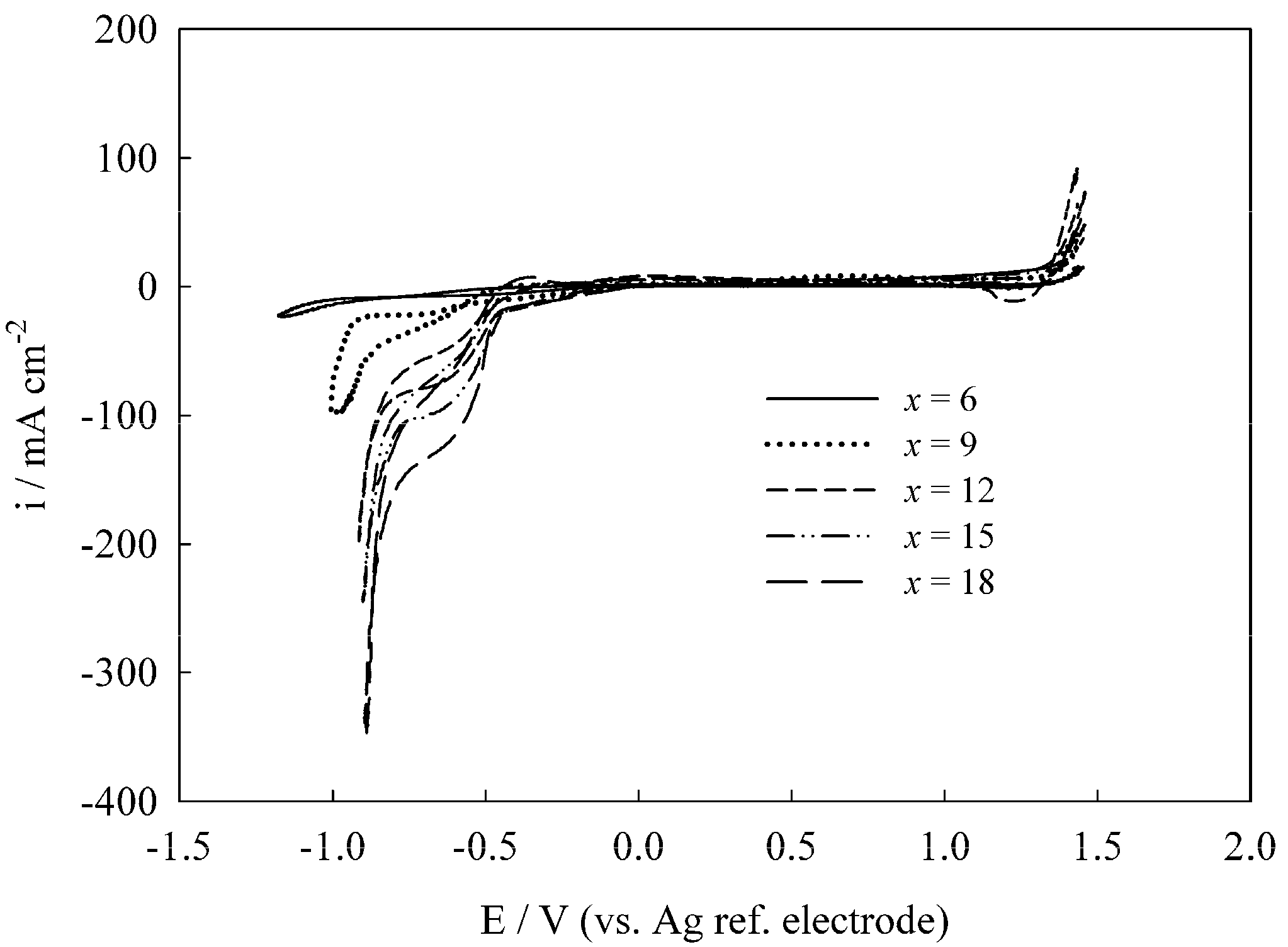
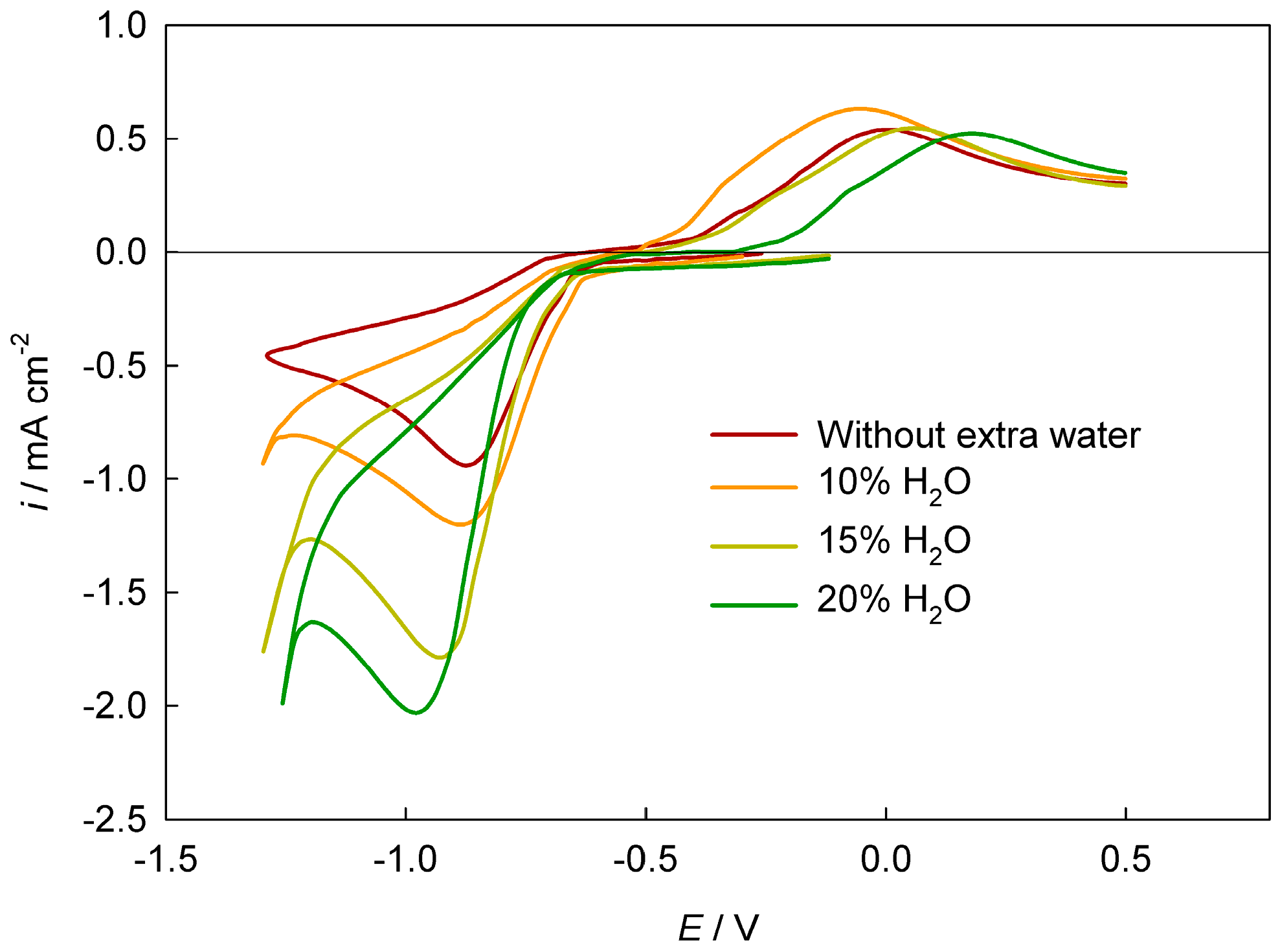
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Protsenko, V.S. Kinetics and Mechanism of Electrochemical Reactions Occurring during the Chromium Electrodeposition from Electrolytes Based on Cr(III) Compounds: A Literature Review. Reactions 2023, 4, 398-419. https://doi.org/10.3390/reactions4030024
Protsenko VS. Kinetics and Mechanism of Electrochemical Reactions Occurring during the Chromium Electrodeposition from Electrolytes Based on Cr(III) Compounds: A Literature Review. Reactions. 2023; 4(3):398-419. https://doi.org/10.3390/reactions4030024
Chicago/Turabian StyleProtsenko, V. S. 2023. "Kinetics and Mechanism of Electrochemical Reactions Occurring during the Chromium Electrodeposition from Electrolytes Based on Cr(III) Compounds: A Literature Review" Reactions 4, no. 3: 398-419. https://doi.org/10.3390/reactions4030024
APA StyleProtsenko, V. S. (2023). Kinetics and Mechanism of Electrochemical Reactions Occurring during the Chromium Electrodeposition from Electrolytes Based on Cr(III) Compounds: A Literature Review. Reactions, 4(3), 398-419. https://doi.org/10.3390/reactions4030024





