Greener and Efficient Epoxidation of 1,5-Hexadiene with tert-Butyl Hydroperoxide (TBHP) as an Oxidising Reagent in the Presence of Polybenzimidazole Supported Mo(VI) Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polymer-Supported Mo(VI) Catalyst
2.3. Characterisation of Polymer-Supported Mo(VI) Catalyst
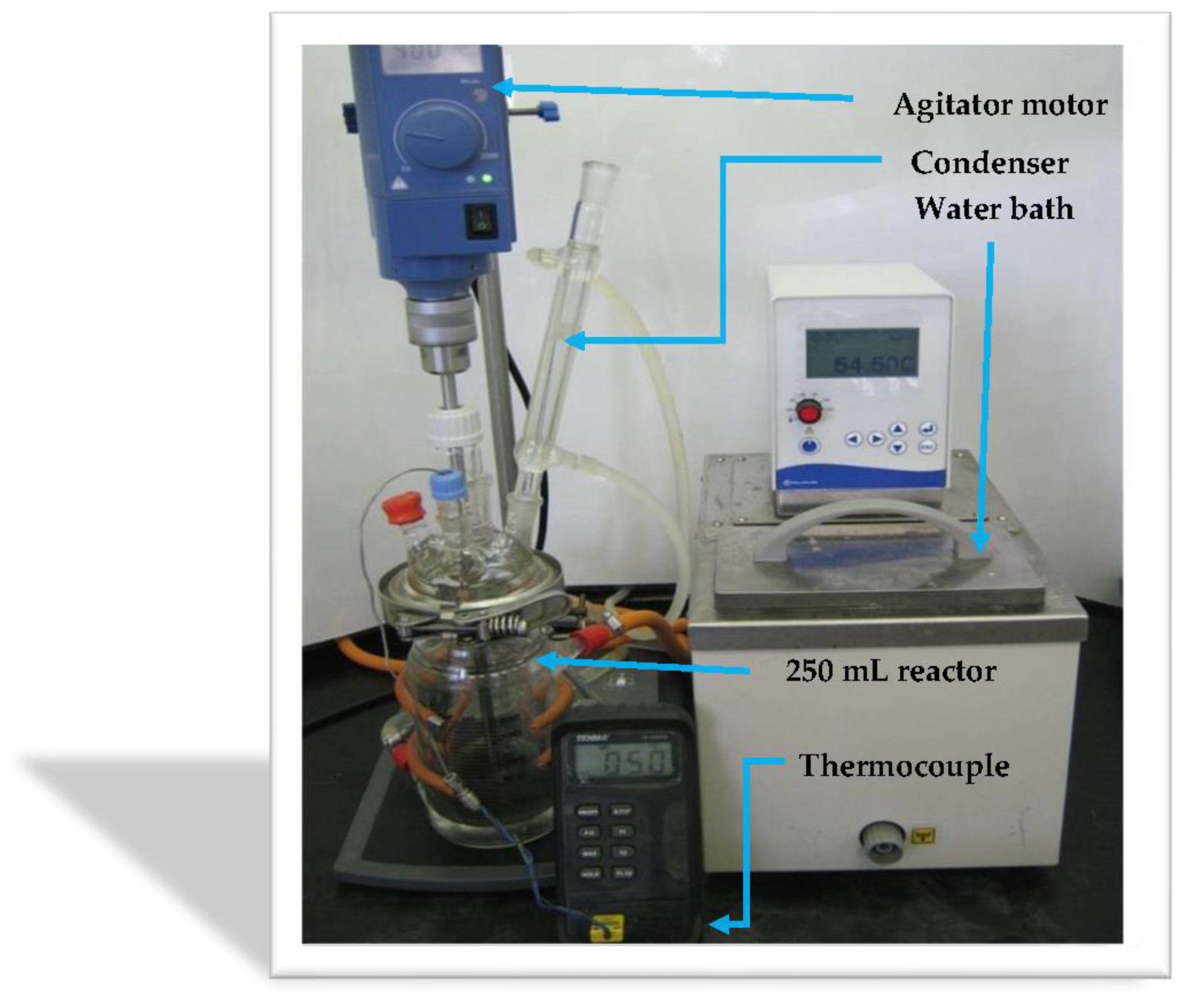
2.4. Batch Epoxidation Studies
2.5. Method of Analysis
2.6. Experimental Design
2.7. Statistical Analysis
3. Results and Discussion
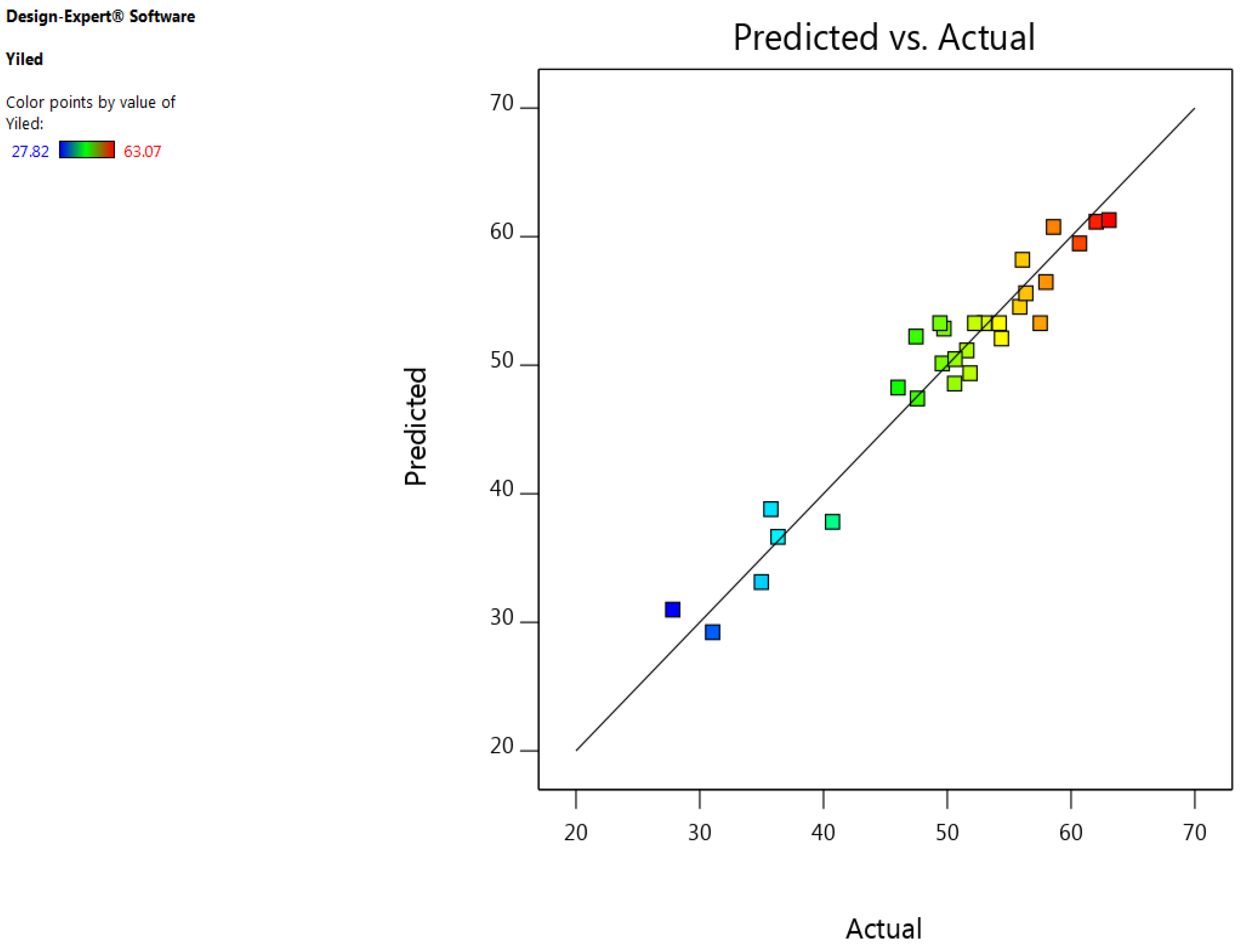
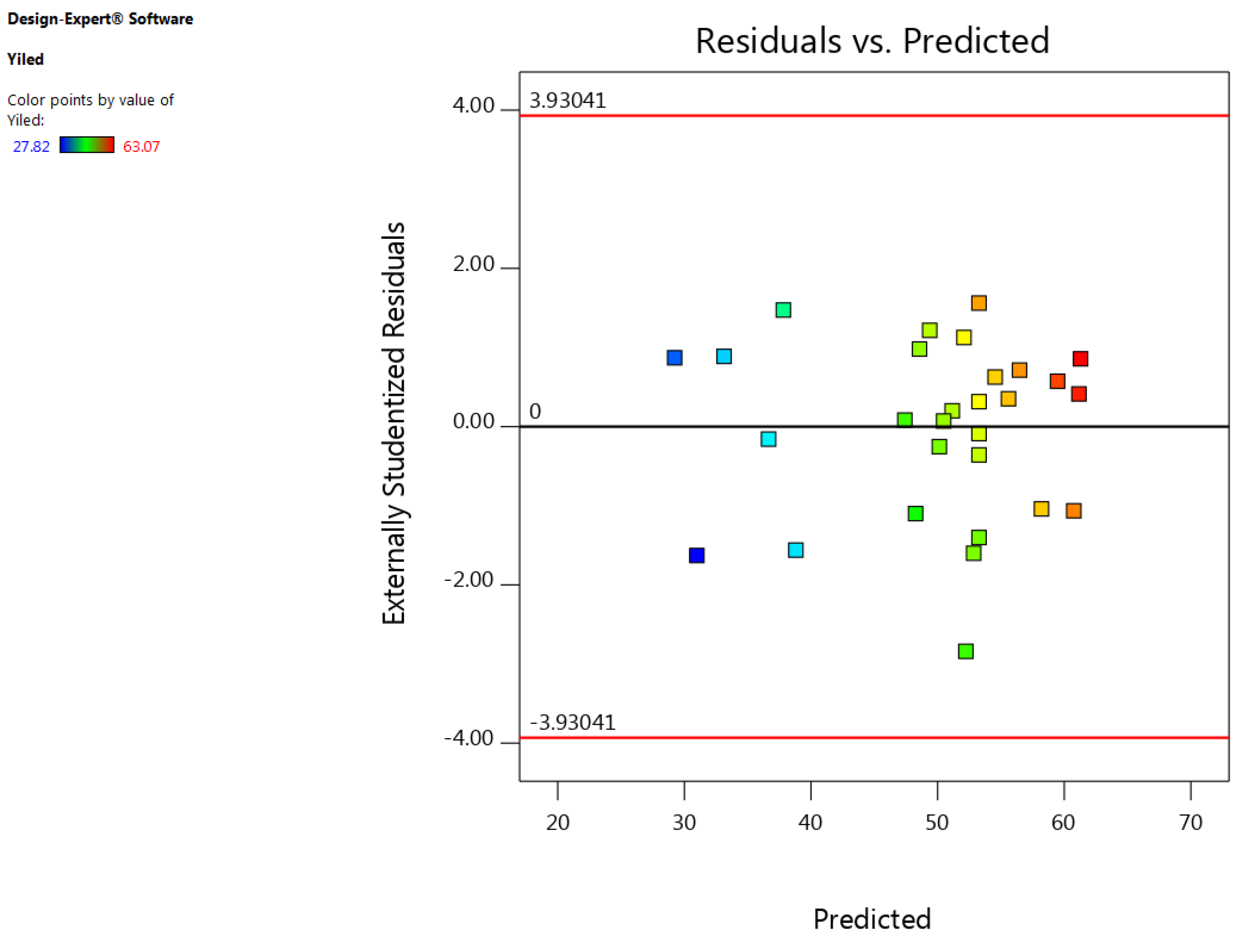
3.1. Development of Regression Model and Adequacy Checking
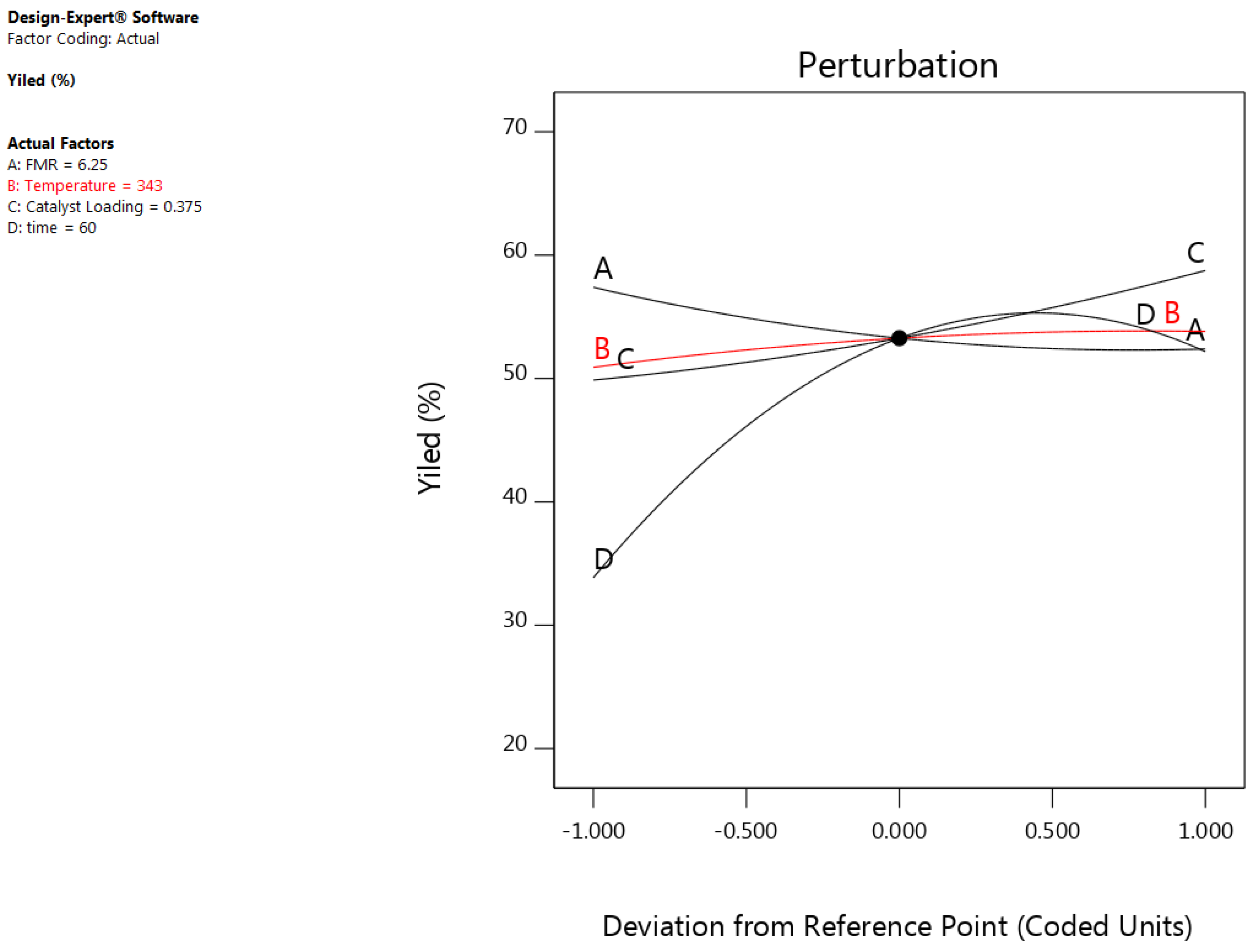
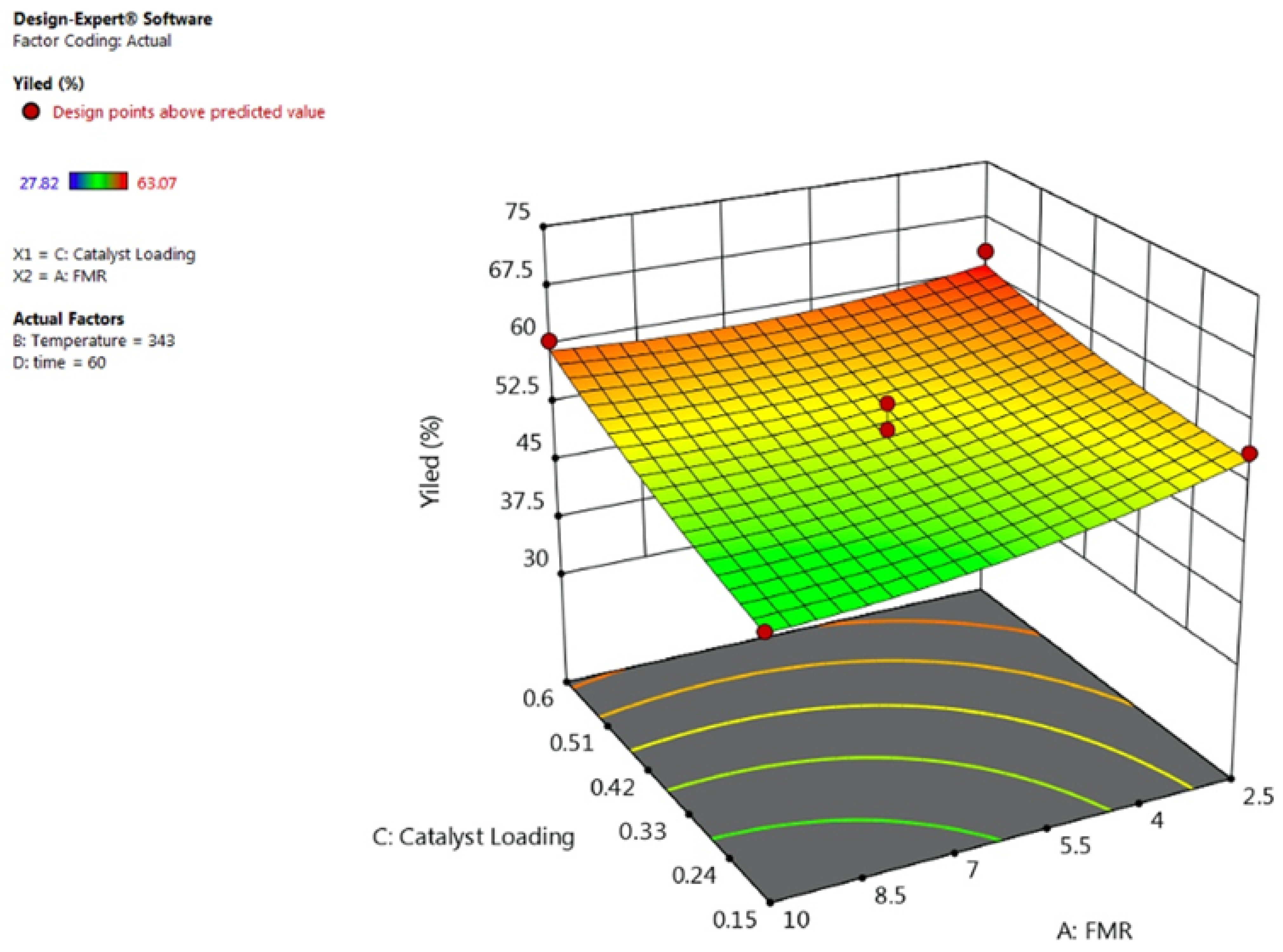
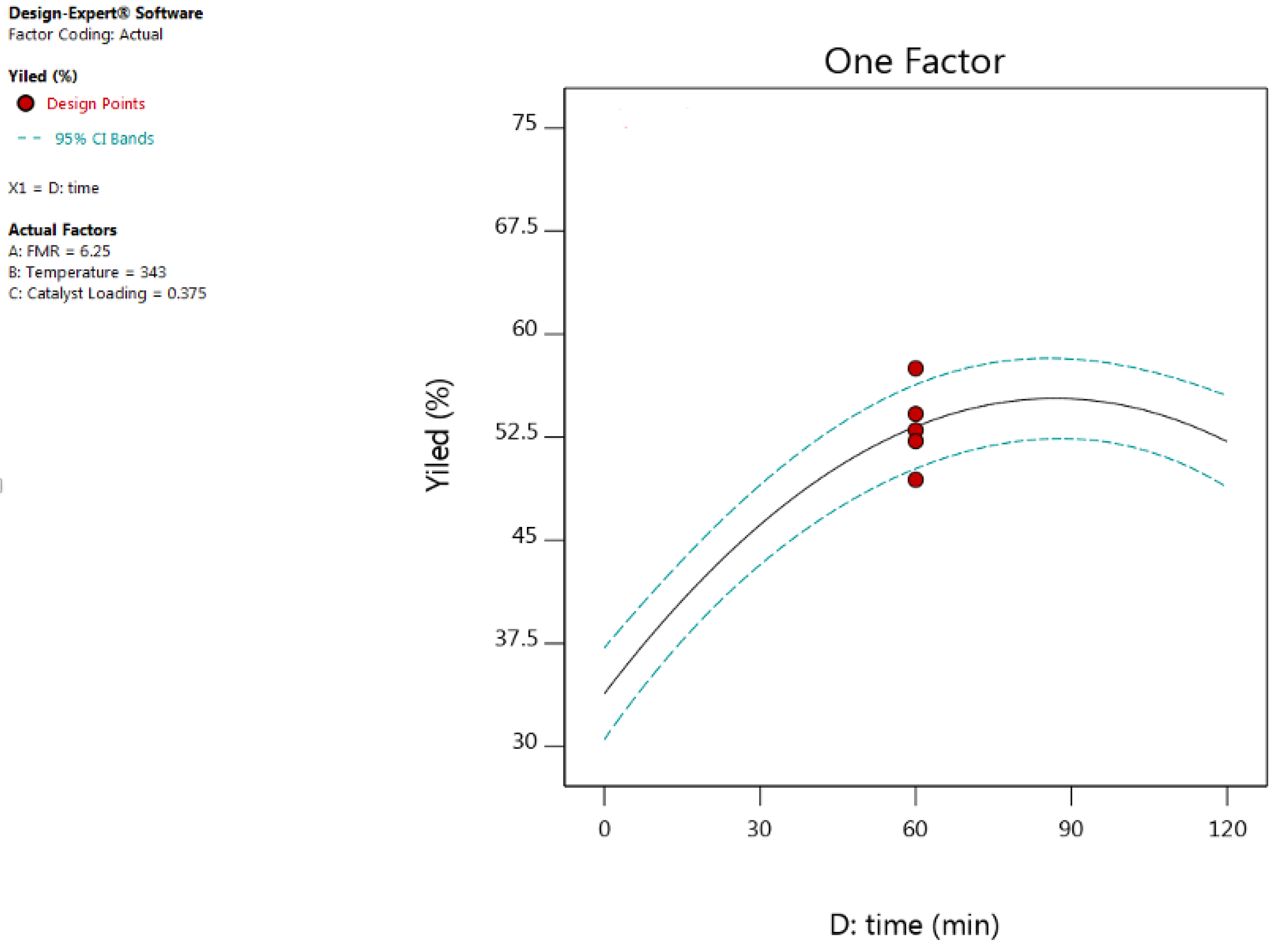
3.2. Effect of Process Variables and Their Interactions
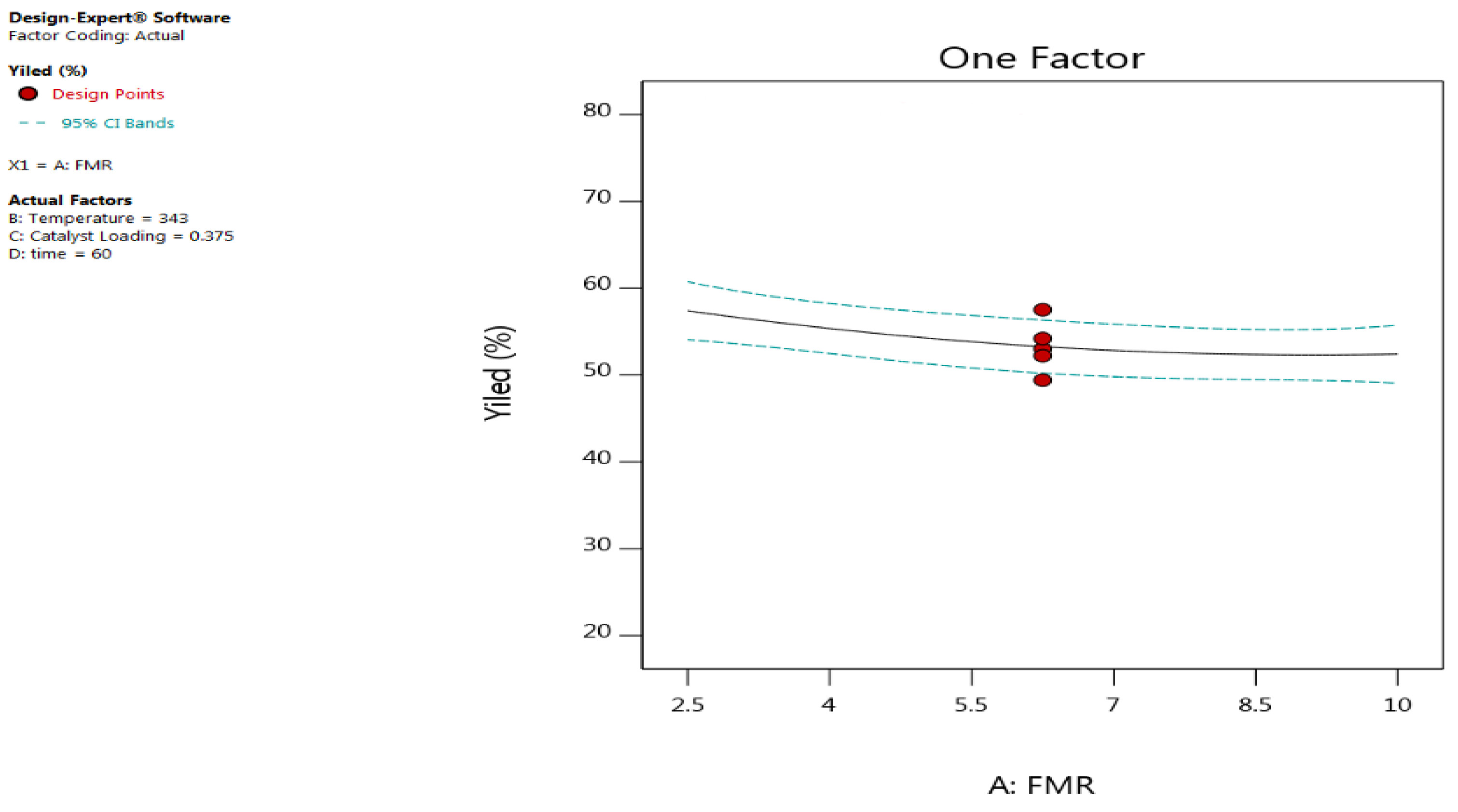
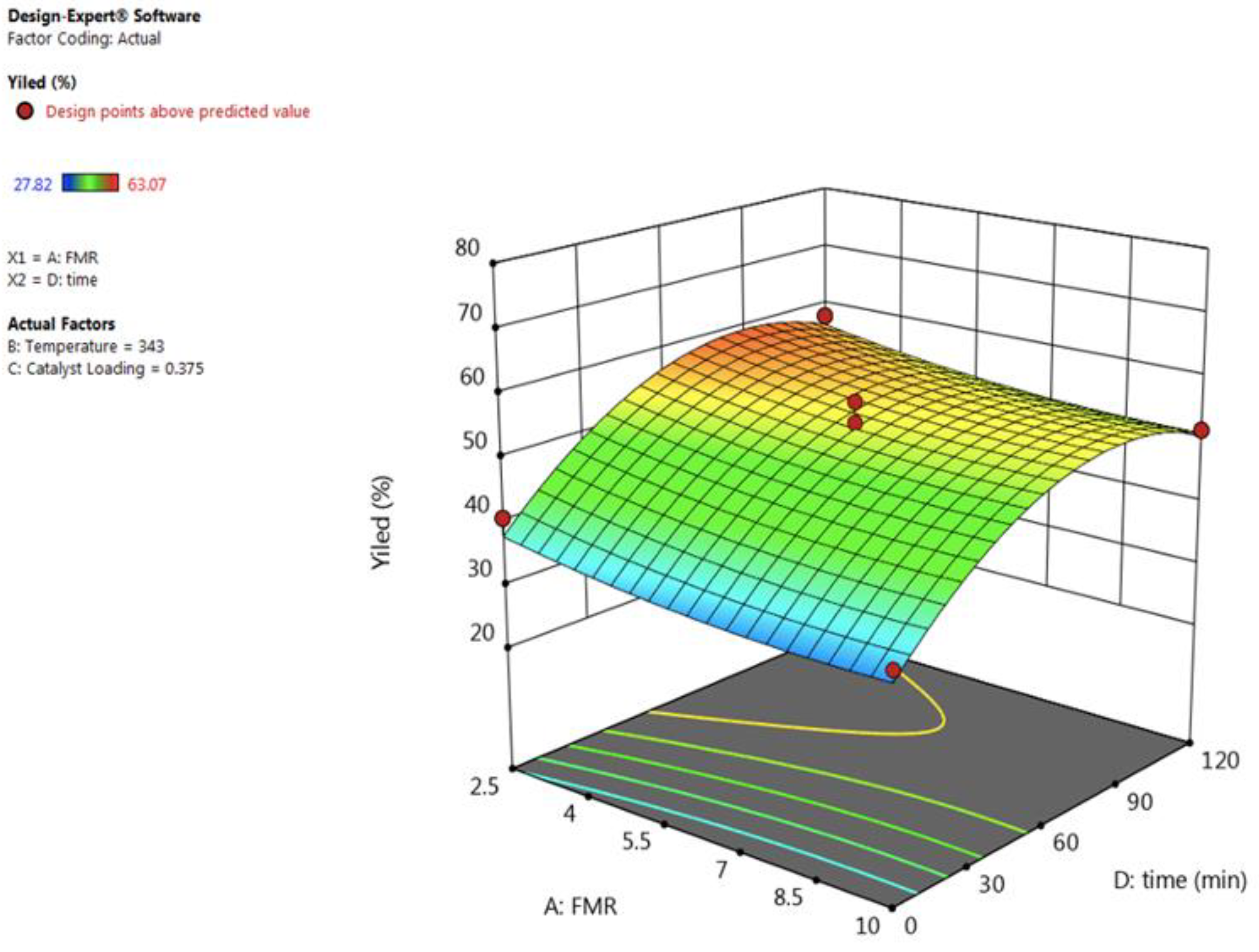
3.2.1. Effect of Feed Molar Ratio (FMR)
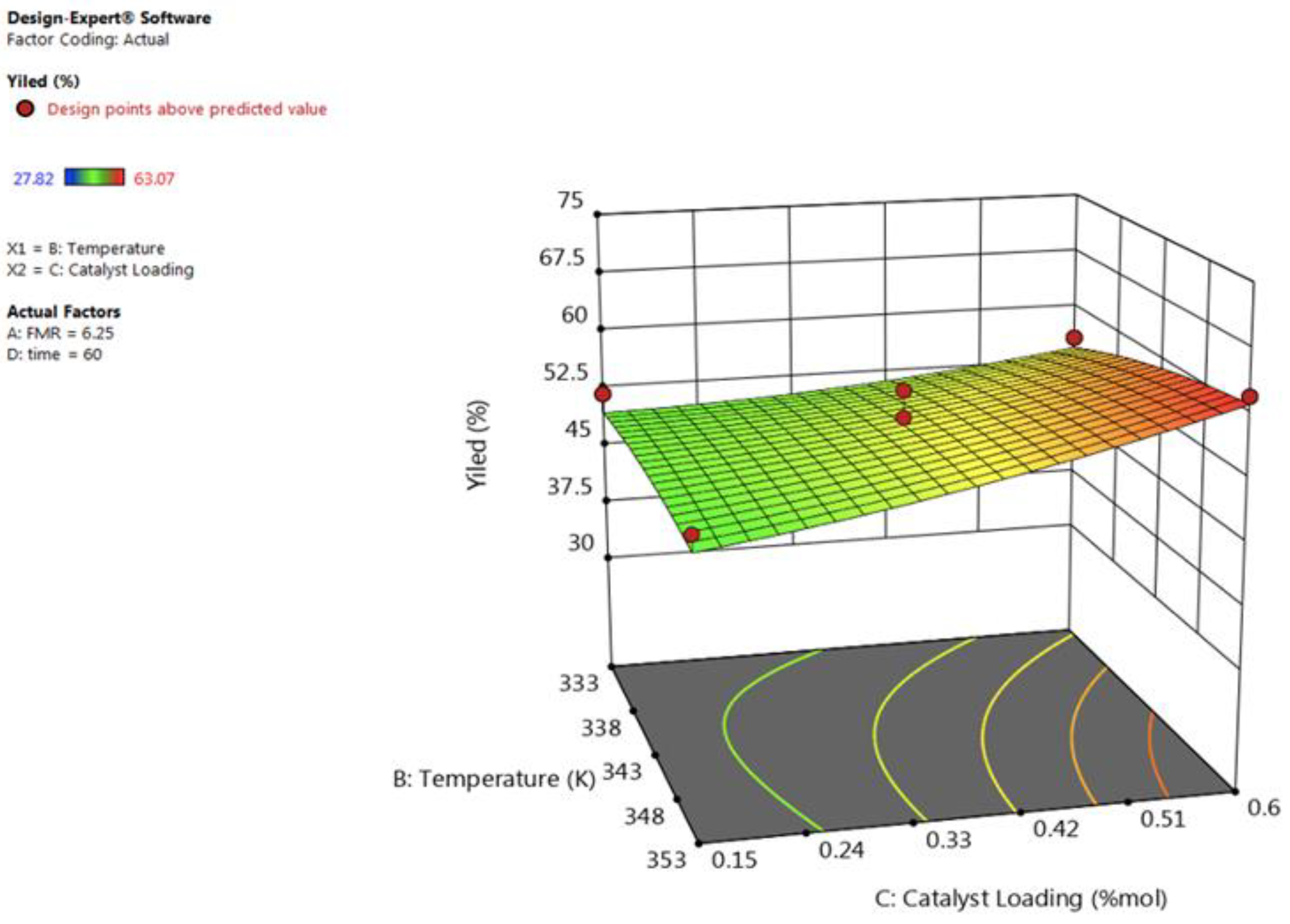
3.2.2. Effect of Reaction Temperature
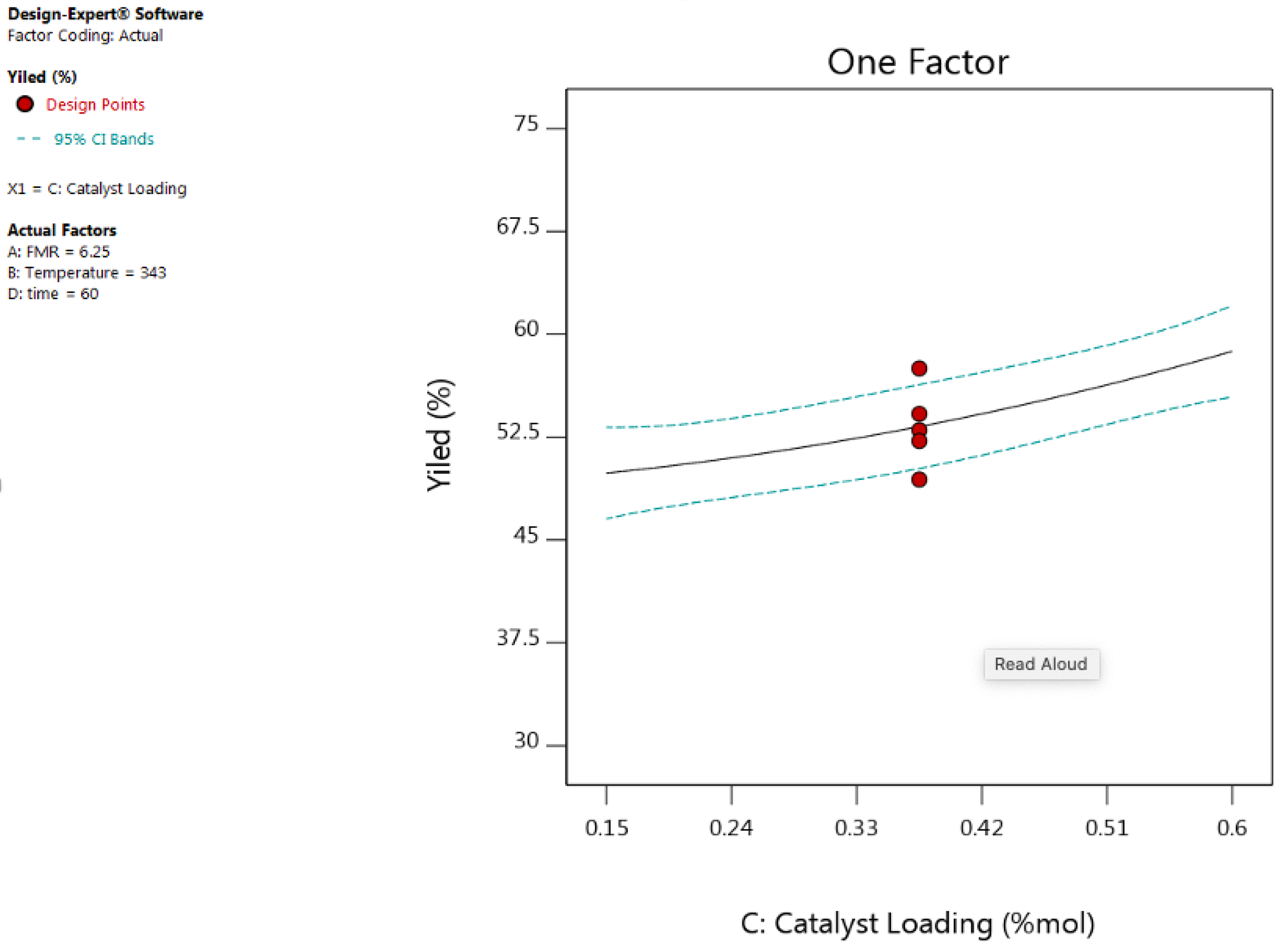
3.2.3. Effect of Catalyst Loading
3.2.4. Effect of Reaction Time
3.3. Optimisation Study of Reaction Variables
3.4. Optimum Conditions Validation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grivani, G.; Tangestaninejad, S.; Habibi, M.H.; Mirkhani, V. Epoxidation of alkenes by a highly reusable and efficient polymer-supported molybdenum carbonyl catalyst. Catal. Commun. 2005, 6, 375–378. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Saha, B. Recent Advances in Greener and Energy Efficient Alkene Epoxidation Processes. Energies 2022, 15, 2858. [Google Scholar] [CrossRef]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. Epoxidation Process. European Patent Number EP2459545B1, 28 February 2019. [Google Scholar]
- Schaus, S.E.; Brandes, B.D.; Larrow, J.F.; Tokunaga, M.; Hansen, K.B.; Gould, A.E.; Furrow, M.E.; Jacobsen, E.N. Highly Selective Hydrolytic Kinetic Resolution of Terminal Epoxides Catalyzed by Chiral (salen)CoIII Complexes. Practical Synthesis of Enantioenriched Terminal Epoxides and 1,2-Diols. J. Am. Chem. Soc. 2002, 124, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Grivani, G.; Tangestaninejad, S.; Habibi, M.H.; Mirkhani, V.; Moghadam, M. Epoxidation of alkenes by a readily prepared and highly active and reusable heterogeneous molybdenum-based catalyst. Appl. Catal. A Gen. 2006, 299, 131–136. [Google Scholar] [CrossRef]
- García, R.; Martínez, M.; Aracil, J. Enzymatic esterification of an acid with an epoxide using immobilized lipase from Mucor miehei as catalyst: Optimization of the yield and isomeric excess of ester by statistical analysis. J. Ind. Microbiol. Biotechnol. 2002, 28, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.A. Synthesis and viscoelastic characterization of novel hydrogels generated via photopolymerization of 1,2-epoxy-5-hexene modified poly(vinyl alcohol) for use in tissue replacement. Acta Biomater. 2008, 4, 967–975. [Google Scholar] [CrossRef]
- Yahya, S.N.; Lin, C.K.; Ramli, M.R.; Jaafar, M.; Ahmad, Z. Effect of cross-link density on optoelectronic properties of thermally cured 1,2-epoxy-5-hexene incorporated polysiloxane. Mater. Des. 2013, 47, 416–423. [Google Scholar] [CrossRef]
- Tangestaninejad, S.; Mirkhani, V.; Moghadam, M.; Grivani, G. Readily prepared heterogeneous molybdenum-based catalysts as highly recoverable, reusable and active catalysts for alkene epoxidation. Catal. Commun. 2007, 8, 839–844. [Google Scholar] [CrossRef]
- Miller, M.M.; Sherrington, D.C. Polybenzimidazole-supported molybdenum(VI) propene epoxidation catalyst. J. Chem. Soc. Chem. Commun. 1994, 1, 55–56. [Google Scholar] [CrossRef]
- Shi, Z.-Q.; Jiao, L.-X.; Sun, J.; Chen, Z.-B.; Chen, Y.-Z.; Zhu, X.-H.; Zhou, J.-H.; Zhou, X.-C.; Li, X.-Z.; Li, R. Cobalt nanoparticles in hollow mesoporous spheres as a highly efficient and rapid magnetically separable catalyst for selective epoxidation of styrene with molecular oxygen. RSC Adv. 2014, 4, 47–53. [Google Scholar] [CrossRef]
- Vondran, J.; Pela, J.; Palczewski, D.; Skiborowski, M.; Seidensticker, T. Curse and Blessing–The Role of Water in the Homogeneously Ru-Catalyzed Epoxidation of Technical Grade Methyl Oleate. ACS Sustain. Chem. Eng. 2021, 9, 11469–11478. [Google Scholar] [CrossRef]
- Santacesaria, E.; Tesser, R.; Di Serio, M.; Turco, R.; Russo, V.; Verde, D. A biphasic model describing soybean oil epoxidation with H2O2 in a fed-batch reactor. Chem. Eng. J. 2011, 173, 198–209. [Google Scholar] [CrossRef]
- Bechtold, K. Versatile and Vexing: The Many Uses and Hazards of Peracetic Acid. Synergist 2016. Available online: https://synergist.aiha.org/201612-peracetic-acid-uses-and-hazards (accessed on 12 April 2022).
- Kollar, J. Epoxidation Process. U.S. Patent Number US53617966A, 11 July 1967. [Google Scholar]
- Salavati-Niasari, M.; Esmaeili, E.; Seyghalkar, H.; Bazarganipour, M. Cobalt(II) Schiff base complex on multi-wall carbon nanotubes (MWNTs) by covalently grafted method: Synthesis, characterization and liquid phase epoxidation of cyclohexene by air. Inorg. Chim. Acta 2011, 375, 11–19. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, P.; Zhang, J.; Bian, G.; Zhang, P.; Dong, Y.; Zhang, W. Highly dispersed molybdenum incorporated hollow mesoporous silica spheres as an efficient catalyst on epoxidation of olefins. Mol. Catal. 2017, 433, 212–223. [Google Scholar] [CrossRef]
- Bisio, C.; Gallo, A.; Psaro, R.; Tiozzo, C.; Guidotti, M.; Carniato, F. Tungstenocene-grafted silica catalysts for the selective epoxidation of alkenes. Appl. Catal. A Gen. 2019, 581, 133–142. [Google Scholar] [CrossRef]
- Cai, L.; Chen, C.; Wang, W.; Gao, X.; Kuang, X.; Jiang, Y.; Li, L.; Wu, G. Acid-free epoxidation of soybean oil with hydrogen peroxide to epoxidized soybean oil over titanium silicalite-1 zeolite supported cadmium catalysts. Ind. Eng. Chem. Res. 2020, 91, 191–200. [Google Scholar] [CrossRef]
- Wu, Z.; He, Z.; Zhou, D.; Yang, Y.; Lu, X.; Xia, Q. One-step synthesis of bi-functional zeolite catalyst with highly exposed octahedral Co for efficient epoxidation of bulky cycloalkenes. Mater. Lett. 2020, 280, 128549. [Google Scholar] [CrossRef]
- Lueangchaichaweng, W.; Singh, B.; Mandelli, D.; Carvalho, W.A.; Fiorilli, S.; Pescarmona, P.P. High surface area, nanostructured boehmite and alumina catalysts: Synthesis and application in the sustainable epoxidation of alkenes. Appl. Catal. A Gen. 2019, 571, 180–187. [Google Scholar] [CrossRef]
- Mikolajska, E.; Calvino-Casilda, V.; Bañares, M.A. Real-time Raman monitoring of liquid-phase cyclohexene epoxidation over alumina-supported vanadium and phosphorous catalysts. Appl. Catal. A Gen. 2012, 421–422, 164–171. [Google Scholar] [CrossRef]
- Borugadda, V.B.; Goud, V.V. Epoxidation of Castor Oil Fatty Acid Methyl Esters (COFAME) as a Lubricant base Stock Using Heterogeneous Ion-exchange Resin (IR-120) as a Catalyst. Energy Procedia 2014, 54, 75–84. [Google Scholar] [CrossRef]
- Peng, C.; Lu, X.H.; Ma, X.T.; Shen, Y.; Wei, C.C.; He, J.; Zhou, D.; Xia, Q.H. Highly efficient epoxidation of cyclohexene with aqueous H2O2 over powdered anion-resin supported solid catalysts. J. Mol. Catal. A-Chem. 2016, 423, 393–399. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Mbeleck, R.; Patel, D.; Sherrington, D.C.; Saha, B. Greener route to 4-vinyl-1-cyclohexane 1,2-epoxide synthesis using batch and continuous reactors. Green Process. Synth. 2014, 3, 411–418. [Google Scholar] [CrossRef]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. Liquid Phase Epoxidation Process. U.S. Patent Number U.S. 9,248,942 B2, 2 February 2016. [Google Scholar]
- Mohammadikish, M.; Yarahmadi, S.; Molla, F. A new water-insoluble coordination polymer as efficient dye adsorbent and olefin epoxidation catalyst. J. Environ. Manag. 2020, 254, 109784. [Google Scholar] [CrossRef] [PubMed]
- Otake, K.-i.; Ahn, S.; Knapp, J.; Hupp, J.T.; Notestein, J.M.; Farha, O.K. Vapor-Phase Cyclohexene Epoxidation by Single-Ion Fe(III) Sites in Metal–Organic Frameworks. Inorg. Chem. 2021, 60, 2457–2463. [Google Scholar] [CrossRef]
- Mbeleck, R.; Mohammed, M.L.; Ambroziak, K.; Sherrington, D.C.; Saha, B. Efficient epoxidation of cyclododecene and dodecene catalysed by polybenzimidazole supported Mo(VI) complex. Catal. Today 2015, 256, 287–293. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Patel, D.; Mbeleck, R.; Niyogi, D.; Sherrington, D.C.; Saha, B. Optimisation of alkene epoxidation catalysed by polymer supported Mo(VI) complexes and application of artificial neural network for the prediction of catalytic performances. Appl. Catal. A-Gen. 2013, 466, 142–152. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Saha, B. Greener and sustainable approach for the synthesis of commercially important epoxide building blocks using polymer-supported Mo(VI) complexes as catalysts. In Ion Exchange and Solvent Extraction, 1st ed.; SenGupta, A.K., Ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group LLC: Milton Park, UK, 2016; p. 33. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. A Continuous Process for the Liquid Phase Epoxidation of an Olefinic Compound. Indian Patent No. 295846, 17 April 2018. [Google Scholar]
- Saha, B. Catalytic Reactors; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2015. [Google Scholar]
- Saha, B.; Ambroziak, K.; Sherrington, D.C.; Mbeleck, R. A Permeable Particle Container. Chinese Patent No. ZL 201410840925.7, 7 December 2016. [Google Scholar]
- Mbeleck, R.; Ambroziak, K.; Saha, B.; Sherrington, D.C. Stability and recycling of polymer-supported Mo(VI) alkene epoxidation catalysts. React. Funct. Polym. 2007, 67, 1448–1457. [Google Scholar] [CrossRef]
- Aboelazayem, O.; Gadalla, M.; Saha, B. Biodiesel production from waste cooking oil via supercritical methanol: Optimisation and reactor simulation. Renew. Energ. 2018, 124, 144–154. [Google Scholar] [CrossRef]
- Onyenkeadi, V.; Aboelazayem, O.; Saha, B. Systematic multivariate optimisation of butylene carbonate synthesis via CO2 utilisation using graphene-inorganic nanocomposite catalysts. Catal. Today 2020, 346, 10–22. [Google Scholar] [CrossRef]
- El-Gendy, N.S.; Deriase, S.F.; Hamdy, A. The Optimization of Biodiesel Production from Waste Frying Corn Oil Using Snails Shells as a Catalyst. Energ. Source Part A. 2014, 36, 623–637. [Google Scholar] [CrossRef]
- Yuan, J.; Huang, J.; Wu, G.; Tong, J.; Xie, G.-Y.; Duan, J.-a.; Qin, M. Multiple responses optimization of ultrasonic-assisted extraction by response surface methodology (RSM) for rapid analysis of bioactive compounds in the flower head of Chrysanthemum morifolium Ramat. Ind. Crop Prod. 2015, 74, 192–199. [Google Scholar] [CrossRef]
- Mohammed, M.L.; Mbeleck, R.; Patel, D.; Niyogi, D.; Sherrington, D.C.; Saha, B. Greener and efficient epoxidation of 4-vinyl-1-cyclohexene with polystyrene 2-(aminomethyl)pyridine supported Mo(VI) catalyst in batch and continuous reactors. Chem. Eng. Res. Des. 2015, 94, 194–203. [Google Scholar] [CrossRef]
- Ambroziak, K.; Mbeleck, R.; He, Y.; Saha, B.; Sherrington, D.C. Investigation of batch alkene epoxidations catalyzed by polymer-supported Mo(VI) complexes. Ind. Eng. Chem. Res. 2009, 48, 3293–3302. [Google Scholar] [CrossRef]




| Catalyst Properties | Values Obtained |
|---|---|
| BET surface area | 18.44 m2/g |
| Pore volume | 0.021986 cm3/g |
| Mo loading (mmol Mo g−1 resin) | 0.825 |
|
Average Pore diameter Average particle size | 21.595 Å 210–295 μm |
| Factors | Code | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| FMR | A | 2.5 | 6.25 | 10 |
| Temperature (K) | B | 333 | 343 | 353 |
| Catalyst loading (mol%) | C | 0.15 | 0.375 | 0.6 |
| Time (min) | D | 0 | 60 | 120 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 2230.55 | 14 | 159.33 | 15.69 | < 0.0001 | Significant |
| A-FMR | 75.00 | 1 | 75.00 | 7.39 | 0.0167 | Significant |
| B-Temperature | 25.35 | 1 | 25.35 | 2.50 | 0.1364 | Not significant |
| C-Catalyst Loading | 236.47 | 1 | 236.47 | 23.29 | 0.0003 | significant |
| D-time | 1008.52 | 1 | 1008.52 | 99.34 | < 0.0001 | significant |
| AB | 31.58 | 1 | 31.58 | 3.11 | 0.0996 | not significant |
| AC | 10.18 | 1 | 10.18 | 1.00 | 0.3337 | not significant |
| AD | 0.1024 | 1 | 0.1024 | 0.0101 | 0.9214 | not significant |
| BC | 13.80 | 1 | 13.80 | 1.36 | 0.2631 | not significant |
| BD | 20.39 | 1 | 20.39 | 2.01 | 0.1783 | not significant |
| CD | 1.12 | 1 | 1.12 | 0.1107 | 0.7443 | not significant |
| A² | 17.20 | 1 | 17.20 | 1.69 | 0.2141 | not significant |
| B² | 5.30 | 1 | 5.30 | 0.5223 | 0.4817 | not significant |
| C² | 7.11 | 1 | 7.11 | 0.7005 | 0.4167 | not significant |
| D² | 681.87 | 1 | 681.87 | 67.17 | < 0.0001 | significant |
| Residual | 142.13 | 14 | 10.15 | |||
| Lack of Fit | 107.22 | 10 | 10.72 | 1.23 | 0.4557 | not significant |
| Pure Error | 34.91 | 4 | 8.73 | |||
| Cor Total | 2372.68 | 28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuiyan, M.M.R.; Mohammed, M.L.; Saha, B. Greener and Efficient Epoxidation of 1,5-Hexadiene with tert-Butyl Hydroperoxide (TBHP) as an Oxidising Reagent in the Presence of Polybenzimidazole Supported Mo(VI) Catalyst. Reactions 2022, 3, 537-552. https://doi.org/10.3390/reactions3040036
Bhuiyan MMR, Mohammed ML, Saha B. Greener and Efficient Epoxidation of 1,5-Hexadiene with tert-Butyl Hydroperoxide (TBHP) as an Oxidising Reagent in the Presence of Polybenzimidazole Supported Mo(VI) Catalyst. Reactions. 2022; 3(4):537-552. https://doi.org/10.3390/reactions3040036
Chicago/Turabian StyleBhuiyan, Md Masud Rana, Misbahu Ladan Mohammed, and Basudeb Saha. 2022. "Greener and Efficient Epoxidation of 1,5-Hexadiene with tert-Butyl Hydroperoxide (TBHP) as an Oxidising Reagent in the Presence of Polybenzimidazole Supported Mo(VI) Catalyst" Reactions 3, no. 4: 537-552. https://doi.org/10.3390/reactions3040036
APA StyleBhuiyan, M. M. R., Mohammed, M. L., & Saha, B. (2022). Greener and Efficient Epoxidation of 1,5-Hexadiene with tert-Butyl Hydroperoxide (TBHP) as an Oxidising Reagent in the Presence of Polybenzimidazole Supported Mo(VI) Catalyst. Reactions, 3(4), 537-552. https://doi.org/10.3390/reactions3040036






