Grafting of Cellulose and Microcrystalline Cellulose with Oligo(L-lactic acid) by Polycondensation Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of OLLA
2.3. Preparation of α- Cellulose from Jute Fiber
2.4. Preparation of MCC
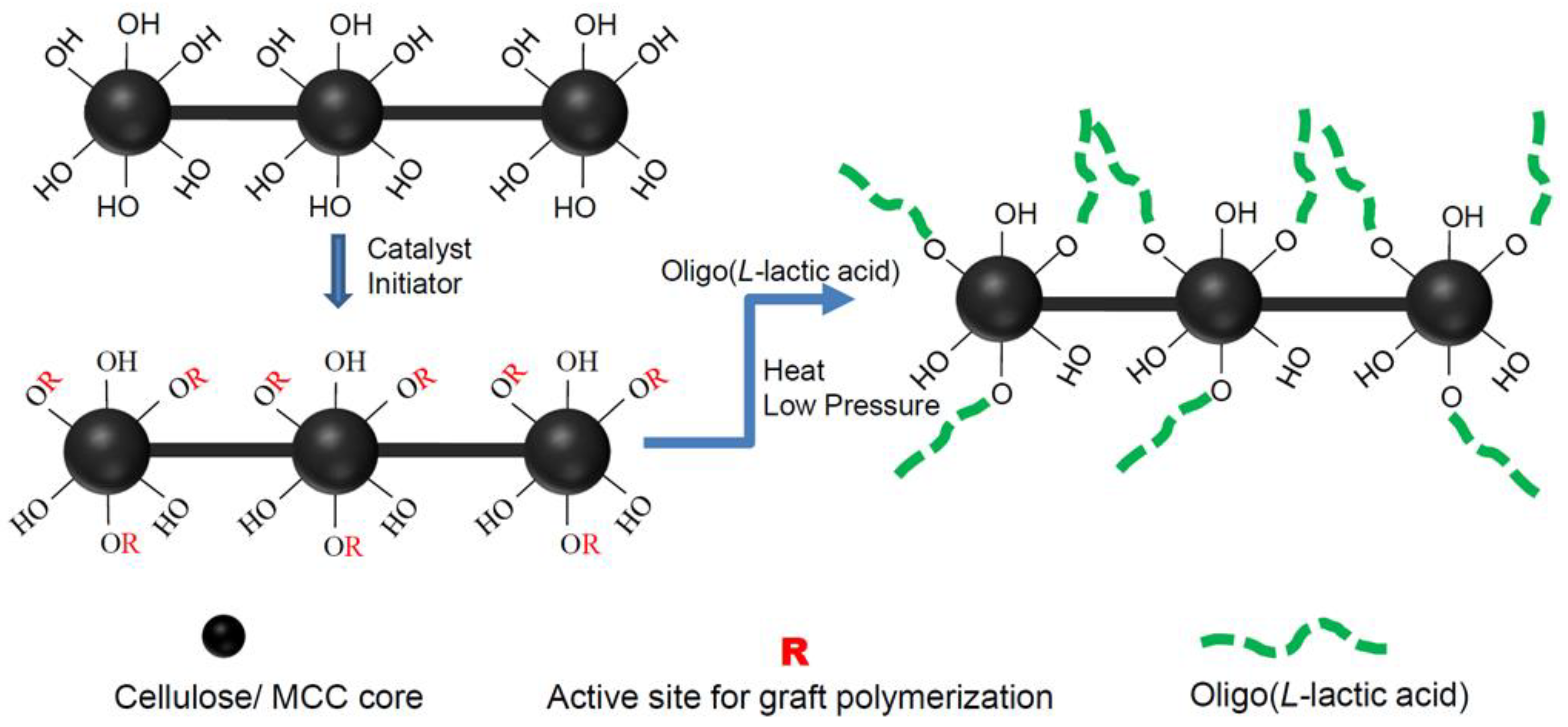
2.5. Grafting of OLLA onto MCC and α-Cellulose
2.6. Measurements
3. Results and Discussion
3.1. Synthesis of OLLA
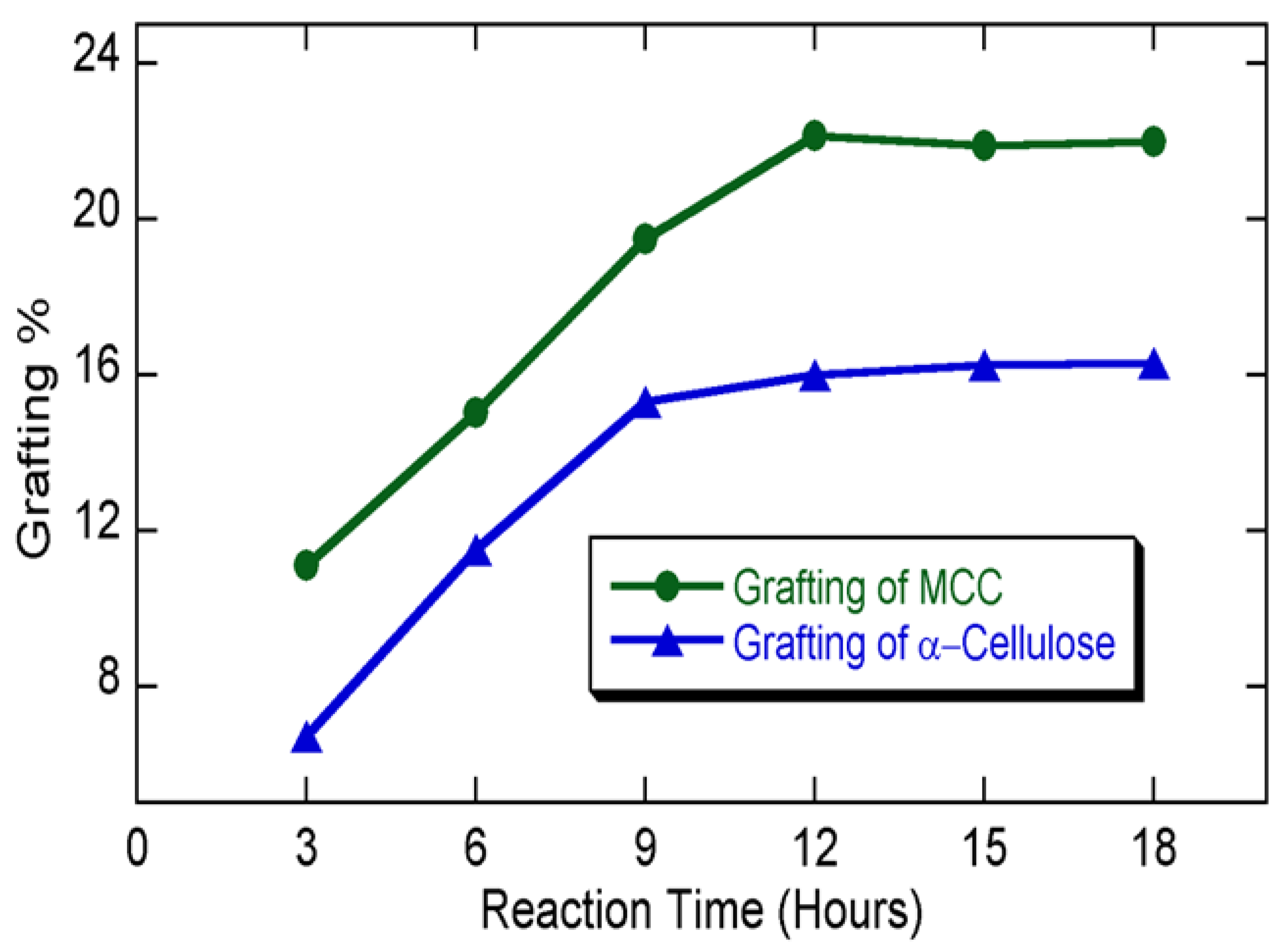
3.2. Grafting
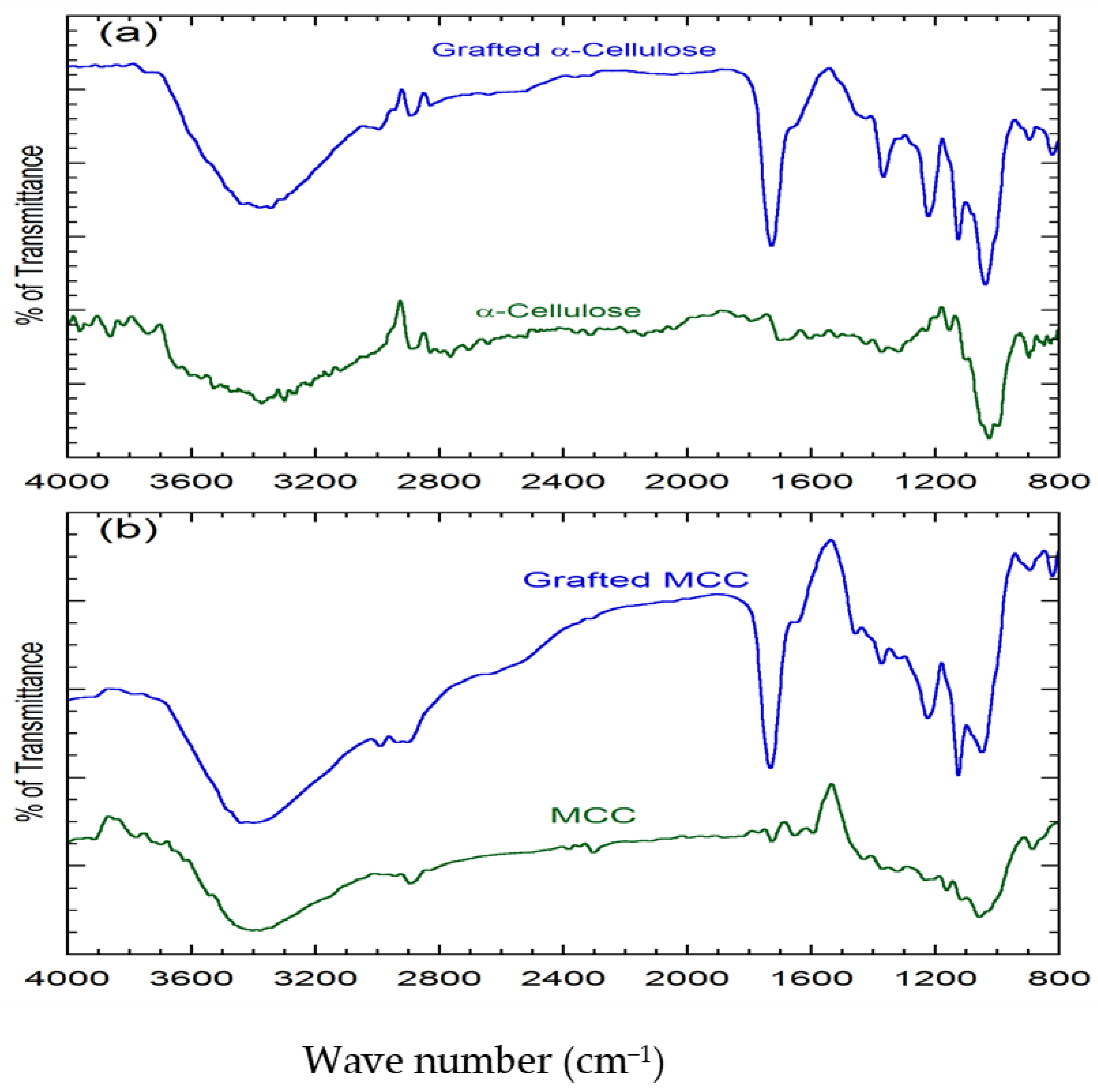
3.3. FTIR Spectroscopy Analysis
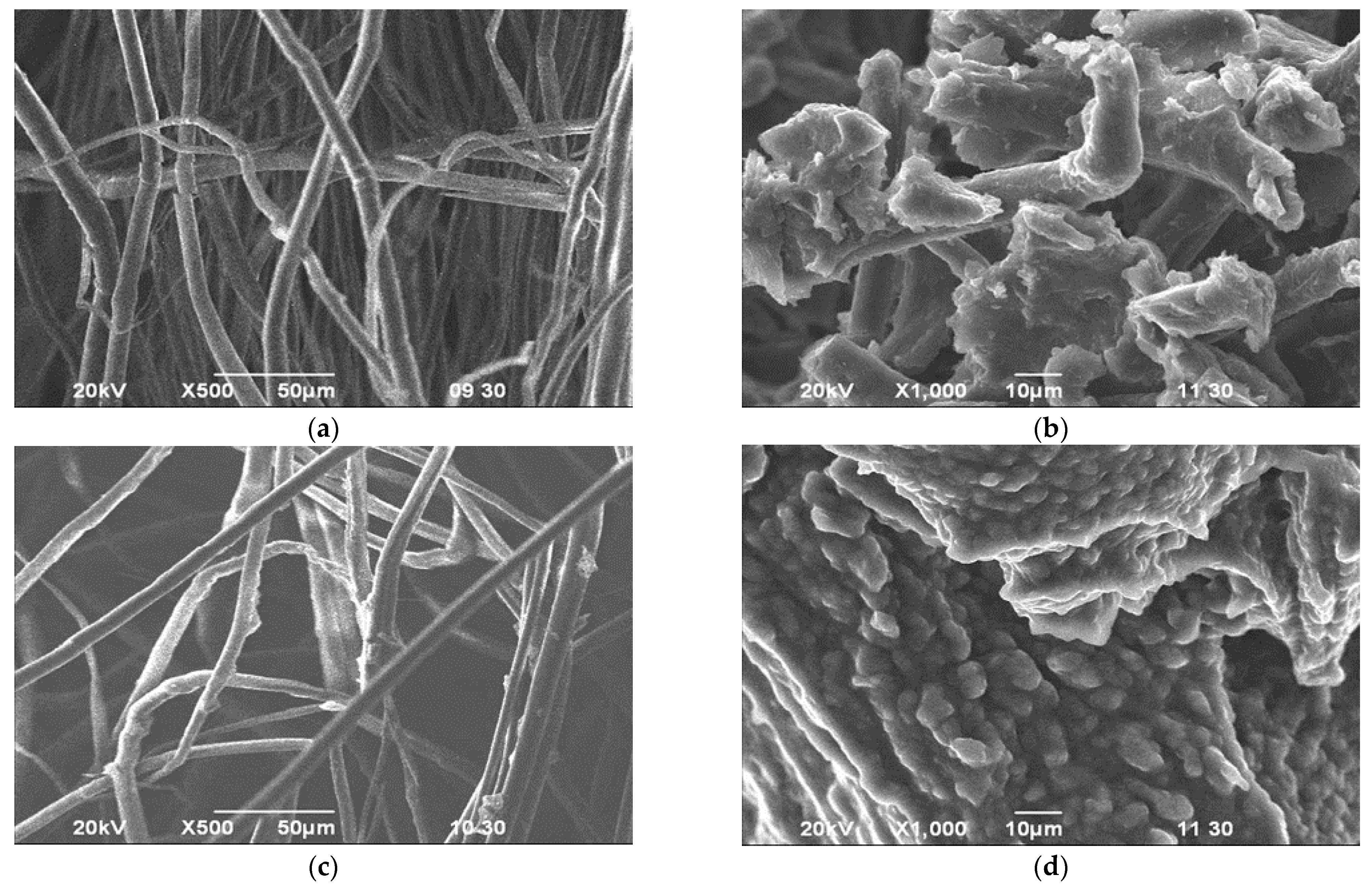
3.4. SEM Analysis
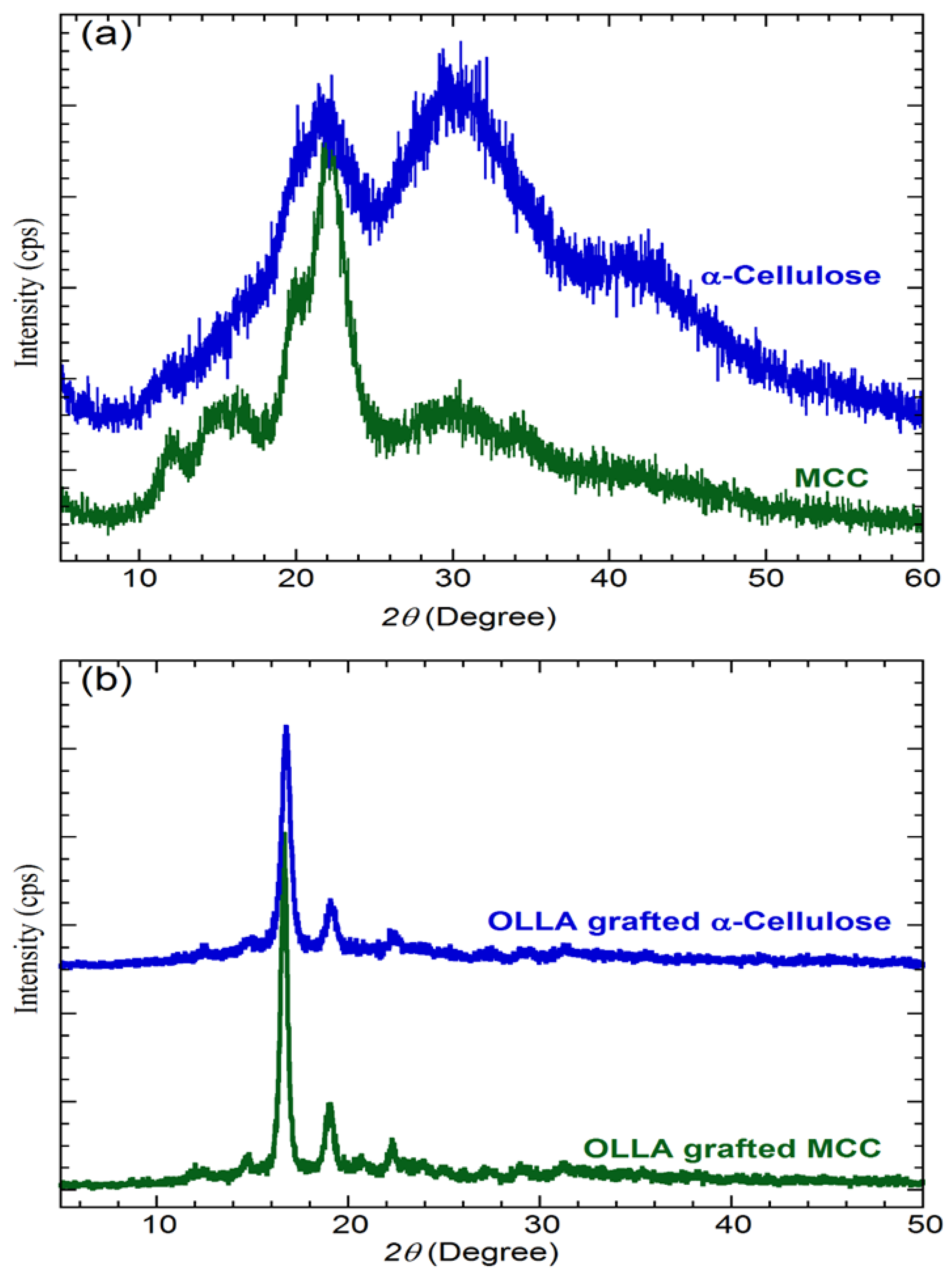
3.5. WAXS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Venkatarajan, S.; Athijayamani, A. An overview on natural cellulose fiber reinforced polymer composites. Mater. Today Proc. 2021, 37, 3620–3624. [Google Scholar] [CrossRef]
- Rahaman, M.H.; Rana, M.M.; Gafur, M.A.; Mohona, A.A. Preparation and analysis of poly (l-lactic acid) composites with oligo (d-lactic acid)-grafted cellulose. J. Appl. Polym. Sci. 2019, 136, 47424. [Google Scholar] [CrossRef]
- Pervin, S.-A.; Ahmed, B.; Zaman, M.A.A.; Rahaman, M.H. Preparation of LDPE-Bleached & Grafted Pulque Fiber Composite and Determination of Its Physicochemical Properties. J. Appl. Sci. Technol. 2013, 9, 81–86. [Google Scholar]
- Rahaman, M.H.; Islam, M.A.; Islam, M.M.; Rahman, M.A.; Alam, N.S. Biodegradable composite adsorbents of modified cellulose and chitosan to remove heavy metal ions from aqueous solution. Curr. Res. Green Sustain. Chem. 2021, 4, 100119. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Ching, Y.C.; Rahman, A.; Ching, K.Y.; Sukiman, N.L.; Cheng, H.C. Preparation and characterization of polyvinyl alcohol-based composite reinforced with nanocellulose and nanosilica. BioResources 2015, 10, 3364–3377. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Long, Z.; Wang, S.; Zhang, J.; Wang, L. Preparation and performance of thermoplastic starch and microcrystalline cellulose for packaging composites: Extrusion and hot pressing. Int. J. Biol. Macromol. 2020, 165, 2295–2302. [Google Scholar] [CrossRef]
- Rahaman, H.; Hosen, S.; Gafur, A.; Habib, R. Small amounts of poly (d-lactic acid) on the properties of poly (l-lactic acid)/microcrystalline cellulose/poly (d-lactic acid) blends. Results Mater. 2020, 8, 100125. [Google Scholar] [CrossRef]
- Rahaman, H.; Habib, R.; Hosen, S.; Gafur, A. Different amounts of chitosan on the morphological, thermal and antibacterial properties of poly (L-lactic acid)/microcrystalline cellulose biopolymer composites. Mater. Int. 2020, 2, 402–411. [Google Scholar]
- Hosen, M.S.; Rahaman, M.H.; Gafur, M.; Habib, R.; Qadir, M. Preparation and characterization of poly (L-lactic acid)/chitosan/microcrystalline cellulose blends. Chem. Sci. Int. J 2017, 21, 1–10. [Google Scholar] [CrossRef]
- Hosen, M.S.; Rahaman, M.H.; Gafur, M.; Ahmed, A.N. Development of thermal properties and surface morphology of poly (L-lactic)/Chitosan blend with microcrystalline cellulose obtained from natural jute fiber. Int. Res. J. Pure Appl. Chem. 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Wohlhauser, S.; Delepierre, G.; Labet, M.; Morandi, G.l.; Thielemans, W.; Weder, C.; Zoppe, J.O. Grafting polymers from cellulose nanocrystals: Synthesis, properties, and applications. Macromolecules 2018, 51, 6157–6189. [Google Scholar] [CrossRef] [Green Version]
- Khan, G.A.; Haque, M.A.; Terano, M.; Alam, M.S. Graft polycondensation of microfibrillated jute cellulose with oligo (L-lactic acid) and its properties. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Stark, N.M. Green esterification: A new approach to improve thermal and mechanical properties of poly (lactic acid) composites reinforced by cellulose nanocrystals. J. Appl. Polym. Sci. 2018, 135, 46468. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Fakhre, N.A.; Ibrahim, B.M. The use of new chemically modified cellulose for heavy metal ion adsorption. J. Hazard. Mater. 2018, 343, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hiraoka, T.; Honda, T.; Hsu, Y.-I.; Asoh, T.-A.; Uyama, H. Oligoether grafting on cellulose microfibers for dispersion in poly (propylene glycol) and fabrication of reinforced polyurethane composite. Compos. Sci. Technol. 2021, 202, 108595. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Minaz-Ul-Haque, M.; Rahman, M.; Rahman, M. Effect of grafting of acrylate monomers on some properties of Agave cantala fiber. J. Polym. Mater. 2006, 23, 279–286. [Google Scholar]
- Maharana, T.; Pattanaik, S.; Routaray, A.; Nath, N.; Sutar, A.K. Synthesis and characterization of poly (lactic acid) based graft copolymers. React. Funct. Polym. 2015, 93, 47–67. [Google Scholar] [CrossRef]
- Xian, X.; Wang, X.; Zhu, Y.; Guo, Y.; Tian, Y. Effects of MCC content on the structure and performance of PLA/MCC biocomposites. J. Polym. Environ. 2018, 26, 3484–3492. [Google Scholar] [CrossRef]
- Carlmark, A.; Larsson, E.; Malmström, E. Grafting of cellulose by ring-opening polymerisation—A review. Eur. Polym. J. 2012, 48, 1646–1659. [Google Scholar] [CrossRef] [Green Version]
- Albertsson, A.-C.; Varma, I.K.; Srivastava, R.K. Polyesters from large lactones. In Handbook of Ring-Opening Polymerization; Jhon Wiley and Sons: Hoboken, NJ, USA, 2009; pp. 287–306. [Google Scholar]
- Roda, J. Polyamides. In Handbook of Ring-Opening Polymerization; Jhon Wiley and Sons: Hoboken, NJ, USA, 2009; pp. 165–195. [Google Scholar]
- Hoogenboom, R. Polyethers and polyoxazolines. In Handbook of Ring-Opening Polymerization; Jhon Wiley and Sons: Hoboken, NJ, USA, 2009; pp. 141–164. [Google Scholar]
- Jérôme, C.; Lecomte, P. Recent advances in the synthesis of aliphatic polyesters by ring-opening polymerization. Adv. Drug Deliv. Rev. 2008, 60, 1056–1076. [Google Scholar] [CrossRef]
- Hafrén, J.; Córdova, A. Direct organocatalytic polymerization from cellulose fibers. Macromol. Rapid Commun. 2005, 26, 82–86. [Google Scholar] [CrossRef]
- Lönnberg, H.; Zhou, Q.; Brumer, H.; Teeri, T.T.; Malmström, E.; Hult, A. Grafting of cellulose fibers with poly (ε-caprolactone) and poly (l-lactic acid) via ring-opening polymerization. Biomacromolecules 2006, 7, 2178–2185. [Google Scholar] [CrossRef]
- Zhu, T.; Guo, J.; Fei, B.; Feng, Z.; Gu, X.; Li, H.; Sun, J.; Zhang, S. Preparation of methacrylic acid modified microcrystalline cellulose and their applications in polylactic acid: Flame retardancy, mechanical properties, thermal stability and crystallization behavior. Cellulose 2020, 27, 2309–2323. [Google Scholar] [CrossRef]
- Hu, Y.; Tang, L.; Lu, Q.; Wang, S.; Chen, X.; Huang, B. Preparation of cellulose nanocrystals and carboxylated cellulose nanocrystals from borer powder of bamboo. Cellulose 2014, 21, 1611–1618. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, J.; Zhang, F.; Xie, X. Syntheses, characterization, and in vitro degradation of ethyl cellulose-graft-poly (ε-caprolactone)-block-poly (l-lactide) copolymers by sequential ring-opening polymerization. Biomacromolecules 2007, 8, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Huang, Y. The synthesis of CDA-g-PMMA copolymers through atom transfer radical polymerization. Polymer 2004, 45, 7091–7097. [Google Scholar] [CrossRef]
- Shen, D.; Yu, H.; Huang, Y. Densely grafting copolymers of ethyl cellulose through atom transfer radical polymerization. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4099–4108. [Google Scholar] [CrossRef]
- Rahaman, M.H.; Tsuji, H. Synthesis and Characterization of Stereo Multiblock Poly (lactic acid) s with Different Block Lengths by Melt Polycondensation of Poly (l-lactic acid)/Poly (d-lactic acid) Blends. Macromol. React. Eng. 2012, 6, 446–457. [Google Scholar] [CrossRef]
- Jayaramudu, J.; Maity, A.; Sadiku, E.; Guduri, B.; Rajulu, A.V.; Ramana, C.V.; Li, R. Structure and properties of new natural cellulose fabrics from Cordia dichotoma. Carbohydr. Polym. 2011, 86, 1623–1629. [Google Scholar] [CrossRef]
- Ray, D.; Sarkar, B.; Basak, R.; Rana, A. Study of the thermal behavior of alkali-treated jute fibers. J. Appl. Polym. Sci. 2002, 85, 2594–2599. [Google Scholar] [CrossRef]
- Auras, R.A.; Lim, L.-T.; Selke, S.E.; Tsuji, H. Poly (Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 10. [Google Scholar]
- Thomas, M.G.; Abraham, E.; Jyotishkumar, P.; Maria, H.J.; Pothen, L.A.; Thomas, S. Nanocelluloses from jute fibers and their nanocomposites with natural rubber: Preparation and characterization. Int. J. Biol. Macromol. 2015, 81, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yue, J.; Liu, S. Preparation of nanocrystalline cellulose via ultrasound and its reinforcement capability for poly (vinyl alcohol) composites. Ultrason. Sonochem. 2012, 19, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Holbery, J.; Li, K. A technique for production of nanocrystalline cellulose with a narrow size distribution. Cellulose 2009, 16, 455–465. [Google Scholar] [CrossRef]
- Satyamurthy, P.; Vigneshwaran, N. A novel process for synthesis of spherical nanocellulose by controlled hydrolysis of microcrystalline cellulose using anaerobic microbial consortium. Enzym. Microb. Technol. 2013, 52, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, D.; He, J. Degradation of graft polymer and blend based on cellulose and poly (L-lactide). J. Appl. Polym. Sci. 2013, 130, 2257–2264. [Google Scholar] [CrossRef]
- Samain, X.; Langlois, V.; Renard, E.; Lorang, G. Grafting biodegradable polyesters onto cellulose. J. Appl. Polym. Sci. 2011, 121, 1183–1192. [Google Scholar] [CrossRef]
- Rahaman, M.H.; Tsuji, H. Hydrolytic degradation behavior of stereo multiblock and diblock poly (lactic acid) s: Effects of block lengths. Polym. Degrad. Stab. 2013, 98, 709–719. [Google Scholar] [CrossRef]
- Jithendra, P.; Rajam, A.M.; Kalaivani, T.; Mandal, A.B.; Rose, C. Preparation and characterization of aloe vera blended collagen-chitosan composite scaffold for tissue engineering applications. ACS Appl. Mater. Interfaces 2013, 5, 7291–7298. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.S.; Guthrie, J.; Law, T.K.; Turkington, P. Chitosan and modified chitosan membranes I. Preparation and characterisation. J. Appl. Polym. Sci. 1987, 33, 641–656. [Google Scholar] [CrossRef]
- Wulandari, W.; Rochliadi, A.; Arcana, I. Nanocellulose prepared by acid hydrolysis of isolated cellulose from sugarcane bagasse. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Solo, Indonesia, 8–9 September 2015; Volume 107, p. 012045. [Google Scholar]
- Wootthikanokkhan, J.; Kasemwananimit, P.; Sombatsompop, N.; Kositchaiyong, A.; Isarankura na Ayutthaya, S.; Kaabbuathong, N. Preparation of modified starch-grafted poly (lactic acid) and a study on compatibilizing efficacy of the copolymers in poly (lactic acid)/thermoplastic starch blends. J. Appl. Polym. Sci. 2012, 126, E389–E396. [Google Scholar] [CrossRef]
- Rahman, M.M.; Afrin, S.; Haque, P.; Islam, M.; Islam, M.S.; Gafur, M. Preparation and characterization of jute cellulose crystals-reinforced poly (l-lactic acid) biocomposite for biomedical applications. Int. J. Chem. Eng. 2014, 2014, 842147. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-M.; Cai, Z.-S.; Yu, J.-Y.; Xia, Z.-P. Changes in composition, structure, and properties of jute fibers after chemical treatments. Fibers Polym. 2009, 10, 776–780. [Google Scholar] [CrossRef]
- Peng, S.; Wang, X.; Dong, L. Special interaction between poly (propylene carbonate) and corn starch. Polym. Compos. 2005, 26, 37–41. [Google Scholar] [CrossRef]
- Hua, S.; Chen, F.; Liu, Z.-Y.; Yang, W.; Yang, M.-B. Preparation of cellulose-graft-polylactic acid via melt copolycondensation for use in polylactic acid based composites: Synthesis, characterization and properties. RSC Adv. 2016, 6, 1973–1983. [Google Scholar] [CrossRef]
- Xiao, L.; Mai, Y.; He, F.; Yu, L.; Zhang, L.; Tang, H.; Yang, G. Bio-based green composites with high performance from poly (lactic acid) and surface-modified microcrystalline cellulose. J. Mater. Chem. 2012, 22, 15732–15739. [Google Scholar] [CrossRef]
- Dai, S.; Wang, M.; Zhuang, Z.; Ning, Z. Crystallization and Alkaline Degradation Behaviors of Poly (l-Lactide)/4-Armed Poly (ε-Caprolactone)-Block-Poly (d-Lactide) Blends with Different Poly (d-Lactide) Block Lengths. Polymers 2020, 12, 2195. [Google Scholar] [CrossRef]
- Rahaman, M.H.; Tsuji, H. Isothermal crystallization and spherulite growth behavior of stereo multiblock poly (lactic acid) s: Effects of block length. J. Appl. Polym. Sci. 2013, 129, 2502–2517. [Google Scholar] [CrossRef]

| Code | Monomer/Co-Initiator | Mn [g·mol−1] | Mw/Mn |
|---|---|---|---|
| L5 | 5 | 1.8 × 102 | 4.2 |
| L7 | 7 | 2.0 × 102 | 4.5 |
| L9 | 9 | 2.6 × 102 | 5.6 |
| Bond for the Absorption (cm−1) | α-Cellulose (cm−1) | Grafted α-Cellulose (cm−1) | MCC (cm−1) | Grafted MCC (cm−1) |
|---|---|---|---|---|
| (3600–2500) –OH stretching | 3385 | 3381 | 3402 | 3439 |
| (3100–2900) –CH stretching (Asym) | 2891 | 2895 | 2893 | 2902 |
| (1780–1650) –C=O stretching | - | 1728 | - | 1732 |
| (1200–1020) –C–O– stretching | 1026 | 1037 | 1049 | 1057 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahaman, M.H.; Haque, M.A.; Rahman, M.A.; Rana, M.M.; Parvez, M.M.; Alam, S.M.N. Grafting of Cellulose and Microcrystalline Cellulose with Oligo(L-lactic acid) by Polycondensation Reaction. Reactions 2022, 3, 213-223. https://doi.org/10.3390/reactions3010016
Rahaman MH, Haque MA, Rahman MA, Rana MM, Parvez MM, Alam SMN. Grafting of Cellulose and Microcrystalline Cellulose with Oligo(L-lactic acid) by Polycondensation Reaction. Reactions. 2022; 3(1):213-223. https://doi.org/10.3390/reactions3010016
Chicago/Turabian StyleRahaman, Md. Hafezur, Md. Anamul Haque, Md. Aminur Rahman, Md. Masud Rana, Md. Masud Parvez, and S. M. Nur Alam. 2022. "Grafting of Cellulose and Microcrystalline Cellulose with Oligo(L-lactic acid) by Polycondensation Reaction" Reactions 3, no. 1: 213-223. https://doi.org/10.3390/reactions3010016
APA StyleRahaman, M. H., Haque, M. A., Rahman, M. A., Rana, M. M., Parvez, M. M., & Alam, S. M. N. (2022). Grafting of Cellulose and Microcrystalline Cellulose with Oligo(L-lactic acid) by Polycondensation Reaction. Reactions, 3(1), 213-223. https://doi.org/10.3390/reactions3010016








