Linear-Rate Reactions for the Thermal Devolatilization of Wheat Straw Based on Pseudo-Components
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thermogravimetric Analysis
3. Results and Discussion
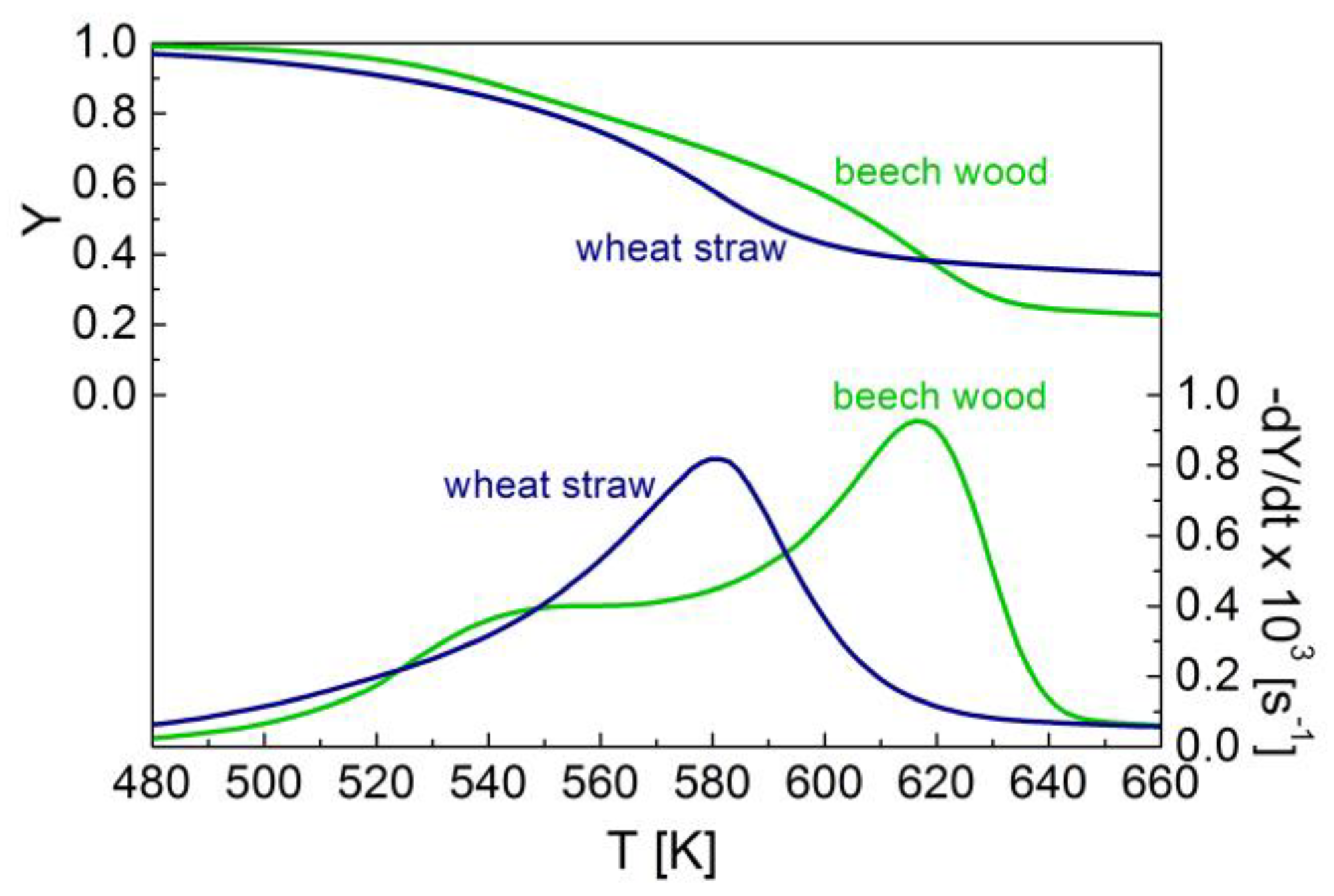
3.1. Thermogravimetric Characteristics
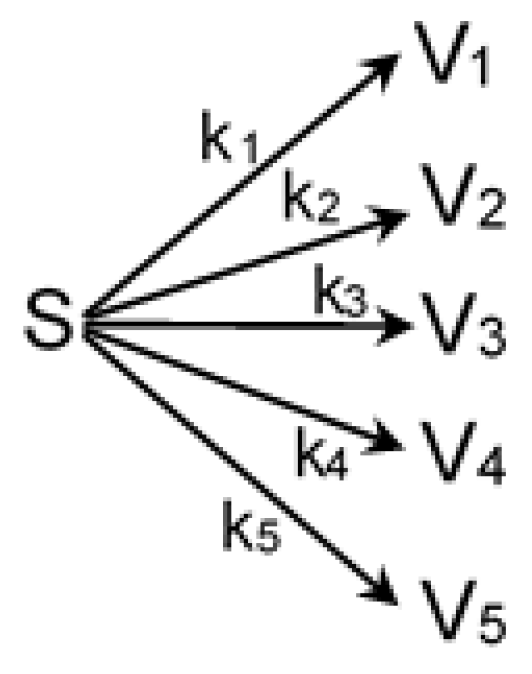
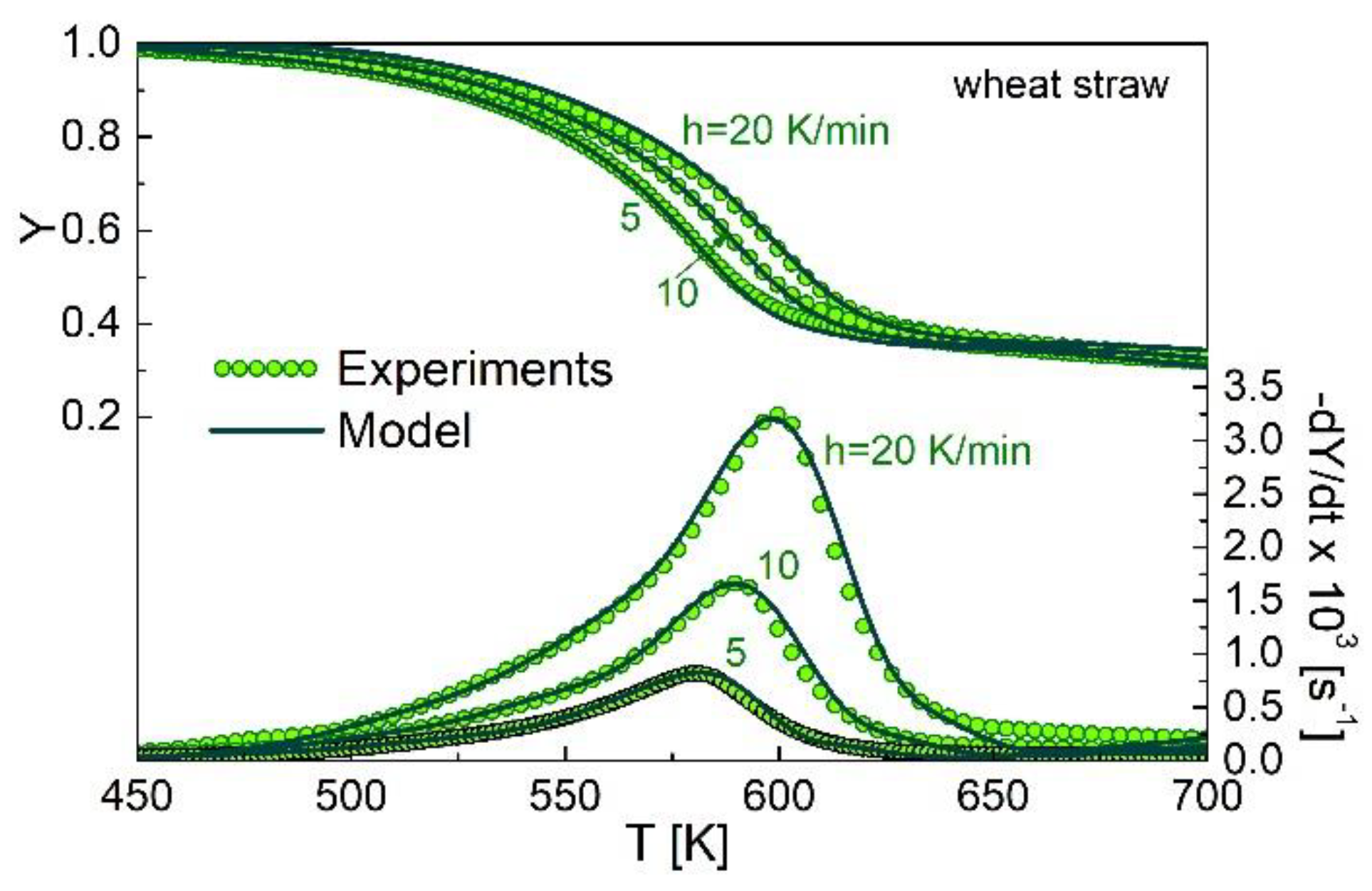
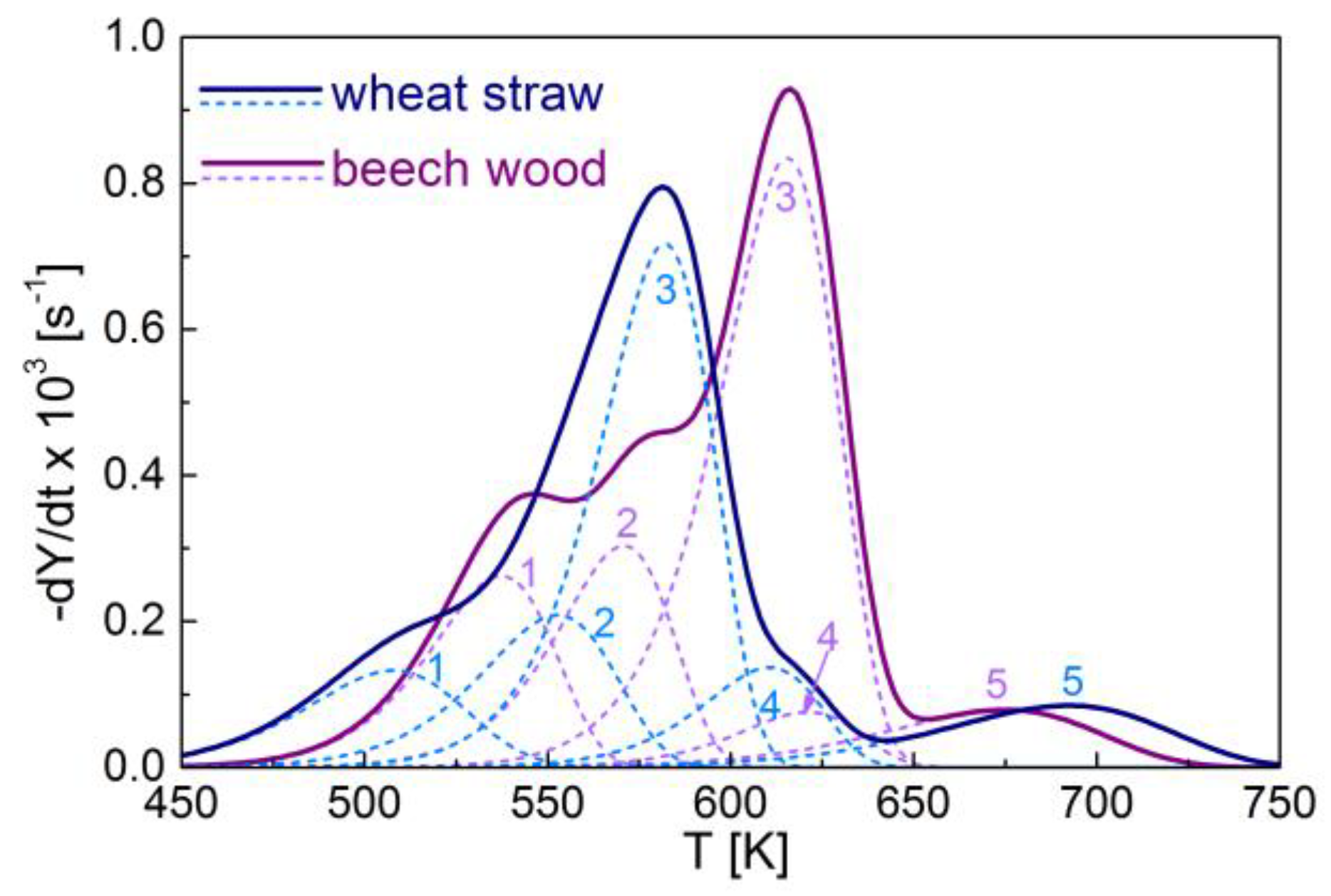
3.2. Kinetic Modeling
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manzano-Agugliaro, F.; Alcayde, A.; Montoya, F.G.; Zapata-Sierra, A.; Gil, C. Scientific production of renewable energies worldwide: An overview. Renew. Sustain. Energy Rev. 2013, 18, 134–143. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog. Energy Combust. Sci. 2008, 34, 47–90. [Google Scholar] [CrossRef]
- Di Blasi, C. Combustion and gasification rates of lignocellulosic chars. Prog. Energy Combust. Sci. 2009, 35, 121–140. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C.; Galgano, A. Pyrolytic conversion of wastes from cereal, protein and oil-protein crops. J. Anal. Appl. Pyrolysis 2017, 127, 426–435. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, X.; Zhao, J.; Wei, X. The alkali metal occurrence characteristics and its release and conversion during wheat straw pyrolysis. Renew. Energy 2020, 151, 255–262. [Google Scholar] [CrossRef]
- Jensen, A.; Dam-Johansen, K. TG-FTIR Study of the influence of potassium chloride on wheat straw pyrolysis. Energy Fuels 1998, 12, 929–938. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; D’Errico, G. Degradation characteristics of straw and washed straw. Thermochim. Acta 2000, 364, 133–142. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Galgano, A. Biomass screening for the production of furfural via thermal decomposition. Ind. Eng. Chem. Res. 2010, 49, 2658–2671. [Google Scholar] [CrossRef]
- Butler, E.; Devlin, G.; Meier, D.; McDonnell, K. Characterization of spruce, salix, miscanthus and wheat straw for pyrolysis applications. Bioresour. Technol. 2013, 131, 202–209. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Sarnataro, F.E.; Gallo, A. Thermal runaway in the pyrolysis of some lignocellulosic biomasses. Energy Fuels 2014, 28, 2684–2696. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Monedero, B.; Bimbela, F.; Arauzo, J.; Faria, J.; Ruiz, M.P. Pyrolysis of Red Eucalyptus, Camelina straw, and wheat straw in an ablative reactor. Energy Fuels 2015, 29, 1766–1775. [Google Scholar] [CrossRef]
- Fonseca, F.G.; Funke, A.; Niebel, A.; Soares Dias, A.P.; Dahmen, N. Moisture content as a design and operational parameter for fast pyrolysis. J. Anal. Appl. Pyrolysis 2019, 139, 73–86. [Google Scholar] [CrossRef]
- Greco, G.; Di Stasi, C.; Rego, F.; Gonzalez, B.; Manya, J.J. Effects of slow-pyrolysis conditions on the products yields and properties and on exergy efficiency: A comprehensive assessment for wheat straw. Appl. Energy 2020, 279, 115842. [Google Scholar] [CrossRef]
- Verma, R.; Verma, S.K.; Verma, S.; Wang, J.; Liu, J.; Jing, B.; Rakesh, K.P. Value-addition of wheat straw through acid treatment and pyrolysisof acid treated residues. J. Clean. Prod. 2021, 282, 124488. [Google Scholar] [CrossRef]
- Branca, C.; Albano, A.; Di Blasi, C. Critical evaluation of wood devolatilization mechanisms. Thermochim. Acta 2005, 429, 133–141. [Google Scholar] [CrossRef]
- Brostrom, M.; Nordin, A.; Pommer, L.; Branca, C.; Di Blasi, C. Influence of torrefaction on the devolatilization and oxidation kinetics of wood. J. Anal. Appl. Pyrolysis 2012, 96, 100–109. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C. A unified mechanism of the combustion reactions of lignocellulosic fuels. Thermochim. Acta 2013, 565, 58–64. [Google Scholar] [CrossRef]
- Moreno, A.I.; Font, R. Pyrolysis of furniture wood waste: Decomposition and gases evolved. J. Anal. Appl. Pyrolysis 2015, 113, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Branca, C.; Di Blasi, C. A summative model for the pyrolysis reaction heats of beech wood. Thermochim. Acta 2016, 638, 10–16. [Google Scholar] [CrossRef]
- Moreno, A.I.; Font, R.; Conesa, J.A. Combustion of furniture wood waste and solid wood: Kinetic study and evolution of pollutants. Fuel 2017, 192, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Barta-Rajnai, E.; Várhegyi, G.; Wang, L.; Skreiberg, Ø.; Grønli, M.; Czégény, Z. Thermal decomposition kinetics of wood and bark and their torrefied products. Energy Fuels 2017, 31, 4024–4034. [Google Scholar] [CrossRef] [Green Version]
- Branca, C.; Di Blasi, C. Thermal degradation behavior and kinetics of industrial hemp stalks and shives. Thermochim. Acta 2021, 697, 178878. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Alvarez, J.; Moreira, R.; Duarte, G.; Nunes, J.; Olazar, M.; Bilbao, J. Pyrolysis kinetics of forestry residues from the Portuguese Central Inland Region. Chem. Eng. Res. Des. 2013, 91, 2682–2690. [Google Scholar] [CrossRef]
- Galgano, A.; Di Blasi, C.; Horvat, A.; Sinai, Y. Experimental validation of a coupled solid- and gas-phase model for combustion and gasification of wood logs. Energy Fuels 2006, 20, 2223–2232. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C. Modeling a stratified downdraft wood gasifier with primary and secondary air entry. Fuel 2013, 104, 847–860. [Google Scholar] [CrossRef]
- Galgano, A.; Di Blasi, C.; De Vita, R. Experimental validation of a wood burning model. Energy Fuels 2018, 32, 8494–8506. [Google Scholar] [CrossRef]
- Zachl, A.; Buchmayr, M.; Gruber, J.; Anca-Couce, A.; Scharler, R.; Hochenauer, C. Evaluation and extension of the load and fuel flexibility limits of a stratified downdraft gasifier. Energy 2022, 239, 122279. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Masotta, F.; De Biase, E. Experimental analysis of reaction heat effects during beech wood pyrolysis. Energy Fuels 2013, 27, 2665–2674. [Google Scholar] [CrossRef] [Green Version]
- Di Blasi, C.; Branca, C.; Galgano, A. Influences of potassium hydroxide on the rate and thermicity of wood pyrolysis reactions. Energy Fuels 2017, 1, 6154–6162. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Galgano, A. Role of the potassium chemical state in the global exothermicity of wood packed-bed pyrolysis. Ind. Eng. Chem. Res. 2018, 57, 11561–11571. [Google Scholar] [CrossRef]
- Gonzalez Martinez, M.; Marlin, N.; Da Silva Perez, D.; Dupont, C.; del Mar Saavedra Rios, C.; Meyer, X.-M.; Gourdon, C.; Mortha, G. Impact of cellulose properties on its behavior in torrefaction: Commercial microcrystalline cellulose versus cotton linters and celluloses extracted from woody and agricultural biomass. Cellulose 2021, 28, 4761–4779. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Santoro, A.; Perez Bermudez, R.A. Weight loss dynamics of wood chips under fast radiative heating. J. Anal. Appl. Pyrolysis 2001, 57, 77–90. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C.; Horacek, H. Analysis of the combustion kinetics and the thermal behavior of an intumescent system. Ind. Eng. Chem Res. 2002, 41, 2107–2114. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C. Self-heating effects in the thermogravimetric analysis of wood char oxidation. Fuel 2020, 276, 118012. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C.; Galgano, A. Experimental analysis about the exploitation of industrial hemp (Cannabis sativa) in pyrolysis. Fuel Proc. Technol. 2017, 162, 20–29. [Google Scholar] [CrossRef]
- Di Blasi, C.; Lanzetta, M. Intrinsic kinetics of isothermal xylan degradation in inert atmosphere. J. Anal. Appl. Pyrolysis 1997, 40, 287–303. [Google Scholar] [CrossRef]
- Varhegyi, G.; Gronli, M.G.; Di Blasi, C. Effects of sample origin, extraction and hot water washing on the devolatilization kinetics of chestnut wood. Ind. Eng. Chem. Res. 2004, 43, 2356–2367. [Google Scholar] [CrossRef] [Green Version]
- Branca, C.; Di Blasi, C.; Mango, C.; Hrablay, I. Products and kinetics of glucomannan pyrolysis. Ind. Eng. Chem. Res. 2013, 52, 5030–5039. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]


| Sample | Tinitial [K] | Tshoulder [K] | Tpeak [K] | Toffset [K] | FWHM [K] |
|---|---|---|---|---|---|
| wheat straw | 465 | 580 | 612 | 46 | |
| 476 | - | 590 | 619 | 46 | |
| 480 | 600 | 628 | 48 | ||
| beech wood | 502 | 558 | 617 | 639 | 47 |
| 511 | 568 | 627 | 651 | 50 | |
| 517 | 576 | 638 | 663 | 54 | |
| Yshoulder | Ypeak | Y773 | −(dY/dt)shoulder × 103 [s−1] | −(dY/dt)peak × 103 [s−1] | |
| wheat straw | 0.58 | 0.29 | 0.82 | ||
| - | 0.57 | 0.29 | - | 1.66 | |
| 0.56 | 0.28 | 3.24 | |||
| beech wood | 0.81 | 0.41 | 0.18 | 0.40 | 0.93 |
| 0.80 | 0.41 | 0.19 | 0.77 | 1.78 | |
| 0.80 | 0.40 | 0.19 | 1.56 | 3.40 |
| (A) Parameters | Straw | Beech |
|---|---|---|
| E1 [kJ/mol] | 101.1 | 138.8 |
| A1 [s−1] | 1.26 × 108 | 1.47 × 1011 |
| E2 [kJ/mol] | 135.9 | 170.1 |
| A2 [s−1] | 3.30 × 1010 | 1.91 × 1013 |
| E3 [kJ/mol] | 179.8 | 194.8 |
| A3 [s−1] | 7.90 × 1013 | 1.70 × 1014 |
| E4 [kJ/mol] | 189.3 | 189.3 |
| A4 [s−1] | 8.60 × 1013 | 4.00 × 1013 |
| E5[kJ/mol] | 125.7 | 125.7 |
| A5 [s−1] | 8.50 × 106 | 1.46 × 107 |
| devTG [%] | 0.87 | 0.83 |
| devDTG [%] | 2.60 | 2.01 |
| (B) Coefficients | Straw | Beech |
| ν1 | 0.090 | 0.140 |
| ν2 | 0.120 | 0.150 |
| ν3 | 0.350 | 0.420 |
| ν4 | 0.070 | 0.040 |
| ν5 | 0.084 | 0.065 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branca, C. Linear-Rate Reactions for the Thermal Devolatilization of Wheat Straw Based on Pseudo-Components. Reactions 2022, 3, 203-212. https://doi.org/10.3390/reactions3010015
Branca C. Linear-Rate Reactions for the Thermal Devolatilization of Wheat Straw Based on Pseudo-Components. Reactions. 2022; 3(1):203-212. https://doi.org/10.3390/reactions3010015
Chicago/Turabian StyleBranca, Carmen. 2022. "Linear-Rate Reactions for the Thermal Devolatilization of Wheat Straw Based on Pseudo-Components" Reactions 3, no. 1: 203-212. https://doi.org/10.3390/reactions3010015
APA StyleBranca, C. (2022). Linear-Rate Reactions for the Thermal Devolatilization of Wheat Straw Based on Pseudo-Components. Reactions, 3(1), 203-212. https://doi.org/10.3390/reactions3010015





