Abstract
Nucleophilic ring-opening reactions of cyclic oxonium derivatives of anionic boron hydrides are a convenient method of their modification which opens practically unlimited prospects for their incorporation into various macro- and biomolecules. This contribution provides an overview of the synthesis and reactivity of cyclic oxonium derivatives of nido-carborane as well as half-sandwich complexes based on it.
1. Introduction
The 7,8-Dicarba-nido-undecaborante anion [7,8-C2B9H12]− (nido-carborane) belongs to a family of polyhedral boron compounds, the discovery of which in the middle of the last century became one of the most significant events in the history of modern inorganic chemistry [1]. This anionic boron cluster with an open pentagonal face was synthesized for the first time by M. F. Hawthorne more than 50 years ago by deboronation of 1,2-dicarba-closo-dodecaborane (ortho-carborane) with potassium hydroxide in methanol [2,3]. Later various milder deboronating agents including amines [4,5,6,7] and fluoride ion [8,9,10,11] were proposed. Deprotonation of nido-carborane with strong bases results in the dicarbollide dianion [7,8-C2B9H11]2−, which was recognized as an isolobal analogue of the cyclopentadienyl anion [12]. Due to this, the dicarbollide dianion is considered as an unusual three-dimensional π-ligand for the synthesis of metal complexes [13,14,15], which can be used as catalysts in a variety of chemical processes [16,17]. Another important area of use for nido-carborane derivatives as water-solubilizing boron moieties is the design of potential drugs for boron neutron capture therapy of cancer [18,19,20,21,22,23]. In addition, based on nido-carborane derivatives, it is possible to obtain reagents for radioimaging of tumors [24,25,26,27,28], new optical and luminescent materials [29,30,31,32,33,34,35,36,37], ionic liquids [38,39], etc. Thus, the development of convenient approaches to the modification of the nido-carborane cluster remains highly relevant.
Currently the main approach to the synthesis of nido-carborane derivatives is based on the deboronation of the corresponding ortho-carborane derivatives. This approach is widely used for the synthesis of C-substituted derivatives of nido-carborane, as well as derivatives containing substituents at the lower belt of the nido-carborane cage [40]. There are several general methods for the synthesis of B-substituted derivatives with substituents in the upper belt of the nido-carborane cage; however, most of them are used to obtain asymmetrically substituted derivatives [9-X-7,8-C2B9H11]− [41,42,43,44,45,46,47,48,49].
Several approaches to the synthesis of symmetrically substituted derivatives of nido-carborane [10-X-7,8-C2B9H11]− with boron–sulfur [50,51] and boron–nitrogen [52,53,54,55] bonds have also been developed recently; however, the greatest interest is the functionalization of nido-carborane via its cyclic oxonium derivatives. Previously, this approach was successfully used for the synthesis of numerous derivatives of closo-decaborate and closo-dodecaborate anions [56,57], as well as sandwich bis(dicarbollide) transition metal complexes [58]. In this contribution synthesis and properties of cyclic oxonium derivatives of nido-carborane as well as half-sandwich complexes based on it will be reviewed.
2. Synthesis of Oxonium Derivatives of nido-Carborane
To date, oxonium derivatives of nido-carborane have been synthesized with all cyclic ethers used as common solvents, including tetrahydrofuran, 1,4-dioxane, and tetrahydropyran. The first oxonium derivative was obtained as early as 1969 by the reaction of the potassium salt of nido-carborane with FeCl3 in a mixture of benzene and tetrahydrofuran. The reaction gave a mixture of asymmetric 9-(CH2)4O-7,8-C2B9H11 and symmetric 10-(CH2)4O-7,8-C2B9H11 which were separated by column chromatography in benzene. Similar mixture of isomers was obtained for the C,C-dimethyl derivative of nido-carborane as well [59] (Scheme 1).
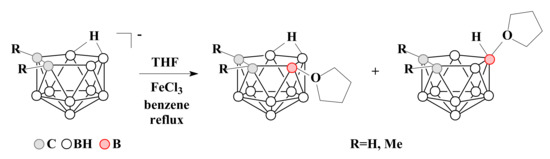
Scheme 1.
The synthesis of 9-(CH2)4O-7,8-C2B9H11 and 10-(CH2)4O-7,8-C2B9H11 by the reaction of nido-carborane with FeCl3 in a THF–benzene mixture.
The proposed mechanism of this reaction includes the initial abstraction of hydrogen hydride from position 9 by iron(III) chloride as a Lewis acid with the formation of a quasi-borinium cation. In the absence of strong nucleophiles, this intermediate can be isomerized to a more stable symmetric form with a quasi-electrophilic center at position 10, which is then attacked by an ether solvent molecule as a weak but most accessible nucleophile. As a result, a mixture of 9- and 10-substituted isomers is formed [60]. This mechanism is known as electrophile-induced nucleophilic substitution (EINS) and is considered as one of the main mechanisms of substitution of hydrogen atoms in polyhedral boron hydrides.
Later, it was found that the reaction of nido-carborane with HgCl2 in tetrahydrofuran leads to the selective formation of the symmetrically substituted product 10-(CH2)4O-7,8-C2B9H11 [60,61]. In a similar way, the derivatives with 1,4-dioxane 10-O(CH2CH2)2O-7,8-C2B9H11 [60] and tetrahydropyran 10-(CH2)5O-7,8-C2B9H11 [62,63] were synthesized.
It is assumed that the selectivity of the reactions in these cases is determined by the fact that the endo-polyhedral hydrogen atom of nido-carborane cage is replaced at the first stage of the reaction by a mercury atom with the formation of η1-metallacarborane, in which mercury is bound to the B(10) atom of the dicarbollide ligand [64,65,66,67]. Heating of this complex results in elimination of mercury and generation of quasi-electrophilic center at position 10 followed by its attack by the nucleophile [60].
Another convenient method for the synthesis of 1,4-dioxane derivative of nido-carborane is to heat in 1,4-dioxane the protonated form of nido-carborane 7,8-C2B9H13, which is formed by treating a suspension of the triethylammonium salt of nido-carborane with concentrated sulfuric acid in toluene [68].
The symmetrically substituted tetrahydrofuran derivative 10-(CH2)4O-7,8-C2B9H11 can be obtained by the reaction of tetramethylammonium salt of nido-carborane with AlCl3 in a mixture of tetrahydrofuran and acetone [69]. The symmetrically substituted tetrahydrofuran and 1,4-dioxane derivatives of nido-carborane were also synthesized by the reactions of nido-carborane with the corresponding ethers in the presence of acetaldehyde or formaldehyde and hydrochloric acid in a mixture of water and toluene [70]. The methods of synthesis of symmetrically substituted oxonium derivatives of nido-carborane are summarized in Scheme 2.
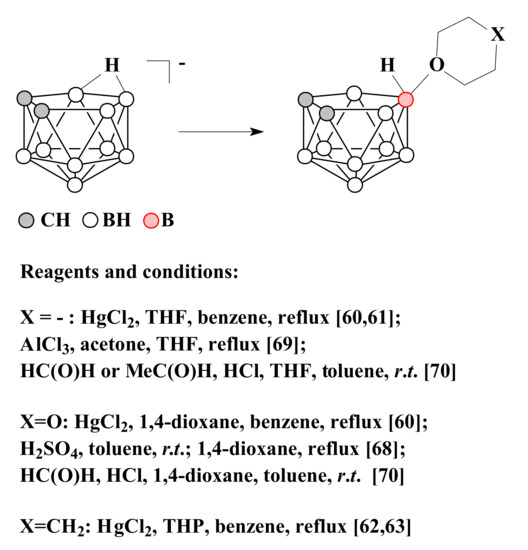
Scheme 2.
The different synthetic pathways to the 10-(CH2)4O-7,8-C2B9H11, 10-O(CH2CH2)2O-7,8-C2B9H11 and 10-(CH2)5O-7,8-C2B9H11.
The molecular structure of the 1,4-dioxane derivative of nido-carborane was determined by single crystal X-ray diffraction [71] (Figure 1).
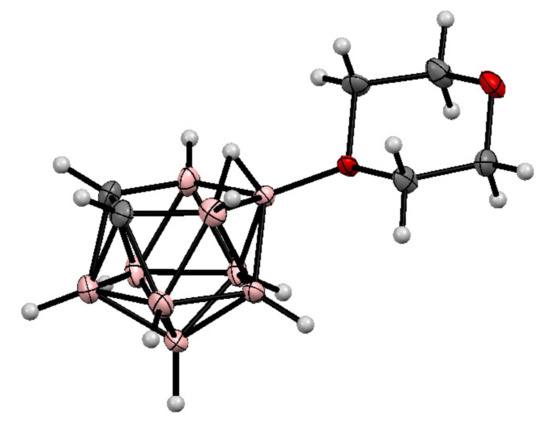
Figure 1.
The molecular structure of 10-O(CH2CH2)2O-7,8-C2B9H11.
It should be noted that, in addition to cyclic oxonium derivatives of nido-carborane, some acyclic oxonium derivatives such as the dimethyloxonium 9-Me2O-7,8-C2B9H11 and 10-Me2O-7,8-C2B9H11 [72] and the diethyloxonium 9-Et2O-7,8-C2B9H11 [73] and 10-Et2O-7,8-C2B9H11 [70,73,74] derivatives are known as well. The molecular structure of the 10-diethyloxonium derivative of nido-carborane was determined by single crystal X-ray diffraction [73] (Figure 2). The formation of the 1,2-dimethoxyethane derivatives 9-MeOCH2CH2(Me)O-7,8-C2B9H11 and 10-MeOCH2CH2(Me)O-7,8-C2B9H11 was also reported, however they have low stability and easily lose the methyl group, giving the corresponding alkoxy derivatives [75].
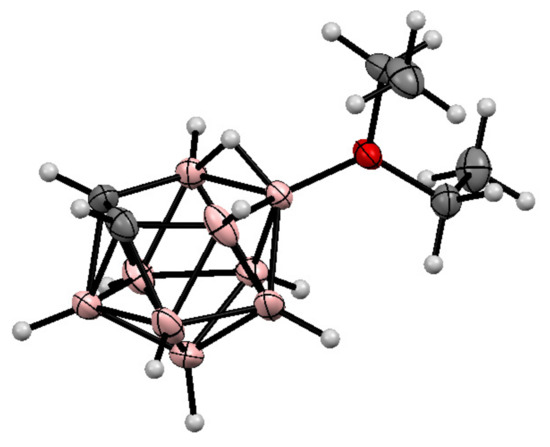
Figure 2.
The molecular structure of 10-Et2O-7,8-C2B9H11.
3. Properties of Oxonium Derivatives of nido-Carborane. Reactions with Nucleophiles
The use of ring opening reactions of cyclic oxonium derivatives of polyhedral boron hydrides under the action of nucleophiles for the preparation of their various derivatives is well known [56,57,58]. In the case of nido-carborane, this method has several advantages. First of all, the substituent is symmetrically located at position 10 of the boron cluster. This avoids the formation of racemic and diastereomeric mixtures of products and greatly facilitates the identification of compounds by NMR, which is one of the main methods for characterization of this type of compounds. Another advantage is that the yield of the target product is in most cases very high or nearly quantitative. In addition, the variety of oxonium derivatives makes it possible to obtain products with different lengths of the spacer between the nido-carborane cage and the substituent.
Thus, by the ring-opening reactions of tetrahydrofuran and 1,4-dioxane derivatives of nido-carborane with phenolates a series of nido-carborane-based carboxylic acids with different spacer lengths between nido-carborane cage and functional group was prepared. Phenolates were generated by the treatment of hydroxybenzoic acids with K2CO3 in acetonitrile [60] (Scheme 3). The reaction of the 1,4-dioxane derivative of nido-carborane with methyl ether of p-hydroxybenzoate in the same conditions led to the corresponding K[10-(4-CH3OOCC6H4O)-CH2CH2OCH2CH2O-7,8-C2B9H11] [76].
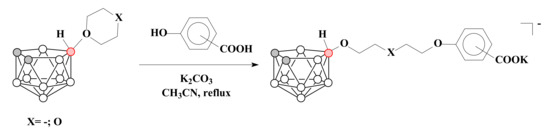
Scheme 3.
The synthesis of nido-carborane derivatives with terminal carboxylic group.
The obtaining of 10-substituted derivatives of nido-carborane in the case of oxonium derivatives and products of their ring-opening reactions leaves free the positions 9 and 11 of nido-carborane cage. This can be used for the following substitution, such as, for example, the halogenation, which was carried out for K[1 0-(4-HOOCC6H4O)-C4H8O-7,8-C2B9H11], K[10-(4-HOOCC6H4O)-CH2CH2OCH2CH2O-7,8-C2B9H11] and K[10-(4-CH3OOCC6H4O)-CH2CH2OCH2CH2O-7,8-C2B9H11] [76] (Scheme 4). The obtained halogenated compounds can find application in the design of drugs for radionuclide imaging and boron neutron capture therapy of cancer.
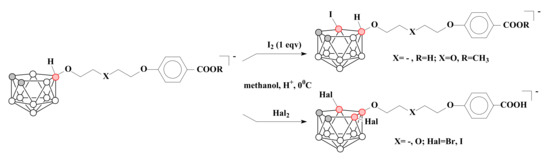
Scheme 4.
The halogenation of K[10-(4-HOOCC6H4O)-C4H8O-7,8-C2B9H11], K[10-(4-HOOCC6H4O)-CH2CH2OCH2CH2O-7,8-C2B9H11] and K[10-(4-CH3OOCC6H4O)-CH2CH2OCH2CH2O-7,8-C2B9H11].
The ring-opening reaction of 1,4-dioxane derivative of nido-carborane with 2-methoxy phenol in the presence of K2CO3 resulted in the compound with a terminal guaiacol group K[10-(2-CH3OC6H4O)-CH2CH2OCH2CH2O-7,8-C2B9H11] [71] (Scheme 5). The boron-containing calixarene [4-t-Bu-calix[4]arene-1,3-(10-CH2CH2OCH2CH2O-7,8-C2B9H11)2]2− was prepared in similar conditions by the treatment of 1,4-dioxane derivative of nido-carborane with 4-t-Bu-calix[4]arene [71].
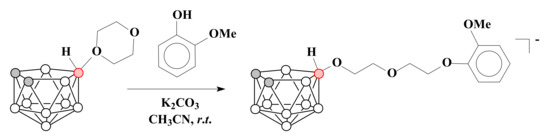
Scheme 5.
The synthesis of the nido-carborane derivative with the terminal guaiacol group.
The ring-opening reactions of 1,4-dioxane and tetrahydropyran derivatives of nido-carborane with 7-diethyl-4-hydroxycoumarin led to the water-soluble conjugates of the nido-carborane cluster with coumarin [77] (Scheme 6). The measurement of the distribution coefficients (log D7,4) of the obtained compounds showed their appropriate lipophilicity for medicinal applications [77].
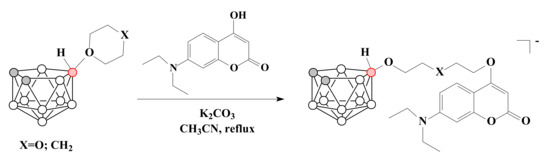
Scheme 6.
The synthesis of nido-carborane conjugates with coumarin.
The reactions of two equivalents of 1,4-dioxane derivative of nido-carborane with 1,2-O,O’- and 1,2-S,S’-dinucleophiles (1,2-dihydroxybenzene [78], 1,2-dimercaptoethane, 1,2-dimercaptobenzene [79], 1,2-dihydroxy-closo-carborane, 1,2-dimercapto-closo-carborane[79]) in the presence of K2CO3 in acetonitrile makes it possible to obtain nido-carborane-based podands related to acyclic crown ether 18-crown-6 [78,79] (Scheme 7).
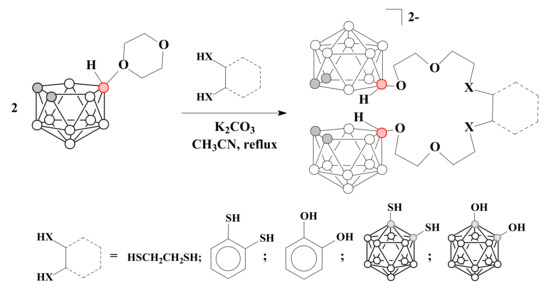
Scheme 7.
The synthesis of nido-carborane-based podands.
The reaction of two equivalents of 1,4-dioxane derivative of nido-carborane with resorcinol proceeds in a similar way, resulting in K2[1,3-(10-OC2H4OC2H4O-7,8-C2B9H11)2C6H4], whereas the reaction with hydroquinone leads to two products K2[1,4-(10-OC2H4OC2H4O-7,8-C2B9H11)2C6H4] and K[10-(4′-KOC6H4O)-CH2CH2OCH2CH2O-7,8-C2B9H11] [78] (Scheme 8).
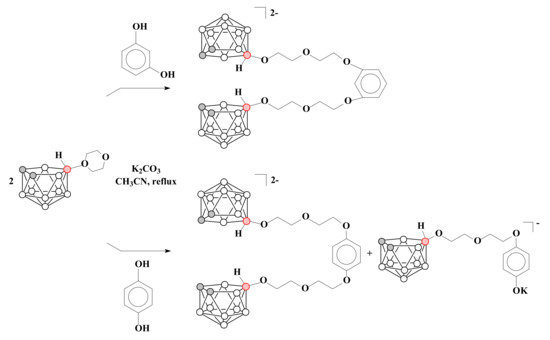
Scheme 8.
The reactions of the 1,4-dioxane derivative of nido-carborane with resorcinol and hydroquinone.
The reaction of two equivalents of 10-O(CH2CH2)2O-7,8-C2B9H11 with ethylene glycol in the presence of K2CO3 leads to the formation of carboranyl triethylene glycol ether [10-HO(CH2CH2O)3-7,8-C2B9H11]− along with a certain amount of carboranyl diethylene glycol formate [10-HC(O)O(CH2CH2O)2-7,8-C2B9H11]− [78] (Scheme 9).
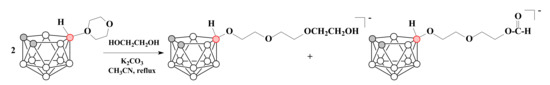
Scheme 9.
The reaction of 1,4-dioxane derivative of nido-carborane with ethylene glycol.
The reaction of a 1,4-dioxane derivative of nido-carborane with thiourea leads to the formation of a thiouronium salt 10-(NH2)2CSCH2CH2OCH2CH2O-7,8-C2B9H11, the subsequent hydrolysis of which gives the corresponding thiol [10-HSCH2CH2OCH2CH2O-7,8-C2B9H11]−. The formed thiol was reacted with one equivalent of 1,4-dioxane derivative of nido-carborane to give a podand [(10-CH2CH2OCH2CH2O-7,8-C2B9H11)2S]2− related to acyclic crown ether 15-crown-5 [79] (Scheme 10).
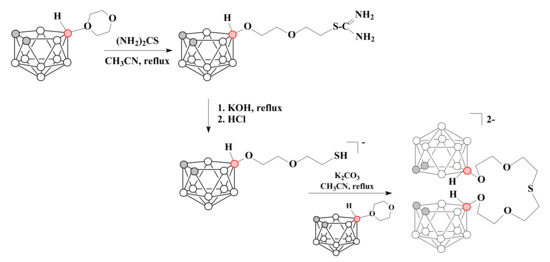
Scheme 10.
The reaction of 1,4-dioxane derivative of nido-carborane with thiourea.
The nido-carborane thiol [10-HSCH2CH2OCH2CH2O-7,8-C2B9H11]− can also be obtained directly by the reaction of 10-O(CH2CH2)2O-7,8-C2B9H11 with excess of aqueous sodium hydrosulfide in tetrahydrofuran. The by-product of this reaction is a thioether [(10-CH2CH2OCH2CH2O-7,8-C2B9H11)2S]2− [71].
Two nido-carborane-based podands [(10-CH2CH2OCH2CH2O-7,8-C2B9H11)2S]2− and [1,2-(10-SCH2CH2OCH2CH2O-7,8-C2B9H11)2C6H4]2− were used for the synthesis of crown ethers with the cobalt bis(dicarbollide) fragment incorporated in heteromacrocycle [79] (Scheme 11).
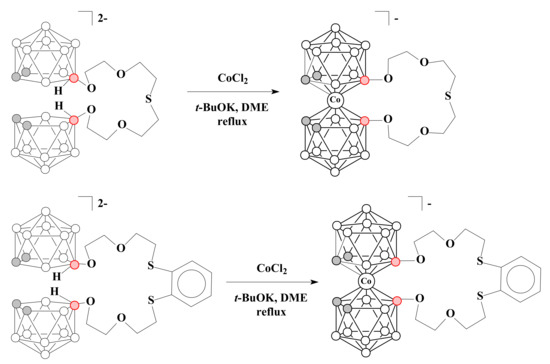
Scheme 11.
Synthesis of crown ethers with the incorporated cobalt bis(dicarbollide) fragment.
The crystal structure of sodium salt of [8,8′-(OCH2CH2OCH2CH2S)2-o-C6H4-3,3′-Co(1,2-C2B9H9)2]−, which was obtained during the drying of the solution of parent potassium salt in diethyl ether over Na2SO4, was determined by X-ray analysis [79] (Figure 3).
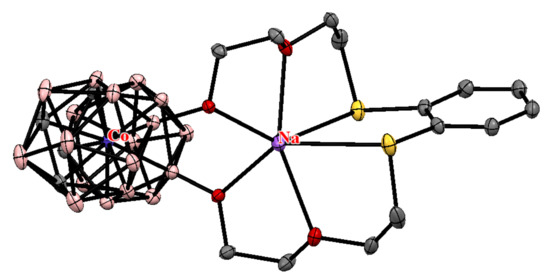
Figure 3.
The molecular structure of Na[8,8′-(OCH2CH2OCH2CH2S)2-o-C6H4-3,3′-Co(1,2-C2B9H9)2].
By the ring-opening reactions of tetrahydrofuran and 1,4-dioxane derivatives of nido-carborane with sodium azide a series of nido-carborane-based azides with different spacer lengths between the nido-carborane cage and functional group was synthesized [60,80,81] (Scheme 12).
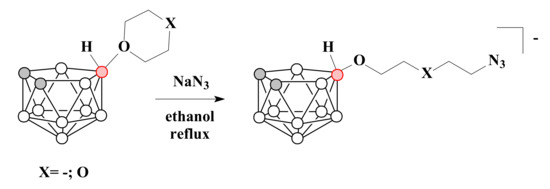
Scheme 12.
The synthesis of nido-carborane derivatives with terminal azido group.
The obtained azides can be used for medical application due to the possibility of their conjugation with biomolecules by standard methods of bioorganic chemistry such as, for example, Cu(I)-catalyzed reaction of 1,3-dipolar [3 + 2] -cycloaddition of terminal azido group with alkynes (“click” reaction) [44,82,83,84,85,86]. Thus, several conjugates with nucleosides were prepared by Cu(I)-catalyzed click reaction of 10-N3CH2CH2OCH2CH2O-7,8-C2B9H11 with modified derivatives of 2′-deoxyadenosine [80], uridine and thymidine [81], containing terminal alkyne group (Scheme 13).
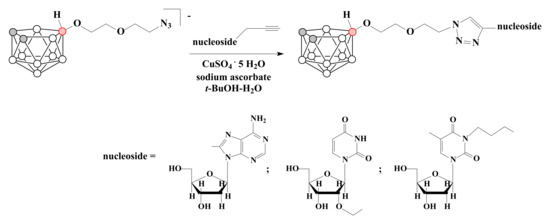
Scheme 13.
The synthesis of nido-carborane-based conjugates with nucleosides.
The reactions of 1,4-dioxane [63,71] and tetrahydropyran [63] derivatives of nido-carborane with ammonia in tetrahydrofuran led to the corresponding ammonium derivatives of nido-carborane. The obtained compounds were used for the conjugation with 3-nitro-1,8-naphthalic anhydride in the presence of Et3N to give water-soluble boron containing 3-nitro-1,8-naphthalimides, which are potential interesting agents for boron neutron capture therapy [63] (Scheme 14).
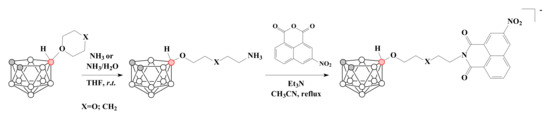
Scheme 14.
The synthesis of ammonium derivatives of nido-carborane and their conjugates with 3-nitro-1,8-naphthalic anhydride.
The molecular structure of 10-NH3CH2CH2OCH2CH2O-7,8-C2B9H11 was determined by single crystal X-ray diffraction [71] (Figure 4).
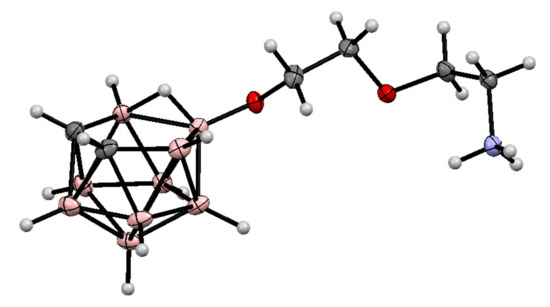
Figure 4.
The molecular structure of 10-NH3CH2CH2OCH2CH2O-7,8-C2B9H11.
The charge-compensated nido-carborane-based conjugates with mitonafide were prepared in mild conditions by the ring-opening reactions of 1,4-dioxane and tetrahydropyran derivatives of nido-carborane with dimethylamino group of mitonafide [63] (Scheme 15).
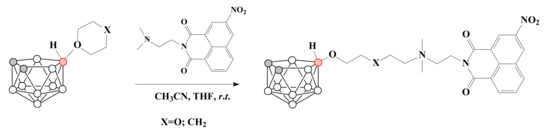
Scheme 15.
The synthesis of nido-carborane-based conjugates with mitonafide.
The reactions of 1,4-dioxane derivative of nido-carborane with 4-aminophenol in tetrahydrofuran in mild conditions led to the formation of the corresponding ammonium derivative 10-(4-OH-C6H4)-NH2CH2CH2OCH2CH2O-7,8-C2B9H11, whereas the treatment of 10-O(CH2CH2)2O-7,8-C2B9H11 with 2-amino-1,3-propanediol resulted in a mixture of 10-(HOCH2)2CHNH2CH2CH2OCH2CH2O-7,8-C2B9H11 and [(HOCH2)2CHNH-N,N-(10,10′-CH2CH2OCH2CH2O-7,8-C2B9H11)2]− [71]. At the same time the reaction with n-butylamine led only to [n-C4H9NH-(10-CH2CH2OCH2CH2O-7,8-C2B9H11)2]− [68] (Scheme 16).
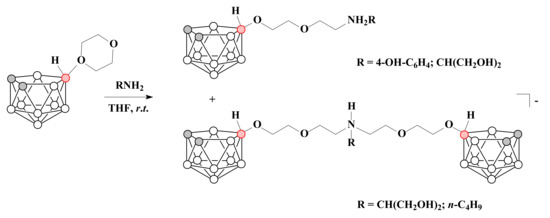
Scheme 16.
The ring-open reactions of 1,4-dioxane derivative of nido-carborane with primary amines.
The treatment of 1,4-dioxane derivative of nido-carborane with 4-aminophenol in the presence of K2CO3 in acetonitrile under reflux conditions gave the product of ring-opening both by amino and hydroxyl groups [7,8-C2B9H11-10-CH2CH2OCH2CH2O-H2N-C6H4-O-10′-CH2CH2OCH2CH2O-7,8-C2B9H11]− [71] (Scheme 17).
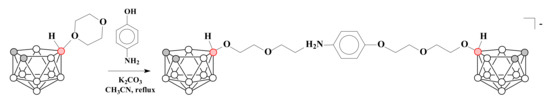
Scheme 17.
The reaction of 1,4-dioxane derivative of nido-carborane with 4-aminophenol in the presence of K2CO3.
The reactions of 1,4-dioxane derivative of nido-carborane with halide ions from tetrabutylammonium salts Bu4NX in diethyl ether at room temperature led to the ring-opening of the cycle with the formation of the corresponding halogen derivatives [10-X-CH2CH2OCH2CH2O-7,8-C2B9H11]− (X = Cl, Br, I) [71] (Scheme 18).
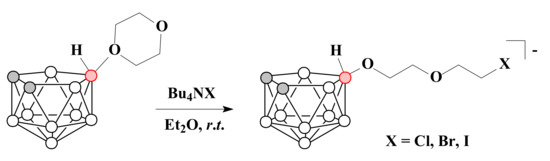
Scheme 18.
The reactions of 1,4-dioxane derivative of nido-carborane with halide ions.
The ring-opening reaction of 1,4-dioxane derivative of nido-carborane with KOH under mild conditions in biphasic system of diethyl ether and 2,5M aqueous KOH proceeded with the formation of [10-HO-CH2CH2OCH2CH2O-7,8-C2B9H11]−, whereas the heating of 10-O(CH2CH2)2O-7,8-C2B9H11 in 2,5M aqueous KOH through deprotonation of bridge hydrogen led to the substitution of the ether by hydroxyl group [10-HO-7,8-C2B9H11]− [71] (Scheme 19).
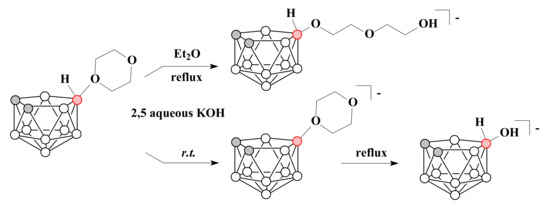
Scheme 19.
The reactions of 1,4-dioxane derivative of nido-carborane with KOH.
The ring-opening reactions of 1,4-dioxane derivative of nido-carborane with dimethyl sulfide or triphenylphosphine gave the corresponding sulfonium and phosphonium derivatives 10-L-CH2CH2OCH2CH2O-7,8-C2B9H11 (L = SMe2, PPh3). Subsequent treatment of these compounds with a base such as potassium tert-butoxide in both cases led to a side chain shortening product [10-HOCH2CH2O-7,8-C2B9H11]− [87] (Scheme 20).
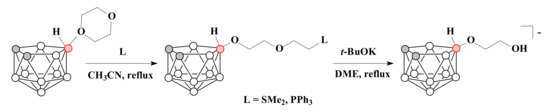
Scheme 20.
The synthetic route to [10-HOCH2CH2O-7,8-C2B9H11]− through the contraction of disclosed 1,4-dioxane ring.
The molecular structure of 10-Me2SCH2CH2OCH2CH2O-7,8-C2B9H11 was determined by single crystal X-ray diffraction [88] (Figure 5).
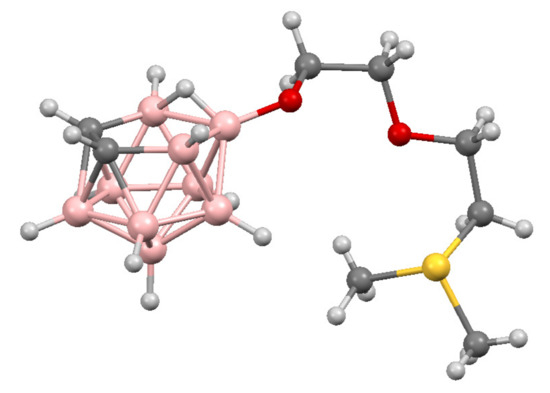
Figure 5.
The molecular structure of 10-Me2SCH2CH2OCH2CH2O-7,8-C2B9H11.
The dimethyl- and diethyloxonium derivatives of nido-carborane are also easily reacted with nucleophiles with the loss of the methyl or ethyl group, respectively, and can be used as alkylating agents [72,73].
4. Synthesis and Properties of Oxonium Derivatives of nido-carborane-Based Half-Sandwich Complexes
At the moment, for nido-carborane-based half-sandwich complexes only derivatives with tetrahydrofuran are known. With rare exceptions, they were obtained randomly, due to the fact that reactions involving metallacarboranes are most often carried out in dry tetrahydrofuran.
Thus, the reaction of protonated nido-carborane 7,8-nido-C2B9H13 with [(CO)5MnX] (X = Me, Et or BF4) in tetrahydrofuran results in [3,3,3-(CO)3-3-Mn(8-(CH2)4O-1,2-C2B9H10)] [89] (Scheme 21). The molecular structure of this complex was determined by X-ray diffraction [89] (Figure 6).
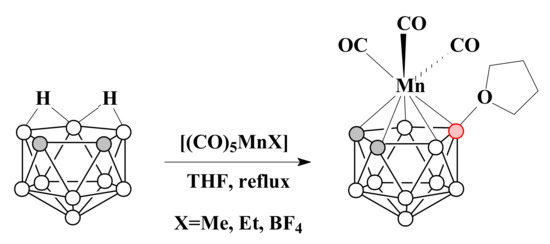
Scheme 21.
The synthesis of [3,3,3-(CO)3-3-Mn(8-(CH2)4O-1,2-C2B9H10)].
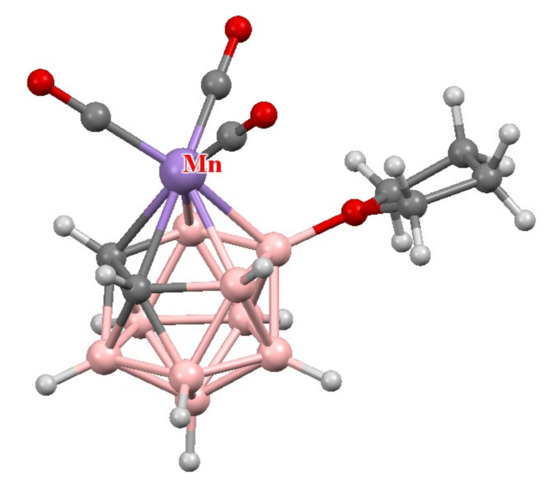
Figure 6.
The molecular structure of [3,3,3-(CO)3-3-Mn(8-(CH2)4O-1,2-C2B9H10)].
The THF derivatives of the same tricarbonyl manganese(I) complex [3,3,3-(CO)3-3-Mn(8-(CH2)4O-1,2-C2B9H10)], as well as its C,C′-dimethyl derivative [3,3,3-(CO)3-3-Mn(8-(CH2)4O-1,2-Me2-1,2-C2B9H8)], can also be prepared directly from the parent metallacarboranes with tetrahydrofuran in the presence of methyl triflate [90] (Scheme 22).
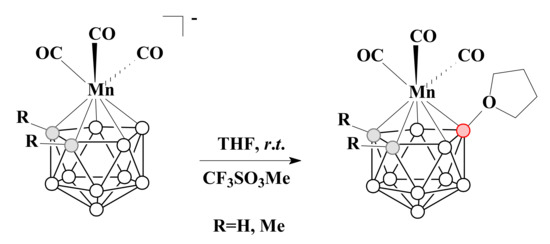
Scheme 22.
The synthetic route to [3,3,3-(CO)3-3-Mn(8-(CH2)4O-1,2-C2B9H10)] and [3,3,3-(CO)3-3-Mn(8-(CH2)4O-1,2-Me2-1,2-C2B9H8)].
It was shown that the reaction of tetrahydrofuran derivative of the tricarbonyl manganese complex [3,3,3-(CO)3-3-Mn(8-(CH2)4O-1,2-C2B9H10)] with NEt4I in THF under reflux conditions resulted in the opening of the tetrahydrofuran cycle with the formation of [3,3,3-(CO)3-3-Mn(8-I-CH2(CH2)3O-1,2-C2B9H10)]− [89] (Scheme 23).
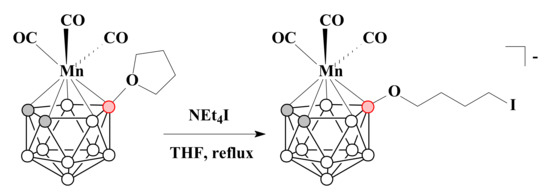
Scheme 23.
The reaction of [3,3,3-(CO)3-3-Mn(8-(CH2)4O-1,2-C2B9H10)] with NEt4I.
The treatment of [3,3,3-(CO)3-3-Mn(8-(CH2)4O-1,2-C2B9H10)] with donor molecules such as PPh3, NEt3 and 4-methylpyridine in THF at room temperature gave the corresponding charge-compensated complexes [3,3,3-(CO)3-3-Mn(8-L-CH2(CH2)3O-1,2-C2B9H10)] (L = PPh3, NEt3, 4-Me-C5H4N) [89] (Scheme 24).
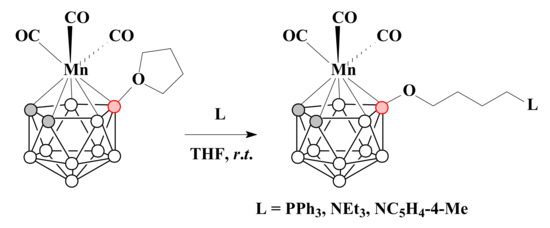
Scheme 24.
The reaction of [3,3,3-(CO)3-3-Mn(8-(CH2)4O-1,2-C2B9H10)] with PPh3, NEt3 and 4-methylpyridine.
The ruthenium(II) complex [3-Cp*-3-Ru(8-(CH2)4O-1,2-μ-O(CH2)2-1,2-C2B9H8)] was prepared by the reaction of thallium salt of parent [3-Cp*-3-Ru(1,2-μ-O(CH2)2-1,2-C2B9H9)]− with [Cp*RuCl]4 in tetrahydrofuran [91] (Scheme 25). It should be noted that, in the absence of the CH2OCH2 bridge between carbon atoms, this reaction leads to the 13-vertex dimetallacarborane [Cp*Ru(C2B9H11)RuCp*] [91].
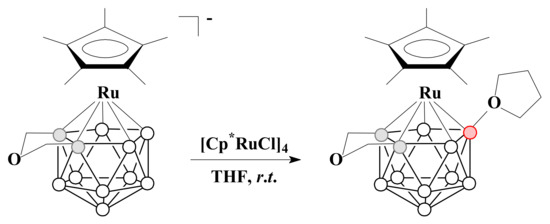
Scheme 25.
The synthetic route to [3-Cp*-3-Ru(8-(CH2)4O-1,2-μ-O(CH2)2-1,2-C2B9H8)].
The allyl dicarbonyl molybdenum complex with THF [3-(η3-C3H5)-3-(CO)2-3-Mo(8-(CH2)4O-1,2-Me2-1,2-C2B9H8)] can be synthesized by the reaction of parent metallacarborane with tetrahydrofuran in the presence of [Ph3C][BF4] [92] (Scheme 26).
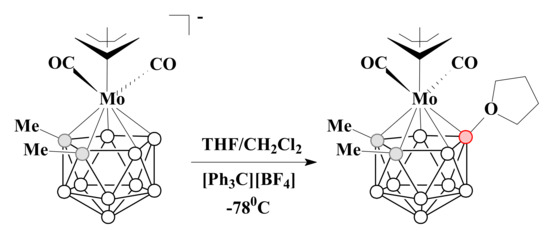
Scheme 26.
The synthetic route to [3-(η3-C3H5)-3-(CO)2-3-Mo(8-(CH2)4O-1,2-Me2-1,2-C2B9H8)].
In similar conditions, but in the presence of Et2O, the acyclic oxonium complexes of molybdenum and tungsten were prepared [92] (Scheme 27).
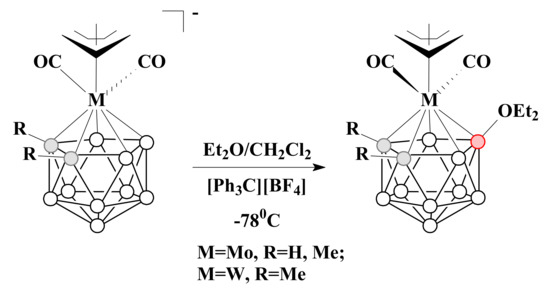
Scheme 27.
The synthetic route to acyclic diethyloxonium nido-carborane-based complexes with molybdenum and tungsten.
The molecular structure of [3-(η3-C3H5)-3-(CO)2-3-Mo(8-Et2O-1,2-Me2-1,2-C2B9H8)] was determined by X-ray diffraction [92] (Figure 7).
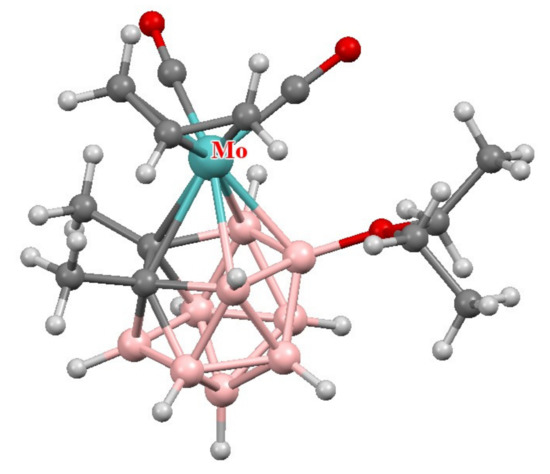
Figure 7.
The molecular structure of [3-(η3-C3H5)-3-(CO)2-3-Mo(8-Et2O-1,2-Me2-1,2-C2B9H8)].
The reaction of a tetrahydrofuran derivative of the allyl dicarbonyl molybdenum complex [3-(η3-C3H5)-3-(CO)2-3-Mo(8-(CH2)4O-1,2-Me2-1,2-C2B9H8)] with NEt4F or NBu4F in THF at room temperature led to the corresponding ammonium salts of [3-(η3-C3H5)-3-(CO)2-3-Mo(8-F-CH2(CH2)3O-1,2-Me2-1,2-C2B9H8)]−. The treatment of tetraethylammonium salt of the obtained complex with HBF4.OEt2 under an atmosphere of CO gave the charge-compensated tetracarbonyl molybdenum complex [3-(CO)4-3-Mo(8-F-CH2(CH2)3O-1,2-Me2-1,2-C2B9H8)] [92] (Scheme 28).
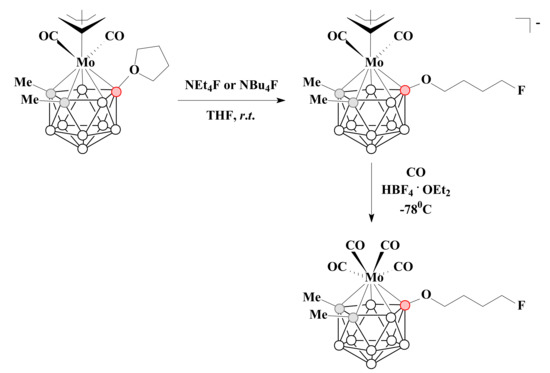
Scheme 28.
The synthetic route to [3-(η3-C3H5)-3-(CO)2-3-Mo(8-F-CH2(CH2)3O-1,2-Me2-1,2-C2B9H8)]− and [3-(CO)4-3-Mo(8-F-CH2(CH2)3O-1,2-Me2-1,2-C2B9H8)].
In summary, the oxonium derivatives of nido-carborane offer a convenient and simple method for modifying the nido-carborane cluster, which allows both the introduction of necessary functional groups into the side substituent and the direct preparation of conjugates with the required molecules. The resulting products of their ring-opening reactions are symmetric derivatives free from enantiomeric and diasteriomeric mixtures.
Author Contributions
Conceptualization and writing M.Y.S.; review and editing, I.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Ministry of Science and Higher Education of the Russian Federation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McCleverty, J.A. Highlights in inorganic chemistry over the last 100 years. Annu. Rep. Prog. Chem. Sect. A 2004, 100, 3–13. [Google Scholar] [CrossRef]
- Wiesboeck, R.A.; Hawthorne, M.F. Dicarbaundecaborane(13) and Derivatives. J. Am. Chem. Soc. 1964, 86, 1642–1643. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Young, D.C.; Garrett, P.M.; Owen, D.A.; Schwerin, S.G.; Tebbe, F.N.; Wegner, P.A. Preparation and characterization of the (3)-1,2- and (3)-1,7-dicarbadodecahydroundecarborate(-1) ions. J. Am. Chem. Soc. 1968, 90, 862–868. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Kalinin, V.I. On the reaction of amines with barenes. Tetrahedron Lett. 1965, 7, 407–409. [Google Scholar] [CrossRef]
- Hawthorne, M.F.; Wegner, P.A.; Stafford, R.C. Comments on the reaction of amines with 1,2-dicarbaclovododecaborane(12). Inorg. Chem. 1965, 4, 1675. [Google Scholar] [CrossRef]
- Davidson, M.G.; Fox, M.A.; Hibbert, T.G.; Howard, J.A.K.; Mackinnon, A.; Neretin, I.S.; Wade, K. Deboronation of ortho-carborane by an iminophosphorane: Crystal structures of the novel carborane adduct nido-C2B10H12·HNP(NMe2)3 and the borenium salt [(Me2N)3PNHBNP(NMe2)3]2O2+(C2B9H12−)2. Chem. Commun. 1999, 1649–1650. [Google Scholar] [CrossRef]
- Wise, S.D.; Au, W.; Getman, T.D. The effect of varying the solvent system on the rate of the deboration of o-carborane by n-butyl amine. Main Group Met. Chem. 2002, 25, 411–413. [Google Scholar] [CrossRef]
- Tomita, H.; Luu, H.; Onak, T. Cage opening of parent closo cage carboranes with fluoride ion: Formation of 5-fluoro-hexahydro-nido-2,4-dicarbahexaborate(1-) ([5-F-nido-2,4-C2B4H6]−). Inorg. Chem. 1991, 30, 812–815. [Google Scholar] [CrossRef]
- Fox, M.A.; Gill, W.R.; Herbertson, P.L.; MacBride, J.A.H.; Wade, K.; Colquhoun, H.M. Deboronation of C-substituted ortho- and meta-closo-carboranes using “wet” fluoride ion solutions. Polyhedron 1996, 15, 565–571. [Google Scholar] [CrossRef]
- Getman, T.D. Investigation of potassium fluoride supported on alumina in the deboronation of o-carborane. Inorg. Chem. 1998, 37, 3422–3423. [Google Scholar] [CrossRef]
- Yoo, J.; Hwang, J.-W.; Do, Y. Facile and mild deboronation of o-carboranes using cesium fluoride. Inorg. Chem. 2001, 40, 568–570. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, M.F.; Young, D.C.; Andrews, T.D.; Howe, D.V.; Pilling, R.L.; Pitts, A.D.; Reintjer, M.; Warren, L.F.; Wegner, P.A. π-Dicarbollyl derivatives of the transition metals. Metallocene analogs. J. Am. Chem. Soc. 1968, 90, 879–896. [Google Scholar] [CrossRef]
- Grimes, R.N. Transitional metal metallacarbaboranes. In Comprehensive Organometallic Chemistry II; Elsevier: Oxford, UK, 1995; Volume 1, pp. 373–430. [Google Scholar]
- Grimes, R.N. Metallacarboranes in the new millennium. Co-ord. Chem. Rev. 2000, 200, 773–811. [Google Scholar] [CrossRef]
- Hosmane, N.S.; Maguire, J.A. Metallacarboranes of d- and f-block metals. In Comprehensive Organometallic Chemistry III; Elsevier: Oxford, UK, 2007; Volume 3, pp. 175–264. [Google Scholar]
- Gozzi, M.; Schwarze, B.; Hey-Hawkins, E. Half- and mixed-sandwich metallacarboranes in catalysis. In Handbook of Boron Chemistry with Applications in Organometallics, Catalysis, Materials and Medicine. Boron in Catalysis; Hosmane, N.S., Eagling, R., Eds.; World Scientific Publishing Europe Ltd.: London, UK, 2019; Volume 2, pp. 27–80. [Google Scholar] [CrossRef]
- Zhu, Y.; Hosmane, N.S. Carborane-based catalysts for polymerization of olefins. In Handbook of Boron Chemistry with Applications in Organometallics, Catalysis, Materials and Medicine. Boron in Catalysis; Hosmane, N.S., Eagling, R., Eds.; World Scientific Publishing Europe Ltd.: London, UK, 2019; Volume 2, pp. 117–134. [Google Scholar] [CrossRef]
- Hawthorne, M.F. The role of chemistry in the development of boron neutron capture therapy of cancer. Angew. Chem. Int. Ed. Engl. 1993, 32, 950–984. [Google Scholar] [CrossRef]
- Soloway, A.H.; Tjarks, W.; Barnum, B.A.; Rong, F.-G.; Barth, R.F.; Codogni, I.M.; Wilson, J.G. The chemistry of neutron capture therapy. Chem. Rev. 1998, 98, 1515–1562. [Google Scholar] [CrossRef] [PubMed]
- Valliant, J.F.; Guenther, K.J.; King, A.S.; Morel, P.; Schaffer, P.; Sogbein, O.O.; Stephenson, K.A. The medicinal chemistry of carboranes. Co-ord. Chem. Rev. 2002, 232, 173–230. [Google Scholar] [CrossRef]
- Armstrong, A.F.; Valliant, J.F. The bioinorganic and medicinal chemistry of carboranes: From new drug discovery to molecular imaging and therapy. Dalton Trans. 2007, 4240–4251. [Google Scholar] [CrossRef]
- Lee, W.; Sarkar, S.; Ahn, H.; Kim, J.Y.; Lee, Y.J.; Chang, Y.; Yoo, J. PEGylated liposome encapsulating nido-carborane showed significant tumor suppression in boron neutron capture therapy (BNCT). Biochem. Biophys. Res. Commun. 2020, 522, 669–675. [Google Scholar] [CrossRef]
- Varaksin, M.V.; Smyshliaeva, L.A.; Rusinov, V.L.; Makeev, O.G.; Melekhin, V.V.; Baldanshirieva, A.D.; Gubina, O.G.; Charushin, V.N.; Chupakhin, O.N. Synthesis, characterization, and in vitro assessment of cytotoxicity for novel azaheterocyclic nido-carboranes—Candidates in agents for boron neutron capture therapy (BNCT) of cancer. Tetrahedron 2021, 102, 132525. [Google Scholar] [CrossRef]
- Tolmachev, V.V.; Sjöberg, S. Polyhedral boron compounds as potential linkers for attachment of radiohalogens to targeting proteins and peptides. A review. Collect. Czech. Chem. Commun. 2002, 67, 913–935. [Google Scholar] [CrossRef]
- Wilbur, D.S.; Chyan, M.-C.; Hamlin, D.K.; Vessella, R.L.; Wedge, T.J.; Hawthorne, M.F. Reagents for astatination of biomolecules. 2. Conjugation of anionic boron cage pendant groups to a protein provides a method for direct labeling that is stable to in vivo deastatination. Bioconjugate Chem. 2007, 18, 1226–1240. [Google Scholar] [CrossRef]
- Green, A.E.C.; Harrington, L.E.; Valliant, J.F. Carborane-carbohydrate derivatives—Versatile platforms for developing targeted radiopharmaceuticals. Can. J. Chem. 2008, 86, 1063–1069. [Google Scholar] [CrossRef]
- Armstrong, A.F.; Lebert, J.M.; Brennan, J.D.; Valliant, J.F. Functionalized carborane complexes of the [M(CO)2(NO)]2+ core (M = 99mTc, Re): A new class of organometallic probes for correlated in vitro and in vivo imaging. Organometallics 2009, 28, 2986–2992. [Google Scholar] [CrossRef]
- El-Zaria, M.E.; Janzen, N.; Valliant, J.F. Room-temperature synthesis of Re(I) and Tc(I) metallocarboranes. Organometallics 2012, 31, 5940–5949. [Google Scholar] [CrossRef]
- Visbal, R.; Ospino, I.; López-de-Luzuriaga, J.M.; Laguna, A.; Gimeno, M.C. N-Heterocyclic carbene ligands as modulators of luminescence in three-coordinate gold(I) complexes with spectacular quantum yields. J. Am. Chem. Soc. 2013, 135, 4712–4715. [Google Scholar] [CrossRef] [PubMed]
- Axtell, J.C.; Kirlikovali, K.O.; Djurovich, P.I.; Jung, D.; Nguyen, V.T.; Munekiyo, B.; Royappa, A.T.; Rheingold, A.L.; Spokoyny, A.M. Blue phosphorescent zwitterionic iridium(III) complexes featuring weakly coordinating nido-carborane-based ligands. J. Am. Chem. Soc. 2016, 138, 15758–15765. [Google Scholar] [CrossRef]
- Shafikov, M.Z.; Suleymanova, A.F.; Czerwieniec, R.; Yersin, H. Design strategy for Ag(I)-based thermally activated delayed fluorescence reaching an efficiency breakthrough. Chem. Mater. 2017, 29, 1708–1715. [Google Scholar] [CrossRef]
- Nghia, N.V.; Jana, S.; Sujith, S.; Ryu, J.Y.; Lee, J.; Lee, S.U.; Lee, M.H. nido-Carboranes: Donors for thermally activated delayed fluorescence. Angew. Chem. Int. Ed. 2018, 57, 12483–12488. [Google Scholar] [CrossRef]
- Nghia, N.V.; Oh, J.; Sujith, S.; Jung, J.; Lee, M.H. Tuning the photophysical properties of carboranyl luminophores by closo- to nido-carborane conversion and application to OFF–ON fluoride sensing. Dalton Trans. 2018, 47, 17441–17449. [Google Scholar] [CrossRef]
- He, T.-F.; Ren, A.-M.; Chen, Y.-N.; Hao, X.-L.; Shen, L.; Zhang, B.-H.; Wu, T.-S.; Zhang, H.-X.; Zou, L.-Y. Molecular-level insight of Cu(I) complexes with the 7,8-bis(diphenylphosphino)-7,8-dicarba-nido-undecaborate ligand as a thermally activated delayed fluorescence emitter: Luminescent mechanism and design strategy. Inorg. Chem. 2021, 59, 12039–12053. [Google Scholar] [CrossRef]
- Li, Q.; Shi, C.; Huang, M.; Zhang, X.; Sun, F.; Zheng, Y.; Yan, H.; Yang, C.; Yuan, A. Three types of charged ligand-based neutral phosphorescent iridium(III) complexes featuring nido-carborane: Synthesis, structures, and solution processed organic light-emitting diode applications. Dalton Trans. 2021, 50, 16304–16310. [Google Scholar] [CrossRef]
- Alconchel, A.A.; Crespo, O.; García-Orduña, P.; Gimeno, M.C. closo- or nido-Carborane diphosphane as responsible for strong thermochromism or time activated delayed fluorescence (TADF) in [Cu(N^N)(P^P)]0. Inorg. Chem. 2021, 60, 18521–18528. [Google Scholar] [CrossRef] [PubMed]
- Lerouge, F.; Ferrer-Ugalde, A.; Viñas, C.; Teixidor, F.; Sillanpää, R.; Abreu, A.; Xochitiotzi, E.; Farfán, N.; Santillan, R.; Núñez, R. Synthesis and fluorescence emission of neutral and anionic di- and tetra-carboranyl compounds. Dalton Trans. 2011, 40, 7541–7550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Hosmane, N.S. Ionic liquids: Recent advances and applications in boron chemistry. Eur. J. Inorg. Chem. 2017, 38-39, 4369–4377. [Google Scholar] [CrossRef] [Green Version]
- Sivaev, I.B. Nitrogen heterocyclic salts of polyhedral borane anions: From ionic liquids to energetic materials. Chem. Heterocycl. Compd. 2017, 53, 638–658. [Google Scholar] [CrossRef]
- Grimes, R.N. Carboranes, 3rd ed.; Academic Press: London, UK, 2016; pp. 179–257. [Google Scholar] [CrossRef]
- Zakharova, M.V.; Sivaev, I.B.; Anufriev, S.A.; Timofeev, S.V.; Suponitsky, K.Y.; Godovikov, I.A.; Bregadze, V.I. A new approach to the synthesis of functional derivatives of nido-carborane: Alkylation of [9-MeS-nido-7,8-C2B9H11]−. Dalton Trans. 2014, 43, 5044–5053. [Google Scholar] [CrossRef]
- Anufriev, S.A.; Zakharova, M.V.; Sivaev, I.B.; Bregadze, V.I. New carborane-containing acids and amines. Russ. Chem. Bull. 2017, 66, 1643–1649. [Google Scholar] [CrossRef]
- Timofeev, S.V.; Zhidkova, O.B.; Prikaznova, E.A.; Sivaev, I.B.; Semioshkin, A.; Godovikov, I.A.; Starikova, Z.A.; Bregadze, V.I. Direct synthesis of nido-carborane derivatives with pendant functional groups by copper-promoted reactions with dimethylalkylamines. J. Organomet. Chem. 2014, 757, 21–27. [Google Scholar] [CrossRef]
- Druzina, A.A.; Zhidkova, O.B.; Dudarova, N.V.; Kosenko, I.D.; Ananyev, I.V.; Timofeev, S.V.; Bregadze, V.I. Synthesis and structure of nido-carboranyl azide and its “click” reactions. Molecules 2021, 26, 530. [Google Scholar] [CrossRef]
- Druzina, A.A.; Zhidkova, O.B.; Dudarova, N.V.; Nekrasova, N.A.; Suponitsky, K.Y.; Timofeev, S.V.; Bregadze, V.I. Synthesis of zwitter-ionic conjugate of nido-carborane with cholesterol. Molecules 2021, 26, 6687. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, W.; Liu, W.; Wei, X.; Chen, M.; Zhang, X.; Zhang, X.; Liang, Y.; Lu, C.; Yan, H. Metal-free oxidative B-N coupling of nido-carborane with N-heterocycles. Angew. Chem. Int. Ed. 2019, 58, 11886–11892. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jei, B.B.; Scheremetjew, A.; Kuniyil, R.; Ackermann, L. Electrochemical B-H nitrogenation: Access to amino acids and BODIPY-labeled nido-carboranes. Angew. Chem. Int. Ed. 2021, 60, 1482–1487. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, D.; Xu, J.; Li, C.; Lu, C.; Yan, H. Electrooxidative B-H functionalization of nido-carboranes. Angew. Chem. Int. Ed. 2021, 60, 7838–7844. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhao, W.; Xu, S.; Xu, J.; Li, C.; Lu, C.; Yan, H. Photoredox B-H functionalization to selective B-N(sp3) coupling of nido-carborane with primary and secondary amines. Chem. Commun. 2021, 57, 8580–8583. [Google Scholar] [CrossRef] [PubMed]
- Anufriev, S.A.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Bregadze, V.I. Synthesis of 10-methylsulfide and 10-alkylmethylsulfonium nido-carborane derivatives: B–H π interactions between the B–H–B hydrogen atom and alkyne group in 10-RC≡CCH2S(Me)-7,8-C2B9H11. Eur. J. Inorg. Chem. 2017, 2017, 4436–4443. [Google Scholar] [CrossRef] [Green Version]
- Erokhina, S.A.; Stogniy, M.Y.; Suponitsky, K.Y.; Kosenko, I.D.; Sivaev, I.B.; Bregadze, V.I. Synthesis of new nido-carborane based carboxylic acids and amines. Polyhedron 2018, 153, 145–151. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Sivaev, I.B.; Bregadze, V.I. Nucleophilic addition reactions to the ethylnitrilium derivative of nido-carborane 10-EtC≡N-7,8-C2B9H11. New J. Chem. 2018, 42, 17958–17967. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. 10-NCCH2CH2OCH2CH2C≡N-7,8-C2B9H11: Synthesis and reactions with various nucleophiles. Polyhedron 2019, 174, 114170. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Godovikov, I.A.; Sivaev, I.B.; Bregadze, V.I. Synthesis of novel carboranyl amidines. J. Organomet. Chem. 2020, 909, 121111. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Markov, V.Y.; Sivaev, I.B. Synthesis and crystal structures of nickel(II) and palladium(II) complexes with o-carboranyl amidine ligands. Dalton Trans. 2021, 50, 4967–4975. [Google Scholar] [CrossRef]
- Semioshkin, A.A.; Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives of polyhedral boron hydrides and their synthetic applications. Dalton Trans. 2008, 977–992. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives as an efficient synthetic tool for the modification of polyhedral boron hydrides. In Boron Science: New Technologies and Applications; Hosmane, N.S., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 623–637. [Google Scholar]
- Druzina, A.A.; Shmalko, A.V.; Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives of cobalt and iron bis(dicarbollide)s and their use in organic synthesis. Russ. Chem. Rev. 2021, 90, 785–830. [Google Scholar] [CrossRef]
- Young, D.C.; Howe, D.V.; Hawthorne, M.F. Ligand derivatives of (3)-1,2-dicarbadodecahydroundecaborate(-1). J. Am. Chem. Soc. 1969, 91, 859–862. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Abramova, E.N.; Lobanova, I.A.; Sivaev, I.B.; Bragin, V.I.; Petrovskii, P.V.; Tsupreva, V.N.; Sorokina, O.V.; Bregadze, V.I. Synthesis of functional derivatives of 7,8-dicarba-nido-undecaborate anion by ring-opening of its cyclic oxonium derivatives. Collect. Czech. Chem. Commun. 2007, 72, 1676–1688. [Google Scholar] [CrossRef]
- Zakharkin, L.I.; Kalinin, V.N.; Zhigareva, G.G. Oxidation of dicarbadodecahydronidoundecaborate anions by mercury chloride in tetrahydrofuran and pyridine. Bull. Acad. Sci. USSR Div. Chem. Sci. 1979, 28, 2198–2199. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Sivaev, I.B.; Malysheva, Y.B.; Bregadze, V.I. Synthesis of tetrahydropyran oxonium derivative of 7,8-dicarba-nido-undecaborane anion [10-(CH2)5O-7,8-C2B9H11]. Vestnik N. I. Lobachevskiy Nizhegorod Univ. 2013, 4, 115–117. Available online: http://www.unn.ru/pages/e-library/vestnik/99999999_West_2013_4(1)/19.pdf (accessed on 30 December 2021).
- Laskova, J.; Kosenko, I.; Serdyukov, A.; Sivaev, I.; Bregadze, V.I. Synthesis of naphthalimide derivatives of closo-dodecaborate and nido-carborane. J. Organomet. Chem. 2022, 959, 122186. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Stogniy, M.Y. Mercury derivatives of polyhedral boranes, carboranes, and metallacarboranes. Russ. Chem. Bull. 2019, 68, 217–253. [Google Scholar] [CrossRef]
- Colquhoun, H.M.; Greenhough, T.J.; Wallbridge, M.G.H. Carbaborane derivatives of the late- and post-transition elements. Part 2. Dicarbaundecaboranyl compounds of copper(I), gold(I), and mercury(II); the crystal and molecular structure of 3-triphenylphosphine-3-mercura-1,2-dicarbadodecaborane(II), a pseudo-σ-bonded metallacarbaborane. J. Chem. Soc. Dalton Trans. 1979, 619–628. [Google Scholar] [CrossRef]
- Teixidor, F.; Ayllon, J.A.; Viñas, C.; Kivekäs, R.; Sillanpää, R.; Casabo, J. Mercury coordination to Exo-dithio-7,8-dicarba-nido-undecaborate derivatives. J. Organomet. Chem. 1994, 483, 153–157. [Google Scholar] [CrossRef]
- Shaw, K.F.; Reid, B.D.; Welch, A.J. Synthesis and characterisation of metal complexes of ether carbaboranes. Molecular structures of d6 ML3, d8 ML2 and d10 ML complexes of mono- and di-ether C2B9 carbaborane ligands, showing the progressive importance of secondary M…O bonding. J. Organomet. Chem. 1994, 482, 207–220. [Google Scholar] [CrossRef]
- Řezácová, P.; Pokorná, J.; Brynda, J.; Kožíšek, M.; Cígler, P.; Lepšík, M.; Fanfrlík, J.; Řezáč, J.; Šašková, K.G.; Sieglová, I.; et al. Design of HIV protease inhibitors based on inorganic polyhedral metallacarboranes. J. Med. Chem. 2009, 52, 7132–7141. [Google Scholar] [CrossRef]
- Frank, R.; Auer, H.; Hey-Hawkins, E. Functionalization of the nido-dicarbaborate anion nido-7,8-C2B9H12− by hydride abstraction. J. Organomet. Chem. 2013, 747, 217–224. [Google Scholar] [CrossRef]
- Plešek, J.; Jelínek, T.; Mareš, F.; Heřmánek, S. Unique dialkylsulfoniomethylation of the 7,8-C2B9H12− ion to the 9-R2S-CH2-7,8-C2B9H11 zwitterions by formaldehyde and dialkyl sulfides. General synthesis of the compounds 10-R2E-7,8-C2B9H11 (E = O, S). Collect. Czech. Chem. Commun. 1993, 58, 1534–1547. [Google Scholar] [CrossRef]
- Bakardjiev, M.; El Anwar, S.; Bavol, D.; Růžičková, Z.; Grüner, B. Focus on chemistry of the 10-dioxane-nido-7,8-dicarba-undecahydrido undecaborate zwitterion; exceptionally easy abstraction of hydrogen bridge and double-action pathways observed in ring cleavage reactions with OH− as nucleophile. Molecules 2020, 25, 814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stogniy, M.Y.; Erokhina, S.A.; Kosenko, I.D.; Semioshkin, A.A.; Sivaev, I.B. Dimethyloxonium and methoxy derivatives of nido-carborane and metal complexes thereof. Inorganics 2019, 7, 46. [Google Scholar] [CrossRef] [Green Version]
- Shmal’ko, A.V.; Anufriev, S.A.; Anisimov, A.A.; Stogniy, M.Y.; Sivaev, I.B.; Bregadze, V.I. Synthesis of cobalt and nickel 6,6′-diphenylbis(dicarbollides). Russ. Chem. Bull. 2019, 68, 1239–1247. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Anufriev, S.A.; Bogdanova, E.V.; Sivaev, I.B.; Bregadze, V.I. Mercury(II) chloride in the synthesis of nido-carborane derivatives with B-N, B-O and B-S bonds. Russ. Chem. Bull. 2022, 71, 91–101. [Google Scholar]
- Stogniy, M.Y.; Anufriev, S.A.; Shmal’ko, A.V.; Antropov, S.M.; Anisimov, A.A.; Suponitsky, K.Y.; Filippov, O.A.; Sivaev, I.B. The unexpected reactivity of 9-iodo-nido-carborane: From nucleophilic substitution reactions to the synthesis of tricobalt tris(dicarbollide) Na[4,4′,4′’-(MeOCH2CH2O)3-3,3′,3′’-Co3(μ3-O)(μ3-S)(1,2-C2B9H10)3]. Dalton Trans. 2021, 50, 2671–2688. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Sivaev, I.B.; Petrovskii, P.V.; Bregadze, V.I. Halogenation of the 7,8-dicarba-nido-undecaborate anion derivatives [10-RO-7,8-C2B9H11]−. Russ. Chem. Bull. 2012, 82, 91–94. [Google Scholar] [CrossRef]
- Serdyukov, A.; Kosenko, I.; Druzina, A.; Grin, M.; Mironov, A.F.; Bregadze, V.I.; Laskova, J. Anionic polyhedral boron clusters conjugates with 7-diethylamino-4-hydroxycoumarin. Synthesis and lipophilicity determination. J. Organomet. Chem. 2021, 946–947, 121905. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Kazakov, G.S.; Sivaev, I.B.; Bregadze, V.I. Synthesis of podands with nido-carboranyl groups as a basis for construction of crown ethers with an incorporated metallacarborane moiety. Russ. Chem. Bull. 2013, 3, 699–704. [Google Scholar] [CrossRef]
- Kazakov, G.S.; Stogniy, M.Y.; Sivaev, I.B.; Suponitsky, K.Y.; Godovikov, I.A.; Kirilin, A.D.; Bregadze, V.I. Synthesis of crown ethers with the incorporated cobalt bis(dicarbollide) fragment. J. Organomet. Chem. 2015, 798, 196–203. [Google Scholar] [CrossRef]
- Olejniczak, A.; Wojtczak, B.; Lesnikowski, Z.J. 2′-Deoxyadenosine bearing hydrophobic carborane pharmacophore nucleosides. Nucleotides Nucleic Acids 2007, 26, 1611–1613. [Google Scholar] [CrossRef] [PubMed]
- Wojtczak, B.A.; Andrysiak, A.; Grüner, B.; Lesnikowski, Z.J. “Chemical ligation”: A versatile method for nucleoside modification with boron clusters. Chem. Eur. J. 2008, 14, 10675–10682. [Google Scholar] [CrossRef] [PubMed]
- Stanislav, I.; Presolski, V.; Hong, P.; Finn, M.G. Copper-catalyzed azide-alkyne click chemistry for bioconjugation. Curr. Protoc. Chem. Biol. 2011, 3, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Jiang, Z.; He, H.; Liu, H.; Thayumanavan, S. Azide-terminated RAFT polymers for biological applications. Curr. Protoc. Chem. Biol. 2020, 12, e85. [Google Scholar] [CrossRef]
- Tiwari, V.K.; Mishra, B.B.; Mishra, K.B.; Mishra, N.; Singh, A.S.; Chen, X. Cu-catalyzed click reaction in carbohydrate chemistry. Chem. Rev. 2016, 116, 3086–3240. [Google Scholar] [CrossRef]
- Druzina, A.A.; Stogniy, M.Y. Synthesis of cholesterol derivatives based on closo- and nido-carboranes. Russ. Chem. Bull. 2021, 70, 527–532. [Google Scholar] [CrossRef]
- Shmal’ko, A.V.; Stogniy, M.Y.; Kazakov, G.S.; Anufriev, S.A.; Sivaev, I.B.; Kovalenko, L.V.; Bregadze, V.I. Cyanide free contraction of disclosed 1,4-dioxane ring as a route to cobalt bis(dicarbollide) derivatives with short spacer between the boron cage and terminal functional group. Dalton Trans. 2015, 44, 9860–9878. [Google Scholar] [CrossRef] [PubMed]
- Šubrtová, V.; Petříček, V.; Hummel, L. Structure of the zwitterionic 10-[2-(dimethylsulfonioethoxy)ethoxy]undecahydro-7,8-dicarba-nido-undecaborate(1-). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1989, 45, 1964–1966. [Google Scholar] [CrossRef]
- Gómez-Saso, M.; Mullica, D.F.; Sappenfield, E.; Stone, F.G.A. Reaction of nido-7,8-C2B9H13 with pentacarbonyl(methyl)manganese: Crystal structure of the charge-compensated complex [Mn(CO)3{η5-7,8-C2B9H10-10-O(CH2)4}]. Polyhedron 1996, 15, 793–801. [Google Scholar] [CrossRef]
- Hata, M.; Kautz, J.A.; Lu, X.L.; McGrath, T.D.; Stone, F.G.A. Revisiting [Mn(CO)3(η5-nido-7,8-C2B9H11)]−, the dicarbollide analogue of [(η5-C5H5)Mn(CO)3]: Reactivity studies leading to boron atom functionalization. Organometallics 2004, 23, 3590–3602. [Google Scholar] [CrossRef]
- Perekalin, D.S.; Lyssenko, K.A.; Kudinov, A.R.; Corsini, M.; Fabrizi de Biani, F. Synthesis of 13-vertex dimetallacarboranes by electrophilic insertion into 12-vertex ruthenacarboranes. Dalton Trans. 2017, 46, 15710–15718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullica, D.F.; Sappenfield, E.L.; Stone, F.G.A.; Woollam, S.F. Allyl carborane complexes of molybdenum and tungsten: Cage-hydride abstraction reactions in the presence of donor molecules. Organometallics 1994, 13, 157–166. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).