Abstract
Dry reforming of methane (DRM) reaction has drawn much interest due to the reduction of greenhouse gases and production of syngas. Coking and sintering have hindered the large-scale operations of Ni-based catalysts in DRM reactions at high temperatures. Smart designs of Ni-based catalysts are comprehensively summarized in fourth aspects: surface regulation, oxygen defects, interfacial engineering, and structural optimization. In each part, details of the designs and anti-deactivation mechanisms are elucidated, followed by a summary of the main points and the recommended strategies to improve the catalytic performance, energy efficiency, and utilization rate.
1. Introduction
Many studies have been focused on the conversion of CO2 and CH4 (two greenhouse gases) to value-added chemicals or petrochemicals in various processes, such as reforming of methane [1,2,3,4,5,6,7,8], CO2 methanation [9,10,11,12,13,14,15,16], membrane-assisted reaction [17,18,19,20,21], chemical looping [22], reversed water-gas-shift [23,24], and catalytic decomposition [25]. Therein, dry reforming of methane (DRM) reaction draws much interest because it contributes to the reduction of greenhouse gases (CO2 and CH4) and generates a mixture of CO and H2 (syngas), which can be used as fuels and further converted to high value-added petrochemicals [26,27,28,29,30,31]. The reaction equation is shown as below:
CH4 + CO2 ↔ 2CO + 2H2 ΔH298K = 261 kJmol−1
The low H2:CO ratio is favorable to Fischer–Tropsch reaction due to the promotion of chain growth and suppression of methanation. Also, the endothermic forward reaction benefits the Solchem process by converting solar energy to chemical energy. Moreover, DRM reaction is applicable for the storage of seasonal energy in the form of CO and H2 [32,33,34,35].
In order to activate the reactants, noble metal catalysts are applied and proved to be highly reactive. However, the high cost, low abundance, and metal sintering hinder their large-scale applications in industry [30]. Ni-based catalysts have been widely studied due to their low cost and good activities, but carbon deposition, metal sintering, and surface oxidation lead to the deactivation of catalysts and impede their further applications [30]. For example, the in situ XPS technique was applied to monitor the evolution of Ni surface during the reaction. Results showed that during the inactive period, 3 nm layer of NiO covered the metallic Ni surface, leading to the decrease of reactivity [36]. Solutions have been provided to address this issue, including the size control of active metals, doping second transition element, adding alkali earth or rare earth promoters to the support, tuning metal-support interaction (MSI), structure designs and optimization of reaction parameters [26,27,28,29,30,31]. Researchers have reviewed the progress of Ni-based catalysts for DRM reaction in terms of the metal doping, porous supports, and kinetics/mechanisms, which shows the high importance of and widespread attention paid towards the DRM reaction [26,27,28,29,30,31]. However, it is rare to see a summary of the structure designs of supports, architectures of the catalysts, surface species control and interfacial strength adjustment in recent years, which greatly affects the active surface area, activities, and anti-deactivation capabilities. In this review, we summarize the recent advances of Ni-based catalysts’ modifications and corresponding catalytic performances for DRM reaction. The following parts consist of surface regulation, oxygen defects, interfacial engineering, and structural optimization, followed by concluding remarks and prospects.
2. Surface Regulation
For most heterogeneous reactions, reactants are adsorbed and activated on the surface active sites to produce the intermediates and final products. Modifying the surface properties has significant effects on the catalytic process and performances. In DRM reaction, CO2 and CH4 are converted to CO and H2 through adsorption, dissociation, and recombination on the surface of Ni-based catalysts. Surface basicity exerts a great influence on the catalytic conversions and coke resistance [30].
Surface acidity and basicity affect the electron environment of metal active sites and the adsorption of reactants, thus determining the catalytic performances. For example, Lewis acid sites reduce electron density of Ni species and balance the activation of CH4 and removal of carbon deposits, leading to a high stability in DRM reaction [37]. Also, enhanced basicity of the supports improves the adsorption of CO2, which subsequently dissociates into CO and oxygen atoms, reacting with the decomposition intermediate CHx to produce CO and inhibit carbon formation [30]. To enhance the surface basicity, various compounds have been utilized, including alkali and alkali earth metal oxides and rare earth metal oxides [30,38,39,40,41].
La2O3 as a rare earth metal oxide, acts as the support to promote the adsorption of CO2 by reacting with CO2 to form La oxycarbonate (La2O2CO3) [42]. According to in-situ DRIFT study, carbon species from the activation of methane reacted with the La oxycarbonate to form CO, removing the surface coke and maintaining the active sites [43]. The reaction mechanism was shown as below [43]:
To further enhance the CO2 adsorption, MgO as a basic oxide is promising due to its successful applications [44,45,46,47]. When MgO was added into the Ni/La2O3, due to its strong basicity, more CO2 adsorbed on the surface to react with La2O3 to form monoclinic La2O3CO3. Interestingly, at a low Mg:La ratio, only hexagonal La2O3 and La2O2CO3 mixtures were formed, which was not effective as monoclinic phase in reacting with carbon species, thus deactivating the catalysts much faster (seen in Table 1) [48]. To further elucidate the mechanism of removing the carbon deposits by La2O3, a theoretical study was conducted to compare the different catalytic pathways of La2O3 and La metal. La2O3-Ni followed the reaction pathway below: CH4 + CO2 + La2O3→CH + CO2(La2O2-O) + 3H→CHO + CO2(La2O2) + 3H→2CO + 2H2 + La2O3. It showed that the oxygen atom dissociated from La oxycarbonate reacted with CH or C species to form CO. On the other hand, La metal dopant was not effective as the oxide since the faster rate of CH→C + H for La-Ni accelerated the coke formation than La2O3-Ni catalysts [49]. As another rare earth metal oxide, CeO2 may decrease the total basicity. However, the intermediate and strong basic sites could be enhanced, thus promoting the CO2 adsorption and modifying the activity (shown in Table 1) [50].
Besides La2O3, CeO2 and MgO, other group metal oxides can also enhance the surface basicity. Considering the acidity of SiO2 support, Ga2O3 (Table 1) was added as a promoter to enhance the basicity and facilitate the adsorption of CO2 to form carbonate or bicarbonate species (Figure 1), which reacted with carbon deposits more easily than the physically or linearly adsorbed CO2 on the pristine SiO2 surface [51].
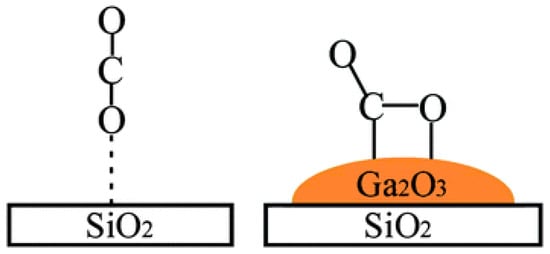
Figure 1.
Activation of CO2 on Ga2O3-promoted SiO2 support. Reproduced with permission from reference [51].
B2O3 as a non-metal oxide has been successfully doped into Ni/Al2O3 to enhance the surface hydroxyl group concentrations. The borated surface was rich in OH group which promoted the oxidation of carbon deposits from CH4 decomposition [52].
In certain situation, if the basicity of the support is too strong, the CO2 adsorption may not inhibit but lead to carbon deposition [53]. As shown in Table 1, to alleviate the basicity of Ni supported on MgO/Al2O3, Y2O3 was added to introduce more weak and medium basic sites on the surface [54,55]. By using 1.5 wt% of Y2O3, the highest conversion of CH4 and CO2 was achieved with the best stability [54]. In addition, La-promoted Ni–Mg–Al hydrotalcite was reported to be anti-coking due to the gasification of carbon deposits where weak and medium basic sites were increased [56].
In a word, adding promoters like MgO, La2O3 and Ga2O3 could enhance the surface basicity, thus facilitating the adsorption of CO2 which dissociated into CO and O and removed carbon deposits. Besides the surface basicity adjustment, surface hydroxyl group concentrations could be increased by adding B2O3 as a promoter, which promoted the carbon oxidation and maintained the stability. But a careful control of the surface basicity may be necessary since a too strong basicity could not inhibit the coke formation.

Table 1.
A summary of surface regulation effects on the catalytic performances of Ni-based catalysts for DRM reaction.
Table 1.
A summary of surface regulation effects on the catalytic performances of Ni-based catalysts for DRM reaction.
| Catalyst a) | Preparation Method | Reaction Condition | Methane Conversion | CO2 Conversion | Carbon Formation | Comments | Ref. |
|---|---|---|---|---|---|---|---|
| Ni/La2O3 | Wet impregnation method | 700 °C; CH4/CO2 = 1 | 70% for CH4 after 50 h | 75% for CO2 after 50 h | 10.3% | Carbon species reacted with the La oxycarbonate to form CO. | [42] |
| Ni/MgO-La2O3 | Co-precipitation and impregnation method | 700 °C; CH4/CO2 = 1 | 63% for CH4 after 200 h | 65% for CO2 after 200 h | 0.031 molC/molCH4 | When MgO was added into the Ni/La2O3, more CO2 adsorbed on the surface to react with La2O3 to form monoclinic La2O3CO3. | [48] |
| Ce-NiMgAl | Co-precipitation method | 550 °C; CH4/CO2 = 1 | 54.8% for CH4 after 5 h | 37.4% for CO2 after 5 h | Around 90% | CeO2 enhanced the amount of medium and strong basic sites. | [50] |
| Ni/SiO2–Ga2O3 | Incipient wetness impregnation method | 700 °C; CH4/CO2 = 1 | 70% for CH4 after 10 h | 79% for CO2 after 10 h | 16.8 mg/gcat | Ga2O3 was added as a promoter to enhance the basicity to facilitate the adsorption of CO2 to form carbonate or bicarbonate. | [51] |
| 5%Ni/B2O3–Al2O3 | Impregnation method | 700 °C; CH4/CO2 = 1 | 75% for CH4 after 65 h | 67% for CO2 after 65 h | 13% | The borated surface was rich in OH group which promoted the oxidation of carbon deposits. | [52] |
| HTNi-Y | Co-precipitation method | 700 °C; CH4/CO2 = 1 | 74% for CH4 after 10 h | 78% for CO2 after 10 h | 75.8% | Y2O3 was added to introduce more weak and medium basic sites on the surface. | [54] |
| Y-NiMgAl | Co-precipitation method | 700 °C; CH4/CO2 = 1 | 85% for CH4 after 5 h | 90% for CO2 after 5 h | 35.1% | Basicity increased with the increase loading of Y. | [55] |
| La-NiMgAl | Co-precipitation method | 550 °C; CH4/CO2 = 1 | 45% for CH4 after 8 h | 37% for CO2 after 8 h | 87.5% | La dopant enhanced the content of medium and weak basic sites. | [56] |
a) HT: hydrotalcite.
3. Oxygen Defects
In addition to the surface basicity and acidity regulation, oxygen defects including lattice and surface oxygen and oxygen vacancies enhance the oxygen mobility and adsorption, which effectively removes the carbon deposits during DRM reaction [30]. Rare earth metal oxides, transition metal oxides, mixed oxides, and perovskites will be discussed as below based on the origin of oxygen defects and corresponding effects on the carbon removal.
3.1. Rare-Earth Metal Oxides
Studies have shown that rare earth metal oxides can generate oxygen defects, promote oxygen mobility, and scavenge carbon species [57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]. As the representative, CeO2 with a good redox property can release lattice oxygen to generate oxygen vacancies, thus accelerating the oxygen mobility. When CeO2 was used as the support for Ni metals, the catalytic performance benefited from the lattice oxygen defects of CeO2 and the reversible conversion between Ce3+ and Ce4+. When Ce4+ was reduced to Ce3+, lattice oxygen was released and oxygen vacancies were formed, which improved the adsorption of oxygen species like O2 or CO2 to generate oxygen radicals, thus reducing the carbon deposition on the surface. However, an excessive amount of CeO2 may cover the Ni active sites and lower the accessibility of reactants to the Ni metal surface, causing a low conversion of CH4 and CO2 [30,65,78,79].
Theoretical study was applied to explore and elucidate the role of CeO2 in Ni catalysts for DRM reaction. Results showed that the activation sites for CH4 and CO2 were different. For CH4 activation, the preferred sites included metal-support interface, oxygen vacancies at the metal-support interface and the metal vacancies. But for CO2, the reactant molecule was mostly adsorbed and dissociated at oxygen vacancies at either the support or the metal-support interface. Moreover, the active sites could be regenerated by the interfacial oxygen of CeO2, maintaining the stability of the catalyst [58]. The detailed mechanistic diagram is shown in Figure 2 below.
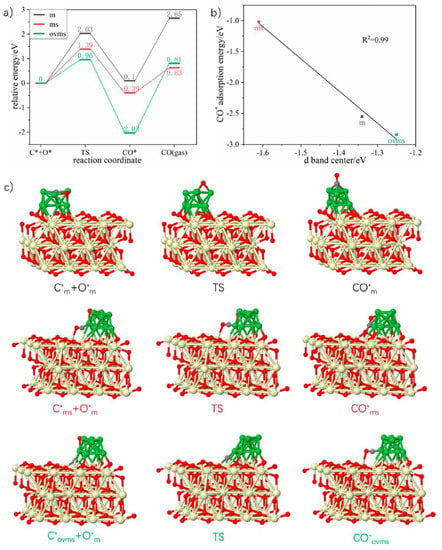
Figure 2.
Three paths of C* elimination. (a) Relative energy of C* elimination at m, ms, and ovms sites. (b) Relation of d band center with CO* adsorption energy. (c) Corresponding configurations of part a. Gray spheres represent C, red spheres represent O, green spheres represent Ni and cream-colored spheres represent Ce. Reproduced with permission from reference [58].
Another possible explanation of the catalytic effect of CeO2 was presented as below:
CeO2 + nCH4→CeO2n + nCO + 2nH2
CeO2-n + nCO2→CeO2 + nCO
The lattice oxygen of CeO2 reacted with CH4 to produce CO and H2, leaving a ceria with oxygen defects, which subsequently grabbed oxygen atoms from CO2 to recover to CeO2 and produce another molecule of CO. The initial high oxidation state of Ce ion is essential to the whole process. However, during the DRM reaction, the existence of hydrogen possibly reduced Ce4+ to Ce3+, decreasing the conversion of CH4 and CO2. Therefore, the immediate removal of hydrogen product from the system determines the catalytic effect of CeO2 [80].
To stabilize the CeO2 in the Ni catalysts, ZrO2 can be added to substitute the Ce in the lattice to form a cubic phase of CexZr1-xO2. This structure contains more oxygen vacancies and higher oxygen storage capacities than tetragonal phase. Because of the enhanced oxygen release capability, carbon deposits around the oxides are easily oxidized to form CO [81]. Likewise, as seen in Table 2, in another catalyst of Ni supported on CeO2-ZrO2 mixed oxides, the bonding degree determined the amount of oxygen vacancies. Also, the different oxidation states of Ce and Zr produced more oxygen vacancies. With the increase of Ni concentration, oxygen vacancies increased accordingly. When Ni reached the optimal molar ratio (9.3 mol%), the highest conversion of CH4 and CO2 was achieved [82].
Besides CeO2, Y2O3 is also proved to be effective in removing the carbon deposits due to its surface oxygen mobility. During the DRM reaction, the movement of surface oxygen was accelerated with the generation of interstitial oxygen ions when lattice defects were produced in Y2O3. Interestingly, Y2O3 with a smaller crystal size exhibited a higher surface oxygen mobility, thus decreasing the carbon formation rate [57]. In another study shown in Table 2, α-oxygen was found on the surface of Y2O3 to activate CH4 and remove the carbon. Considering the limited amount of α-oxygen, it was necessary to feed a small amount of oxygen to maintain the stability of the catalyst by regenerating the α-oxygen [83].
Another rare earth metal oxide La2O3 also draws attention in recent years as a promoter for Ni catalysts to inhibit coke formation in DRM reaction. As presented in Table 2, besides the effect of La oxycarbonate formed with adsorbed CO2 mentioned previously, the addition of small amount of Sr could enhance the surface oxygen species mobility, which facilitated the C-H activation and coke removal, leading to a high CO2 conversion and low carbon formation even at 600 °C reaction temperature [84].
In addition to the mixed oxides of CeO2 and ZrO2 above, two rare earth metal oxides can also be mixed to form a support for Ni catalyst, such as Ce0.70La0.20Ni0.10O2-δ. During the reaction, the lattice of CeO2 expanded due to the partial dissolution of La3+ into the lattice structure to generate oxygen vacancies. Moreover, with the increase of reduction temperature, the change of Ce oxidation state further increased the amount of oxygen vacancies. Under the condition of GHSV = 26,400 h−1, no carbon deposition was found, demonstrating the excellent coke resistance (seen in Table 2) [85].
In a word, owing to the redox property and high oxygen storage capacity, rare earth metal oxides can produce oxygen defects (vacancies) with the change of oxidation state and release of oxygen atoms. With the addition of second metals, the synergetic effect may generate more oxygen defects so that the activation of CH4 and CO2 as well as the carbon removal can be enhanced simultaneously, leading to the high activity and stability of Ni catalysts for the DRM reaction.
3.2. Transition Metal Oxides
Due to the incompletely occupied d orbitals, transition metal oxides have been widely studied as a support to enhance the coke resistance by producing lattice oxygens to reduce the carbon depositions during DRM reaction. ZrO2 and TiO2 are two representatives which will be discussed in detail below [86,87,88,89]. As for Fe, considering its main existing form as a Ni-Fe alloy instead of metal oxides, the effect of Fe will be illustrated in the interfacial engineering part.
The lattice oxygen species of ZrO2 plays a crucial role in the anti-coking property of Ni catalysts in DRM reaction. As seen in Table 2, the results of XRD and XPS showed that CH4 decomposed into CHx fragments and H atoms. The latter combined with each other to produce H2; the former adsorbed on the Ni metal to form NiCHx species. Because of the intimate interaction between Ni and ZrO2, the lattice oxygen of ZrO2 reacted with CHx to form CO and H2 and regenerated the Ni active sites. In the meanwhile, the coke formation was inhibited due to the release of lattice oxygen of ZrO2 and its affinity for the CO2 adsorption [59].
To enhance the interaction between Ni and ZrO2, a core-shell structure of Ni-ZrO2@SiO2 was prepared by hydrolysis of TEOS to form a mesoporous SiO2 shell and thermal treatment of NiZr(OH)x to form a Ni-ZrO2 core as shown in Figure 3. More active sites were formed at the interface and perimeter of Ni-ZrO2 with the stabilization effect of ZrO2 on Ni. As presented in Table 2, more abundant active oxygen provided by ZrO2 easily accessed the carbon species at Ni active sites and oxidized the coke to form CO, maintaining the stability of the catalyst [89].
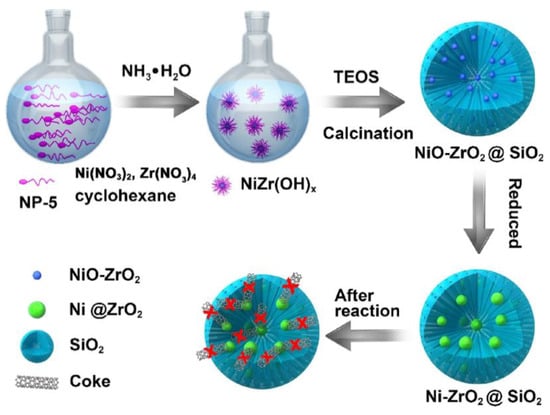
Figure 3.
Illustration of the fabrication method of the Ni-ZrO2@SiO2 catalyst with ultra-high coking and sintering resistance for the DRM reaction. Reproduced with permission from reference [89].
In order to elucidate the effect of different oxygen species in ZrO2 on the carbon removal for Ni catalysts in DRM reaction, three types of calcination atmospheres were applied. Results showed that the optimal ratio of surface adsorbed oxygen to lattice oxygen was achieved under H2 treatment, most favorable for CO2 and CH4 adsorption and activation, thus leading to the highest conversions and stability. Relative speaking, catalysts calcined under N2 and oxidizing atmospheres presented a lower ratio of surface oxygen to lattice oxygen, suffering from the serious carbon formation shown in Table 2 [90].
Besides ZrO2, another transition metal oxide TiO2 is proved to be effective in removing the carbon due to the lattice oxygen. Two oxygen species were found in TiO2, that is, oxygen ions inside TiO2 and lattice oxygen at the interface. The later could react with the coke formed on the surface of Ni to produce CO, thus maintaining the active surface area for the reactants to continue dissociation. Moreover, water vapor provided by the humid environment showed a high affinity for the oxygen vacancies and dissociated on TiO2 to reduce the carbon deposits [86].
In a word, the lattice oxygen of ZrO2 and TiO2 can react with surface carbon species such as CHx and carbon deposits to form CO. A careful control of the ratio between surface and lattice oxygen may affect the coke resistance. Further strengthening the MSI can lead to the intimate contact between abundant oxygen species and carbon residues, resulting in a high conversion and low carbon deposition.
3.3. Perovskites
Represented by the formula ABO3, perovskite structures have been widely investigated in heterogeneous catalysis at high temperatures due to its good thermal stability [91,92,93,94,95,96,97]. By substitution of A-site ions, the dimension of unit cells changes, thus changing the covalence of B-O bond accordingly, resulting in a lattice defect formation. These defects cause the increased amount of oxygen adsorption and high concentration of oxygen vacancies, leading to a promoted oxygen fluidity in the crystal structure [98,99,100]. Therefore, perovskites are extensively applied in DRM reaction to enhance the coke resistance of Ni catalysts.
Various elements have been added into LaNiO3 to replace A-site or B-site ions [101,102,103,104]. For example, Sr was doped in the perovskite structure to partially substitute A-site La ions to tune the cell dimension and oxygen states. The lattice distortions generated high lattice oxygen mobility, activating C-H bond in CH4 and adsorbing CO2 molecules to form La2O2CO3 [104]. For B-site replacement as presented in Table 2, Cu partially substituted Ni ions to enhance the mobility of lattice oxygen species, leading to a high conversion of CH4 but a low catalytic stability because agglomeration of Ni particle took place with a weak MSI. On the contrary, Fe-substituted LaSrNiFeO3 catalyst exhibited a long-term stability in DRM reaction due to the abundance of lattice oxygen and strong MSI [104]. The modification effect of Fe is elucidated in another work [105]. When Fe was doped into LaNiO3 structure, Fe could be converted to FeO and Fe3O4 by CO2 and Fe3O4 reacted with La2O3 to form LaFeO3-δ, which distorted the lattice structure and produced oxygen vacancies via compensation of the low valency of Fe ions. To modify the A-site of LaNiFeO3, Ce can be added to partially replace the La to introduce more oxygen vacancies by both activating the B-site ions and reversibly shuttling between Ce3+ and Ce4+. In detail, unlike Sr2+ with a lower valency than La3+, Ce3+ was not possible to directly provide oxygen vacancies when doped into the perovskite structure. But according to the TPR profiles as Figure 4 below, the reduction peak of Ni(Fe) ions shifted to lower temperatures with the increase of Ce amount, reflecting an improved reducibility and more oxygen vacancies indirectly offered by Ce. On the other hand, due to the local redox fluctuations of Ce ions, the regeneration of transition metals may be facilitated with the promoted migration inside/outside the perovskite structure. Therefore, more oxygen vacancies brought by Ce enhanced the oxygen mobility and backfilling of lattice oxygen and promoted the adsorption and activation of CO2 to CO and oxygen atoms which in turn replenished the oxygen vacancies (seen in Table 2) [106]. As for the B-site of LaNiFeO3, in another study, Co was doped into LaNiFeO3 to improve the oxygen mobility due to the spin state, multivalent property and oxygen affinity. When the molar ratio of Co equaled to 0.1 and 0.3, the modified perovskite structure presented a structural stability and high conversions [101].
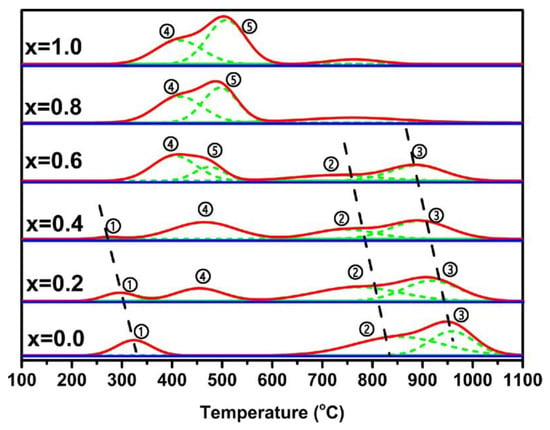
Figure 4.
TPR profiles of fresh La1-xCexNi0.5Fe0.5O3 with different x values. Reproduced with permission from reference [106].
Besides LaNiO3 structure, LaAlO3 perovskite catalysts are proved to be more resistant to coke formation than α-Al2O3 due to the higher thermal stability and more oxygen vacancies [107]. Two possible mechanisms were illustrated in Figure 5 below, which differed in the location of oxygen vacancies and NiO in the perovskite. When oxygen ions of NiO happened to be in the oxygen vacancies, two electrons of H2 were released to reduce Ni ions to form metallic Ni and produce H2O by reacting with O2−, enhancing the dispersion of Ni particles on the surface. When oxygen ions were located adjacent to the oxygen vacancies, H2 would be heterolytically dissociate into H+ and H−. The later adsorbed on the surface of acidic support while the former combined with O2- to produce water. In this case, the reduction of NiO was not preferred and the metal dispersion became poor. These two mechanisms reflected the promotional effect of oxygen vacancies on the migration of lattice oxygen of NiO and coke removal at the perovskite interface.
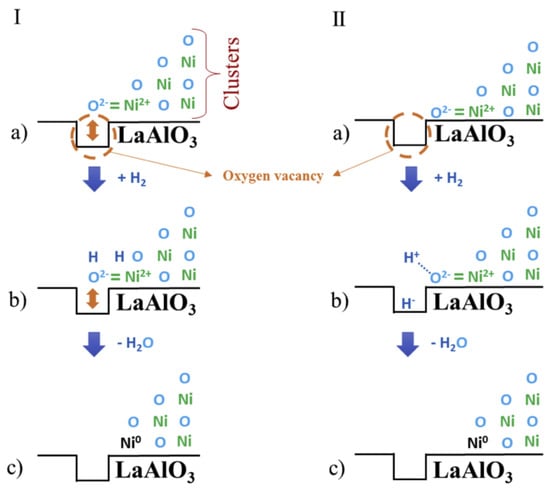
Figure 5.
Scheme of the reduction/activation mechanism of the Ni catalyst supported on LaAlO3 catalysts. (I)—Terminal oxygen ion of the NiO clusters located on the surface oxygen vacancy. (II)—Terminal oxygen ion of the NiO clusters located adjacent to the surface oxygen vacancy. Reproduced with permission from reference [107].

Table 2.
A summary of oxygen defects effects on the catalytic performances of Ni-based catalysts for DRM reaction.
Table 2.
A summary of oxygen defects effects on the catalytic performances of Ni-based catalysts for DRM reaction.
| Catalyst | Preparation Method | Reaction Condition | Methane Conversion | CO2 Conversion | Carbon Formation | Comments | Ref. |
|---|---|---|---|---|---|---|---|
| Ni–CaO–ZrO2 | Co-precipitation method | 700 °C; CH4/CO2 = 1 | 73% for CH4 after 35 h | 83% for CO2 after 35 h | NA | The lattice oxygen of ZrO2 reacted with CHx to form CO and H2 and regenerated the Ni active sites. | [59] |
| NiRhCe2Zr1.51 | Pseudo sol–gel method | 700 °C; CH4/CO2 = 7:3 | 16% for CH4 after 25 h | 37.5% for CO2 after 25 h | 4–10 mgc/100 mgcat | ZrO2 and CeO2 formed a cubic phase of CexZr1-xO2, generating more oxygen vacancies and higher oxygen storage capacities than tetragonal phase. | [81] |
| Ni/CeO2−ZrO2 | Co-precipitation/molten salt synthesis | 750 °C; CH4/CO2 = 1 | 2.56 mmol/(m2·h) | NA | 3.75 × 10−6 mgcoke/(m2·h) | With the increase of Ni concentration, oxygen vacancies increased accordingly. | [82] |
| PdO–NiO/Y2O3 | Wet impregnation method | 700 °C; CH4/CO2/O2 = 2/1/1 | 91.11% for CH4 after 24 h | 44.98% for CO2 after 24 h | 16.8 mg/gcat/h | α-oxygen was found on the surface of Y2O3 to activate CH4 and remove the carbon. | [83] |
| Ni-SDL (Sr doped La) | Wet impregnation method | 600 °C; CH4/CO2 = 1 | 78% for CH4 after 10 h | 60% for CO2 after 10 h | 50 mg/gcat/h | The addition of small amount of Sr could enhance the surface oxygen species mobility. | [84] |
| Ce0.70La0.20Ni0.10O2-δ | Combustion synthesis | 750 °C; CH4/CO2 = 1 | 71% for CH4 after 50 h | 83% for CO2 after 50 h | n.d. | The lattice of CeO2 expanded due to the partial dissolution of La3+ into the lattice structure to generate oxygen vacancies. | [85] |
| Ni-ZrO2@SiO2 | Microemulsion method | 800 °C; CH4/CO2 = 1 | 90.5% for CH4 after 240 h | 93.2% for CO2 after 240 h | n.d. | More abundant active oxygen provided by ZrO2 easily accessed to the carbon species and oxidized the coke to form CO. | [89] |
| Ni/ZrO2 | Deposition-precipitation method | 700 °C; CH4/CO2 = 1 | 52% for CH4 after 300 min | 59% for CO2 after 300 min | 26.7% | The optimal ratio of surface adsorbed oxygen to lattice oxygen was achieved under H2 treatment. | [90] |
| La(CoxNi1-x)0.5Fe0.5O3 | Sol-gel self-combustion method | 750 °C; CH4/CO2 = 1 | 70% for CH4 after 30 h | 80% for CO2 after 30 h | <3 mgc/gcat | Co was doped into LaNiFeO3 to improve the oxygen mobility due to the spin state, multivalent property and oxygen affinity of Co. | [102] |
| La0.8Sr0.2Ni0.8Cu0.2O3 | Sol-gel method | 600–800 °C; CH4/CO2 = 1 | 80% for CH4 after 24 h | 80% for CO2 after 24 h | 70 mg/gcat/h | Partial substitution by Sr and Cu generated higher lattice oxygen mobility. | [105] |
| La1-xCexNi0.5Fe0.5O3 | Sol-gel self-combustion method | 750 ℃; CH4/CO2 = 1 | 62% for CH4 after 25 h | 72% for CO2 after 25 h | NA | More oxygen vacancies brought by Ce enhanced the oxygen mobility and backfilling of lattice oxygen. | [107] |
In a word, partial substitution of A-site and B-site can generate oxygen vacancies by lattice distortion or redox property, which promotes the lattice oxygen mobility and adsorption of CO2 to form CO and oxygen atoms. Carbon species derived from the methane activation can be removed by the oxygen atoms, presenting the potential of substituted perovskites as a coke scavenger in the DRM reaction.
For the utilization of oxygen defects, rare earth metal oxides, transition metal oxides and perovskites could be adopted as the supports or promoters which generated oxygen vacancies, surface oxygen or lattice oxygen species, facilitating the mobility of oxygen and oxidizing the coke in time.
4. Interfacial Engineering
Either the metal-metal synergy or metal-support interaction affects the electron density, surface composition and metal dispersion, thus determining the activation of reactants anti-deactivation properties of Ni catalysts [108,109]. In the following, two strategies of interfacial engineering will be discussed in detail, namely Ni-metal alloy formation and Ni–support interaction.
4.1. Ni-Metal Alloy Formation
Bimetallic Ni catalysts enjoy the benefits of synergetic effects between Ni and another metal, such as oxygen affinity, redox cycle, alleviated agglomeration, produced reducibility, dilution effect [109,110,111,112,113,114,115]. For example, Cu can modify the Ni environments and prevent carbon formation [116]. Co can inhibit sintering of Ni by formation NiCo alloy and enhance the resistance to oxidation [117]. Nobel metals can improve the Ni dispersion on the surface of the support and increase the tendency of CH oxygenation instead of dehydrogenation [118,119]. The following part will cover the effects of Ni alloys with different groups of metals on the physicochemical properties and catalytic performances of Ni-based catalysts in DRM reaction.
4.1.1. Alloy of Ni and Noble Metals
When noble metals are added to Ni catalysts, the reducibility of Ni is enhanced due to the spillover of H2 on the surface of noble metals. In the presence of Pt, the reduction temperatures of Ni-Pt alloy shifted to lower regions in the TPR profiles. This was due to the ready adsorption of H2 molecules on the Pt surface and dissociated into H species which subsequently reduced NiO to form metallic Ni [120]. Besides the promotional effect on the reducibility of Ni ions, noble metals can also alleviate the oxidation of Ni by forming alloys. It is well known that a layer of Ni2+ covering the metallic Ni particles reduces the active sites, thus leading to a poor activation of CH4. Due to the resistance to oxidation under atmospheric or pressurized conditions, noble metals prevent Ni metals from being oxidized when forming alloys [121]. Moreover, Ni dispersion can be enhanced due to the dilution effect of noble metals when forming alloy with them. The resulting small particles contributed to the enhanced coke resistance in DRM reaction [113].
A mechanistic study was to explore the surface composition and reconstruction of Ni and Pt under oxidative environment as shown in Figure 6. When heating a layer of Pt atoms covering Ni bulk, Pt atoms would form a Ni-Pt alloy on the surface. Under oxidative treatment, segregation of Ni from the alloy was observed and the Ni-rich surface was exposed to outside to form O/Ni-Pt-Pt structure. Similarly, when a layer of Ni atoms covered Pt bulk, Ni atoms would diffuse into the sublayers to form a stable Pt-Ni-Pt structure upon being heated. When treated with oxidative atmospheres, Ni again segregated to the surface to form the same O/Ni-Pt-Pt structure. By using EXAFS to characterize the Pt coated Ni catalysts and analyze the coordination number of Pt-Ni and Pt-Pt, an increased value of N (Pt-Ni) and decreased value of N (Pt-Pt) reflected the migration of Pt to sublayers and Ni to the surface, transforming the Pt monolayer to a core-shell structure with Ni as the core and Ni-Pt alloy as the shell. This resulting structure facilitated the oxidation of CH and carbon removal (seen in Table 3) [119]. Moreover, recent operando studies suggested that the formation of PtNi alloys could promote the formation of Ni oxide surface clusters, which related to the strong MSI effect, thus leading to a catalytic performance enhancement [122,123].
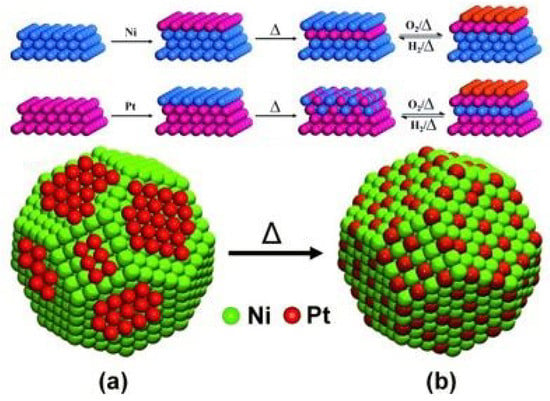
Figure 6.
(a) Surface restructuring of Ni/Pt bimetallic systems induced by temperature and adsorbates. (b) Evolution of the surface structure of the Ni/Pt bimetallic NPs upon thermal treatment. Reproduced with permission from reference [119].
Preparation methods may affect the surface composition. When co-impregnation was applied to prepare Ni-Pd/Al2O3 catalyst, a uniform Ni-Pd alloy was formed due to the strong interaction between two metals. When sequential impregnation was utilized, segregation of Pd atoms was observed because of the weak interaction. In this case, Pd atoms preferred to locate on the surface instead of diffusing into the sublayers [124].
4.1.2. Alloy of Ni and Transition Metals
Similar with noble metals, transition metals can enhance the anti-sintering and anti-coking properties of Ni catalysts by forming alloys [39,77,125,126,127,128,129]. But the driver force may differ from that of noble metals.
After the addition of Co, Ni-Co alloys exhibited an improved affinity for oxygen but a stronger resistance to oxidation than Co alone [130]. First principle calculations showed that Co was very sensitive to oxygen which was proved by the experimental results that when Co loading was below 9 wt% on Al2O3, the Co metals suffered from oxidation, thus losing the active sites and deactivating DRM reaction [131]. However, when Ni-Co alloy was formed, due to the synergy between two metals, both the oxidation of metals and carbon formation were inhibited due to the adsorption of CO2. At 800 °C, only 1.7 wt% of carbon deposits were produced after 100 h DRM reaction [130,132]. One possible explanation for the inhibition effect of Co on oxidation is demonstrated by Gao et al. [133] in the XPS study of reduced Ni-Co/SiO2, whereby the shift of the binding energies of Ni and Co reflected the donation of electrons from Co to Ni, increasing the electron density of Ni and prevented Ni from being oxidized. Meanwhile, metal sintering was also impeded. As for the anti-coking ability, DFT calculations showed that the activation of CH4 on Ni clusters produced mainly CH3 and H species; while for Ni-Co alloy, due to the high affinity of Co for oxygen, CH4 reacted with oxygen atoms to form CH3 and OH species, which removed surface carbon deposits more readily (shown in Table 3) [134].
Compared with Co, the addition of Fe mostly reduces the carbon deposits via redox cycle. In the Ni-Fe/MgAl2O4 catalyst, after pulse injection of CO2, Ni-Fe decomposed to form metallic Ni and Fe. Different from stable Ni phase, Fe was easily to be oxidized to FeOx. Following the Mars–van Krevelen (MvK) mechanism, carbon species on the surface of Ni were oxidized by FeOx to form CO and Fe [135]. Likewise, in another study, dealloying and realloying process took place during the DRM reaction. In detail as shown in Table 3, CO2 adsorbed on the Ni-Fe alloy oxidized the Fe to form FeO, which reacted with the carbon derived from the CH4 decomposition to form CO and regenerate metallic Fe. The redox cycle of Fe/Fe2+ was crucial in inhibiting the carbon formation [136].
As another transition metal, Cu has been proved effective in DRM reaction when forming alloy with Ni. On one hand, Cu alleviated the metal sintering after 30 h reaction; on the other hand, with a molar ratio of Cu:Ni equaling to 1:3, a high conversion of reactants with minimal carbon formation was achieved [137]. A possible mechanism was elucidated by DFT calculation that O(OH) species preferred to adsorb on the CuNi (111) phase, which promoted the elimination of carbon deposits. Besides the DFT calculations, experimental evidence is expected [26].
As for the Cu amount, it was found that by increasing the concentration of Cu, methane activation may be inhibited since Ni active sites were covered by Cu which exhibited slower dissociation kinetics than Ni. As shown in Table 3, this competitive adsorption can be minimized by a careful control of the amount of Cu added in the Ni catalysts [137].
4.2. Ni-Support Interaction
Metal-support interaction (MSI) influences the metal size, dispersion, surface area and reducibility of Ni-catalysts, in turn determining the reactivity and stability in DRM reaction [26,30]. MSI can be turned by the support materials and promoters/dopants. For example, though silica is an inert support, by adopting a modified prepared method, a stable Ni phyllosilicate structure can be formed which enhances the MSI and controls the size and dispersion of Ni [29]. Ca as a promoter promotes the interaction between Ni and Al2O3, thus impeding the metal sintering and improving the dispersion of Ni [138]. However, a too strong MSI may exert a negative impact on the metal surface area since a high reduction temperature is needed [30].
When CeO2 was used as the support for Ni, strong MSI was formed, which facilitated the dissociation of methane in DRM reaction [65,67]. By DFT calculations, the activation barrier for Ni alone was 0.9 eV while for Ni-CeO2-x, the value dropped to 0.15 eV only. It is widely known that the rate determining step for DRM reaction is the activation of C-H bond in CH4 molecules [139], therefore, a lower value of activation energy enhanced the reaction rate and energy efficiency. After 80 h reaction at 450 °C, the conversion was 12%, close to the equilibrium value at this condition. As for the reaction pathway, once the first H was released from CH4, the following transformation of CHx species proceeded very quickly. The carbon species was oxidized by the O adatoms of CO2 to form CO, and H desorbed the surface in the form of H2 and H2O. Interestingly, the non-defective CeO2 exhibited no signs of CO2 activation. Only when the Ni2+ and Ce4+ were reduced by the decomposition products of CH4 to form metallic Ni and Ce3+, could the CO2 reacted with oxygen vacancies and H adatoms produced by methane activation to form CO. This was proved by the decreased amount of Ce3+ exposed to CH4 and CO2 atmospheres compared with CH4 alone according to Ce 4d XPS results as shown in Figure 7 below. In summary as presented in Table 3, Ni and O atoms cooperatively adsorbed the CH4 molecules due to the Ni-Ce solid solution formation, where a strong MSI tuned the electronic or chemical properties of Ni, thus enhancing the activation of methane by Ni metals and inhibiting the carbon formation proved by the lack of C or NiCx peaks as below [65].
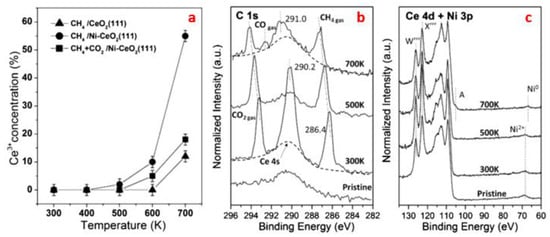
Figure 7.
(a) Ce3+ concentration measured in XPS as a function of temperature under reaction conditions (i.e., exposure to 100 mTorr of methane or a mixture of 100 mTorr of methane and 100 mTorr of CO2). (b,c) C 1s and Ce 4d + Ni 3p spectra of the Ni-CeO2(111) (θNi ≈ 0.1 ML) surface under 100 mTorr CH4 + 100 mTorr of CO2 at 27, 227, and 427 °C. Reproduced with permission from reference [65].
Similarly, in another work, under a small Ni coverage, Ni and O atoms collaboratively activated the C-H bond in CH4, leading to a good conversion at low reaction temperature 377 °C. Due to the strong MSI and electrons transfer from Ni to CeO2, Ni2+ and Ce3+ formed, generating the most stable site Ni2+@O-hollow. Ni2+ was the only Ni species either before or after the adsorption of CH4 at room temperatures. When the coverage of Ni increased to 0.5 ML, NiCx species appeared on the surface; when the Ni coverage was below 0.2 ML, the small Ni atoms or particles contacted closely with the CeO2 support, resulting in a much lower activation energy of CH4 than that with a Ni coverage of 0.5 ML. A small loading of Ni formed small particles, which experienced large electronic perturbations from CeO2. These small Ni particles and O atoms in CeO2 promoted the activation of CH4 in a cooperative way. When Ni2+/CeO2 was transformed to Ni0/CeO2-x by reacting with C species from CH4 decomposition, CO and oxygen vacancies were generated [67,140,141]; the oxygen vacancies formed on the reduced CeO2-x facilitated the adsorption and activation of CO2, thus leading to an enhanced conversion and stability in DRM reaction [67].
In certain situations, however, strong MSI may not be a benefit. As shown in Table 3, when a layer of CeO2 covered the Ni-Co alloys, the promotional effect of Co on the enhanced MSI was cancelled off by the reduction of active sites exposed to the reactants [63]. Similar findings were shown presented for the Ni/TiO2. Despite the strong MSI, the surface free energy may be reduced due to the coverage of Ni active sites by the migration of TiOx species over the surface.
In addition to CeO2, owing to the strong basicity of MgO, a strong MSI between Ni and MgO derived from the solid solution probably enhance the anti-coking property [142,143]. However, the surface and under-coordinated Ni still exhibited a sensitivity to coke formation [144]. MgAl2O4 spinel structure may be a promising candidate to produce a highly dispersed Ni particles due to the strong MSI; together with the basicity of the support, CO2 adsorption will be promoted, and carbon deposition will be alleviated (seen in Table 3) [145]. But an extraordinarily strong MSI may bring a high reduction temperature, possibly leading to the metal sintering. To further modify the dispersion of Ni, Co was added to the Ni/MgAl2O4 to slow the reduction of Ni and generate a small and uniform Ni particle, resulting in an enhanced DRM activity [146].
Besides the rare earth metal oxides, alkali earth metal oxides and transition metal oxides, SiO2 as a support can also form a stable structure with Ni species with a modified preparation method [147,148]. With the addition of NH3 to control the pH, Ni phyllosilicate formed between the Ni and SiO2 under thermal treatment of Ni precursors. Upon decomposition of the phyllosilicate, highly dispersed Ni particles interacted strongly with the SiO2, achieving a high and stable conversion of CH4 (74%) and CO2 (82%) over 50 h at 700 °C as seen in Table 3 [149].
In addition to the ammonia-assisted method, thermal treatment also benefited the Ni phyllosilicate formation. Compared with air calcination or H2 treatment, a sequential calcination method was advantageous in terms of the size and MSI of Ni/SiO2 catalysts. In detail, the first step of H2 calcination at 400 °C scavenged oxygen radicals produced from the nitrate decomposition, reducing the Ni particle size and enhance the dispersion. The subsequent air calcination at 700 °C strengthened the MSI by forming the Ni phyllosilicate, anchoring Ni particles and preventing the sintering. As presented in Table 3, the Ni/SiO2 catalyst prepared with the facile modified incipient wetness impregnation method exhibited an outstanding catalytic performance (80% for CH4 and 85% for CO2) with little sintering and no coke formation after 100 h DRM reaction [150].
In a word, by adjusting the Ni-metal interface and Ni-support interaction, the physicochemical properties of Ni catalysts can be improved due to the synergy between Ni and dopant metals and the electronic and chemical perturbation by the support. In turn, catalytic performances and anti-deactivation abilities in DRM reaction can be enhanced by introducing oxygen vacancies, surface basicity, oxygen affinity, redox cycle, low activation barrier, steric hindrance, and electron transfer upon interfacial engineering in Ni-based catalysts.

Table 3.
A summary of interfacial engineering effects on the catalytic performances of Ni-based catalysts for DRM reaction.
Table 3.
A summary of interfacial engineering effects on the catalytic performances of Ni-based catalysts for DRM reaction.
| Catalyst | Preparation Method | Reaction Condition | Methane Conversion | CO2 Conversion | Carbon Formation | Comments | Ref. |
|---|---|---|---|---|---|---|---|
| NiCo/CeO2 | Incipient wetness impregnation method | 700 °C; CH4/CO2 = 1 | 64% for CH4 after 300 min | 69% for CO2 after 300 min | 11.5% | When a layer of CeO2 covered the Ni-Co alloys, the promotional effect of Co on the enhanced MSI was cancelled off. | [63] |
| Ni/CeO2 | NA | 450 °C; CH4/CO2 = 1 | 10−7 torr CH4 after 80 h | 10−7 torr CO2 after 80 h | NA | Ni and O atoms cooperatively adsorbed the CH4 molecules due to the Ni-Ce solid solution formation. | [65] |
| Ni/TiO2 | Impregnation method | 790 °C; CH4/CO2 = 1 | 41% for CH4 initially | 67% for CO2 initially | 8.0 × 10−4 gC/gcat | The surface free energy may be reduced due to the coverage of Ni active sites by the migration of TiOx species. | [108] |
| NiPt/Al2O3 | NA | 700 °C; CH4/CO2 = 1 | 85.8% for CH4 after 18 h | 91.2% for CO2 after 18 h | 0.1 molC/molsurface metal/h | Pt monolayer in a core-shell structure with Ni facilitated the oxidation of CH and carbon removal. | [119] |
| NiCo/MgO-ZrO2 | Co-precipitation and impregnation method | 600 °C; CH4/CO2 = 1 | 6.6 mol·(g·atom·NiCosurface·s)−1 | NA | NA | For Ni-Co alloy, due to the high affinity of Co for oxygen, CH4 reacted with oxygen atoms to form CH3 and OH species. | [134] |
| Fe-Ni/MgAl2O4 | Co-precipitation and incipient wetness impregnation method | 750 °C; CH4/CO2 = 1 | 0.13 molCH4 s−1 molNi−1 after third cycle | NA | 5.02 molCO molNi+Fe−1 | Carbon species on the surface of Ni were oxidized by FeOx to form CO and Fe. | [135] |
| Fe-Ni/MgAl2O4 | Co-precipitation and incipient wetness impregnation method | 750 °C; CH4/CO2 = 1 | NA | NA | NA | The redox cycle of Fe/Fe2+ was crucial in inhibiting the carbon formation. | [136] |
| Cu–Ni/SiO2 | Hydrothermal process | 700 °C; CH4/CO2 = 1 | 70% for CH4 after 30 h | NA | 3% | When Cu formed alloy with Ni, the metal sintering and carbon formation were alleviated. | [137] |
| Ca-promoted Ni/α-Al2O3 | Wetness co-impregnation method | 800 °C; CH4/CO2 = 1 | 84.8% for CH4 after 240 min | 85.5% for CO2 after 240 min | 24.9 mg/gcat/h | Ca promoted the interaction between Ni and Al2O3, thus impeding the metal sintering and improving the dispersion of Ni. | [138] |
| AuNi/MgAl2O4 | Sol preparation method | 650 °C; CH4/CO2 = 69:30 | 20% for CH4 after 1000 min | 48% for CO2 after 1000 min | 3 mg | MgAl2O4 spinel structure increased the basicity of the support, so CO2 adsorption will be promoted, and carbon deposition will be alleviated | [145] |
| Ni@Ni embedded SiO2 | Self-templating method | 700 °C; CH4/CO2 = 1 | 74% for CH4 after 50 h | 82% for CO2 after 50 h | n.d. | Upon decomposition of the phyllosilicate, highly dispersed Ni particles interacted strongly with the SiO2. | [149] |
| Ni/SiO2 | Incipient wetness impregnation method | 700 °C; CH4/CO2 = 1 | 80% for CH4 after 100 h | 85% for CO2 after 100 h | n.d. | Sequential H2-air calcination enhanced Ni dispersion and MSI with SiO2. | [150] |
5. Structural Optimization
A small particle size can enhance the activity of Ni-based catalysts [151]. To alleviate the Ni sintering at high temperatures during DRM reactions, optimizations have been made on the structure of supports, such as porous materials which enhance the surface area and providing the anchoring points for metal active sites [30,31]. Moreover, hierarchical designs of the catalyst including core-shell, yolk-shell, embedded and hollow structures, sterically hinder the migration of Ni and prevent the agglomeration [27,30].
5.1. Porous Supports
High porosity of carbon materials inhibits the movement of metal particles and enhances the dispersion on the carbon support. Also, porous carbon materials can be prepared into various physical forms, such as pellets, cloth, and fiber [30]. Li et al. [152] synthesized porous carbon from renewable hydrochar as the precursor and successfully anchored Ni2+ ions on the surface, leading to highly dispersed Ni nanoparticles with the diameter of less than 8 nm. Methane conversions reached 52% and 70% for 700 °C and 800 °C pretreated catalyst respectively.
In addition to porous carbon materials, silica with high porosities and ordered structures is widely used to control the particle size and enhance the metal dispersion by confining metal particles in the pores (seen in Table 4) [153,154,155,156,157,158]. SBA-15 with silanol groups on its surface was able to trap the Ni ions by bonding with amine groups of polyethylenimine (PEI) in the Ni-PEI complexes (Figure 8). Little sintering took place due to the confinement effect of SBA-15 with ordered channels, leading to a high stability over 40 h of reaction [155]. When ethylene glycol (EG) was used as the solvent, Ni-EG complexes were pumped into the pores of SBA-15 by capillary force, hindering the migration of Ni during the drying and calcination process [154].
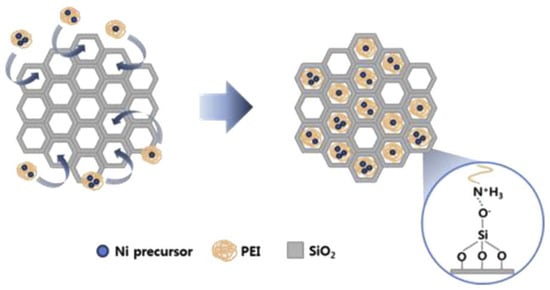
Figure 8.
Schematic diagram of PEI-aided route. Reproduced with permission from reference [155].
As shown in Table 4, besides Ni monometallic catalysts, bimetallic Ni-Co supported on SBA-15 maintained the activity over 50 h. The small particle size (4–5 nm) and high metal dispersions (17–20%) were achieved by the confinement effect of ordered channel of SBA-15 [159]. Perovskite structures LaNiO3 were also impregnated into the mesoporous SBA-15 supports. With the help of the stable structures, the conversions were kept stable over 60 h without agglomeration of Ni particles [160].
Compared with SBA-15, MCM-41 and KIT-6 supported Ni catalysts exhibited lower conversions and higher carbon depositions in DRM reaction. For KIT-6, the micropores were occupied by Ni particles so that the pathways of reactants were blocked despite the high Ni dispersion [157]. For MCM-41, it suffered poorer structural stability than SBA-15 especially when TiOx and MnOx hindered the channels for reactants to pass through and the amorphous form of silica was transformed into crystals [161,162].
To enhance the diffusion efficiency and thermal stability, as shown in Table 4, hexagonal mesoporous silica (HMS) with thicker pore walls and more interconnected channels was applied as the support of Ni to improve the metal dispersion and inhibit sintering by forming Si-O-Ni bonds, leading to a stable conversion over 100 h at 700 °C [158].
For other mesoporous silica supports, one way to enhance the metal dispersion on porous silica supports is to utilize a novel method (seen in Table 4). Colloidal approach was applied by converting Ni-silicide colloids into highly dispersed Ni nanoensembles supported on porous silica under thermal treatment, achieving a 60% higher syngas yields [163]. Polyols precursors were treated under inert atmospheres and decomposed to produce carbon residues, protecting Ni particles from migrating from internal pores to external surfaces. The near-equilibrium conversions at 750 °C resulted from the well-dispersed Ni particles inside the mesoporous channels exposed completely to the reactants [164].
In addition to the colloidal and polyols approaches, ligand-assisted incipient wetness impregnation (IWI) method is applied to control the size and MSI (presented in Table 4). Similar with PEI and EG forming complexes with Ni ions on SBA-15 supports, a combination of oleic acid (OA) and oleylamine (OAm) were used as co-ligands to protect Ni from agglomeration by ligand bonds between Ni ions and amine groups of OAm and hydrogen bonds between carboxylic groups of OA and silanol groups of mesoporous silica. Together with the steric hindrance of the hydrocarbon chain network, a high dispersion of Ni particles and strong MSI were achieved, leading to a stable activity in DRM reaction [165]. Moreover, this method proved effective for reducing the size bimetallic Ni-Co to 3.1 nm averagely, resulting in a high conversion of methane and CO2 over 30 h at 700 °C [133]. Furthermore, in a systematic study of the amine effect, the organic amines with a long hydrocarbon chain and high concentration produced a small size of Ni and a high dispersion as well as a strong MSI [166].
Compared with inert materials like carbon and silica, alkali earth metal oxides, rare earth metal oxides and alumina presented both a porous structure and a surface modification effect [167,168,169,170]. NiO-MgO-Al2O3 mesoporous frameworks provided more active centers for the reactants to be activated and converted to syngas products. Ni particles were anchored within the pores to alleviate the sintering effect and prolong the lifetime in the reaction; with the basic promoters, carbon removal was facilitated due to the adsorption of CO2 which was dissociated into CO and oxygen radicals (seen in Table 4) [167]. In another example as shown in Figure 9, mesoporous NiO-CeO2-Al2O3 materials was prepared by evaporation-induced self-assembly method with the introduction of CeO2 into Ni/Al2O3. The excellent thermal stability of this mesoporous structures (preserved over 80 h at 700 °C) effectively protected Ni from sintering. Also, the redox property of CeO2 oxidized carbon deposits to help maintain the exposure of active sites to reactants during the reaction [171].
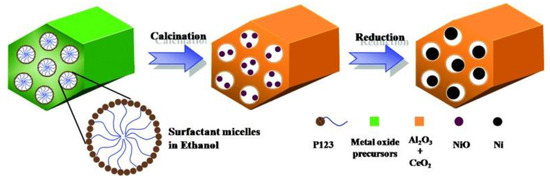
Figure 9.
Schematic illustration of the NiCeAl (R) preparation. Reproduced with permission from reference [171].
In a word, various porous materials such as carbon, silica and alumina provide accommodations for Ni nanoparticles to be protected from migrating from internal pores to external surfaces of the supports. Organic ligands, novel methods and promoters are utilized to further enhance the anti-sintering and anti-coking capabilities, leading to a high conversion of methane and CO2 and long-term stability.
5.2. Hierarchical Designs
Besides the rational design of the porous supports to enhance the surface area and confine the metal nanoparticles inside the pores or channels, architecture optimizations of the catalysts have become an effective method to improve the sintering and coking resistance. Owing to the uniform microporosity, high thermal stability and unique shape selectivity, zeolites draw attentions in stabilizing Ni particles and prevent the sintering during DRM reaction [172,173]. Moreover, other smart designs emerge in recent years, such as core-shell structures. With the first design of “nanoreactor” Pt@CoO core-shell nanostructures, various nanocomposites containing metal and metal oxides protected by silica shells are developed in recent years, including core-shell, yolk-shell, hollow and embedded structures, to partially separate metal particles from each other and enhance the dispersion [30,174,175,176,177,178,179,180,181,182].
Ni@SiO2 core-shell catalysts with Ni as the core and SiO2 as the shell exhibited a strong resistance towards filamentous carbon deposition (shown in Table 4). However, amorphous carbon still formed within the nanocavities between the core and shell. Minimizing the nanocavities was recommended to modify the structure and improve the coke resistance [183].
To enhance the catalytic performance, a thick SiO2 shell was formed to transform the core-shell structure to yolk-shell structures with Ni as the core surrounded by Ni nanoparticles embedded in the SiO2 shell (Figure 10). With a 11.2 nm of thickness, this unique structure exhibited an enhanced conversion and stability in DRM reaction with the help of strong MSI and small satellite Ni particles [184]. However, the turnover frequency (TOF) can be increased by tuning surface area and Ni exposure. Instead of using micro-emulsion method, a self-templating method was utilized to successfully prepared a yolk-shell Ni@SiO2 catalyst, increasing the TOF by 4.5 times of the previous one (shown in Table 4) [149].
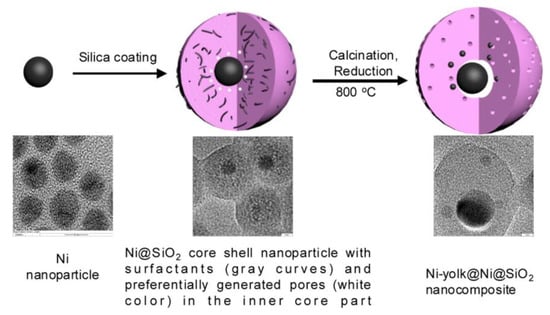
Figure 10.
Schematic illustration of the formation process of Ni−yolk@Ni@SiO2 nanocomposite. Reproduced with permission from reference [184].
To further enhance the active surface area and metal loadings, as presented in Table 4, multi-core shell catalysts with multiple Ni cores and a mesoporous SiO2 shell were synthesized by reversed micelle method. During the reaction, Ni particles migrated into the SiO2 shell, leading to the formation of uni-nuclear Ni core embedded in the shell [185]. To achieve a better anti-sintering property, a layer of silica was coated on the multiple Ni cores supported on the mesoporous silica as shown in Figure 11. With the protective layer of silica, 5.2 nm of Ni particles with a narrow size distribution were prevented from agglomeration due to the strong MSI and steric hindrance, reaching a high stability over 170 h at 800 °C [186].
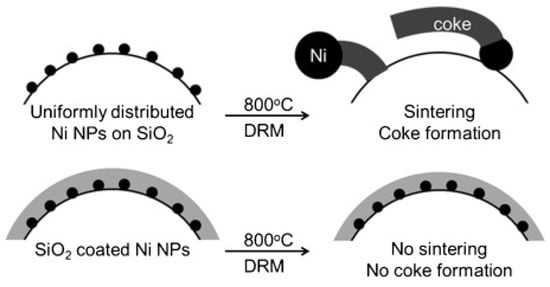
Figure 11.
DRM with highly coke-resistant silica-coated nickel nanoparticle catalysts. Reproduced with permission from reference [186].
To improve the MSI between Ni and SiO2, Ni phyllosilicate structures were prepared by ammonia evaporation method, which generated highly dispersed Ni nanoparticles with the size of 6 nm supported on silica. With a layer of SiO2 covering the Ni metals, a good activity was achieved at 600 °C over 24 h because of the enhanced MSI and confinement effect of mesoporous SiO2 [182].
As for the controlled synthesis of Ni phyllosilicate, Ni precursors exerted an influence on the size of Ni particles, thus affecting the diffusion speed through the Ni phyllosilicate layer to form a new phyllosilicate species with silicate ions (seen in Table 4). Ni acetylacetonate, among various precursors, reacted with mesoporous silica spheres to form Ni phyllosilicates by hydrothermal method, exhibiting a unique pore structure with the smallest pore volume and pore size. This strong MSI between Ni and the support retarded carbon nanotube formation by anchoring Ni on the silica [147].
Besides the effect of precursors on the size of Ni, as shown in Table 4, modification of the porosity and basicity can further enhance the adsorption and accessibility of reactants. Therefore, Ni-Mg phyllosilicate were synthesized via facile hydrothermal method as shown in Figure 12. By adjusting the duration of treatment, an ideal porosity and basicity could be realized [187]. On one hand, due to the high affinity of MgO for CO2, more reactants were adsorbed on the surface more easily through a modified pore structure, accelerating the conversion of methane and the removal of carbon deposits. On the other hand, because of the strong MSI between Ni and MgO, metal sintering could be prevented to a large extent. Together with the protective layer of mesoporous silica, this catalyst maintained active for 72 h at 750 °C [188].
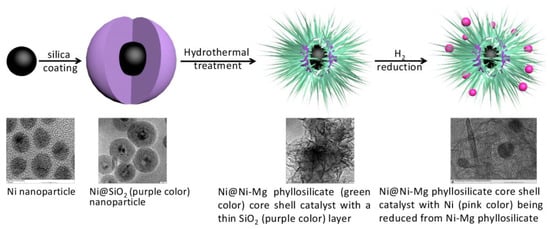
Figure 12.
Schematic illustration of the formation process of Ni@Ni–Mg phyllosilicate core–shell catalyst. Reproduced with permission from reference [187].
Likewise, as shown in Table 4, NiMgAl layer double hydroxide was firstly synthesized by hydrothermal method, followed by coating a layer of mesoporous silica (Figure 13). After calcination and reduction, Ni nanoparticles were generated inside the cavity, which were confined by both MgO and silica layer. The synergies between the confinement effect of mesoporous silica and basicity of MgO resulted in an 8 h stable conversion at 750 °C with little carbon deposition [189].
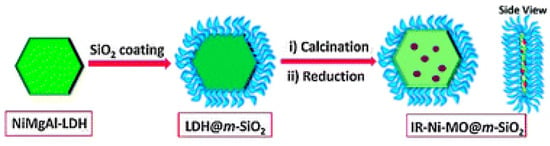
Figure 13.
Schematic illustration of the modular catalysts. Reproduced with permission from reference [189].
Besides Ni monometallic catalysts, the core–shell structures can be extended to Ni-based bimetallic catalysts. 12 nm Ni-Cu alloys were successfully synthesized by tuning the precursor concentrations. Being covered by the silica shell, the sintering of alloys could be alleviated, presenting a higher conversion than impregnated samples. Moreover, higher H2 selectivity could be achieved by the suppression effect on RWGS reaction with the addition of Cu (shown in Table 4) [190].
Ni-Co@silica core-shell structures calcined at 500 °C exhibited poor activities and stabilities. However, as presented in Table 4, when calcined at 800 °C, with the increase of crystallinity of SiO2 and enhanced MSI, the activity could be maintained stable over 1000 h, reflecting the high thermal stability of the support [191].
In a word, by designing the hierarchical structures of Ni-based catalysts, Ni particle size can be controlled, metal sintering can be alleviated, and coke resistance can be enhanced due to the confinement effect of silica shell and strong MSI derived from the Ni phyllosilicate. By doping another metal (Co, Cu, and Fe) to form alloys with Ni and adding Mg precursors to prepare Ni-Mg phyllosilicates, the activity and stability of the catalysts for DRM reaction can be further enhanced because of the promotional effects, such as the adsorption of CO2 and suppression of RWGS reaction.

Table 4.
A summary of structural optimization effects on the catalytic performances of Ni-based catalysts for DRM reaction.
Table 4.
A summary of structural optimization effects on the catalytic performances of Ni-based catalysts for DRM reaction.
| Catalyst a) | Preparation Method | Reaction Condition | Methane Conversion | CO2 Conversion | Carbon Formation | Comments | Ref. |
|---|---|---|---|---|---|---|---|
| NiCo/SiO2 | Incipient wetness impregnation method | 700 °C; CH4/CO2 = 1 | 73% for CH4 after 30 h | 77% for CO2 after 30 h | n.d. | Homogeneous Ni-Co alloy supported on mesoporous silica was synthesized by oleylamine/oleic acid organic pair. | [133] |
| NiPhy (Ni phyllosilicate) hollow sphere (HS) | Hydrothermal method | 700 °C; CH4/CO2 = 1 | 77.5% for CH4 after 70 h | 86.6% for CO2 after 70 h | <5% | Ni phyllosilicates exhibited a unique pore structure with the smallest pore volume and pore size. | [147] |
| Ni yolk@Ni@SiO2 | Self-templating method | 700 °C; CH4/CO2 = 1 | 74% for CH4 after 50 h | 82% for CO2 after 50 h | 1.4% | A yolk-shell Ni@SiO2 catalyst increased the TOF by tuning surface area and Ni exposure | [149] |
| Ni@C | Wet impregnation method | 700 °C; CH4/CO2 = 1 | 68% for CH4 after 800 min | 80% for CO2 after 800 min | 79.3% | Porous carbon anchored Ni2+ and enhanced dispersion. | [151] |
| Ni/SBA-15 | Modified impregnation method | 750 °C; CH4/CO2 = 1 | 87% for CH4 after 34 h | 92% for CO2 after 34 h | 3.8% | Ni-EG complexes were pumped into the pores of SBA-15 by capillary force, hindering the migration of Ni. | [154] |
| Ni/SBA-15 | Modified impregnation method | 750 °C; CH4/CO2 = 1 | 85% for CH4 after 40 h | 88% for CO2 after 40 h | 3.63 mgc/gcat | Little sintering took place due to the confinement effect of SBA-15 with ordered channels. | [155] |
| Ni/KIT-6 | Impregnation method | 750 °C; CH4/CO2 = 1 | 60% for CH4 after 20 h | 78% for CO2 after 20 h | NA | The micropores were occupied by Ni particles so that the pathways of reactants were blocked despite the high Ni dispersion. | [157] |
| Ni/HMS | S0I0 assembly pathways | 700 °C; CH4/CO2 = 1 | 72% for CH4 after 100 h | 84% for CO2 after 100 h | 6.22% | Hexagonal mesoporous silica (HMS) improved the metal dispersion and inhibit sintering by forming Si-O-Ni bonds. | [158] |
| NiCo/SBA-15 | Co-precipitation method | 700 °C; CH4/CO2 = 1 | 70% for CH4 after 50 h | 78% for CO2 after 50 h | n.d. | Small particle size (4–5 nm) and high metal dispersions (17–20%) were achieved by the confinement effect of ordered channel of SBA-15. | [159] |
| LaxNiyOz/KIT-6 | Sol-gel method | 800 °C; CH4/CO2 = 1 | 88% for CH4 after 60 h | 100% for CO2 after 60 h | 3.41% | Perovskite structures LaNiO3 were also impregnated into the mesoporous SBA-15 supports. | [160] |
| Ni (SiyOz)/SiO2 | Colloidal approach | 500 °C; CH4/CO2 = 1.3 | 465 molCO/ h·moltot.Ni | 450 molCO2/ h·moltot.Ni | NA | Colloidal approach was applied by converting Ni-silicide colloids into highly dispersed Ni nanoensembles supported on porous silica. | [163] |
| Ni/SiO2 | Incipient wetness impregnation method | 700 °C; CH4/CO2 = 1 | 70% for CH4 after 1000 min | 72% for CO2 after 1000 min | n.d. | Co-ligands to protect Ni from agglomeration by ligand and hydrogen bond and steric hindrance. | [165] |
| Ni/SiO2 | Incipient wetness impregnation method | 700 °C; CH4/CO2 = 1 | 70% for CH4 after 20 h | 78% for CO2 after 20 h | 1.8 mgc/gcat h | With a small size, high dispersion and strong MSI, Ni/SiO2 prepared with oleylamine showed the most stable catalytic performances. | [166] |
| NiO–MgO–Al2O3 | evaporation induced self-assembly strategy | 700 °C; CH4/CO2 = 1 | 78% for CH4 after 100 h | 83% for CO2 after 100 h | 7.0% | Ni particles were anchored within the pores to alleviate the sintering effect and basicity of support could enhance CO2 adsorption and remove coke. | [167] |
| NiO-CeO2-Al2O3 | evaporation induced self-assembly strategy | 700 °C; CH4/CO2 = 1 | 78% for CH4 after 80 h | 73% for CO2 initially | 21.5% | The excellent thermal stability of this mesoporous structures protected Ni from sintering and the redox property of CeO2 oxidized carbon deposits. | [171] |
| Silica@Ni@Silica | Ammonia evaporation method | 600 °C; CH4/CO2 = 1 | 44% for CH4 after 24 h | 54% for CO2 after 24 h | 7.6% | With a layer of SiO2 covering the Ni metals, a good activity was achieved at 600 °C over 24 h | [180] |
| Ni@SiO2 | Sol-gel process | 750 °C; CH4/CO2 = 1 | 54.1% for CH4 after 25 h | 65.6% for CO2 after 25 h | 1.2% | Strong resistance towards filamentous carbon deposition was exhibited; however, amorphous carbon still formed within the nanocavities | [183] |
| Ni@SiO2 | Microemulsion method | 800 °C; CH4/CO2 = 1 | 90% for CH4 after 90 h | 95% for CO2 after 90 h | 4.8% | With a 11.2 nm of thickness, this unique structure exhibited an enhanced conversion and stability with the strong MSI and small satellite Ni particles. | [184] |
| Ni@SiO2 | Reverse micelle method | 800 °C; CH4/CO2 = 1 | 87% for CH4 after 100 h | 88% for CO2 after 100 h | NA | Ni particles migrated into the SiO2 shell, leading to the formation of uni-nuclear Ni core embedded in the shell. | [185] |
| Silica coated Ni/SiO2 | Microemulsion method | 800 °C; CH4/CO2 = 1 | 42.5% for CH4 after 170 h | 56.6% for CO2 after 170 h | n.d. | To achieve a better anti-sintering property, a layer of silica was coated. | [186] |
| Ni@Ni-MgPhy | Hydrothermal method | 700 °C; CH4/CO2 = 1 | 78% for CH4 after 95 h | 81% for CO2 after 95 h | 4.4% after 20 h | By adjusting the duration of treatment, an ideal porosity and basicity could be realized. | [187] |
| Ni@Ni-MgPhy | Hydrothermal method | 750 °C; CH4/CO2 = 1 | 85% for CH4 after 72 h | 89% for CO2 after 72 h | n.d. | Strong MSI and the protective layer of mesoporous silica prevented metal sintering. | [188] |
| NiMgAl-(LDH)@mesoporous-SiO2 | Hydrothermal and sol-gel method | 750 °C; CH4/CO2 = 1 | 79% for CH4 after 8 h | 88% for CH4 after 8 h | 2.5% | Ni nanoparticles were generated inside the cavity, which were confined by both MgO and silica layer. | [189] |
| Cu–Ni@SiO2 | Microemulsion method | 700 °C; CH4/CO2 = 1 | 75% for CH4 after 16 h | NA | NA | Being covered by the silica shell, the sintering of alloys could be alleviated. | [190] |
| NiCo@SiO2 | Microemulsion method | 800 °C; CH4/CO2 = 1 | 90% for CH4 after 1000 h | 90% for CO2 after 1000 h | n.d. after 150 h | With the increase of crystallinity of SiO2 and enhanced MSI, the activity could be maintained stable. | [191] |
a) AC: active carbon; HMS: Hexagonal mesoporous silica; LDH: layered double hydroxides.
6. Conclusions and Prospects
In this review, smart designs of Ni-based catalysts are discussed in four categories, including surface regulation, oxygen defects and interfacial engineering and structural optimization. In surface regulation, enhanced basicity can promote the CO2 adsorption, resulting in active oxygen atoms which reacts with carbon species to form CO. Various oxides like La2O3, MgO, Ga2O3, and B2O3 can tune the surface basicity but too strong a basicity may inhibit carbon deposition. In oxygen defects, lattice distortion in perovskite structures, redox property of rare earth and lattice oxygen of transition metal oxides can promote the oxygen mobility, thus facilitating the oxidation of CHx and C deposits to form CO and H2. In interfacial engineering, doping noble metals can enhance the reducibility of Ni, prevent Ni from agglomeration and impede the metal oxidation. Transition metals with oxygen affinity or redox cycle can absorb more CO2 to produce CO and oxygen atoms, accelerating the carbon removal. Besides the Ni-metal synergy, a strong MSI can slow the reduction and retard the migration of Ni, thus leading to a high dispersion on the support, such as MgO and SiO2. Moreover, electron perturbations of Ni from CeO2 generate active Ni sites to adsorb and dissociate CH4. On the other hand, the Ce3+ promotes the C-O bond activation in CO2 molecules to produce oxygen atoms, leading to an improved reactivity and stability in DRM reaction. In structural optimization, porous supports and core-shell structures can enhance the surface area and provide steric hindrance, leading to a highly dispersed Ni particle and more exposure to the reactants. In this case, the activation of reactants is facilitated, and metal sintering is inhibited. Despite the progress in designing Ni-based catalysts to resist coke and sintering, there is still room for further enhancement of the catalytic performance and a clear explanation of the mechanism.
First, a facile, economical, and green preparation strategy will be beneficial to the large-scale operations of DRM reaction in industry. Multi-step reaction processes may limit the yields of the catalyst materials. Also, the cost of the raw materials should be carefully controlled to an acceptable level. Furthermore, production and dispose of waste streams are another issue to be considered.
Second, novel techniques such as plasma, light and electricity assisted catalytic process can be a promising way to avoid the high temperature activation and metal sintering. Energy efficiency and stability may be enhanced in this way.
Third, combining with membrane, CO2 can be separated from the raw feed and simultaneously converted into valuable syngas by reforming of methane. The recent advancement of highly CO2-permeable membranes paves the way for developing an integrated system for both CO2 capture and conversions [192,193,194].
Fourth, single-site or single-atom catalysts have drawn great interest in recent years due to the high efficiency of expensive catalysts and enhanced reactivity and selectivity in various reactions. For DRM reaction, single Ni atom catalysts have been proved to be highly active. However, how to prevent the agglomeration is still waiting to be resolved.
Fifth, by using CeO2 as the support, low temperature or even room temperature activation of the DRM reactions have been reported. This strategy can save the energy input and alleviate the metal sintering. Considering the low equilibrium conversion at these conditions, an integrated system is to be developed for an efficient and immediate recovery of the unreacted molecules to realize the chemical looping process.
Author Contributions
X.G.: Conceptualization, Data curation, Investigation, Writing—original draft, Writing—review & editing. J.A.: Writing—review & editing, Project administration, Supervision, Validation. S.K.: Funding acquisition, Resources, Project administration, Supervision, Validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Education in Singapore (MOE) Tier 2 grant (MOE2017-T2-2-130), Singapore Agency for Science, Technology and Research (A*STAR) AME IRG grant (No. A1783c0016), National Environment Agency (NEA) in Singapore (WTE-CRP 1501-103) and Youth Innovation Talents Project of Guangdong Universities (natural science) in China (2019KQNCX098).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Das, S.; Ashok, J.; Xi, S.; Borgna, A.; Hidajat, K.; Kawi, S. Highly Dispersed Ni/Silica by Carbonization–Calcination of a Chelated Precursor for Coke-Free Dry Reforming of Methane. ACS Appl. Energy Mater. 2020, 3, 7719–7735. [Google Scholar] [CrossRef]
- Mo, L.; Leong, K.K.M.; Kawi, S. A highly dispersed and anti-coking Ni–La2O3/SiO2 catalyst for syngas production from dry carbon dioxide reforming of methane. Catal. Sci. Technol. 2014, 4, 2107–2114. [Google Scholar] [CrossRef]
- Ashok, J.; Bian, Z.; Wang, Z.; Kawi, S. Ni-phyllosilicate structure derived Ni–SiO2–MgO catalysts for bi-reforming applications: Acidity, basicity and thermal stability. Catal. Sci. Technol. 2018, 8, 1730–1742. [Google Scholar] [CrossRef]
- Oemar, U.; Hidajat, K.; Kawi, S. High catalytic stability of Pd-Ni/Y2O3 formed by interfacial Cl for oxy-CO2 reforming of CH4. Catal. Today 2017, 281, 276–294. [Google Scholar] [CrossRef]
- Dewangan, N.; Hui, W.M.; Jayaprakash, S.; Bawah, A.-R.; Poerjoto, A.J.; Jie, T.; Ashok, J.; Hidajat, K.; Kawi, S. Recent progress on layered double hydroxide (LDH) derived metal-based catalysts for CO2 conversion to valuable chemicals. Catal. Today 2020, 356, 490–513. [Google Scholar] [CrossRef]
- Oemar, U.; Hidajat, K.; Kawi, S. Pd–Ni catalyst over spherical nanostructured Y2O3 support for oxy-CO2 reforming of methane: Role of surface oxygen mobility. Int. J. Hydrogen Energy 2015, 40, 12227–12238. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Z.; Jiang, B.; Hongmanorom, P.; Zhong, W.; Kawi, S. A review on perovskite catalysts for reforming of methane to hydrogen production. Renew. Sustain. Energy Rev. 2020, 134, 110291. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Q.; Li, M.; Cao, J.; Liu, F.; Pan, H.; Wang, Z.; Kawi, S. Recent advances in process and catalyst for CO2 reforming of methane. Renew. Sustain. Energy Rev. 2020, 134, 110312. [Google Scholar] [CrossRef]
- Das, S.; Pérez-Ramírez, J.; Gong, J.; Dewangan, N.; Hidajat, K.; Gates, B.C.; Kawi, S. Core–shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 2020, 49, 2937–3004. [Google Scholar] [CrossRef] [PubMed]
- Hongmanorom, P.; Ashok, J.; Zhang, G.; Bian, Z.; Wai, M.H.; Zeng, Y.; Xi, S.; Borgna, A.; Kawi, S. Enhanced performance and selectivity of CO2 methanation over phyllosilicate structure derived Ni-Mg/SBA-15 catalysts. Appl. Catal. B 2021, 282, 119564. [Google Scholar] [CrossRef]
- Wai, M.H.; Ashok, J.; Dewangan, N.; Das, S.; Xi, S.; Borgna, A.; Kawi, S. Design of Ceria Catalysts for Low-Temperature CO Oxidation. ChemCatChem 2020, 12, 1–11. [Google Scholar]
- Ashok, J.; Ang, M.L.; Kawi, S. Enhanced activity of CO2 methanation over Ni/CeO2-ZrO2 catalysts: Influence of preparation methods. Catal. Today 2017, 281, 304–311. [Google Scholar] [CrossRef]
- Ashok, J.; Pati, S.; Hongmanorom, P.; Zhang, T.; Chen, J.; Kawi, S. A review of recent catalyst advances in CO2 methanation processes. Catal. Today 2020, 356, 471–489. [Google Scholar] [CrossRef]
- Bian, Z.; Chan, Y.M.; Yu, Y.; Kawi, S. Morphology dependence of catalytic properties of Ni/CeO2 for CO2 methanation: A kinetic and mechanism study. Catal. Today 2020, 347, 31–38. [Google Scholar] [CrossRef]
- Yu, Y.; Chan, Y.M.; Bian, Z.; Song, F.; Wang, J.; Zhong, Q.; Kawi, S. Enhanced performance and selectivity of CO2 methanation over g-C3N4 assisted synthesis of Ni-CeO2 catalyst: Kinetics and DRIFTS studies. Int. J. Hydrogen Energy 2018, 43, 15191–15204. [Google Scholar] [CrossRef]
- Yu, Y.; Bian, Z.; Song, F.; Wang, J.; Zhong, Q.; Kawi, S. Influence of Calcination Temperature on Activity and Selectivity of Ni–CeO2 and Ni–Ce0.8Zr0.2O2 Catalysts for CO2 Methanation. Top. Catal. 2018, 61, 1514–1527. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Dewangan, N.; Li, Z.; Das, S.; Pati, S.; Li, Z.; Lin, J.Y.S.; Kawi, S. Catalytic mixed conducting ceramic membrane reactors for methane conversion. React. Chem. Eng. 2020, 5, 1868–1891. [Google Scholar] [CrossRef]
- Wang, Z.; Ashok, J.; Pu, Z.; Kawi, S. Low temperature partial oxidation of methane via BaBi0.05Co0.8Nb0.15O3−δ-Ni phyllosilicate catalytic hollow fiber membrane reactor. Chem. Eng. J. 2017, 315, 315–323. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Z.; Liu, L.; Pati, S.; Wai, M.H.; Kawi, S. Coupling CO2 separation with catalytic reverse water-gas shift reaction via ceramic-carbonate dual-phase membrane reactor. Chem. Eng. J. 2020, 379, 122182. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Wang, Z.; Kawi, S. Oxidative CO2 Reforming of Methane in La0.6Sr0.4Co0.8Ga0.2O3-δ (LSCG) Hollow Fiber Membrane Reactor. Environ. Sci. Technol. 2013, 47, 14510–14517. [Google Scholar] [CrossRef]
- Subhasis, P.; Jangam, A.; Nikita, D.; Chen, T.; Kawi, S. Ultra-thin (~1 μm) Pd–Cu membrane reactor for coupling CO2 hydrogenation and propane dehydrogenation applications. J. Membr. Sci. 2020, 595, 117496. [Google Scholar]
- Hu, J.; Poelman, H.; Marin, G.B.; Detavernier, C.; Kawi, S.; Galvita, V.V. FeO controls the sintering of iron-based oxygen carriers in chemical looping CO2 conversion. J. CO2 Util. 2020, 40, 101216. [Google Scholar] [CrossRef]
- Liu, L.; Das, S.; Chen, T.; Dewangan, N.; Ashok, J.; Xi, S.; Borgna, A.; Li, Z.; Kawi, S. Low temperature catalytic reverse water-gas shift reaction over perovskite catalysts in DBD plasma. Appl. Catal. B 2020, 265, 118573. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Das, S.; Xi, S.; Kawi, S. LaNiO3 as a precursor of Ni/La2O3 for reverse water-gas shift in DBD plasma: Effect of calcination temperature. Energy Convers. Manag. 2020, 206, 112475. [Google Scholar] [CrossRef]
- Maneerung, T.; Hidajat, K.; Kawi, S. Co-production of hydrogen and carbon nanofibers from catalytic decomposition of methane over LaNi(1−x)MxO3−α perovskite (where M = Co, Fe and X = 0, 0.2, 0.5, 0.8, 1). Int. J. Hydrogen Energy 2015, 40, 13399–13411. [Google Scholar] [CrossRef]
- Bian, Z.F.; Das, S.; Wai, M.H.; Hongmanorom, P.; Kawi, S. A Review on Bimetallic Nickel-Based Catalysts for CO2 Reforming of Methane. ChemPhysChem 2017, 18, 3117–3134. [Google Scholar] [CrossRef]
- Li, Z.W.; Li, M.; Bian, Z.F.; Kathiraser, Y.; Kawi, S. Design of highly stable and selective core/yolk–shell nanocatalysts-A review. Appl. Catal. B 2016, 188, 324–341. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Oemar, U.; Saw, E.T.; Li, Z.W.; Kawi, S. Kinetic and mechanistic aspects for CO2 reforming of methane over Ni based catalysts. Chem. Eng. J. 2015, 278, 62–78. [Google Scholar] [CrossRef]
- Bian, Z.F.; Kawi, S. Preparation, characterization and catalytic application of phyllosilicate: A review. Catal. Today 2020, 339, 3–23. [Google Scholar] [CrossRef]
- Kawi, S.; Kathiraser, Y.; Ni, J.; Oemar, U.; Li, Z.W.; Saw, E.T. Progress in Synthesis of Highly Active and Stable Nickel-Based Catalysts for Carbon Dioxide Reforming of Methane. ChemSusChem 2015, 8, 3556–3575. [Google Scholar] [CrossRef]
- Li, Z.W.; Das, S.; Hongmanorom, P.; Dewangan, N.; Wai, M.H.; Kawi, S. Silica-based micro- and mesoporous catalysts for dry reforming of methane. Catal. Sci. Technol. 2018, 8, 2763–2778. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. Catalytic reforming of methane with carbon dioxide over nickel catalysts I. Catalyst characterization and activity. Appl. Catal. A 1996, 142, 73–96. [Google Scholar] [CrossRef]
- Gadalla, A.M.; Bower, B. The role of catalyst support on the activity of nickel for reforming methane with CO2. Chem. Eng. Sci. 1988, 43, 3049–3062. [Google Scholar] [CrossRef]
- Chubb, T.A. Characteristics of CO2/CH4 reforming-methanation cycle relevant to the solchem thermochemical power system. Sol. Energy 1980, 24, 342–345. [Google Scholar] [CrossRef]
- McCrary, J.H.; McCrary, G.E.; Chubb, T.A.; Nemecek, J.J.; Simmons, D.E. An experimental study of the CO2-CH4 reforming-methanation cycle as a mechanism for converting and transporting solar energy. Sol. Energy 1982, 29, 141–151. [Google Scholar] [CrossRef]
- Kaichev, V.V.; Gladky, A.Y.; Prosvirin, I.P.; Saraev, A.A.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; Bukhtiyarov, V.I. In situ XPS study of self-sustained oscillations in catalytic oxidation of propane over nickel. Surf. Sci. 2013, 609, 113–118. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, J.; Chen, L.W.; Lin, J.Y.; Kawi, S. Lewis Acid Sites Stabilized Nickel Catalysts for Dry (CO2) Reforming of Methane. ChemCatChem 2016, 8, 3732–3739. [Google Scholar] [CrossRef]
- Wang, C.; Sun, N.; Kang, M.; Wen, X.; Zhao, N.; Xiao, F.; Wei, W.; Zhao, T.; Sun, Y. The bi-functional mechanism of CH4 dry reforming over a Ni–CaO–ZrO2 catalyst: Further evidence via the identification of the active sites and kinetic studies. Catal. Sci. Technol. 2013, 3, 2435–2443. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Atia, H.; Ibrahim, A.A.; Ha, Q.L.M.; Schneider, M.; M-Pohl, M.; Fakeeha, A.H. CO2-reforming of methane to produce syngas over Co-Ni/SBA-15 catalyst: Effect of support modifiers (Mg, La and Sc) on catalytic stability. J. CO2 Util. 2017, 21, 395–404. [Google Scholar] [CrossRef]
- Phan, T.S.; Sane, A.R.; de Vasconcelos, B.R.; Nzihou, A.; Sharrock, P.; Grouset, D.; Minh, D.P. Hydroxyapatite supported bimetallic cobalt and nickel catalysts for syngas production from dry reforming of methane. Appl. Catal. B 2018, 224, 310–321. [Google Scholar] [CrossRef]
- Zhang, L.; Lian, J.; Li, L.; Peng, C.; Liu, W.M.; Xu, X.L.; Fang, X.Z.; Wang, Z.; Wang, X.; Peng, H.E. LaNiO3 nanocube embedded in mesoporous silica for dry reforming of methane with enhanced coking resistance. Microporous Mesoporous Mater. 2018, 266, 189–197. [Google Scholar] [CrossRef]
- Li, X.Y.; Li, D.; Tian, H.; Zeng, L.; Zhao, J.-Z.; Gong, J.L. Dry reforming of methane over Ni/La2O3 nanorod catalysts with stabilized Ni nanoparticles. Appl. Catal. B 2017, 202, 683–694. [Google Scholar] [CrossRef]
- Zhang, Z.; Verykios, X.E.; MacDonald, S.; Affrossman, S. Comparative Study of Carbon Dioxide Reforming of Methane to Synthesis Gas over Ni/La2O3 and Conventional Nickel-Based Catalysts. J. Phys. Chem. 1996, 100, 744–752. [Google Scholar] [CrossRef]
- Jabbour, K.; Massiani, P.; Davidson, A.; Casale, S.; El Hassan, N. Ordered mesoporous “one-pot” synthesized Ni-Mg(Ca)-Al2O3 as effective and remarkably stable catalysts for combined steam and dry reforming of methane (CSDRM). Appl. Catal. B 2017, 201, 527–542. [Google Scholar] [CrossRef]
- Fang, X.Z.; Zhang, J.; Liu, J.J.; Wang, C.; Huang, Q.; Xu, X.L.; Peng, H.G.; Liu, W.M.; Wang, X.; Zhou, W.F. Methane dry reforming over Ni/Mg-Al-O: On the significant promotional effects of rare earth Ce and Nd metal oxides. J. CO2 Util. 2018, 25, 242–253. [Google Scholar] [CrossRef]
- Zuo, Z.J.; Liu, S.Z.; Wang, Z.C.; Liu, C.; Huang, W.; Huang, J.; Liu, P. Dry Reforming of Methane on Single-Site Ni/MgO Catalysts: Importance of Site Confinement. ACS Catal. 2018, 8, 9821–9835. [Google Scholar] [CrossRef]
- Feng, X.Q.; Liu, J.; Zhang, P.; Zhang, Q.; Xu, L.Y.; Zhao, L.P.; Song, X.F.; Gao, L. Highly coke resistant Mg–Ni/Al2O3 catalyst prepared via a novel magnesiothermic reduction for methane reforming catalysis with CO2: The unique role of Al–Ni intermetallics. Nanoscale 2019, 11, 1262–1272. [Google Scholar] [CrossRef]
- Ni, J.; Chen, L.W.; Lin, J.Y.; Schreyer, M.K.; Wang, Z.; Kawi, S. High performance of Mg–La mixed oxides supported Ni catalysts for dry reforming of methane: The effect of crystal structure. Int. J. Hydrogen Energy 2013, 38, 13631–13642. [Google Scholar] [CrossRef]
- Li, K.; He, F.; Yu, H.M.; Wang, Y.; Wu, Z.J. Theoretical study on the reaction mechanism of carbon dioxide reforming of methane on La and La2O3 modified Ni(111) surface. J. Catal. 2018, 364, 248–261. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Da Costa, P.; Grzybek, T. Catalytic activity of hydrotalcite-derived catalysts in the dry reforming of methane: On the effect of Ce promotion and feed gas composition. Reac. Kinet. Mech. Cat. 2017, 121, 185–208. [Google Scholar] [CrossRef]
- Pan, X.Y.; Kuai, P.; Liu, Y.; Ge, Q.; Liu, C.J. Promotion effects of Ga2O3 on CO2 adsorption and conversion over a SiO2-supported Nicatalyst. Energy Environ. Sci. 2010, 3, 1322–1325. [Google Scholar] [CrossRef]
- Ni, J.; Chen, L.; Lin, J.; Kawi, S. Carbon deposition on borated alumina supported nano-sized Ni catalysts for dry reforming of CH4. Nano Energy 2012, 1, 674–686. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Influence of Ce/Zr molar ratio on catalytic performance of hydrotalcite-derived catalysts at low temperature CO2 methane reforming. Int. J. Hydrogen Energy 2017, 42, 23556–23567. [Google Scholar] [CrossRef]
- Świrk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Yttrium promoted Ni-based double-layered hydroxides for dry methane reforming. J. CO2 Util. 2018, 27, 247–258. [Google Scholar] [CrossRef]
- Świrk, K.; Zhang, H.; Li, S.; Chen, Y.; Rønning, M.; Motak, M.; Grzybek, T.; Da Costa, P. Carbon-resistant NiO-Y2O3-nanostructured catalysts derived from double-layered hydroxides for dry reforming of methane. Catal. Today 2020, in press. [Google Scholar]
- Liu, H.; Wierzbicki, D.; Debek, R.; Motak, M.; Grzybek, T.; Da Costa, P.; Gálvez, M.E. La-promoted Ni-hydrotalcite-derived catalysts for dry reforming of methane at low temperatures. Fuel 2016, 182, 8–16. [Google Scholar] [CrossRef]
- Sun, G.B.; Hidajat, K.; Wu, X.S.; Kawi, S. A crucial role of surface oxygen mobility on nanocrystalline Y2O3 support for oxidative steam reforming of ethanol to hydrogen over Ni/Y2O3 catalysts. Appl. Catal. B 2008, 81, 303–312. [Google Scholar] [CrossRef]
- Lian, Z.; Olanrele, S.O.; Si, C.W.; Yang, M.; Li, B. Critical Role of Interfacial Sites between Nickel and CeO2 Support in Dry Reforming of Methane: Revisit of Reaction Mechanism and Origin of Stability. J. Phys. Chem. C 2020, 124, 5118–5124. [Google Scholar] [CrossRef]
- Sun, N.; Wen, X.; Wang, F.; Peng, W.; Zhao, N.; Xiao, F.K.; Wei, W.; Sun, Y.H.; Kang, J.T. Catalytic performance and characterization of Ni–CaO–ZrO2 catalysts for dry reforming of methane. Appl. Surf. Sci. 2011, 257, 9169–9176. [Google Scholar] [CrossRef]
- Tao, Q.Q.; Wang, Z.D.; Bandara, J.; Guo, C.Q.; Gan, Y.; Zhang, L.; Lu, Z.X.; Tan, H.Y.; Yan, C.F. Enhanced catalytic activity of Ni–Mo2C/La2O3–ZrO2 bifunctional catalyst for dry reforming of methane. J. Mater. Sci. 2018, 53, 14559–14572. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Yang, J.; Zhang, J.; Zhang, L.; Li, H.; Zhao, Y.; Li, H.X.; Wu, K.; Xu, G.Q.; et al. Enhanced catalytic performance of Ir catalysts supported on ceria-based solid solutions for methane dry reforming reaction. Catal. Today 2017, 281, 295–303. [Google Scholar] [CrossRef]
- Ay, H.; Üner, D. Dry reforming of methane over CeO2 supported Ni, Co and Ni–Co catalysts. Appl. Catal. B 2015, 179, 128–138. [Google Scholar] [CrossRef]
- Elsayed, N.H.; Roberts, N.R.M.; Joseph, B.; Kuhn, J.N. Low temperature dry reforming of methane over Pt–Ni–Mg/ceria–zirconia catalysts. Appl. Catal. B 2015, 179, 213–219. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Grinter, D.C.; Lustemberg, P.G.; Nguyen-Phan, T.-D.; Zhou, Y.H.; Luo, S.; Waluyo, I.; Crumlin, E.J.; Stacchiola, D.J.; Zhou, J.; et al. Dry Reforming of Methane on a Highly-Active Ni-CeO2 Catalyst: Effects of Metal-Support Interactions on C−H Bond Breaking. Angew. Chem. Int. Ed. 2016, 55, 7455–7459. [Google Scholar] [CrossRef] [PubMed]
- Vasiliades, M.A.; Makri, M.M.; Djinović, P.; Erjavec, B.; Pintar, A.; Efstathiou, A.M. Dry reforming of methane over 5 wt% Ni/Ce1-xPrxO2-δ catalysts: Performance and characterisation of active and inactive carbon by transient isotopic techniques. Appl. Catal. B 2016, 197, 168–183. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Ramírez, P.J.; Liu, Z.Y.; Gutiérrez, R.A.; Grinter, D.G.; Carrasco, J.; Senanayake, S.D.; Rodriguez, J.A.; Ganduglia-Pirovano, M.V. Room-Temperature Activation of Methane and Dry Re-forming with CO2 on Ni-CeO2(111) Surfaces: Effect of Ce3+ Sites and Metal–Support Interactions on C–H Bond Cleavage. ACS Catal. 2016, 6, 8184–8191. [Google Scholar] [CrossRef]
- Löfberg, A.; Guerrero-Caballero, J.; Kane, T.; Rubbens, A.; Jalowiecki-Duhamel, L. Ni/CeO2 based catalysts as oxygen vectors for the chemical looping dry reforming of methane for syngas production. Appl. Catal. B 2017, 212, 159–174. [Google Scholar] [CrossRef]
- Akri, M.; Pronier, S.; Chafik, T.; Achak, O.; Granger, P.; Simon, P.; Trentesaux, M.; Batiot-Dupeyrat, C. Development of nickel supported La and Ce-natural illite clay for autothermal dry reforming of methane: Toward a better resistance to deactivation. Appl. Catal. B 2017, 205, 519–531. [Google Scholar] [CrossRef]
- Gurav, H.R.; Dama, S.; Samuel, V.; Chilukuri, S. Influence of preparation method on activity and stability of Ni catalysts supported on Gd doped ceria in dry reforming of methane. J. CO2 Util. 2017, 20, 357–367. [Google Scholar] [CrossRef]
- Shamskar, F.R.; Meshkani, F.; Rezaei, M. Preparation and characterization of ultrasound-assisted co-precipitated nanocrystalline La-, Ce-, Zr –promoted Ni-Al2O3 catalysts for dry reforming reaction. J. CO2 Util. 2017, 22, 124–134. [Google Scholar] [CrossRef]
- Le Saché, E.; Santos, J.L.; Smith, T.J.; Centeno, M.A.; Arellano-Garcia, H.; Odriozola, J.A.; Reina, T.R. Multicomponent Ni-CeO2 nanocatalysts for syngas production from CO2/CH4 mixtures. J. CO2 Util. 2018, 25, 68–78. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Palcheva, R.; Karakirova, Y.; Sanchez, M.C.C.; Tyuliev, G.; Gaigneaux, E.; Fierro, J.L.G. Structure and surface properties of ceria-modified Ni-based catalysts for hydrogen production. Appl. Catal. B 2018, 225, 340–353. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Akhtamberdinova, Z.; Chen, X.P.; Jiang, G.D.; Liu, D. Dry Reforming of Shale Gas and Carbon Dioxide with Ni-Ce-Al2O3 Catalyst: Syngas Production Enhanced over Ni-CeOx Formation. ChemCatChem 2018, 10, 4689–4698. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E.; Kesavan, J.K.; Kumar, S.M.S.; Selvakumar, K. Dry reforming of methane over Ni supported on doped CeO2: New insight on the role of dopants for CO2 activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Yan, X.L.; Hu, T.; Liu, P.; Li, S.; Zhao, B.; Zhang, Q.; Jiao, W.Y.; Chen, S.; Wang, P.F.; Lu, J.J.; et al. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO2. Appl. Catal. B 2019, 246, 221–231. [Google Scholar] [CrossRef]
- Sagar, T.V.; Padmakar, D.; Lingaiah, N.; Prasad, P.S.S. Influence of Solid Solution Formation on the Activity of CeO2 Supported Ni–Cu Mixed Oxide Catalysts in Dry Reforming of Methane. Catal. Lett. 2019, 149, 2597–2606. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Hamid, M.Y.S. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Macario, A.; Frontera, P.; Candamano, S.; Crea, F.; De Luca, P.; Antonucci, P.L. Nanostructured Catalysts for Dry-Reforming of Methane. J. Nanosci. Nanotechnol. 2019, 19, 3135–3147. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic dry reforming of methane over high surface area ceria. Appl. Catal. B 2005, 60, 107–116. [Google Scholar] [CrossRef]
- Horváth, A.; Stefler, G.; Geszti, O.; Kienneman, A.; Pietraszek, A.; Guczi, L. Methane dry reforming with CO2 on CeZr-oxide supported Ni, NiRh and NiCo catalysts prepared by sol–gel technique: Relationship between activity and coke formation. Catal. Today 2011, 169, 102–111. [Google Scholar] [CrossRef]
- Safavinia, B.; Wang, Y.M.; Jiang, C.Y.; Roman, C.; Darapaneni, P.; Larriviere, J.; Cullen, D.A.; Dooley, K.M.; Dorman, J.A. Enhancing CexZr1–xO2 Activity for Methane Dry Reforming Using Subsurface Ni Dopants, Role of catalyst support over PdO–NiO catalysts on catalyst activity and stability for oxy-CO2 reforming of methane. ACS Catal. 2020, 10, 4070–4079. [Google Scholar] [CrossRef]
- Oemar, U.; Hidajat, K.; Kawi, S. Role of catalyst support over PdO–NiO catalysts on catalyst activity and stability for oxy-CO2 reforming of methane. Appl. Catal. A 2011, 402, 176–187. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Kawi, S. Promotional effect of alkaline earth over Ni–La2O3 catalyst for CO2 reforming of CH4: Role of surface oxygen species on H2 production and carbon suppression. Int. J. Hydrogen Energy 2011, 36, 14435–14446. [Google Scholar] [CrossRef]
- Pino, L.; Italiano, C.; Vita, A.; Laganà, M.; Recupero, V. Ce0.70La0.20Ni0.10O2-δ catalyst for methane dry reforming: Influence of reduction temperature on the catalytic activity and stability. Appl. Catal. B 2017, 218, 779–792. [Google Scholar] [CrossRef]
- Yang, W.Q.; Wang, Z.B.; Tan, W.Z.; Peng, R.R.; Wu, X.J.; Lu, Y.L. First principles study on methane reforming over Ni/TiO2(110) surface in solid oxide fuel cells under dry and wet atmospheres. Sci. China Mater. 2020, 63, 364–374. [Google Scholar] [CrossRef]
- Lou, Y.; Steib, M.; Zhang, Q.; Tiefenbacher, K.; Horváth, A.; Jentys, A.; Liu, Y.; Lercher, J.A. Design of stable Ni/ZrO2 catalysts for dry reforming of methane. J. Catal. 2017, 356, 147–156. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, Y.; Wang, S.H.; Zhao, Q.; Mao, D.H.; Hu, C.W. Low-Temperature Catalytic CO2 Dry Reforming of Methane on Ni-Si/ZrO2 Catalyst. ACS Catal. 2018, 8, 6495–6506. [Google Scholar] [CrossRef]
- Liu, W.M.; Li, L.; Zhang, X.H.; Wang, Z.; Wang, X.; Peng, H.G. Design of Ni-ZrO2@SiO2 catalyst with ultra-high sintering and coking resistance for dry reforming of methane to prepare syngas. J. CO2 Util. 2018, 27, 297–307. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.F.; Wu, Y.Q.; Pan, J.X.; Zhang, Q.D.; Tan, Y.S.; Han, Y.Z. Insight into the effects of the oxygen species over Ni/ZrO2 catalyst surface on methane reforming with carbon dioxide. Appl. Catal. B 2019, 244, 427–437. [Google Scholar] [CrossRef]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Kim, C.H.; Qi, G.; Dahlbery, K.; Li, W. Strontium-Doped Perovskites Rival Platinum Catalysts for Treating NOx in Simulated Diesel Exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cuaspud, J.; Perez, C.A.; Schmal, M. Nanostructured La0.8Sr0.2Fe0.8Cr0.2O3 Perovskite for the Steam Methane Reforming. Catal. Lett. 2016, 146, 2504–2515. [Google Scholar] [CrossRef]
- Gomez-Cuaspud, J.A.; Vera-Lopez, E.; Carda-Castello, J.B.; Barrachina-Albert, E. One-step hydrothermal synthesis of LaFeO3 perovskite for methane steam reforming. React. Kinet. Mech. Catal. 2016, 120, 167–179. [Google Scholar] [CrossRef]
- Di Bartolomeo, E.; Basoli, F.; Luisetto, I.; Tuti, S.; Zurlo, F.; Salehi, Z.; Licoccia, S. Ni and Ni-Co La0.8Sr0.2Ga0.8Mg0.2O3−δ infiltrated cells in H2 and CH4/CO2 mixture. Appl. Catal. B 2016, 191, 1–7. [Google Scholar] [CrossRef]
- Messaoudi, H.; Thomas, S.; Djaidja, A.; Slyemi, S.; Barama, A. Study of LaxNiOy and LaxNiOy/MgAl2O4 catalysts in dry reforming of methane. J. CO2 Util. 2018, 24, 40–49. [Google Scholar] [CrossRef]
- Kang, D.; Lim, H.S.; Lee, M.; Lee, J.W. Syngas production on a Ni-enhanced Fe2O3/Al2O3 oxygen carrier via chemical looping partial oxidation with dry reforming of methane. Appl. Energy 2018, 211, 174–186. [Google Scholar] [CrossRef]
- Fan, M.S.; Abdullah, A.Z.; Bhatia, S. Catalytic Technology for Carbon Dioxide Reforming of Methane to Synthesis Gas. ChemCatChem 2009, 1, 192–208. [Google Scholar] [CrossRef]
- Pakhare, D.; Schwartz, V.; Abdelsayed, V.; Haynes, D.; Shekhawat, D.; Puston, J.; Spivey, J. Kinetic and mechanistic study of dry (CO2) reforming of methane over Rh-substituted La2Zr2O7 pyrochlores. J. Catal. 2014, 316, 78–92. [Google Scholar] [CrossRef]
- Wang, H.Q.; Dong, X.L.; Zhao, T.T.; Yu, H.R.; Li, M. Dry reforming of methane over bimetallic Ni-Co catalyst prepared from La(CoxNi1-x)0.5Fe0.5O3 perovskite precursor: Catalytic activity and coking resistance. Appl. Catal. B 2019, 245, 302–313. [Google Scholar] [CrossRef]
- Yang, E.H.; Noh, Y.S.; Hong, G.H.; Moon, D.J. Combined steam and CO2 reforming of methane over La1-xSrxNiO3 perovskite oxides. Catal. Today. 2018, 299, 242–250. [Google Scholar] [CrossRef]
- Rynkowski, J.; Samulkiewicz, P.; Ladavos, A.; Pomonis, P. Catalytic performance of reduced La2−xSrxNiO4 perovskite-like oxides for CO2 reforming of CH4. Appl. Catal. A 2004, 263, 1–9. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Maneerung, T.; Kathiraser, Y.; Kawi, S. CO2 dry-reforming of methane over La0.8Sr0.2Ni0.8M0.2O3 perovskite (M = Bi, Co, Cr, Cu, Fe): Roles of lattice oxygen on C–H activation and carbon suppression. Int. J. Hydrogen Energy 2012, 37, 11195–11207. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Imtiaz, Q.; Kim, S.M.; Abdala, P.M.; Yoon, S.; Müller, C.R. Dry-reforming of methane over bimetallic Ni–M/La2O3 (M = Co, Fe): The effect of the rate of La2O2CO3 formation and phase stability on the catalytic activity and stability. J. Catal. 2016, 343, 208–214. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.T.; Dong, X.L.; Li, M.; Wang, H.Q. Effects of Ce substitution at the A-site of LaNi0.5Fe0.5O3 perovskite on the enhanced catalytic activity for dry reforming of methane. Appl. Catal. B 2018, 224, 214–221. [Google Scholar] [CrossRef]
- Figueredo, G.P.; Medeiros, R.L.B.A.; Macedo, H.P.; De Oliveira, Â.A.S.; Braga, R.M.; Mercury, J.M.R.; Melo, M.A.F. A comparative study of dry reforming of methane over nickel catalysts supported on perovskite-type LaAlO3 and commercial α-Al2O3. Int. J. Hydrogen Energy 2018, 43, 11022–11037. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Hu, Y.H. Role of Support in CO2 Reforming of CH4 to Syngas over Ni Catalysts. J. Catal. 1996, 162, 230–238. [Google Scholar] [CrossRef]
- Foley, H.C.; Hong, A.J.; Brinen, J.S.; Allard, L.F.; Garrat-Reed, A.J. Bimetallic catalysts comprised of dissimilar metals for the reduction of carbon monoxide with hydrogen. Appl. Catal. 1990, 61, 351–375. [Google Scholar] [CrossRef]
- Fan, C.; Zhu, Y.A.; Xu, Y.; Zhou, Y.; Zhou, X.G.; Chen, D. Origin of synergistic effect over Ni-based bimetallic surfaces: A density functional theory study. J. Chem. Phys. 2012, 137, 014703. [Google Scholar] [CrossRef]
- De, S.; Zhang, J.; Luque, R.; Yan, N. Ni-based bimetallic heterogeneous catalysts for energy and environmental applications. Energy Environ. Sci. 2016, 9, 3314–3347. [Google Scholar] [CrossRef]
- Tanksale, A.; Beltramini, J.N.; Dumesic, J.A.; Lu, G.Q. Effect of Pt and Pd promoter on Ni supported catalysts—A TPR/TPO/TPD and microcalorimetry study. J. Catal. 2008, 258, 366–377. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, B.; Wang, X.; Li, W.; Han, Y.; Gu, J.; Zhang, J. Non-mercury catalytic acetylene hydrochlorination over bimetallic Au–Co(III)/SAC catalysts for vinyl chloride monomer production. Green Chem. 2013, 15, 829–836. [Google Scholar] [CrossRef]
- Sankar, M.; Dimitratos, N.; Miedziak, P.J.; Wells, P.P.; Kiely, C.J.; Hutchings, G.J. Designing bimetallic catalysts for a green and sustainable future. Chem. Soc. Rev. 2012, 41, 8099–8139. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hariharan, S.; Tiwari, A.K. Pt–Ni Subsurface Alloy Catalysts: An Improved Performance toward CH4 Dissociation. J. Phys. Chem. C 2018, 122, 10857–10870. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, E.G.; Joo, O.S.; Jung, K.D. Stabilization of Ni/Al2O3 catalyst by Cu addition for CO2 reforming of methane. Appl. Catal. A 2004, 269, 1–6. [Google Scholar] [CrossRef]
- Fan, M.S.; Abdullah, A.Z.; Bhatia, S. Utilization of Greenhouse Gases through Dry Reforming: Screening of Nickel-Based Bimetallic Catalysts and Kinetic Studies. ChemSusChem 2011, 4, 1643–1653. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Li, L.; Zhou, L.; Ould-Chikh, S.; Anjum, D.H.; Kanoun, M.B.; Scaranto, J.; Hedhili, M.N.; Khalid, S.; Laveille, V.P.; D’Souza, L.; et al. Controlled Surface Segregation Leads to Efficient Coke-Resistant Nickel/Platinum Bimetallic Catalysts for the Dry Reforming of Methane. ChemCatChem 2015, 7, 819–829. [Google Scholar] [CrossRef]
- Lucci, F.R.; Marcinkowski, M.D.; Lawton, T.J.; Sykes, E.C.H. H2 Activation and Spillover on Catalytically Relevant Pt–Cu Single Atom Alloys. J. Phys. Chem. C 2015, 119, 24351–24357. [Google Scholar] [CrossRef]
- Tomishige, K.; Kanazawa, S.; Ito, S.-I.; Kunimori, K. Catalyst development for direct heat supply from combustion to reforming in methane reforming with CO2 and O2. Appl. Catal. A 2003, 244, 71–82. [Google Scholar] [CrossRef]
- Kim, J.; Park, W.H.; Doh, W.H.; Lee, S.W.; Noh, M.C.; Gallet, J.-J.; Bournel, F.; Kondoh, H.; Mase, K.; Jung, Y.; et al. Adsorbate-driven reactive interfacial Pt-NiO1−x nanostructure formation on the Pt3Ni(111) alloy surface. Sci. Adv. 2018, 4, eaat3151. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Kim, J.; Song, H.C.; Kim, D.; Jeong, B.; Lee, J.; Shin, J.W.; Ryoo, R.; Park, J.Y. Catalytic Synergy on PtNi Bimetal Catalysts Driven by Interfacial Intermediate Structures. ACS Catal. 2020, 10, 10459–10467. [Google Scholar] [CrossRef]
- Mukainakano, Y.; Yoshida, K.; Okumura, K.; Kunimori, K.; Tomishige, K. Catalytic performance and QXAFS analysis of Ni catalysts modified with Pd for oxidative steam reforming of methane. Catal. Today 2008, 132, 101–108. [Google Scholar] [CrossRef][Green Version]
- AlSabban, B.; Falivene, L.; Kozlov, S.M.; Aguilar-Tapia, A.; Ould-Chikh, S.; Hazemann, J.-L.; Cavallo, L.; Basset, J.-M.; Takanabe, K. In-operando elucidation of bimetallic CoNi nanoparticles during high-temperature CH4/CO2 reaction. Appl. Catal. B 2017, 213, 177–189. [Google Scholar] [CrossRef]
- Margossian, T.; Larmier, K.; Kim, S.M.; Krumeich, F.; Muller, C.; Copéret, C. Supported Bimetallic NiFe Nanoparticles through Colloid Synthesis for Improved Dry Reforming Performance. ACS Catal. 2017, 7, 6942–6948. [Google Scholar] [CrossRef]
- Bian, Z.F.; Kawi, S. Highly carbon-resistant Ni–Co/SiO2 catalysts derived from phyllosilicates for dry reforming of methane. J. CO2 Util. 2017, 18, 345–352. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, M.; Bag, A.; Shah, M.; Bordoloi, A. Facile synthesis of highly disperse Ni–Co nanoparticles over mesoporous silica for enhanced methane dry reforming. Nanoscale 2018, 10, 6409–6425. [Google Scholar] [CrossRef]
- Wu, Z.X.; Yang, B.; Miao, S.; Liu, W.; Xie, J.L.; Lee, S.; Pellin, M.J.; Xiao, D.Q.; Su, D.S.; Ma, D. Lattice Strained Ni-Co alloy as a High-Performance Catalyst for Catalytic Dry Reforming of Methane. ACS Catal. 2019, 9, 2693–2700. [Google Scholar] [CrossRef]
- Nagaoka, K.; Takanabe, K.; Aika, K.-I. Modification of Co/TiO2 for dry reforming of methane at 2 MPa by Pt, Ru or Ni. Appl. Catal. A 2004, 268, 151–158. [Google Scholar] [CrossRef]
- Jones, G.; Jakobsen, G.J.; Shim, S.S.; Kleis, J.; Andersson, M.P.; Rossmeisl, J.; Abild-Pedersen, F.; Bligaard, T.; Helveg, S.; Hinnemann, B.; et al. First principles calculations and experimental insight into methane steam reforming over transition metal catalysts. J. Catal. 2008, 259, 147–160. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Z.; Zhu, Y.-A.; Tong, G.; Zhang, J.; Engelbrekt, C.; Ulstrup, J.; Zhu, K.; Zhou, X. Tuning the composition of metastable CoxNiyMg100−x−y(OH)(OCH3) nanoplates for optimizing robust methane dry reforming catalyst. J. Catal. 2015, 330, 106–119. [Google Scholar] [CrossRef]
- Gao, X.Y.; Ashok, J.; Widjaja, S.; Hidajat, K.; Kawi, S. Ni/SiO2 catalyst prepared via Ni-aliphatic amine complexation for dry reforming of methane: Effect of carbon chain number and amine concentration. Appl. Catal. A 2015, 503, 34–42. [Google Scholar] [CrossRef]
- Tu, W.; Ghoussoub, M.; Singh, C.V.; Chin, C.Y.-H. Molecularly Tailored Nickel Precursor and Support Yield a Stable Methane Dry Reforming Catalyst with Superior Metal Utilization. J. Am. Chem. Soc. 2017, 139, 6928–6945. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced Carbon-Resistant Dry Reforming Fe-Ni Catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Batchu, R.; Galvita, V.V.; Poelman, H.; Marin, G.B. Carbon gasification from Fe–Ni catalysts after methane dry reforming. Appl. Catal. B 2016, 185, 42–55. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Q.; Cai, W.; Zhang, P.; Song, X.; Sun, Z.; Gao, L. Phyllosilicate evolved hierarchical Ni- and Cu–Ni/SiO2 nanocomposites for methane dry reforming catalysis. Appl. Catal. A 2015, 503, 94–102. [Google Scholar] [CrossRef]
- Hou, Z.; Yokota, O.; Tanaka, T.; Yashima, T. Characterization of Ca-promoted Ni/α-Al2O3 catalyst for CH4 reforming with CO2. Appl. Catal. A 2003, 253, 381–387. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J.Y. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Arbag, H.; Yasyerli, S.; Yasyerli, N.; Dogu, G.; Dogu, T. Enhancement of catalytic performance of Ni based mesoporous alumina by Co incorporation in conversion of biogas to synthesis gas. Appl. Catal. B 2016, 198, 254–265. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Łamacz, A.; Krztoń, A.; Djéga-Mariadassou, G. Partial oxidation of methane over Ni0/La2O3 bifunctional catalyst IV: Simulation of methane total oxidation, dry reforming and partial oxidation using the Quasi-Steady State Approximation. Appl. Catal. B 2016, 199, 424–432. [Google Scholar] [CrossRef]
- García-Vargas, J.M.; Valverde, J.L.; Lucas-Consuegra, A.D.; Gomez-Monedero, B.; Dorado, F.; Sanchez, P. Methane tri-reforming over a Ni/β-SiC-based catalyst: Optimizing the feedstock composition. Int. J. Hydrogen Energy 2013, 38, 4524–4532. [Google Scholar] [CrossRef]
- Tomishige, K.; Yamazaki, O.; Chen, Y.G.; Yokoyama, K.; Li, X.H.; Fujimoto, K. Development of ultra-stable Ni catalysts for CO2 reforming of methane. Catal. Today 1998, 45, 35–39. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, K.; Liu, Z.; Xiao, H.; Deng, W.; Zhou, X. Carbon dioxide reforming of methane over promoted NixMg1−xO (1 1 1) platelet catalyst derived from solvothermal synthesis. Appl. Catal. B 2014, 148–149, 177–190. [Google Scholar] [CrossRef]
- Horváth, A.; Guczi, L.; Kocsonya, A.; Safran, G.; Parola, V.L.; Liotta, L.F.; Pantaleo, G.; Venezia, A.M. Sol-derived AuNi/MgAl2O4 catalysts: Formation, structure and activity in dry reforming of methane. Appl. Catal. A 2013, 468, 250–259. [Google Scholar] [CrossRef]
- Wang, H.; Miller, J.T.; Shakouri, M.; Xi, C.; Wu, T.; Zhao, H.; Akatay, M.C. XANES and EXAFS studies on metal nanoparticle growth and bimetallic interaction of Ni-based catalysts for CO2 reforming of CH4. Catal. Today 2013, 207, 3–12. [Google Scholar] [CrossRef]
- Li, Z.W.; Kawi, S. Multi-Ni@Ni phyllosilicate hollow sphere for CO2 reforming of CH4: Influence of Ni precursors on structure, sintering, and carbon resistance. Catal. Sci. Technol. 2018, 8, 1915–1922. [Google Scholar] [CrossRef]
- Li, Z.W.; Jiang, B.; Wang, Z.G.; Kawi, S. High carbon resistant Ni@Ni phyllosilicate@SiO2 core shell hollow sphere catalysts for low temperature CH4 dry reforming. J. CO2 Util. 2018, 27, 238–246. [Google Scholar] [CrossRef]
- Li, Z.; Kathiraser, Y.; Kawi, S. Facile Synthesis of High Surface Area Yolk–Shell Ni@Ni Embedded SiO2 via Ni Phyllosilicate with Enhanced Performance for CO2 Reforming of CH4. ChemCatChem 2015, 7, 160–168. [Google Scholar] [CrossRef]
- Gao, X.Y.; Hidajat, K.; Kawi, S. Facile synthesis of Ni/SiO2 catalyst by sequential hydrogen/air treatment: A superior anti-coking catalyst for dry reforming of methane. J. CO2 Util. 2016, 15, 146–153. [Google Scholar] [CrossRef]
- Choi, H.; Oh, S.; Tran, S.B.T.; Park, J.Y. Size-controlled model Ni catalysts on Ga2O3 for CO2 hydrogenation to methanol. J. Catal. 2019, 376, 68–76. [Google Scholar] [CrossRef]
- Li, Y.M.; Wang, Z.J.; Zhang, B.; Liu, Z.G.; Yang, T.X. Dry Reforming of Methane (DRM) by Highly Active and Stable Ni Nanoparticles on Renewable Porous Carbons. Catalysts 2020, 10, 501. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.; Shi, Y.; Zhao, B.; Wang, M.; Liu, Q.; Wang, J.; Long, K.; Duan, Y.; Ning, P. A sintering and carbon-resistant Ni-SBA-15 catalyst prepared by solid-state grinding method for dry reforming of methane. J. CO2 Util. 2017, 17, 10–19. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Sun, M.H.; Ning, P.; Long, K.X.; Wang, J.; Tang, T.; Fan, J.; Sun, H.Y.; Yin, L.T.; Lin, Q. Effect of thermal induction temperature on re-dispersion behavior of Ni nanoparticles over Ni/SBA-15 for dry reforming of methane. Appl. Surf. Sci. 2019, 469, 368–377. [Google Scholar] [CrossRef]
- Kang, D.; Lim, S.H.; Lee, J.W. Enhanced catalytic activity of methane dry reforming by the confinement of Ni nanoparticles into mesoporous silica. Int. J. Hydrogen Energy 2017, 42, 11270–11282. [Google Scholar] [CrossRef]
- Baktash, E.; Littlewood, P.; Pfrommer, J.; Schomäcker, R.; Driess, M.; Thomas, A. Controlled Formation of Nickel Oxide Nanoparticles on Mesoporous Silica using Molecular Ni4O4 Clusters as Precursors: Enhanced Catalytic Performance for Dry Reforming of Methane. ChemCatChem 2015, 7, 1280–1284. [Google Scholar] [CrossRef]
- Qian, L.; Huang, K.; Wang, H.; Kung, M.C.; Kung, H.H.; Li, J.; Chen, G.; Du, Q. Evaluation of the catalytic surface of Ni impregnated meso-microporous silica KIT-6 in CH4 dry reforming by inverse gas chromatography. Microporous Mesoporous Mater. 2017, 243, 301–310. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Q.; Zhang, T.; Wang, Y.; Wang, J.; Long, K.; Song, Z.; Liu, X.; Ning, P. Facile one-pot synthesis of highly dispersed Ni nanoparticles embedded in HMS for dry reforming of methane. Chem. Eng. J. 2017, 313, 1370–1381. [Google Scholar] [CrossRef]
- Xin, J.; Cui, H.; Cheng, Z.; Zhou, Z. Bimetallic Ni-Co/SBA-15 catalysts prepared by urea co-precipitation for dry reforming of methane. Appl. Catal. A 2018, 554, 95–104. [Google Scholar] [CrossRef]
- Guo, Y.H.; Xia, C.; Liu, B.S. Catalytic properties and stability of cubic mesoporous LaxNiyOz/KIT-6 catalysts for CO2 reforming of CH4. Chem. Eng. J. 2014, 237, 421–429. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.Y.; Cheo, W.N.E.; Lau, R.; Borgna, A.; Yang, Y. MCM-41 supported nickel-based bimetallic catalysts with superior stability during carbon dioxide reforming of methane: Effect of strong metal–support interaction. J. Catal. 2009, 266, 380–390. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.Y.; Adeline, H.H.; Zeng, G.; Li, Y.; Yang, Y. Carbon dioxide reforming of methane over nickel-grafted SBA-15 and MCM-41 catalysts. Catal. Today 2009, 148, 243–250. [Google Scholar] [CrossRef]
- Baudouin, D.; Szeto, K.C.; Laurent, P.; Mallman, A.D.; Fenet, B.; Veyre, L.; Rodemerck, U.; Copéret, C.; Thieuleux, C. Nickel–Silicide Colloid Prepared under Mild Conditions as a Versatile Ni Precursor for More Efficient CO2 Reforming of CH4 Catalysts. J. Am. Chem. Soc. 2012, 134, 20624–20627. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Shi, L.; Zhang, J.; Zhang, D. Immobilizing Ni nanoparticles to mesoporous silica with size and location control via a polyol-assisted route for coking- and sintering-resistant dry reforming of methane. Chem. Commun. 2014, 50, 7250–7253. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y.; Liu, H.J.; Hidajat, K.; Kawi, S. Anti-Coking Ni/SiO2 Catalyst for Dry Reforming of Methane: Role of Oleylamine/Oleic Acid Organic Pair. ChemCatChem 2015, 7, 4188–4196. [Google Scholar] [CrossRef]
- Gao, X.Y.; Tan, Z.W.; Hidajat, K.; Kawi, S. Highly reactive Ni-Co/SiO2 bimetallic catalyst via complexation with oleylamine/oleic acid organic pair for dry reforming of methane. Catal. Today 2017, 281, 250–258. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Chou, L. Carbon dioxide reforming of methane over ordered mesoporous NiO–MgO–Al2O3 composite oxides. Appl. Catal. B 2011, 108–109, 177–190. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Chou, L. Mesoporous nanocrystalline ceria–zirconia solid solutions supported nickel based catalysts for CO2 reforming of CH4. Int. J. Hydrogen Energy 2012, 37, 18001–18020. [Google Scholar] [CrossRef]
- Xu, L.; Miao, Z.; Song, H.; Chen, W.; Chou, L. Significant roles of mesostructure and basic modifier for ordered mesoporous Ni/CaO–Al2O3 catalyst towards CO2 reforming of CH4. Catal. Sci. Technol. 2014, 4, 1759–1770. [Google Scholar] [CrossRef]
- Ashcroft, A.T.; Cheetham, A.K.; Green, M.L.H.; Vernon, P.D.F. Partial oxidation of methane to synthesis gas using carbon dioxide. Nature 1991, 352, 225–226. [Google Scholar] [CrossRef]
- Wang, N.; Xu, Z.; Deng, J.; Shen, K.; Yu, X.; Qian, W.; Chu, W.; Wei, F. One-pot Synthesis of Ordered Mesoporous NiCeAl Oxide Catalysts and a Study of Their Performance in Methane Dry Reforming. ChemCatChem 2014, 6, 1470–1480. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Khan, W.U.; Al-Fatesh, A.S.; Abasaeed, A.E. Stabilities of zeolite-supported Ni catalysts for dry reforming of methane. Chin. J. Catal. 2013, 34, 764–768. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, S.; Zhang, A.; Song, C.; Shi, C.; Guo, X. Hollow zeolite encapsulated Ni–Pt bimetals for sintering and coking resistant dry reforming of methane. J. Mater. Chem. A 2015, 3, 16461–16468. [Google Scholar] [CrossRef]
- Yin, Y.; Rioux, R.M.; Erdonmez, C.K.; Hughes, S.; Somorjai, G.A.; Alivisatos, A.P. Formation of Hollow Nanocrystals Through the Nanoscale Kirkendall Effect. Science 2004, 304, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Bang, J.U.; Lee, J.; Ko, C.H.; Song, H. Ni@SiO2yolk-shell nanoreactor catalysts: High temperature stability and recyclability. J. Mater. Chem. 2010, 20, 1239–1246. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.Y.; Han, S.W.; Cho, Y.K.; Jeong, M.-G.; Park, E.J.; Kim, Y.D. The catalytic stability of TiO2-shell/Ni-core catalysts for CO2 reforming of CH4. Appl. Catal. A 2015, 495, 184–191. [Google Scholar]
- Lu, Y.; Guo, D.; Ruan, Y.Z.; Zhao, Y.J.; Wang, S.P.; Ma, X.B. Facile one-pot synthesis of Ni@HSS as a novel yolk-shell structure catalyst for dry reforming of methane. J. CO2 Util. 2018, 24, 190–199. [Google Scholar] [CrossRef]
- Li, Z.W.; Kawi, S. Facile Synthesis of Multi-Ni-Core@Ni Phyllosilicate@CeO2 Shell Hollow Spheres with High Oxygen Vacancy Concentration for Dry Reforming of CH4. ChemCatChem 2018, 10, 2994–3001. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, M.R.; Fang, J.H.; Shi, L.Y.; Zhang, D.S. Hexagonal boron nitride supported mesoSiO2-confined Ni catalysts for dry reforming of methane. Chem. Commun. 2017, 53, 7549–7552. [Google Scholar] [CrossRef]
- Tian, J.Q.; Ma, B.; Bu, S.Y.; Yuan, Q.H.; Zhao, C. One-pot synthesis of highly sintering- and coking-resistant Ni nanoparticles encapsulated in dendritic mesoporous SiO2 for methane dry reforming. Chem. Commun. 2018, 54, 13993–13996. [Google Scholar] [CrossRef]
- Dou, J.; Zhang, R.G.; Hao, X.B.; Bao, Z.H.; Wu, T.P.; Wang, B.J.; Yu, F. Sandwiched SiO2@Ni@ZrO2 as a coke resistant nanocatalyst for dry reforming of methane. Appl. Catal. B 2019, 254, 612–623. [Google Scholar] [CrossRef]
- Bian, Z.F.; Kawi, S. Sandwich-Like Silica@Ni@Silica Multicore–Shell Catalyst for the Low-Temperature Dry Reforming of Methane: Confinement Effect Against Carbon Formation. ChemCatChem 2018, 10, 320–328. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F. Coke-resistant Ni@SiO2 catalyst for dry reforming of methane. Appl. Catal. B 2015, 176–177, 513–521. [Google Scholar] [CrossRef]
- Li, Z.; Mo, L.; Kathiraser, Y.; Kawi, S. Yolk–Satellite–Shell Structured Ni–Yolk@Ni@SiO2 Nanocomposite: Superb Catalyst toward Methane CO2 Reforming Reaction. ACS Catal. 2014, 4, 1526–1536. [Google Scholar] [CrossRef]
- Peng, X.Z.H.; Zhang, L.; Rao, C.; Lian, J.; Liu, W.; Ying, G.Z.J.; Wang, Z.; Zhang, N.; Wang, X. One-Pot Facile Fabrication of Multiple Nickel Nanoparticles Confined in Microporous Silica Giving a Multiple-Cores@Shell Structure as a Highly Efficient Catalyst for Methane Dry Reforming. ChemCatChem 2017, 9, 127–136. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, C.; Park, J.S.; Lee, H. Highly Coke-Resistant Ni Nanoparticle Catalysts with Minimal Sintering in Dry Reforming of Methane. ChemSusChem 2014, 7, 451–456. [Google Scholar] [CrossRef]
- Li, Z.W.; Kathiraser, Y.; Ashok, J.; Oemar, U.; Kawi, S. Simultaneous Tuning Porosity and Basicity of Nickel@Nickel–Magnesium Phyllosilicate Core–Shell Catalysts for CO2 Reforming of CH4. Langmuir 2014, 30, 14694–14705. [Google Scholar] [CrossRef]
- Bian, Z.F.; Suryawinata, I.Y.; Kawi, S. Highly carbon resistant multicore-shell catalyst derived from Ni-Mg phyllosilicate nanotubes@silica for dry reforming of methane. Appl. Catal. B 2016, 195, 1–8. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Gao, R.; Huang, L.; Shi, L.; Zhang, J. Design of modular catalysts derived from NiMgAl-LDH@m-SiO2 with dual confinement effects for dry reforming of methane. Chem. Commun. 2013, 49, 6770–6772. [Google Scholar] [CrossRef]
- Wu, T.; Cai, W.; Zhang, P.; Song, X.; Gao, L. Cu–Ni@SiO2 alloy nanocomposites for methane dry reforming catalysis. RSC Adv. 2013, 3, 23976–23979. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.; Li, H. NiCo@SiO2 core-shell catalyst with high activity and long lifetime for CO2 conversion through DRM reaction. Nano Energy 2018, 45, 101–108. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Z.; Hu, J.; Wai, M.H.; Kawi, S.; Lin, Y.S. High CO2 permeability of ceramic-carbonate dual-phase hollow fiber membrane at medium-high temperature. J. Membr. Sci. 2020, 597, 117770. [Google Scholar] [CrossRef]
- Yang, T.N.; Kathiraser, Y.; Kawi, S. La0.6Sr0.4Co0.8Ni0.2O3−δ hollow fiber membrane reactor: Integrated oxygen separation—CO2 reforming of methane reaction for hydrogen production. Int. J. Hydrogen Energy 2013, 38, 4483–4491. [Google Scholar] [CrossRef]
- Chen, T.; Wang, Z.; Das, S.; Liu, L.; Li, Y.; Kawi, S.; Lin, Y.S. A novel study of sulfur-resistance for CO2 separation through asymmetric ceramic-carbonate dual-phase membrane at high temperature. J. Membr. Sci. 2019, 581, 72–81. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).