Proximate Composition of Rice Grains Grown in Brazil Assessed Using Near-Infrared Spectroscopy: A Strategy for Selecting Superior Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Post-Harvesting Processing
2.3. Proximate Composition Analysis
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Zhang, T.; Zhou, X.; Xu, T.; Li, C.; Li, Z.; Wang, S.; Li, M.; Hong, J. Insights into the Environmental–Economic Sustainability of Rice Production in China. J. Clean. Prod. 2025, 498, 145205. [Google Scholar] [CrossRef]
- Xavier, A.I.S.; Arbage, A.P.; da Silva, M.R.; Ribas, G.G.; Meus, L.D.; dos Santos, G.A.d.A.; Streck, N.A.; Zanon, A.J. Economic and Productive Analysis of Irrigated Rice Crops Using a Multicase Study. Pesq. Agropec. Bras. 2021, 56, e02037. [Google Scholar] [CrossRef]
- Costa, J.C.; de Jesus, A.C.d.S.; de Jesus, J.G.L.; Madruga, M.F.; Souza, T.N.; Louzada, M.L.d.C. Diferenças no Consumo Alimentar Dda População Brasileira por Raça/Cor da Pele em 2017–2018. Rev. Saúde Pública 2023, 57, 4. [Google Scholar] [CrossRef] [PubMed]
- Siregar, S.; Nurhikmat, A.; Amdani, R.Z.; Hatmi, R.U.; Kobarsih, M.; Kusumaningrum, A.; Karim, M.A.; Dameswari, A.H.; Siswanto, N.; Siswoprayogi, S.; et al. Estimation of Proximate Composition in Rice Using ATR-FTIR Spectroscopy and Chemometrics. ACS Omega 2024, 9, 32760–32768. [Google Scholar] [CrossRef]
- Leal, M.M.; Bilhalva, N.d.S.; de Moraes, R.S.; Coradi, P.C. Physical Classification of Soybean Grains Based on Physicochemical Characterization Using Near-Infrared Spectroscopy. AgriEngineering 2025, 7, 194. [Google Scholar] [CrossRef]
- Bilhalva, N.d.S.; Coradi, P.C.; de Oliveira, D.P.; Nunes, M.T.; Lombardi, B.P.; Beskow, A. Evaluation of the Quality and Classification of Parboiled Rice Using Near-Infrared Spectroscopy and Multivariate Statistical Analyses. Eng. Agrícola 2025, 45, e202470211. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, W.; Ma, Y.; Cao, C.; Zhang, G.; Jiang, Y. Near-Infrared Spectroscopy Combined with Effective Variable Selection Algorithm for Rapid Detection of Rice Taste Quality. Biosyst. Eng. 2024, 237, 214–219. [Google Scholar] [CrossRef]
- Debra Sandilands. Univariate Analysis. In Encyclopedia of Quality of Life and Well-Being Research; Springer International Publishing: Cham, Switzerland, 2023; pp. 7391–7394. [Google Scholar]
- de Barros, H.E.A.; Alexandre, A.C.S.; Campolina, G.A.; Alvarenga, G.F.; Silva, L.M.d.S.F.e.; Natarelli, C.V.L.; Carvalho, E.E.N.; Vilas Boas, E.V.d.B. Edible Seeds Clustering Based on Phenolics and Antioxidant Activity Using Multivariate Analysis. LWT 2021, 152, 112372. [Google Scholar] [CrossRef]
- Moura, F.S.H.; de Souza Dias, F.; Beatriz Souza e Souza, L.; de Magalhães, B.E.A.; de Aragão Tannus, C.; Correia de Carvalho, W.; Cardoso Brandão, G.; dos Santos, W.N.L.; Graças Andrade Korn, M.; Cristina Muniz Batista dos Santos, D.; et al. Evaluation of Multielement/Proximate Composition and Bioactive Phenolics Contents of Unconventional Edible Plants from Brazil Using Multivariate Analysis Techniques. Food Chem. 2021, 363, 129995. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araújo Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa Solos: Brasília, Brazil, 2018. [Google Scholar]
- Wrege, M.S.; Steinmetz, S.; Júnior, C.R.; Almeida, I.R. Atlas Climático da Região Sul do Brasil; Embrapa: Brasília, Brazil, 2012. [Google Scholar]
- Sosbai—Sociedade Sul-Brasileira de Arroz Irrigado. Arroz irrigado: Recomendações técnicas da pesquisa para o Sul do Brasil. In Proceedings of the XXXIII Reunião Técnica Da Cultura Do Arroz Irrigado, Restinga Seca, Brazil, 25–26 July 2022. [Google Scholar]
- Zaccaria. AZ-1- DTA- Testing Rice Mill. Available online: https://www.zaccaria.com.br/en-US/produtos/equipamentos-para-arroz/laboratorio-de-arroz-h97ha/paz-1dta-provador-para-arroz-amxsw (accessed on 1 April 2025).
- Perten. Perten Instruments Laboratory Mill: 3100. Available online: https://contact.perkinelmer.com/perten-food-quality-testing (accessed on 1 April 2025).
- Foss Analytics. NIRS TM DS2500 L. The Reliable and Versatile Lab NIR. Available online: https://www.fossanalytics.com/pt-br/products/nirs-ds2500-l (accessed on 1 April 2025).
- Salinas Ruíz, J.; Montesinos López, O.A.; Hernández Ramírez, G.; Crossa Hiriart, J. Generalized Linear Mixed Models with Applications in Agriculture and Biology; Springer International Publishing: Cham, Switzerland, 2023; ISBN 978-3-031-32799-5. [Google Scholar]
- Rennberger, G.; Turechek, W.W.; Keinath, A.P. Dynamics of the Ascospore Dispersal of Stagonosporopsis Citrulli, a Causal Agent of Gummy Stem Blight of Cucurbits. Plant Pathol. 2021, 70, 1908–1919. [Google Scholar] [CrossRef]
- R Core Team-R. R Core Team-R: A Language and Environment for Statistical Computing. Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 1 July 2025).
- SAS. SAS OnDemand for Academics. Available online: https://www.sas.com/pt_br/software/on-demand-for-academics.html (accessed on 1 July 2025).
- Verma, D.K.; Srivastav, P.P. Proximate Composition, Mineral Content and Fatty Acids Analyses of Aromatic and Non-Aromatic Indian Rice. Rice Sci. 2017, 24, 21–31. [Google Scholar] [CrossRef]
- Dias, L.G.; Hacke, A.; dos Santos Souza, E.; Nath, S.; Canesin, M.R.; Vilella, O.V.; Geloneze, B.; Pallone, J.A.L.; Cazarin, C.B.B.; Blakeslee, J.J.; et al. Comparison of Chemical and Nutritional Compositions between Aromatic and Non-Aromatic Rice from Brazil and Effect of Planting Time on Bioactive Compounds. J. Food Compos. Anal. 2022, 111, 104608. [Google Scholar] [CrossRef]
- Nath, S.; Bhattacharjee, P.; Bhattacharjee, S.; Datta, J.; Dolai, A.K. Grain Characteristics, Proximate Composition, Phytochemical Capacity, and Mineral Content of Selected Aromatic and Non-Aromatic Rice Accessions Commonly Cultivated in the North-East Indian Plain Belt. Appl. Food Res. 2022, 2, 100067. [Google Scholar] [CrossRef]
- Brasil. Ministério Da Agricultura, Pecuária e Abastecimento. Instrução Normativa No 6, de 16 de Fevereiro de 2009. Available online: https://sistemasweb.agricultura.gov.br/sislegis/action/detalhaAto.do?method=visualizarAtoPortalMapa&chave=1687046295 (accessed on 1 August 2025).
- M, F.; John, D.; Raman, M. Physicochemical Properties, Eating and Cooking Quality and Genetic Variability: A Comparative Analysis in Selected Rice Varieties of South India. Food Prod. Process. Nutr. 2023, 5, 49. [Google Scholar] [CrossRef]
- Panda, K.K.; Bisht, S.S.; Mishra, R.; Sahu, P.K.; Panda, A.K.; Subedi, R. Nutritional Analysis of Rice Landraces from Southern Odisha, India. Food Sci. Nutr. 2024, 12, 227–238. [Google Scholar] [CrossRef]
- Jiang, P.; Li, R.; Tian, X.; Liu, Y.; Zhang, D.; Ren, F.; Liu, M.; Tan, B. Effect of milling on proximate composition, γ-oryzanol, vitamin B1, polyphenolic, and bioaccessibility of phenolic of brown rice. J. Food Process. Preserv. 2022, 46, e17215. [Google Scholar] [CrossRef]
- Yu, L.; Fan, J.; Yan, C.; Xu, C. Starch Deficiency Enhances Lipid Biosynthesis and Turnover in Leaves. Plant Physiol. 2018, 178, 118–129. [Google Scholar] [CrossRef]
- Chang, L.; Liu, Z.; Ying, X.; Kalandarov, B.; Ergashev, M.; Tong, X.; Zhang, J.; Jin, J.; Ying, J. Molecular Basis of Lipid Metabolism in Oryza sativa L. Plants 2024, 13, 3263. [Google Scholar] [CrossRef]
- Wang, B.; Guo, T.; Wang, H.; Li, J.; Sun, Z.; Liu, Y.; Xing, Y.; Xu, B.; Yang, B.; Li, J.; et al. Characterization and Expression Profile Analysis of the 3-phosphoglycerate Dehydrogenase Family in Rice. Agron. J. 2021, 113, 1039–1049. [Google Scholar] [CrossRef]
- Ouyang, N.; Hu, W.; Meng, J.; Wang, B. Amino Acid Derivatives as Regulatory Molecules: Mechanisms in Plant Growth and Stress Tolerance. Crop J. 2025, 13, 668–680. [Google Scholar] [CrossRef]
- Ying, Y.; Deng, B.; Zhang, L.; Hu, Y.; Liu, L.; Bao, J.; Xu, F. Multi-Omics Analyses Reveal Mechanism for High Resistant Starch Formation in an Indica Rice SSIIIa Mutant. Carbohydr. Polym. 2025, 347, 122708. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Y.; Chen, H.; An, H.; Liu, Y.; Xu, J. Effect of the Degree of Milling on the Microstructure and Composition of Japonica Rice. Grain Oil Sci. Technol. 2022, 5, 194–203. [Google Scholar] [CrossRef]
- Oh, S.; Lee, S.; Park, S.; Lee, S.; Lee, S.; Cho, H.; Chung, Y.; Park, S. Statistical Study on the Environmental Effects on the Natural Variation of Nutritional Components in Rice Varieties. Food Sci. Nutr. 2019, 7, 163–172. [Google Scholar] [CrossRef] [PubMed]


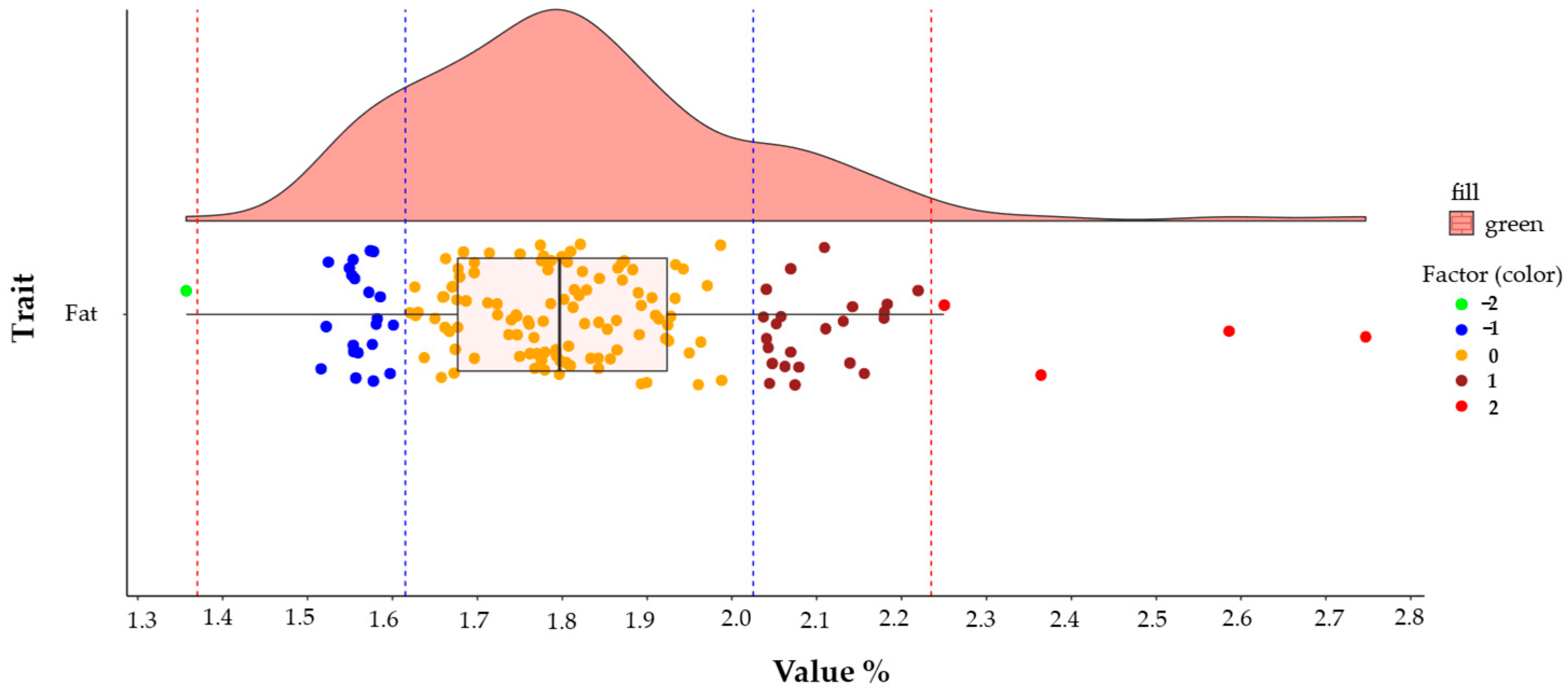
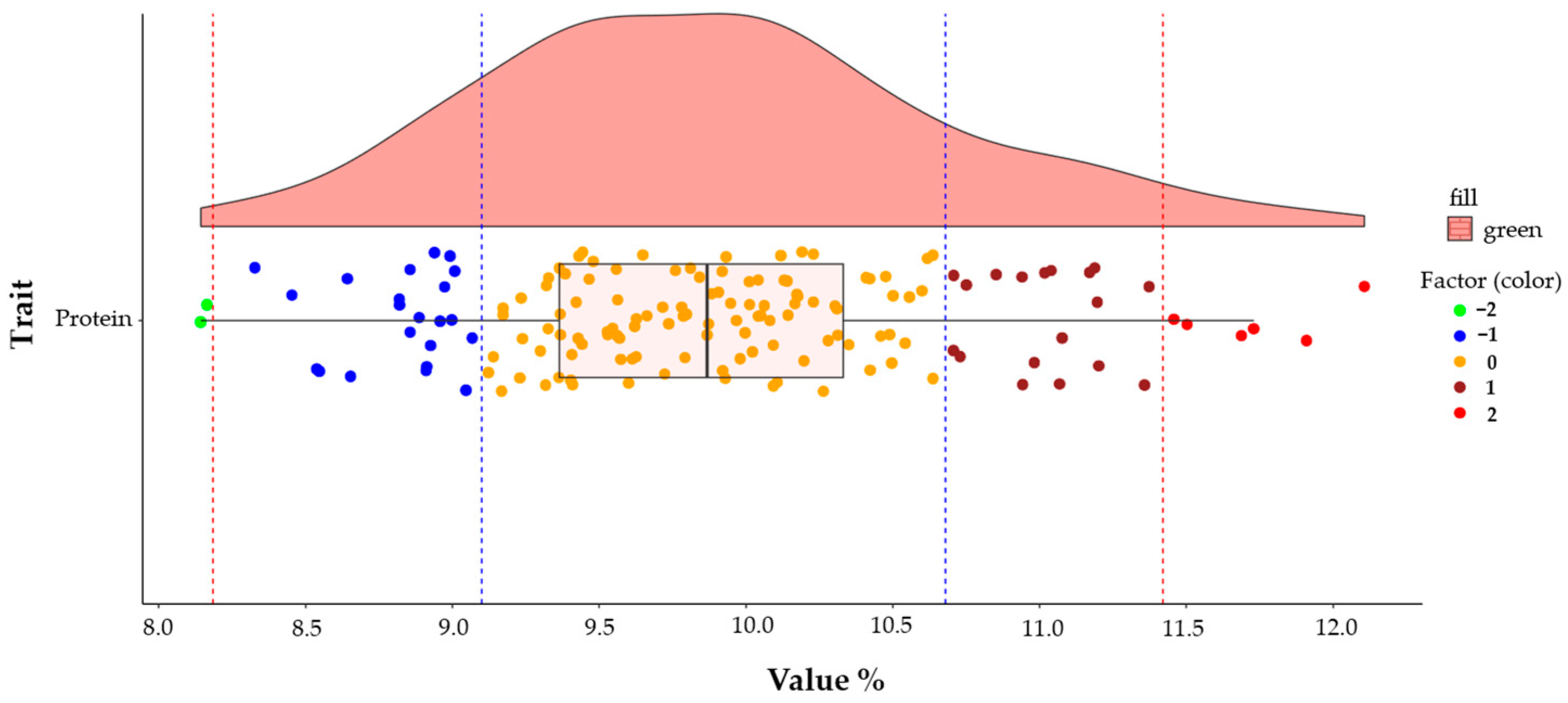


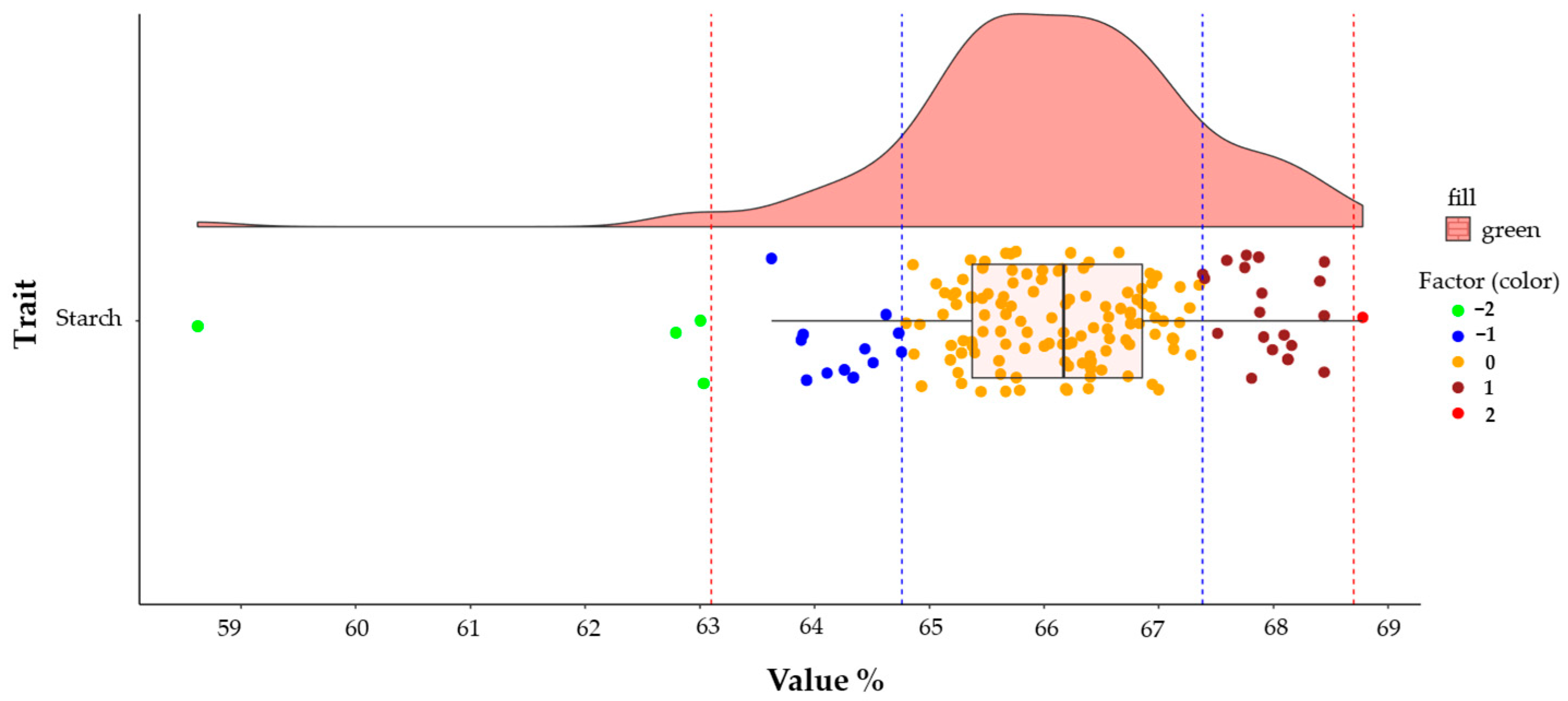
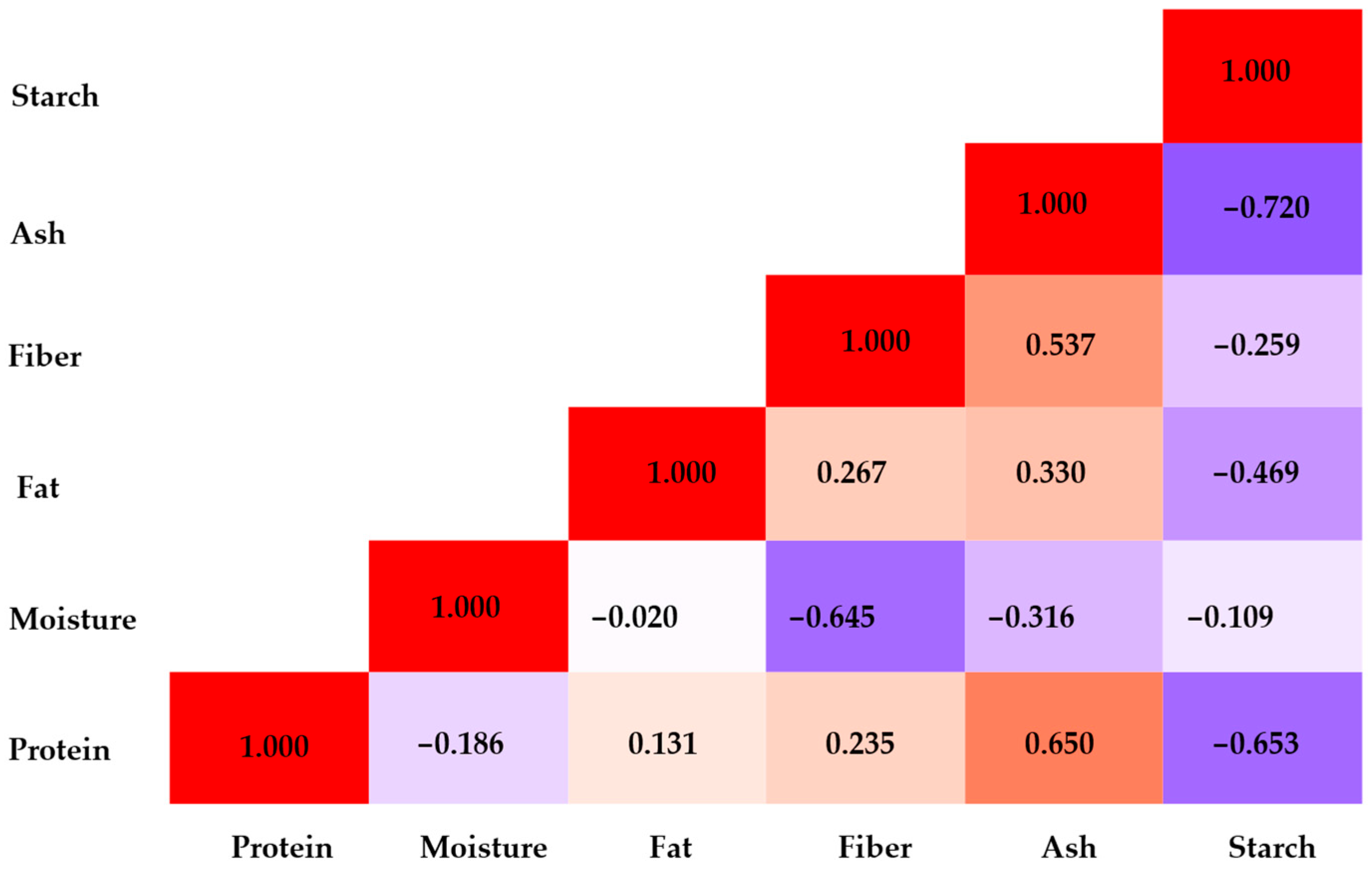

| Eigenvalues | Eigenvectors | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variance | Components | ||||||||
| PC | Eigenvalue | Proportion | Cumulative | Protein | Moisture | Fat | Fiber | Ash | Starch |
| PC1 * | 3.022 | 0.504 | 0.504 | 0.4338 | −0.2472 | 0.2862 | 0.4209 | 0.5307 | −0.4583 |
| PC2 * | 1.485 | 0.248 | 0.751 | 0.1957 | 0.7043 | 0.2115 | −0.4712 | 0.0446 | −0.4438 |
| PC3 | 0.931 | 0.155 | 0.906 | −0.5192 | 0.0305 | 0.8246 | 0.1761 | −0.1354 | 0.0119 |
| PC4 | 0.308 | 0.051 | 0.958 | 0.5840 | −0.3909 | 0.4008 | −0.3949 | −0.3818 | 0.2091 |
| PC5 | 0.156 | 0.026 | 0.984 | −0.2109 | −0.1953 | 0.1050 | −0.5475 | 0.7185 | 0.3006 |
| PC6 | 0.099 | 0.016 | 1.000 | 0.3441 | 0.5009 | 0.1473 | 0.3378 | 0.1896 | 0.6774 |
| Group | Protein | Fiber | Ash | Fat | Starch | Moisture |
|---|---|---|---|---|---|---|
| Q1 | 0.0951 | 0.0184 | 0.0154 | 0.0182 | 0.6618 | 0.1331 |
| Q2 | 0.0927 | 0.0198 | 0.0153 | 0.0174 | 0.6733 | 0.1227 |
| Q3 | 0.1030 | 0.0210 | 0.0165 | 0.0183 | 0.6592 | 0.1156 |
| Q4 | 0.1066 | 0.0197 | 0.0167 | 0.0191 | 0.6463 | 0.1289 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariano, A.A.; Chagas, G.B.d.; Rodrigues, L.A.; de Brito Leal, A.; da Silveira, M.C.; de Oliveira, M.; Costa de Oliveira, A.; da Maia, L.C.; Pegoraro, C. Proximate Composition of Rice Grains Grown in Brazil Assessed Using Near-Infrared Spectroscopy: A Strategy for Selecting Superior Genotypes. AgriEngineering 2025, 7, 305. https://doi.org/10.3390/agriengineering7090305
Mariano AA, Chagas GBd, Rodrigues LA, de Brito Leal A, da Silveira MC, de Oliveira M, Costa de Oliveira A, da Maia LC, Pegoraro C. Proximate Composition of Rice Grains Grown in Brazil Assessed Using Near-Infrared Spectroscopy: A Strategy for Selecting Superior Genotypes. AgriEngineering. 2025; 7(9):305. https://doi.org/10.3390/agriengineering7090305
Chicago/Turabian StyleMariano, Aguiar Afonso, Gabriel Brandão das Chagas, Larissa Alves Rodrigues, Andreza de Brito Leal, Michel Cavalheiro da Silveira, Maurício de Oliveira, Antonio Costa de Oliveira, Luciano Carlos da Maia, and Camila Pegoraro. 2025. "Proximate Composition of Rice Grains Grown in Brazil Assessed Using Near-Infrared Spectroscopy: A Strategy for Selecting Superior Genotypes" AgriEngineering 7, no. 9: 305. https://doi.org/10.3390/agriengineering7090305
APA StyleMariano, A. A., Chagas, G. B. d., Rodrigues, L. A., de Brito Leal, A., da Silveira, M. C., de Oliveira, M., Costa de Oliveira, A., da Maia, L. C., & Pegoraro, C. (2025). Proximate Composition of Rice Grains Grown in Brazil Assessed Using Near-Infrared Spectroscopy: A Strategy for Selecting Superior Genotypes. AgriEngineering, 7(9), 305. https://doi.org/10.3390/agriengineering7090305






