Plant-Based Bioherbicides: Review of Eco-Friendly Strategies for Weed Control in Organic Bean and Corn Farming
Abstract
1. Introduction
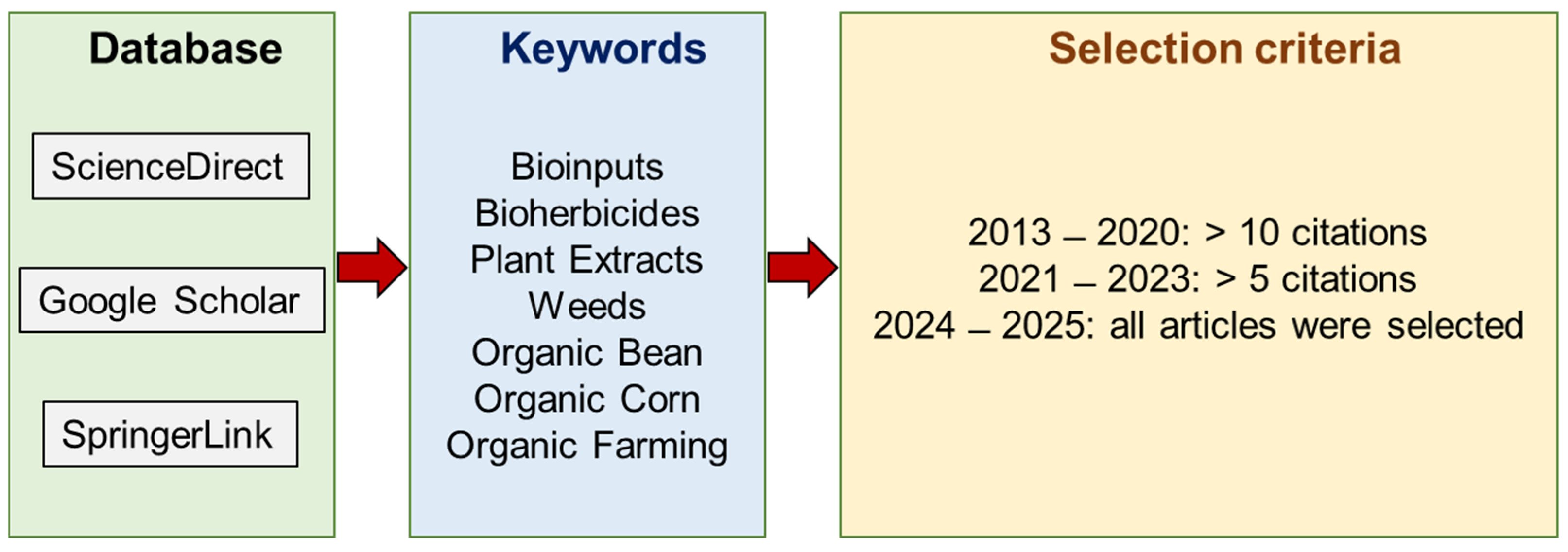
2. Methodology of Review Preparation
3. Main Weeds That Infest Corn and Beans
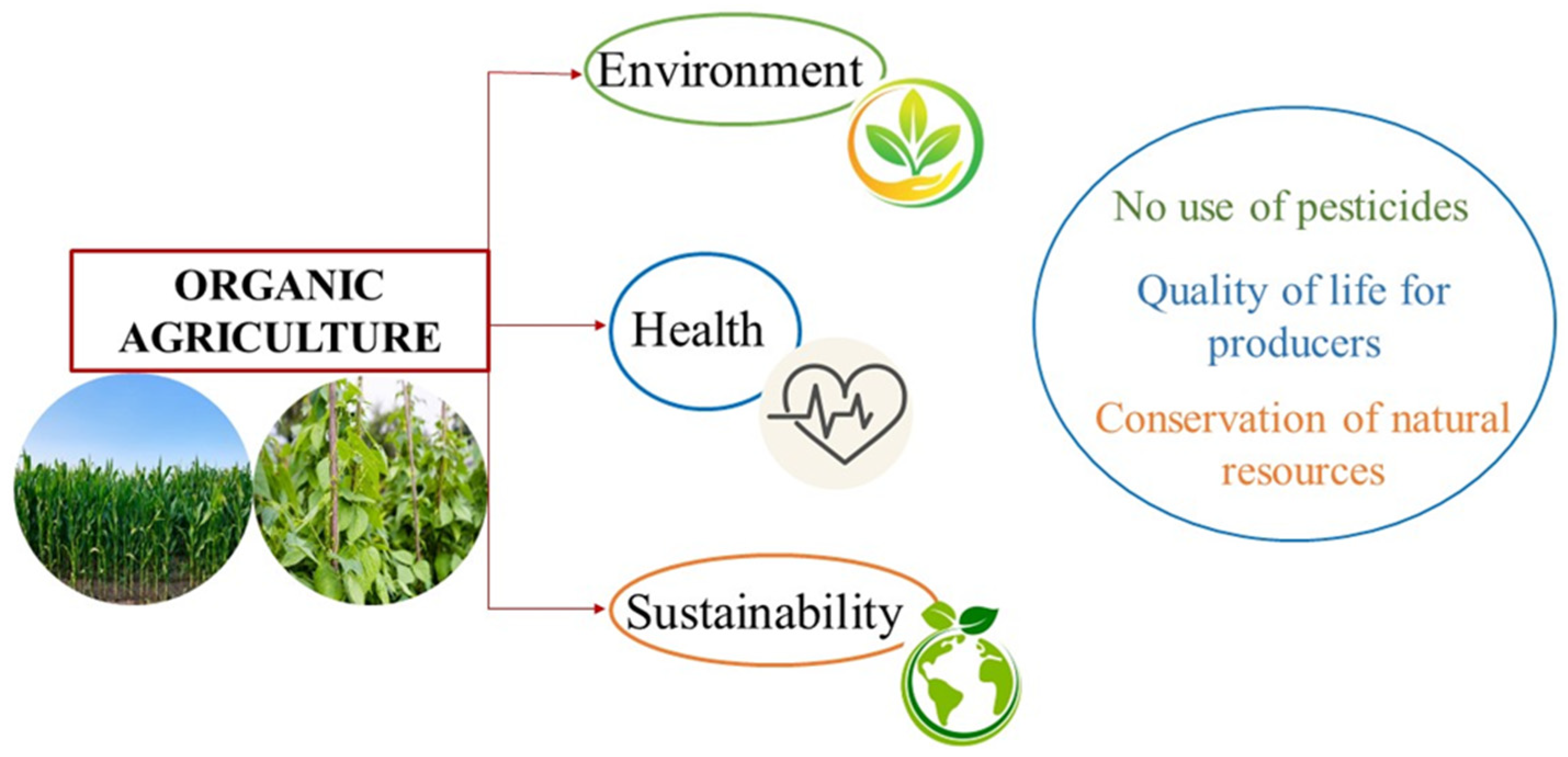
4. Organic Farming
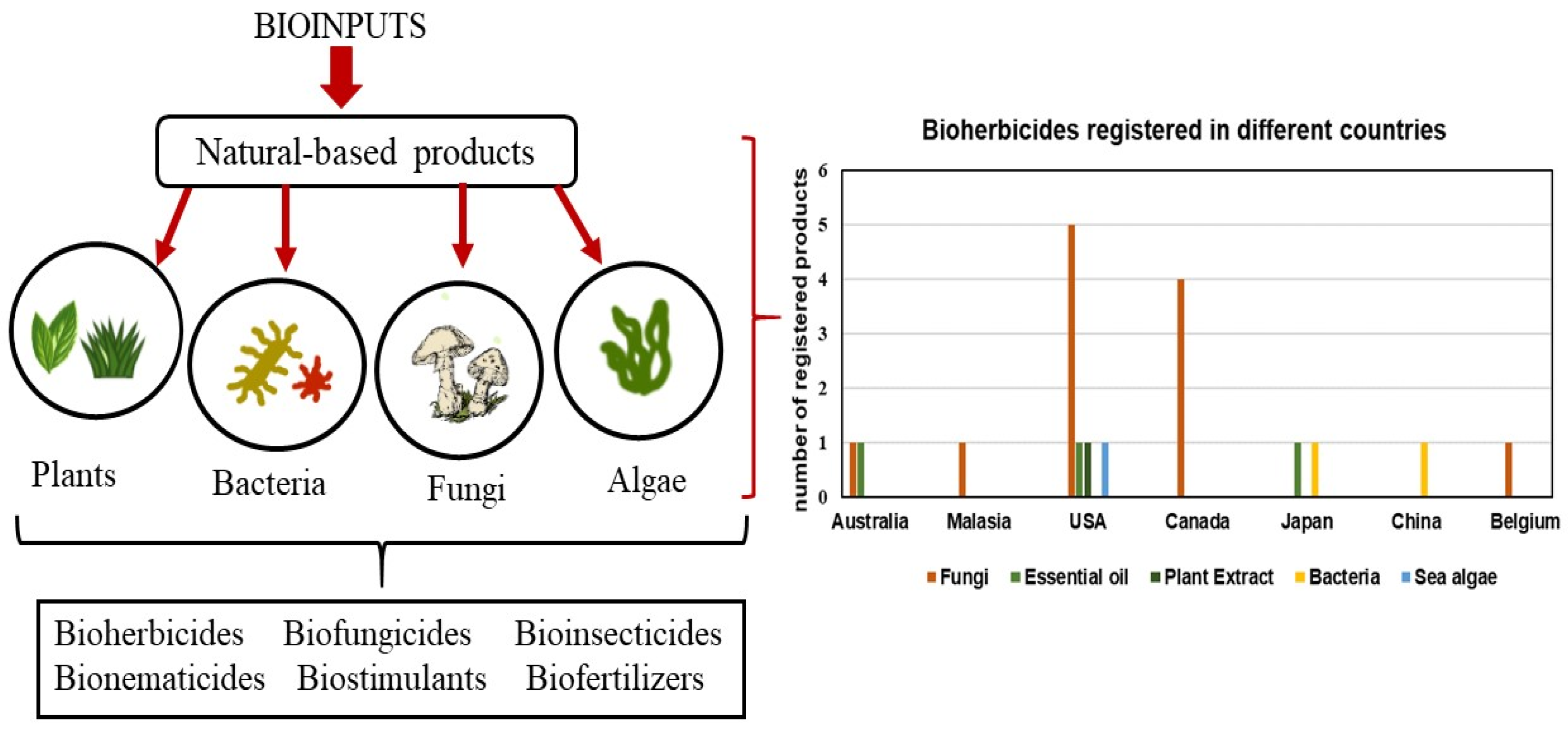
4.1. Bioinputs
Bioherbicides
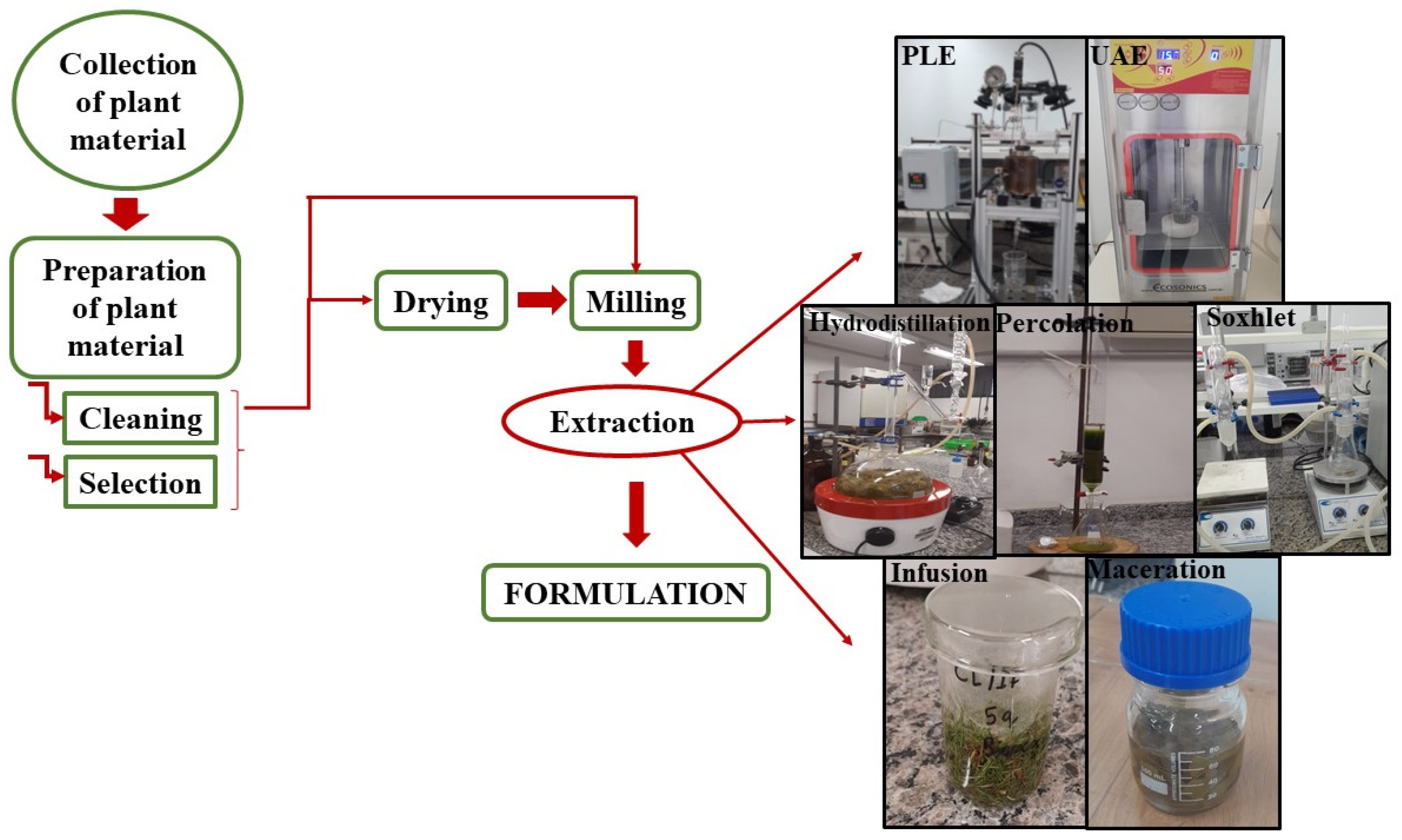
5. Obtaining Plant Extracts
5.1. Main Plants
5.2. Main Methods for Obtaining Plant Extracts
- Infusion: This is one of the simplest methods used, similar to making tea. It involves placing the heated solvent, usually distilled water, but other solvents such as ethanol can also be used, in contact with the plant material for several minutes to allow infusion. The advantage lies in rapid extraction. In the environmental aspect, the use of GRAS (Generally Recognized As Safe) solvents is a benefit. The main variables are temperature and extraction time. The infusion method is used to obtain volatile and water-soluble compounds.
- Maceration: The plant material, usually fresh or dried, is crushed or ground with a solvent, usually ethanol or hydroethanolic mixtures, and left for a long time. In the environmental aspect, the use of GRAS solvents is an advantage. A long extraction time is a disadvantage. The main variables are temperature and extraction time. Maceration is recommended for extracting plant materials rich in extracts of interest and without a defined cellular structure, including thermolabile compounds.
- Soxhlet: It is a continuous method, where plant material is placed on a filter paper and inserted into the apparatus. Then, a distillation flask is filled with solvent. A siphon aspirates the solvent, such as n-hexane, from the filter paper and returns it to the distillation flask, transporting and extracting the substances of interest. This process is repeated until complete extraction is achieved. It is used in the extraction of non-volatile compounds. It needs a long extraction time and often relies on hazardous organic solvents like n-hexane. The advantage of Soxhlet lies in enabling efficient solvent recycling within the system, thereby reducing overall solvent waste compared to open percolation setups, though its total solvent volume may still be significant. The variables involved in this process are the biomass-to-solvent ratio and extraction time.
- Percolation: This is a continuous method where the solvent at room temperature (approximately 25 °C) passes through the dried and ground plant material. The method is used for extracting compounds soluble in liquid solvents. The main advantage is a rapid extraction time, while the high solvent consumption is a disadvantage. The efficiency depends on factors such as the particle size of the plant material, the shape and dimensions of the percolator, and the solvent flow rate. The absence of heating represents a positive environmental aspect, as it eliminates the need for fossil fuel consumption.
- Hydrodistillation: This method is used for extracting essential oils. It involves the contact of plant material with water, which, upon boiling, carries volatile compounds. Upon condensation, a heterogeneous mixture is formed, consisting of the essential oil and hydrosol (a by-product of the essential oil). The separation of these compounds is performed to obtain the essential oil. The Clevenger-type distillation apparatus is commonly used for this type of extraction. The main variable is the extraction time. The use of water is a benefit in the environmental aspect.
- PLE: This method involves subjecting the solvent to moderate temperatures (50–90 °C) and moderate to high pressures (2–20 MPa) to extract desired metabolites from the plant material. The method is considered a green technology as it uses water or organic solvents such as ethanol. The main variables are temperature, pressure, solvent/biomass ratio (g/g), and extraction time. This type of method is not recommended for extracting heat-sensitive compounds, as they may be degraded due to the temperature used in the process.
- UAE: This method involves the propagation of low-frequency mechanical waves where the plant material, mixed with the solvent, comes into contact with an ultrasonic probe. This probe ruptures the plant cell wall, enhancing contact with the solvent and facilitating the extraction of desired compounds. It is suitable for extracting heat-sensitive compounds. Low solvent consumption and extraction time are advantages, aligning with the principles of green chemistry and sustainable processing. The main variables include power (Watts) or energy density (Joules per cubic centimeter), pulse cycle (dimensionless), extraction time (minutes), and, in some cases, frequency (Hertz).
- SFE: In this method, the solvent is raised to temperatures and pressures above its critical point, which can be either liquids or gases. CO2 is the most commonly used solvent in this type of extraction because it is low-cost, non-toxic, and non-flammable. It is used in the extraction of polar or slightly polar compounds. SFE with supercritical CO2 is widely considered a green and safe method due to the non-toxic and non-flammable nature of the solvent. The main variables are temperature, pressure, solvent/biomass ratio (g/g), addition of polarity modifiers, and extraction time.
- MAE: The extraction occurs through uniform microwave heating. Extraction is carried out quickly and effectively due to the high pressure and temperature of the system. Compounds that are soluble in polar and non-polar solvents used in extraction can be recovered by this method. It offers advantages such as short extraction time and the possibility of extracting multiple samples simultaneously. The main variables are microwave power, irradiation time, extraction time, frequency, and solvent/biomass ratio (g/g).
- Turbolysis: The method involves extraction with agitation, reducing the size of the particles of the plant material used due to the high shear force applied in the process, leading to the rapid dissolution of the substances of interest. The method is indicated for obtaining water-soluble compounds. It offers advantages such as speed, efficiency, and simplicity of extraction, while its disadvantages include heat generation, filtration difficulties, and limitations in using materials of high hardness. The main variables are extraction time, temperature, and speed.
- MHG: This method is based on the combination of microwaves with hydrodiffusion, typically used for the extraction of essential oils and phenolic compounds. In the environmental aspect, the process occurs at atmospheric pressure and does not require the use of solvents. It offers advantages such as rapid extraction, making it a clean technology, and providing a high extraction yield. The main variables are power, frequency, and extraction time.
6. Bioactive Compounds Against Weeds in Organic Bean and Corn Farming
7. Plant Extracts for Weed Control in Organic Bean and Corn Farming
Formulation
8. Patents
9. Summary of Findings, Concluding Remarks, and Future Outlooks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CONAB Levantamento da Safra de Grãos 2024/2025. Available online: https://www.gov.br/conab/pt-br/assuntos/noticias/aviso-de-pauta-2013-9o-levantamento-da-safra-de-graos-2024-2025 (accessed on 16 June 2025).
- De Mastro, G.; El Mahdi, J.; Ruta, C. Bioherbicidal Potential of the Essential Oils from Mediterranean Lamiaceae for Weed Control in Organic Farming. Plants 2021, 10, 818. [Google Scholar] [CrossRef]
- Dolianitis, B.M.; Pfeifenberg, R.; Frescura, V.D.; Tres, M.V.; Zabot, G.L. Phytochemicals from Eucalyptus camaldulensis and Coleus barbatus Control eragrostis plana in Horticulture. Horticulturae 2025, 11, 291. [Google Scholar] [CrossRef]
- Lopes, A.M.; Ribeiro, L.K.; Cogo, M.R.D.M.; Frescura, L.M.; de Rosa, M.B.; Schulz, A.; Abaide, E.R.; Tres, M.V.; Zabot, G.L. Herbicidal Activity of Baccharis trimera Extract on Oryza sativa L. and Cyperus ferax. Agriculture 2025, 15, 1431. [Google Scholar] [CrossRef]
- Ody, L.P.; Santos, M.S.N.; Lopes, A.M.; Mazutti, M.A.; Tres, M.V.; Zabot, G.L. Review on biological and biochemical pesticides as a sustainable alternative in organic agriculture. Biocatal. Agric. Biotechnol. 2025, 67, 103655. [Google Scholar] [CrossRef]
- Ferreira, S.; Oliveira, F.; de Silva, F.G.; Teixeira, M.; Gonçalves, M.; Eugénio, R.; Damásio, H.; Gonçalves, J.M. Assessment of Factors Constraining Organic Farming Expansion in Lis Valley, Portugal. AgriEngineering 2020, 2, 111–127. [Google Scholar] [CrossRef]
- Choudhary, C.S.; Behera, B.; Raza, M.B.; Mrunalini, K.; Bhoi, T.K.; Kumar, M.L.; Nongmaithem, D.; Pradhan, S.; Song, B.; Kumar Das, T. Mechanisms of Allelopathic Interactions for Sustainable Weed Management. Rhizosphere 2023, 25, 100667. [Google Scholar] [CrossRef]
- Gil, G.; Casagrande, D.E.; Cortés, L.P.; Verschae, R. Why the low adoption of robotics in the farms? Challenges for the establishment of commercial agricultural robots. Smart Agric. Technol. 2023, 3, 100069. [Google Scholar] [CrossRef]
- Amaral, G.D.S.; Alcántara-de la Cruz, R.; Martinelli, R.; Junior, L.R.R.; de Carvalho, L.B.; de Azevedo, F.A.; de Silva, M.F.D.G.F. Occurrence of Multiple Glyphosate-Resistant Weeds in Brazilian Citrus Orchards. AgriEngineering 2023, 5, 1068–1078. [Google Scholar] [CrossRef]
- Hasan, M.; Mokhtar, A.S.; Mahmud, K.; Berahim, Z.; Rosli, A.M.; Hamdan, H.; Motmainna, M.; Saiful, A.-H.M. Physiological and Biochemical Responses of Selected Weed and Crop Species to the Plant-Based Bioherbicide WeedLock. Sci. Rep. 2022, 12, 19602. [Google Scholar] [CrossRef]
- Lopes, R.W.N.; Morais, E.M.; Lacerda, J.J.D.J.; da Silva Araújo, F.D. Bioherbicidal Potential of Plant Species with Allelopathic Effects on the Weed Bidens bipinnata L. Sci. Rep. 2022, 12, 13476. [Google Scholar]
- Silva, L.C.V.; da Silva Braulio, C.; de Jesus Correia, A.; Oliveira, A.S.; de Sousa, C.B.D.C.; Vieira, J.D.L.S.; Machado, J.P.; da Silva Novaes, A.P. Allelopathic Effect of Eucalyptus Foliar Extract on Germination of Seed of Tiririca (Cyperus rotundus L.). Braz. J. Anim. Environ. Res. 2021, 4, 1315–1320. [Google Scholar] [CrossRef]
- Ootani, M.A.; Dos Reis, M.R.; Cangussu, A.S.R.; Capone, A.; Fidelis, R.R.; Oliveira, W.; Barros, H.B.; Portella, A.C.F.; de Souza Aguiar, R.; Dos Santos, W.F. Phytotoxic Effects of Essential Oils in Controlling Weed Species Digitaria horizontalis and Cenchrus echinatus. Biocatal. Agric. Biotechnol. 2017, 12, 59–65. [Google Scholar] [CrossRef]
- Pannacci, E.; Masi, M.; Farneselli, M.; Tei, F. Evaluation of Mugwort (Artemisia vulgaris L.) Aqueous Extract as a Potential Bioherbicide to Control Amaranthus retroflexus L. in Maize. Agriculture 2020, 10, 642. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, S.; Spinozzi, E.; Sánchez-Moreiras, A.M.; López-González, D.; Ferrati, M.; Lucchini, G.; Maggi, F.; Petrelli, R.; Araniti, F. Investigating the Phytotoxic Potential of Carlina acaulis Essential Oil against the Weed Bidens pilosa through a Physiological and Metabolomic Approach. Ind. Crops Prod. 2023, 203, 117149. [Google Scholar] [CrossRef]
- Sharma, N.; Rayamajhi, M. Different Aspects of Weed Management in Maize (Zea mays L.): A Brief Review. Adv. Agric. 2022, 2022, 7960175. [Google Scholar] [CrossRef]
- Roberts, J.; Florentine, S.; Dilantha, F.W.G.; Kushan, T.U. Developments and Future Challenges in the Field of Bioherbicides for Weed Control: A Global Review. Plants 2022, 11, 2242. [Google Scholar] [CrossRef] [PubMed]
- Chaves Neto, J.R.; Mazutti, M.A.; Zabot, G.; Tres, M.V. Bioherbicidal Action of Phoma dimorpha Fermented Broth on Seeds and Plants of Senna obtusifolia. Pesq. Agropec. Trop. 2020, 50, e56894. [Google Scholar] [CrossRef]
- Ferreira, E.A.; Paiva, M.C.G.; Pereira, G.A.M.; Oliveira, M.C.; de Barros Silva, E. Phytosociology of weed plants in corn crop submitted to application of nitrogen fertilizer rates. Rev. Agric. Neotrop. 2019, 6, 109–116. [Google Scholar]
- Ahmad, Z.; Khan, S.M.; Abd-Allah, E.F.; Alqarawi, A.A.; Hashem, A. Weed Species Composition and Distribution Pattern in the Maize Crop under the Influence of Edaphic Factors and Farming Practices: A Case Study from Mardan, Pakistan. Saudi J. Biol. Sci. 2016, 23, 741–748. [Google Scholar] [CrossRef]
- Portugal, J.; Alves, P.L.C.A.; Lemos, L.B. Comparison of methods to determine the period prior to weed interference in bean plants with different types of growth habits. Planta Daninha 2014, 32, 719–726. [Google Scholar]
- Idziak, R.; Woznica, Z. Impact of tembotrione and flufenacet plus isoxaflutole application timings, rates, and adjuvant type on weeds and yield of maize. Chil. J. Agric. Res. 2014, 74, 129–134. [Google Scholar] [CrossRef]
- Poonpaiboonpipat, T.; Udomporn, P.; Umporn, S.; Montinee, T.; Patchanee, C.; Chamroon, L. Phytotoxic Effects of Essential Oil from Cymbopogon citratus and Its Physiological Mechanisms on Barnyardgrass (Echinochloa crus-galli). Ind. Crops Prod. 2013, 41, 403–407. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for Weed Control in Agricultural Systems. Crop Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Raniro, H.R.; Oliveira, F.; Araujo, J.O.; Christoffoleti, P.J. Broadcast nitrogen application can negatively affect maize leaf area index and grain yield components under weed competition. Farming Syst. 2023, 1, 100047. [Google Scholar] [CrossRef]
- Ramesh, K.; Rao, A.N.; Chauhan, B.S. Role of Crop Competition in Managing Weeds in Rice, Wheat, and Maize in India: A Review. Crop Prot. 2017, 95, 14–21. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Travaini, M.L.; Bajsa-Hirschel, J.; Svendsen, L.D.; Reichley, A.; Sosa, G.M.; Kim, S.J.; Tamang, P.; Meepagala, K.; Duke, S.O. Synthesis, Herbicidal Activity, and Structure-Activity Relationships of O-Alkyl Analogues of Khellin and Visnagin. J. Agric. Food Chem. 2023, 71, 14593–14603. [Google Scholar] [CrossRef] [PubMed]
- Travaini, M.L.; Sosa, G.M.; Ceccarelli, E.A.; Walter, H.; Cantrell, C.L.; Carrillo, N.J.; Dayan, F.E.; Meepagala, K.M.; Duke, S.O. Khellin and Visnagin, Furanochromones from Ammi visnaga (L.) Lam., as Potential Bioherbicides. J. Agric. Food Chem. 2016, 21, 9475–9487. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.L.S.; Teixeira, I.R.; Timossi, P.C.; Silvério, J.G.D.; Benett, C.G.S. Phytosociological survey of weed plants in intercrops of common beans and castor beans. Planta Daninha 2017, 35, e017162166. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic Agriculture in the Twenty-First Century. Nat. Plants 2016, 2, 15221. [Google Scholar] [CrossRef]
- Yaseen, A.; Mama, S. Research comparing the nutritional content of organic and conventionally grown fruits and vegetables in relation to human health: Review. Org. Agric. 2024, 14, 481–502. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Van Acker, R.; Grohs, R.; Riddle, R. Improved herbicide efficacy for organically grown vegetables. Org. Agric. 2015, 5, 315–322. [Google Scholar] [CrossRef]
- Lu, H.-L.; Chang, Y.-H.; Wu, B.-Y. The compare organic farm and conventional farm to improve sustainable agriculture, ecosystems, and environment. Org. Agric. 2020, 10, 409–418. [Google Scholar] [CrossRef]
- Islam, A.K.M.M.; Karim, S.M.R.; Kheya, S.A.; Yeasmin, S. Unlocking the potential of bioherbicides for sustainable and environment friendly weed management. Heliyon 2024, 10, e36088. [Google Scholar] [CrossRef]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.P. Bioherbicides: Dead in the Water? A Review of the Existing Products for Integrated Weed Management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- Bibi, G.; Nayyar, G.B.; Ajmal, M.; Mehak, A.; Seerat, W.; Shahbaz, M.; Mukhtar, T.; Akram, A. Effect of Culture Filtrates of Alternaria alternata on Seed Germination and Seedling Growth of Sesame. Arch. Phytopathol. Plant Prot. 2023, 56, 625–635. [Google Scholar] [CrossRef]
- Maldaner, J.; Oliveira, M.N.; Santos, D.D.A.; Silva, S.Y.S.; Da Cruz Silva, S.; Lima, T.D.C.; Da Silva, M.L.; Silva, H.T.L.; Siqueira-Silva, D.H.; kist Steffen, G.P.; et al. Bioherbicide and Anesthetic Potential of Aniba canelilla Essential Oil, a Contribution to the Demands of the Agricultural Sector. Biocatal. Agric. Biotechnol. 2022, 42, 102353. [Google Scholar] [CrossRef]
- Marques, M.E.M.; De Carvalho, A.C.; Yendo, A.C.A.; Magedans, Y.V.S.; Zachert, E.; Fett-Neto, A.G. Phytotoxicity of Quillaja lancifolia Leaf Saponins and their Bioherbicide Potential. Plants 2023, 12, 663. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd Allah, E.F. Bioherbicides: Current Knowledge on Weed Control Mechanism. Ecotoxicol. Environ. Saf. 2018, 158, 131–138. [Google Scholar] [CrossRef]
- Lopes, A.M.; Ribeiro, L.K.; Cogo, M.R.D.M.; Frescura, L.M.; de Rosa, M.B.; Schulz, A.; Mayer, F.D.; Abaide, E.R.; Tres, M.V.; Zabot, G.L. Ricinus communis L. Leaf Extracts as a Sustainable Alternative for Weed Management. Sustainability 2025, 17, 6942. [Google Scholar] [CrossRef]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 331. [Google Scholar] [CrossRef]
- Andreani Junior, R.; Otero, M.; Silva, M. Effect of plant extracts aqueous on the weed germination. Encicl. Biosf. 2018, 15, 188. [Google Scholar]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxic Effects of Commercial Eucalyptus citriodora, Lavandula angustifolia, and Pinus sylvestris Essential Oils on Weeds, Crops, and Invasive Species. Molecules 2019, 24, 2847. [Google Scholar] [CrossRef]
- Sarić-Krsmanović, M.; Gajić Umiljendić, J.; Radivojević, L.; Šantrić, L.; Đorđević, T.; Đurović-Pejčev, R. Sensitivity of Cuscuta species and their hosts to Anethum graveolens essential oil. Pestic. I Fitomed. = Pestic. Phytomed. 2023, 38, 33–39. [Google Scholar] [CrossRef]
- Rueda-Ayala, V.; Ramos-Guerrero, L.; Vargas-Jentzsch, P.; Hernández, B.; Höglind, M.; Toscano, I.; Borja, D.; Goetschel, I.; Andújar, D. Allelopathic Properties of Calliandra haematocephala Hassk. Extracts and Fractions as an Alternative for Weed Management in Quinoa and Rice Crops. Acta Physiol. Plant. 2020, 42, 53. [Google Scholar] [CrossRef]
- Mekky, M.S.; Hassanien, A.M.A.; Kamel, E.M.; Ismail, A.E.A. llelopathic Effect of Ocimum basilicum L. Extracts on Weeds and Some Crops and Its Possible Use as New Crude Bio-Herbicide. Ann. Agric. Sci. 2019, 64, 211–221. [Google Scholar] [CrossRef]
- Morra, M.J.; Popova, I.E.; Boydston, R.A. Bioherbicidal Activity of Sinapis alba Seed Meal Extracts. Ind. Crops Prod. 2018, 115, 174–181. [Google Scholar] [CrossRef]
- Scavo, A.; Pandino, G.; Restuccia, A.; Mauromicale, G. Leaf Extracts of Cultivated Cardoon as Potential Bioherbicide. Sci. Horticult. 2020, 261, 109024. [Google Scholar] [CrossRef]
- Tiwari, P.; Kumar, B.; Kaur, M.; Kaur, G.; Kaur, H. Phytochemical screening and Extraction: A Review. Int. Pharm. Sci. 2011, 1, 98–106. [Google Scholar]
- Jintawiwat, R.; Punamorntarakul, N.; Hirunyasiri, R.; Jarupoom, P.; Pankasemsuk, T.; Supasin, S.; Kawee-ai, A. Testing the Efficacy of a Prototype That Combines Ultrasound and Pulsed Electric Field for Extracting Valuable Compounds from Mitragyna speciosa Leaves. AgriEngineering 2023, 5, 1879–1892. [Google Scholar] [CrossRef]
- Confortin, T.C.; Todero, I.; Luft, L.; Ugalde, G.A.; Mazutti, M.A.; Oliveira, Z.B.; Bottega, E.L.; Knies, A.E.; Zabot, G.L.; Tres, M.V. Oil yields, protein contents, and cost of manufacturing of oil obtained from different hybrids and sowing dates of canola. J. Environ. Chem. Eng. 2019, 7, 102972. [Google Scholar] [CrossRef]
- Tăbărașu, A.-M.; Nenciu, F.; Anghelache, D.-N.; Vlăduț, V.-N.; Găgeanu, I. Hybrid Percolation–Ultrasound Method for Extracting Bioactive Compounds from Urtica dioica and Salvia officinalis. Agriculture 2024, 14, 1561. [Google Scholar] [CrossRef]
- Confortin, T.C.; Todero, I.; Luft, L.; Schmaltz, S.; Ferreira, D.F.; Barin, J.S.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Extraction of bioactive compounds from Senecio brasiliensis using emergent technologies. 3 Biotech 2021, 11, 284. [Google Scholar] [CrossRef]
- Farias, C.A.A.; Camponogara, J.A.; Dos Reis, A.R.; Schlesner, S.K.; Zabot, G.L.; De Moraes, D.P.; Bettio, L.; Schmiele, M.; Barin, J.S.; Ballus, C.A.; et al. Combined use of microwaves in the simultaneous production of dehydrated blueberries and aqueous extract. Food Chem. 2025, 486, 144606. [Google Scholar] [CrossRef]
- Limberis, J.D.; Metcalfe, J.Z. Turbolysis: A low-cost, small footprint alternative to commercial bead beaters for cell lysis. HardwareX 2024, 19, e00576. [Google Scholar] [CrossRef]
- Farias, C.A.A.; Moraes, D.P.; Lazzaretti, M.; Ferreira, D.F.; Zabot, G.L.; Barin, J.S.; Ballus, C.A.; Barcia, M.T. Microwave hydrodiffusion and gravity as pretreatment for grape dehydration with simultaneous obtaining of high phenolic grape extract. Food Chem. 2021, 337, 127723. [Google Scholar] [CrossRef]
- Kanatas, P. Potential Role of Eucalyptus Spp. and Acacia Spp. Allelochemicals in Weed Management. Chil. J. Agric. Res. 2020, 80, 452–458. [Google Scholar] [CrossRef]
- Razavi, S.M.; Badihi, M.; Nasrollahi, P. Inhibitory Potential of (-)-Carvone and Carvone-PLGA Composite on Plant Pathogens and Common Weeds. Arch. Phytopathol. Plant Prot. 2022, 55, 926–936. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Karabörklü, S.; Bozok, F.; Ates, B.; Erdogan, S.; Menderes, C.; Karaaslan, M.G. Chemical Composition, Antimicrobial, Insecticidal, Phytotoxic and Antioxidant Activities of Mediterranean Pinus brutia and Pinus pinea Resin Essential Oils. Chin. J. Nat. Med. 2014, 12, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Hosni, K.; Hassen, I.; Sebei, H.; Casabianca, H. Secondary metabolites from Chrysanthemum coronarium (Garland) flowerheads: Chemical composition and biological activities. Ind. Crops Prod. 2013, 44, 263–271. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Khorram, M. Formulation and Assessment of Nano-Encapsulated Bioherbicides Based on Biopolymers and Essential Oil. Ind. Crops Prod. 2020, 149, 112348. [Google Scholar] [CrossRef]
- Kaab, S.B.; Rebey, I.B.; Hanafi, M.; Hammi, K.M.; Smaoui, A.; Fauconnier, M.L.; Clerck, C.D.; Jijakli, M.H.; Ksouri, R. Screening of Tunisian Plant Extracts for Herbicidal Activity and Formulation of a Bioherbicide Based on Cynara cardunculus. S. Afr. J. Bot. 2020, 128, 67–76. [Google Scholar] [CrossRef]
- Scavo, A.; Rial, C.; Molinillo, J.M.G.; Varela, R.M.; Mauromicale, G.; Macias, F.A. The Extraction Procedure Improves the Allelopathic Activity of Cardoon (Cynara cardunculus Var. Altilis) Leaf Allelochemicals. Ind. Crops Prod. 2019, 128, 479–487. [Google Scholar] [CrossRef]
- Kaur, P.; Gupta, S.; Kaur, K.; Kaur, N.; Kumar, R.; Bhullar, M.S. Nanoemulsion of Foeniculum vulgare Essential Oil: A Propitious Striver against Weeds of Triticum aestivum. Ind. Crops Prod. 2021, 168, 113601. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.; El Gendy, A.E.N.; El-Amier, Y.; Gaara, A.; Omer, E.; Al-Rowaily, S.; Assaeed, A.; Al-Rashed, S.; Elshamy, A. Essential Oil of Bassia muricata: Chemical Characterization, Antioxidant Activity, and Allelopathic Effect on the Weed Chenopodium murale. Saudi J. Biol. Sci. 2020, 27, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Algandaby, M.M.; El-Darier, S.M. Management of the Noxious Weed; Medicago polymorpha L. via Allelopathy of Some Medicinal Plants from Taif Region, Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El-Amier, Y.A.; El Gendy, A.E.N.G.; Al-Barati, S.A.; Dar, B.A.; Al-Rowaily, S.L.; Assaeed, A.M. Chemical Composition Variations, Allelopathic, and Antioxidant Activities of Symphyotrichum squamatum (Spreng.) Nesom Essential Oils Growing in Heterogeneous Habitats. Arab. J. Chem. 2020, 13, 4237–4245. [Google Scholar] [CrossRef]
- Ladhari, A.; Gaaliche, B.; Zarrelli, A.; Ghannem, M.; Mimoun, M.B. Allelopathic Potential and Phenolic Allelochemicals Discrepancies in Ficus carica L. Cultivars. S. Afr. J. Bot. 2020, 130, 30–44. [Google Scholar] [CrossRef]
- Araniti, F.; Landi, M.; Lupini, A.; Sunseri, F.; Guidi, L.; Abenavoli, M.R. Origanum vulgare Essential Oils Inhibit Glutamate and Aspartate Metabolism Altering the Photorespiratory Pathway in Arabidopsis thaliana Seedlings. J. Plant Physiol. 2018, 231, 297–309. [Google Scholar] [CrossRef]
- Hamdi, A.; Majouli, K.; Heyden, Y.V.; Flamini, G.; Marzouk, Z. Phytotoxic Activities of Essential Oils and Hydrosols of Haplophyllum tuberculatum. Ind. Crops Prod. 2017, 97, 440–447. [Google Scholar] [CrossRef]
- Feng, G.; Chen, M.; Ye, H.C.; Zhang, Z.K.; Li, H.; Chen, L.L.; Chen, X.L.; Yan, C.; Zhang, J. Herbicidal Activities of Compounds Isolated from the Medicinal Plant Piper sarmentosum. Ind. Crops Prod. 2019, 132, 41–47. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; El-Amier, Y.A.; Assaeed, A.M.S.; Al-Rowaily, S.L. Interspecific Variations in the Habitats of Reichardia tingitana (L.) Roth Leading to Changes in Its Bioactive Constituents and Allelopathic Activity. Saudi J. Biol. Sci. 2020, 27, 489–499. [Google Scholar] [CrossRef]
- Koodkaew, I.; Senaphan, C.; Sengseang, N.; Suwanwong, S. Characterization of Phytochemical Profile and Phytotoxic Activity of Mimosa pigra L. Agric. Nat. Resour. 2018, 52, 162–168. [Google Scholar]
- Anese, S.; Jatobá, L.J.; Grisi, P.U.; Gualtieri, S.C.J.; Santos, M.F.C.; Berlinck, R.G.S. Bioherbicidal Activity of Drimane Sesquiterpenes from Drimys brasiliensis Miers Roots. Ind. Crops Prod. 2015, 74, 28–35. [Google Scholar] [CrossRef]
- Lim, C.J.; Basri, M.; Ee, L.; Cheng, G.; Dzolkhifli, O. Phytoinhibitory Activities and Extraction Optimization of Potent Invasive Plants as Eco-Friendly Weed Suppressant against Echinochloa colona (L.) Link. Ind. Crops Prod. 2017, 100, 19–34. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; Souto, X.C.; Pedrol, N. Water-soluble phenolic acids and flavonoids involved in the bioherbicidal potential of Ulex europaeus and Cytisus scoparius. S. Afr. J. Bot. 2020, 133, 201–211. [Google Scholar] [CrossRef]
- Soto-Maldonado, C.; Caballero-Valdés, E.; Santis-Bernal, J.; Jara-Quezada, J.; Fuentes-Viveros, L.; Zúñiga-Hansen, M.E. Potential of Solid Wastes from the Walnut Industry: Extraction Conditions to Evaluate the Antioxidant and Bioherbicidal Activities. Electron. J. Biotechnol. 2022, 58, 25–36. [Google Scholar] [CrossRef]
- Semerdjieva, I.; Atanasova, D.; Maneva, V.; Zheljazkov, V.; Radoukova, T.; Astatkie, T.; Dincheva, I. Allelopathic Effects of Juniper Essential Oils on Seed Germination and Seedling Growth of Some Weed Seeds. Ind. Crops Prod. 2022, 180, 114768. [Google Scholar] [CrossRef]
- El-Kenany, E.T.; El-Darier, S.M. Suppression Effects of Lantana camara L. Aqueous Extracts on Germination Efficiency of Phalaris minor Retz. and Sorghum bicolor L. (Moench). J. Taibah Univ. Sci. 2013, 7, 64–71. [Google Scholar] [CrossRef]
- Tubeileh, A.M.; Souikane, R.T. Effect of Olive Vegetation Water and Compost Extracts on Seed Germination of Four Weed Species. Curr. Plant Biol. 2020, 22, 100150. [Google Scholar] [CrossRef]
- Kueh, B.W.B.; Yusuo, S.; Osman, N.; Ramli, N.H. Analysis of Melaleuca cajuputi Extract as the Potential Herbicides for Paddy Weeds. Sustain. Chem. Pharm. 2019, 11, 36–40. [Google Scholar] [CrossRef]
- Rhioui, W.; Figuigui, J.A.; Boutagayout, A.; Zouhar, M.; Belmalha, S. Effects of organic and inorganic mulching, nettle extract, and manual weeding on weed management under direct-seeded lentil in Meknes region, Morocco. Crop Prot. 2023, 173, 106376. [Google Scholar] [CrossRef]
- Puig, C.; Valencia, F.; Pardo Muras, M.; Souto, C.; Guinjuan, J.; Pedrol, N. Predictive phytotoxic value of water-soluble allelochemicals in plant extracts for choosing a cover crop or mulch for specific weed control. Ital. J. Agron. 2021, 16, 1872. [Google Scholar] [CrossRef]
- Ullah, H.; Naeem, K.; Khan, I.A. Complementing cultural weed control with plant allelopathy: Implications for improved weed management in wheat crop. Acta Ecol. Sin. 2023, 43, 27–33. [Google Scholar] [CrossRef]
- Alam, T.; Chaudhry, A.; Ahmad, M.; Aziz, R.; Ahmad, R. Terpenes and phenolics in alcoholic extracts of pine needles exhibit biocontrol of weeds (Melilotus albus and Asphodelus tenuifolius) and insect-pest (Plutella xylostella). J. King Saud. Univ. Sci. 2022, 34, 101913. [Google Scholar] [CrossRef]
- Siyyar, S.; Sarim, F.M.; Majeed, A. Unveiling the allelopathic potential of common weeds extracts for effective management of Parthenium hysterophorus. Ecol. Front. 2025, 45, 362–371. [Google Scholar] [CrossRef]
- Boukhalfa, R.; Ruta, C.; Messgo-Moumene, S.; Calabrese, G.J.; Argentieri, M.P.; De Mastro, G. Assessment of the bioherbicidal potential of Thymus sp. pl. essential oils in weed control. Ind. Crops Prod. 2025, 227, 120856. [Google Scholar] [CrossRef]
- Kostina-Bednarz, M.; Płonka, J.; Barchanska, H. Allelopathy as a source of bioherbicides: Challenges and prospects for sustainable agriculture. Rev. Environ. Sci. Bio/Technol. 2023, 22, 471–504. [Google Scholar] [CrossRef]
- Almeida, T.C.; Spannemberg, S.S.; Brun, T.; Schmaltz, S.; Escobar, O.; Sanchotene, D.M.; Dornelles, S.H.B.; Zabot, G.L.; Tres, M.V.; Kuhn, R.C.; et al. Development of a Solid Bioherbicide Formulation by Spray Drying Technology. Agriculture 2020, 10, 215. [Google Scholar] [CrossRef]
- Duke, S.O.; Twitty, A.; Baker, C.; Sands, D.; Boddy, L.; Travaini, M.L.; Sosa, G.; Polidore, A.L.; Jhala, A.J.; Kloeber, J.M.; et al. New approaches to herbicide and bioherbicide discovery. Weed Sci. 2024, 72, 444–464. [Google Scholar] [CrossRef]
- Hasan, M.; Mokhtar, A.S.; Motmainna, M.; Ahmad-Hamdani, M.S. Development of a nanoemulsion bioherbicide and its herbicidal efficacy. Pest Manag. Sci. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Sabarivasan, R.; Murali Arthanari, P. Application of nanoencapsulation technology in agriculture for effective and sustainable weed management: A critical review. Com. Soil. Sci. Plant Anal. 2025, 56, 277–291. [Google Scholar] [CrossRef]
- Vindas-Reyes, E.; Chacón-Cerdas, R.; Rivera-Méndez, W. Trichoderma Production and Encapsulation Methods for Agricultural Applications. AgriEngineering 2024, 6, 2366–2384. [Google Scholar] [CrossRef]
- Bastos, B.O.; Deobald, G.A.; Brun, T.; Prá, V.D.; Junges, E.; Kuhn, R.C.; Pinto, A.K.; Mazutti, M.A. Solid-state fermentation for production of a bioherbicide from Diaporthe sp. and its formulation to enhance the efficacy. 3 Biotech 2017, 3, 135. [Google Scholar] [CrossRef] [PubMed]
- Sobiech, Ł.; Grzanka, M.; Skrzypczak, G.; Idziak, R.; Włodarczak, S.; Ochowiak, M. Effect of Adjuvants and pH Adjuster on the Efficacy of Sulcotrione Herbicide. Agronomy 2020, 10, 530. [Google Scholar] [CrossRef]
- Todero, I.; Confortin, T.C.; Luft, L.; Brun, T.; Ugalde, G.A.; Almeida, T.C.; Arnemann, J.A.; Zabot, G.L.; Mazutti, M.A. Formulation of a bioherbicide with metabolites from Phoma sp. Sci. Horticult. 2018, 241, 285–292. [Google Scholar] [CrossRef]
- Weston, L.A.; Bertin, C.; Schroeder, F. Bioherbicide from Festuca spp. U.S. Patent US8461085B2, 11 June 2013. [Google Scholar]
- Johannes, C.P. Use of an Aqueous Solution, Suspension or Mixture of the Same, Obtained from Air Parts and/or Parts Below the Tulbaghia violacea Soil and Agapanthus africanus Soil to Inhibit Fungic Infection of a Harvest Change. Patent BRPI0612708B1, 12 May 2013. [Google Scholar]
- Fernandez, L.; Campbell, B.; Koivunen, M.; Marrone, P.G.; Huang, H. A Natural Herbicide Containing Lemongrass Essential Oil. U.S. Patent WO2009049153A2, 16 April 2013. [Google Scholar]
- Marilis, D.M.; Lima, C.P.D.; Obdulio, G.M.; Silva, C.B.D.; Friedrich, S.; Hirota, B.C.K.; Dias, J.D.F.G.; Zanin, S.M.W. Allelopathic Activity of Components and Products from the Seeds of Euterpe edulis Martius, Arecaceae. Patent BR102012014233A2, 6 May 2014. [Google Scholar]
- Mathers, H.M.; Case, L.T. Natural Bioherbicides and Related Materials and Methods. U.S. Patent WO2014194260A1, 12 April 2014. [Google Scholar]
- University, Z.A.F. Natural Herbicide Containing Wood Tar Oil and Production Method Thereof. Patent CN107296060A, 27 October 2017. [Google Scholar]
- Caron, B.O.; Schmidt, D.; Souza, V.Q.D.; Elli, E.F.; Prochnow, D.; Baldisserotto, B.; Heinzmann, B.M. Use of Essential Oil from Aloysia triphylla and the Process of Controlling Dicotyledonous Plants and/or Seeds. Patent BR 10 2016 029366 9 B1, 17 July 2018. [Google Scholar]
- Fagundes, O.F. Process of Obtaining a Natural Herbicide for Spraying Without Harmful Effects on Human, Animal, and Plant Health. Patent BR 10 2018 016302 7 A2, 15 January 2019. [Google Scholar]
- Qi, B. Biological Herbicide for Extinguishing Ageratina adenophora. Patent 201910946784.X, 27 October 2019. [Google Scholar]
- Shao, H.; Han, C.X.; Zhang, C.; Zhou, S.X.; Wei, C.X.; Mei, Y.; Shi, K.; Cheng, Z.R.; Huang, L. Application of Ambrosia artemisiifolia Essential Oil as Herbicide. Patent 201910959911.X, 30 October 2019. [Google Scholar]
- Buonamici, G. Herbicide Comprising Plant Extracts. Patent WO/2020/049401, 12 March 2020. [Google Scholar]
- Herdener, C.A. Herbicide Compositions and Methods to Control Weeds in Organic Crop Production. Patent WO/2022/084933, 22 October 2021. [Google Scholar]
- Oro, V.; Savić, A.; Tabaković, M. Bioherbicide for Regweed Control and Method of Application. Patent 20220926, 22 September 2022. [Google Scholar]
- Liborio, G.A.; Budemberg, E.R.; Liborio, B.U. 100% Non-Toxic Natural Organic Natural Bioherbicide to Combat Undesired Vegetation on Plants. Patent BR102021008235A2, 8 November 2022. [Google Scholar]
- Brommer, C.L. Herbicidal Mentha pantsd, Extract Compositions and Methods of Using Same. U.S. Patent US11771095B2, 3 October 2023. [Google Scholar]
- Meschede, D.K. Herbicidal, Bioherbicidal or Herbicide Additive Compositions, Process for Producing the Compositions, Method for Controlling Weed Pests, and Use of Salicylic Acid and Plant Extract as a Bioherbicide. Patent BR 10 2023 016803 5, 29 August 2023. [Google Scholar]
- Zan, P.A. Composition of Contact Desiccant Bioherbicide. Patent BR 10 2023 023945 5 A2, 7 January 2025. [Google Scholar]


| Weed | Common Name | Family | Life Cycle | Morphological Group |
|---|---|---|---|---|
| Bidens pilosa | Blackjack | Asteraceae | Annual | Broadleaf |
| Digitaria spp. | Crabgrass | Poaceae | Annual | Grasses |
| Eleusine indica | Crowsfoot grass | Poaceae | Annual | Grasses |
| Amaranthus viridis | Caruru | Amaranthaceae | Annual | Broadleaf |
| Digitaria insularis | Sourgrass | Poaceae | Perennial | Grasses |
| Ipomoea purpurea | Morning-glory | Convolvulaceae | Annual | Broadleaf |
| Conyza bonariensis | Buva | Asteraceae | Annual | Broadleaf |
| Euphoria hirta | Saint Lucia grass | Commelinaceae | Annual | Broadleaf |
| Cenchrus echinatus | Crabgrass | Poaceae | Annual | Grasses |
| Cyperus rotundus | Tiririca | Cyperaceae | Perennial | Sedges |
| Amaranthus dubius | Thorn caruru | Amaranthaceae | Annual | Broadleaf |
| Parthenium hysterophos | Ragweed parthenium | Asteraceae | Annual | Broadleaf |
| Urochloa plantaginea | Alexandergrass | Poaceae | Annual | Grasses |
| Acanthospermum hispidum | Bristly starbur | Asteraceae | Annual | Broadleaf |
| Galinsoga parviflora | White Beggar | Asteraceae | Annual | Broadleaf |
| Urochloa decumbens | Brachiaria grass | Poaceae | Perennial | Grasses |
| Echinochloa colona | Jungle rice | Poaceae | Annual | Grasses |
| Ageratum conyzoides | St John’s wort | Asteraceae | Annual | Broadleaf |
| Senna obtusifolia | Forest-pasture | Fabaceae | Annual | Broadleaf |
| Portulaca oleracea | Purslane | Portulacaceae | Annual | Broadleaf |
| Sorghum halepense | Marsh grass | Poaceae | Perennial | Grasses |
| Alternanthea tenella | Joyweed | Amaranthaceae | Perennial | Broadleaf |
| Commelina benghalensis | Asiatic dayflower | Commelinaceae | Perennial | Broadleaf |
| Ambrosia artemisiifolia | Common ragweed | Asteraceae | Annual | Broadleaf |
| Spermacoce latifolia Aubl | Broadleaf Buttonweed | Rubiaceae | Annual | Broadleaf |
| Spermacoce verticillata | False buttonweed | Rubiaceae | Perennial | Broadleaf |
| Tridax procumbens | Coatbuttons | Asteraceae | Annual | Broadleaf |
| Cynodon dactylon | Coast-cross | Poaceae | Perennial | Grasses |
| Bioinput Name * | Effect | Active Ingredient | Main Target |
|---|---|---|---|
| Excellence Mig-66 | Bioinsecticide | Beauveria bassiana | Corn leafhopper |
| Bovenat | Bioinsecticide | Beauveria bassiana | Corn leafhopper |
| Isatrix | Bioinsecticide | Paecilomyces fumosoroseus | Corn leafhopper |
| Octane | Bioinsecticide | Isaria fumosorosea | Corn leafhopper |
| Canabovebio | Bioinsecticide | Beauveria bassiana, isolado IBCB 66 | Corn leafhopper |
| Bv-Bio WP | Bioinsecticide | Beauveria bassiana, isolado IBCB 66 | Corn leafhopper |
| Bravo | Bioinsecticide | Beauveria bassiana, isolado IBCB 66 | Corn leafhopper |
| BTP 078-20 | Bioinsecticide | Beauveria bassiana, isolado IBCB 66 | Euschistus heros |
| Tricobio SC | Biofungicide | Trichoderma harzianum | White rot |
| Lepthure | Bioinsecticide | Bacillus thuringiensis | Pod caterpillar |
| BTP 010-19A | Biofungicide | Bacillus pumilus + Bacillus subtilis + Bacillus velezensis | White rot |
| Crisobase-E | Bioinsecticide | Chrysoperla externa | Green cereal aphid |
| Vestix | Bioinsecticide | Beauveria bassiana | White fly |
| Bio-imune | Biofungicide | Bacillus subtilis | Anthracnose |
| Bio-imune | Biofungicide | Bacillus subtilis | Rust |
| Camperico | Bioherbicide | Xanthomonas campestris | Poa annua |
| Lubao | Bioherbicide | Colletotrichum gloeosporioides | Echinochloa crus-galli |
| Stumpout | Bioherbicide | Cylindrobasidium laeve | Poa annua |
| Phoma | Bioherbicide | Phoma macrostoma | Taraxacum officinale |
| Sarritor | Bioherbicide | Sclerotinia minor | Araxacum officeinale |
| NatureCur | Bioherbicide | Juglans nigra | Portulaca oleraceae Echinochloa crus-galli Portulaca oleraceae Conyza bonariensis Ipomoea purpurea |
| Bioweed | Bioherbicide | Pine oil | Nassella trichotoma |
| Avenger Organic | Bioherbicide | d-Limonene and castor oil | Grass and broadleaf weeds |
| Bialaphos | Bioherbicide | Streptomyces hygroscopicus | Broad-spectrum |
| GreenMatch | Bioherbicide | Lemon grass oil | Broadleaf and grassy weeds |
| Lockdown/Collego | Bioherbicide | Flumioxazin and Colletotrichum gloeosporioides | Residual control of various broadleaf weeds |
| Opportune | Bioherbicide | Streptomyces strain RL-110 T | Broadleaf and sedges |
| Weed Slayer | Bioherbicide | Eugenol, clove oil, molasses | Grassy weeds |
| Stump out | Bioherbicide | Sodium bicarbonate and Cylindrobasidium leave | Poa species |
| Plant | Botanical Part | Weed | Effects | Ref. |
|---|---|---|---|---|
| Eucalyptus camaldulensis | Leaves | Bidens pilosa | Germination inhibition | [42] |
| Dipteryx lacunifera Ducke, Ricinus communis L., Piper tuberculatum, and Jatropha gossypiifolia L. | Leaves | Bidens bipinnata L. | Germination inhibition and reduction in seedling growth | [11] |
| Eucalyptus citriodora, Lavandula angustifolia, and Pinus sylvestris | Leaves | Portulaca oleracea flowers and needles | Germination inhibition and reduction in seedling growth | [43] |
| Eucalyptus citriodora and Cymbopogon nardus | - | Digitaria horizontalis and Cenchrus echinatus | Reduction in dry mass accumulation and reduction in chlorophyll content | [13] |
| Artemisia vulgaris L. | - | Amaranthus retroflexus | Inhibition of seed germination, seedling emergence, and plant growth of redroot pigweed | [14] |
| Cuscuta campestris | Stalk | Amaranthus retroflexus | Germination inhibition and reduction in seedling growth | [44] |
| Eucalyptus camaldulensis | - | Digitaria insularis | Germination inhibition | [42] |
| Calliandra haematocephala | Leaves and inflorescences | Echinochloa crus-galli | Germination inhibition | [45] |
| Eucalyptus grandis | Leaves | Cyperus rotundus | Germination inhibition | [12] |
| Carlina acaulis | Roots | Bidens pilosa L. | Leaf necrosis and interference with photosynthesis | [15] |
| Ocimum basilicum L. | Leaves and flowers | Amaranthus spp. and Portulaca oleraceae | Germination and growth inhibition, and a reduction in dry weight and length of roots | [46] |
| Sinapis alba L. | Seeds | Amaranthus powellii and Setaria viridis | Germination inhibition, necroses, and reduction in plant height | [47] |
| Cynara cardunculus L. var. altilis DC | Leaves | Amaranthus retroflexus L., Portulaca oleracea L., and Stellaria media | Inhibition of germination | [48] |
| Cymbopogon citratus | Leaves | Echinochloa crus-galli | Inhibition of seed germination and seedling growth, reduction in chlorophyll, interference with seed amylase activity, and leaf wilting | [23] |
| Compound | Plants | Mechanisms of Action | Ref. |
|---|---|---|---|
| Carvacrol | Thymus algeriensis and Origanum vulgare | They disrupt the cell membrane and influence metabolic pathways, photosynthesis, cellular respiration, and mitosis | [41] |
| α-Terpinene | Origanum onites and Origanum vulgare | ||
| Thymol | Thymus algeriensis | ||
| Eugenol | Syzygium aromaticum and Eugenia caryophyllus | It causes damage to cell membranes and inhibits photosynthesis | |
| Geranial | Ocimum basilicum | It interferes with germination and growth | |
| Monoterpenes and sesquiterpenes | Artemisia scoparia Waldst et Kit | They inhibit growth, cellular respiration, and the occurrence of chlorosis and necrosis | [39] |
| Rocaglaol | Aglaia odorata Lour. | It inhibits the growth of weeds | |
| Drimane sesquiterpenes | Drimys brasiliensis Miers | They inhibit seed germination, seedling growth, and cell division of the metaxylem in roots | |
| α-Pinene and 1,8-cineole | Eucalyptus tereticornis | They inhibit the growth and vigor of seedlings, respiration, and pigment synthesis | |
| Carvone | Genera of the Lamiaceae family | It inhibits germination, and radicle and pedicle lengths | [58] |
| Flavonoids and terpenoids | Artemisia vulgaris L. | They inhibit seed germination, seedling emergence, and plant growth of redroot pigweed | [14] |
| Citations | Year | Title | Affiliation Country | Plant | Weed | Main Findings | Ref. |
|---|---|---|---|---|---|---|---|
| 87 | 2014 | Chemical composition, antimicrobial, insecticidal, phytotoxic, and antioxidant activities of Mediterranean Pinus brutia and Pinus pinea resin essential oils | Turkey | Pinus pinea and Pinus brutia | Portulaca oleracea L. | The highest dose of the essential oils of P. brutia and P. pinea caused inhibitory effects on the germination of P. oleracea by 13% and 3%, respectively | [59] |
| 79 | 2013 | Secondary metabolites from Chrysanthemum coronarium (Garland) flowerheads: Chemical composition and biological activities | Tunisia and France | Chrysanthemum coronarium | Sinapis arvensise Phalaris canariensis | The essential oil showed good antimicrobial activity against B. aereus and S. aureus; the extract exhibited phytotoxic effects | [60] |
| 69 | 2020 | Formulation and assessment of nano-encapsulated bioherbicides based on biopolymers and essential oil | Iran | Satureja hortensis L. | Amaranthus retroflexus L. | The encapsulation of essential oil with biopolymers and natural crosslinkers was effective and increased its herbicidal activity compared to the non-nanometric essential oil emulsion without polymer | [61] |
| 65 | 2020 | Screening of Tunisian plant extracts for herbicidal activity and formulation of a bioherbicide based on Cynara cardunculus | Tunisia | L. guyonianum, P. harmala, R. chalepensis, R. communis, N. retusa, C. cardunculus, A. herba-alba, M. edule, T. gallica, and D. stramonium | Trifolium incarnatum, Silybum marianum, and Phalaris minor | C. cardunculus was the only plant that showed bioherbicidal potential in both pre- and post-emergence weed control | [62] |
| 57 | 2020 | Leaf extracts of cultivated cardoon as potential bioherbicide | Italy | Cynara cardunculus L. var. altilis DC. | Amaranthus retroflexus L., Portulaca oleracea L., Stellaria media (L.) Vill., and Anagallis arvensis L. | Significant allelopathic effect on the germination of weed seeds, with extracts from dried leaves and ethanol being the most effective | [48] |
| 55 | 2021 | Chemical composition and phytotoxicity of essential oil from invasive plant Ambrosia artemisiifolia L. | China | Ambrosia artemisiifolia | Poa annua, Setaria viridis, Amaranthus retroflexus, and Medicago sativa | The essential oil of A. artemisiifolia inhibited seed germination and seedling development in the weeds, even at low concentrations | [63] |
| 47 | 2021 | Nanoemulsion of Foeniculum vulgare essential oil: A propitious striver against weeds of Triticum aestivum | India | Foeniculum vulgare Mill | Phalaris minor Retz., Avena ludoviciana Durieu, Rumex dentatus L., and Medicago denticulata | F. vulgare showed bioherbicidal potential, being effective even at low doses | [64] |
| 43 | 2020 | Essential oil of Bassia muricata: Chemical characterization, antioxidant activity, and allelopathic effect on the weed Chenopodium murale | Egypt and Saudi Arabia | Bassia muricata | Chenopodium murale | The essential oil of B. muricata significantly reduced the germination and seedling development of the weed Chenopodium murale | [65] |
| 41 | 2018 | Management of the noxious weed; Medicago polymorpha L. via allelopathy of some medicinal plants from Taif region, Saudi Arabia | Saudi Arabia | Achillea santolina L., Pituranthus tortuosus L., and Thymus capitatus L. | Medicago polymorpha L. | The allelopathic potential of the species on Medicago polymorpha L. was confirmed; the species with the highest allelopathic potential were A. monosperma, T. capitatus, and A. santolina | [66] |
| 39 | 2020 | Chemical composition variations, allelopathic, and antioxidant activities of Symphyotrichum squamatum (Spreng.) Nesom essential oils growing in heterogeneous habitats | Egypt | Symphyotrichum squamatum | Bidens pilosa | Significant allelopathic activity against the root and shoot growth of Bidens pilosa; the inhibition was dose-dependent, with the root system of B. pilosa being more inhibited than the shoot system | [67] |
| 38 | 2017 | Phytotoxic effects of essential oils in controlling weed species Digitaria horizontalis and Cenchrus echinatus | Brazil | Eucalyptus citriodora and Cympobogon nardus | Digitaria horizontalis and Cenchrus echinatus | Strong phytotoxic effects on seed germination, plant development, and reduction in chlorophyll and protein content | [13] |
| 37 | 2018 | Bioherbicidal activity of Sinapis alba seed meal extracts | USA | Sinapis alba | Amaranthus powellii and Setaria viridis | The powder from extracts of S. alba containing SCN- as the active ingredient can be reconstituted in water and applied as a spray in the form of a bioherbicide in pre- and post-emergence | [47] |
| 37 | 2020 | Allelopathic potential and phenolic allelochemicals discrepancies in Ficus carica L. cultivars | Tunisia | Ficus carica L. cultivars | Peganum harmala L. and Silybum marianum L. | Leaf extracts were more toxic and significantly influenced seedling elongation compared to branch extracts | [68] |
| 36 | 2018 | Origanum vulgare essential oils inhibit glutamate and aspartate metabolism altering the photorespiratory pathway in Arabidopsis thaliana seedlings | Italy | Origunum vulgare | Arabidopsis thaliana | Interference in glutamine metabolism, excessive accumulation of toxic inorganic nitrogen in the leaves associated with oxidative stress and damage, and decreased efficiency of the photosynthetic apparatus associated with reduced CO2 fixation | [69] |
| 32 | 2017 | Phytotoxic activities of essential oils and hydrosols of Haplophyllum tuberculatum | Tunisia, Belgium, and Italy | Haplophyllum tuberculatum | Raphanus sativus L. | Essential oils from H. tuberculatum have a significant phytotoxic effect on the species | [70] |
| 28 | 2019 | Herbicidal activities of compounds isolated from the medicinal plant Piper sarmentosum | China | Piper sarmentosum | Echinochloa crus-galli, Digitaria sanguinalis, Poa annua L., Eleusine indica (L.), Echinochloa phyllopogon, and Chloris virgata | Sarmentosin could effectively control E. crusgalli, Chloris virgata, Pharbitis nil, A. retroflexus, and Abutilon theophrasti | [71] |
| 27 | 2020 | Interspecific variations in the habitats of Reichardia tingitana (L.) Roth leading to changes in its bioactive constituents and allelopathic activity | Egypt and Saudi Arabia | Reichardia tingitana Roth | Amaranthus lividius and Chenopodium murale | The methanolic extract of R. tingitana exhibited significant allelopathic activity against Chenopodium and Amaranthus, where germination was completely inhibited at concentrations of 75 mg L−1 and 50 mg L−1 | [72] |
| 27 | 2018 | Characterization of phytochemical profile and phytotoxic activity of Mimosa pigra L. | Thailand | Mimosa pigra L. | Ruellia tuberosa L. | Root growth of the plants was inhibited in a concentration-independent manner, significantly inhibiting mitosis | [73] |
| 25 | 2015 | Bioherbicidal activity of drimane sesquiterpenes from Drimys brasiliensis Miers roots | Brazil | Drimys brasiliensis Miers | Barbarea verna (Mill.), Echinochloa crus-galli (L.), and Ipomoea grandifolia | The sesquiterpenes identified as polygodial, polygodial acetal, dendocarbina L., and (+)-fuegin presented the highest levels of activity at low concentrations on all target species | [74] |
| 25 | 2017 | Phytoinhibitory activities and extraction optimization of potent invasive plants as eco-friendly weed suppressant against Echinochloa colona (L.) | Malaysia | Ageratum conyzoides (L.), Asystasia gangetica (L.), Clidemia hirta (L.), Dicranopteris linearis, Imperata cylindrica (L.) Melastoma malabathricum (L.), Mikania micrantha, Ottochloa nodosa, and Pennisetum polystachion | Echinochloa colona (L.) | Leaf extracts of M. micrantha, C. hirta, D. lienaris, and A. conyzoides promoted the highest inhibitory activities | [75] |
| 24 | 2020 | Water-soluble phenolic acids and flavonoids involved in the bioherbicidal potential of Ulex europaeus and Cytisus scoparius | Spain | Ulex europaeus and Cytisus scoparius | Amaranthus retroflexus and Digitaria sanguinalis | Eleven and seventeen phenolic compounds were identified in the aqueous extracts of U. europaeus and C. scoparius, respectively | [76] |
| 22 | 2022 | Potential of solid wastes from the walnut industry: Extraction conditions to evaluate the antioxidant and bioherbicidal activities | Chile | Juglans regia | Broadleaf and narrowleaf weeds | High presence of phenolic compounds with antioxidant activity, which can be effectively recovered using ethanol | [77] |
| 21 | 2022 | Allelopathic effects of Juniper essential oils on seed germination and seedling growth of some weed seeds | Bulgaria | Juniperus sabina L. and J. excelsa Bieb. | Melilotus officinalis L., Trigonella besseriana Ser., and Myosotis arvensis (L.) Hill. | The essential oils exhibited inhibitory effects on weed seeds | [78] |
| 20 | 2013 | Suppression effects of Lantana camara L. aqueous extracts on germination efficiency of Phalaris minor Retz. and Sorghum bicolor L. (Moench) | Egypt | Lantana camara L. | Phalaris minor Retz. | The inhibitory effect on the percentage of germination and germination index of seeds from the weed species was proportional to the concentration of the extract | [79] |
| 16 | 2020 | Effect of olive vegetation water and compost extracts on seed germination of four weed species | USA | Olea europaea L. | Amaranthus retroflexus L., Malva parviflora L., Portulaca oleracea L., and Sonchus oleraceus L. | Complete inhibition or delay in the germination of weed seeds during the first week after application | [80] |
| 15 | 2019 | Analysis of Melaleuca cajuputi extract as the potential herbicides for paddy weeds | Malaysia | Melaleuca cajuputi | Echinochloa crus-galli | The extract of Melaleuca cajuputi at a concentration of 0.05 M exhibited necrosis and chlorosis | [81] |
| 12 | 2023 | Effects of organic and inorganic mulching, nettle extract, and manual weeding on weed management under direct-seeded lentil in Meknes region, Morocco | Marocco | Urtica dioica L. | Weed plants of lentil | Germination inhibition of more than 50% of Glebionis coronaria (L.) seeds and more than 80% of Avena stirilis (L.) | [82] |
| 12 | 2021 | Predictive phytotoxic value of water-soluble a lelochemicals in plant extracts for choosing a cover crop or mulch for specific weed control | Spain | Bromus species mixture; Festuca arundinacea Schreb., Hordeum murinum L., H. vulgare L., Vulpia ciliata Dumort., Medicago rugosa Desr., M. sativa L., Trifolium subterraneum L., T. incarnatum L., Phacelia tanacetifolia Benth., Sinapis alba L., and Pinus sylvestris L. | Conyza bonariensis (L.) Cronquist, Aster squamatus (Spreng.) Hieron, and Bassia scoparia (L. A. J.) | Germination of A. squamatus and C. bonariensis was reduced by 80–100% by the extracts applied at 50% concentration and completely blocked at 100% concentration; C. bonariensis root growth showed only some tolerance to the crude extracts of F. arundinacea and P. sylvestris; Bassia scoparia was relatively tolerant to the aqueous plant extracts, except for T. subterraneum crude extract, which reduced total germination by 80%; B. scoparia showed higher general sensitivity of shoot growth than the other two weed species | [83] |
| 9 | 2023 | Complementing cultural weed control with plant allelopathy: Implications for improved weed management in wheat crop | Pakistan | Parthenium hysterophorus L., Sorghum halepense (L.) Pers., Helianthus annuus L., Triticum aestivum L., and Eucalyptus globulus | Phalaris minor Retz., Avena fatua L.; Poa annua L.; Fumaria indica L., Euphorbia helioscopia, and Chenopodium album L. | Weed density reduction, increasing wheat quality and production | [84] |
| 9 | 2022 | Terpenes and phenolics in alcoholic extracts of pine needles exhibit biocontrol of weeds (Melilotus albus and Asphodelus tenuifolius) and insect-pest (Plutella xylostella) | Pakistan | Pinus roxburghii | Melilotus albus and Asphodelus tenuifolius | Inhibition of the germination of weeds | [85] |
| 0 | 2025 | Unveiling the allelopathic potential of common weeds extracts for effective management of Parthenium hysterophorus | Pakistan | Parthenium hysterophorus | S. viridis, Chenopodium album, C. rotundus, E. helioscopia, R. dentatus, C. tinctorius, X. strumarium, E. bonariensis, P. australis, and I. cylindrica | The extracts inhibited the germination, shoot and root lengths, biomass, and pigment contents of weeds; E. bonariensis and I. cylindrica effectively suppressed shoot and root growth; C. album demonstrated the most potent impact on dry biomass, leaf chlorophyll, and leaf carotenoid content | [86] |
| 0 | 2025 | Assessment of the bioherbicidal potential of Thymus sp. pl. essential oils in weed control | Italy and Algeria | Thymus algeriensis Boiss. et Reut. and Thymus ciliatus Desf. | Lolium perenne L. and Amaranthus retroflexus L. | All essential oils were effective against weeds in the pre-emergence stage; Lolium perenne L. showed resistance to the essential oils, while A. retroflexus was highly sensitive | [87] |
| Title | Year | Country | Summary of the Invention | Ref. |
|---|---|---|---|---|
| Bioherbicide from Festuca spp. | 2013 | United States | Identification of plants with herbicidal potential and methods of using m-tyrosine compounds from Festuca spp. for weed control | [97] |
| Use of an aqueous solution, suspension or mixture of the same, obtained from air parts and/or parts below the Tulbaghia violacea soil and Agapanthus africanus soil to inhibit fungal infection of a harvest change | 2013 | Brazil | Product based on plant extracts from Agapanthus for use in plant protection | [98] |
| A natural herbicide containing lemongrass essential oil | 2013 | United States | The use of compounds in lemongrass oil for controlling broadleaf weeds and grasses | [99] |
| Allelopathic activity of components and products from the seeds of Euterpe edulis Martius, Arecaceae | 2014 | Brazil | Identification of the allelopathic properties of seeds from the species Euterpe edulis in environmental and agricultural contexts | [100] |
| Natural bioherbicides and related materials and methods | 2014 | United States | Weed control through bioherbicidal compositions based on plant extracts (Russian olive, Austrian pine, tree of heaven, autumn olive, and black walnut extract) | [101] |
| Natural herbicide containing wood tar oil and production method thereof | 2017 | China | Production of a natural herbicide containing wood tar oil for weed control | [102] |
| Use of essential oil from Aloysia triphylla and the process of controlling dicotyledonous plants and/or seeds | 2018 | Brazil | Bioherbicide developed from the essential oil of Aloysia triphylla for controlling dicotyledonous weeds | [103] |
| Process of obtaining a natural herbicide for spraying without harmful effects on human, animal, and plant health | 2019 | Brazil | Natural products for weed control developed from the fermentation in vinegar and alcohol of medicinal plants such as banana leaves, jabuticaba leaves, guava leaves, and lemons | [104] |
| Biological herbicide for extinguishing Ageratina adenophora | 2019 | China | Bioherbicide for controlling Ageratina adenophora | [105] |
| Application of Ambrosia artemisiifolia essential oil as herbicide | 2019 | China | Application of Ambrosia artemisiifolia essential oil as an herbicide in monocotyledon and dicotyledon weeds | [106] |
| Herbicide comprising plant extracts | 2020 | Italy | Herbicide based on plant extracts of Sorghum bicolor, Nospospondia, Artemisia absinthium, Prunus laurocerasus, Atropa belladonna, Arabidopsis, Beta vulgaris, and Stachys tuberifera | [107] |
| Herbicide compositions and methods to control weeds in organic crop production | 2021 | Chile | Composition, preparation, and utilization of bioherbicide from extracts of plants from the Myrtaceae family for weed control | [108] |
| Bioherbicide for regweed control and method of application | 2022 | Serbia | Bioherbicide containing Litsea citrata essential oil as the active ingredient, which can be used for the control of both monocotyledonous and dicotyledonous weeds | [109] |
| 100% non-toxic natural organic natural bioherbicide to combat undesired vegetation on plants | 2022 | Brazil | Formulation of a 100% non-toxic natural organic composition, prepared from various components formulated in an aqueous solution, with biodegradable characteristics | [110] |
| Herbicidal Mentha pantsd, extract compositions and methods of using same | 2023 | United States | Composition of herbicide based on plant extract of Mentha sp. | [111] |
| Herbicidal, bioherbicidal or herbicide additive compositions, process for producing the compositions, method for controlling weed pests, and use of salicylic acid and plant extract | 2023 | Brazil | Salicylic acid and/or its derivatives are used, whether or not associated with cinnamon extracts or their active ingredients (cinnamaldehyde or cinnamic aldehyde), for the production of bioherbicides; a process for producing compositions for weed control in crops such as soybeans, corn, beans, and sugarcane | [112] |
| Composition of contact desiccant bioherbicide | 2025 | Brazil | The invention refers to an organic composition produced from the development of a syrup based on organic citrus fruits (Citrus × Limonia) and leaves of the Eucalyptus citriodora through fermentation using the cultivation of fungi known as yeasts of the genus Saccharomyces, subsequently added to the combination of organic inputs, for application as a desiccant in soybean cultivation, in direct combat against weeds | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolianitis, B.M.; Frescura, V.D.S.; Furtado, G.d.F.; Tres, M.V.; Zabot, G.L. Plant-Based Bioherbicides: Review of Eco-Friendly Strategies for Weed Control in Organic Bean and Corn Farming. AgriEngineering 2025, 7, 288. https://doi.org/10.3390/agriengineering7090288
Dolianitis BM, Frescura VDS, Furtado GdF, Tres MV, Zabot GL. Plant-Based Bioherbicides: Review of Eco-Friendly Strategies for Weed Control in Organic Bean and Corn Farming. AgriEngineering. 2025; 7(9):288. https://doi.org/10.3390/agriengineering7090288
Chicago/Turabian StyleDolianitis, Bianca Motta, Viviane Dal Souto Frescura, Guilherme de Figueiredo Furtado, Marcus Vinícius Tres, and Giovani Leone Zabot. 2025. "Plant-Based Bioherbicides: Review of Eco-Friendly Strategies for Weed Control in Organic Bean and Corn Farming" AgriEngineering 7, no. 9: 288. https://doi.org/10.3390/agriengineering7090288
APA StyleDolianitis, B. M., Frescura, V. D. S., Furtado, G. d. F., Tres, M. V., & Zabot, G. L. (2025). Plant-Based Bioherbicides: Review of Eco-Friendly Strategies for Weed Control in Organic Bean and Corn Farming. AgriEngineering, 7(9), 288. https://doi.org/10.3390/agriengineering7090288








