Abstract
Thyme (Thymus vulgaris) was dried using a tray dryer, recirculating tray dryer, and vacuum dryer at 35 °C, 40 °C, and 45 °C, respectively. The dried thyme after attaining 5% moisture content was subjected to a grinding process to obtain powder using a hammer mill for further analysis of physiochemical properties, bioactive compounds, and techno-functional properties. The ash content was 10.21%, fiber content was 13.57%, fat content was 1.69%, protein content was 5.61%, and carbohydrate content was 22.91% for the thyme sample dried at 35 °C via vacuum drying. Meanwhile, regarding the functional properties, the swelling power was 0.31%, dispersibility was 27.72%, emulsion capacity was 35.44%, foam capacity was 35.47%, and foam stability was 1.84% for the thyme sample dried at 40 °C in the vacuum dryer. The total chlorophyll content, ascorbic acid content, and bioactive compounds were retained best in the vacuum-dried sample at 40 °C. Bioactive compound retention for VDT among the selected three techniques at 35 °C was considerably better. The color values were found to be similar to those of freshly harvested thyme (hue, 93.39; chroma, 3.47) for the thyme sample dried at 40 °C in a vacuum dryer. Based on the analysis, it was found that vacuum drying at 40 °C gave better results, followed by the recirculating tray dryer at 40 °C and the tray dryer at 40 °C. The adequately dried thyme samples with the time–temperature combinations for different drying techniques used can be used further for product development and studies on their shelf life.
1. Introduction
The herb Thymus vulgaris, referred to as thyme, has a primary chemical composition consisting of a wide range of bioactive compounds and essential oil components, which differs depending on variety. According to investigations, thyme leaves contain significant amounts of potassium, iron, calcium, manganese, magnesium, and selenium. Thymol is the primary compound in thyme oil that is produced from the leaves, consisting of 1.84% oxygenated sequester, 5.04% sesquiterpene hydrocarbons, 28.69% monoterpene hydrocarbons, and 56.53% monoterpenes [1]. The chemical compositions of different types of thyme are becoming complicated and diverse as a result of the wide variety and ecological environments, as well as the frequent hybridization between various populations. Phenols, such as thymol, carvacrol, linalool, borneol, eucalyptol, and other phenols, are the most prevalent compounds among them. Lemon, Silver, and Odae are the type of thyme that belong to thymol chemotype and amounts to 43.91%, 66.24%, and 30.54%, thymol respectively. According to the study findings of Dong et al. [2]. The geraniol type is represented by Creeping, Golden, Orange, and Wolchul which have geraniol concentrations of 29.57%, 65.99%, 44.70%, and 42.94%, respectively. Carpet and Jiri, which have linalool concentrations of 48.16% and 47.89%, respectively, are known as linalool-type [2]. Numerous flavonoids and phenolic antioxidants, including lutein, pigenin, naringenin, luteolin, and thymonin, are also present in thyme [3]. The primary phenolic component that gives thyme its antioxidant properties is thymol. The flowering stem of thyme is rich in tannins, phenolic acids, including rosmarinic and cafeic acids, and flavonoid derivatives, like luteolol and apigenol [4].
Approximately 250 species, including 36 subspecies, of thyme are known today, which are divided into eight categories: Mikantes, Mastihcina, Piperella, Teucrioides, Pseudothymbra, Thymus, Hyphodromy, and Serpyllum. Thyme is primarily found in the Mediterranean region, where it originated, and is now grown in Spain, France, Italy, Portugal, Germany, Algeria, Egypt, UK, the Caribbean, and the United States [5]. Agrometeorological (geographical origin, climatic conditions, soil composition, etc.) and technological factors (cultivation, species collection, storage, processing technology, etc.) all have an impact on the essential oil composition of thyme. Studies on herbs have shown that the amounts of essential oil in herbs are significantly reduced by drying, even when the herbs are air-dried at ambient temperature. Some studies on the amount of essential oil were conducted, and the amounts were found to be 36–45% in basil, 23–33% in marjoram, and 6–17% in oregano, which implies the effect of drying on the essential oil content of herbs [6]. Alcohols, aldehydes, peroxides, and ketones are examples of secondary aroma molecules that are developed during the drying process, which can significantly alter the volatile characteristics of the essential oil. These secondary products contribute to a significant portion of the dried herb’s overall volatile content, hence resulting in a difference in the final essential oil composition. A study conducted on air-dried dill leaves (dried at 25, 40, and 50 °C) showed that more than 50% of their volatile content contributed to secondary fragrance compounds developed during drying [6]. The hydrolysis of glycosylated volatile compounds, oxidation processes, or the release of compounds through cell wall rupture contribute to the changes in the essential oil components of herbs throughout the drying process. The drying factors, such as drying technique, temperature, vacuum level (for procedures like vacuum-drying or freeze-drying), drying duration, and amount of water evaporated during drying, all affect how much the volatile compounds in dried herbs alter or decrease during the drying process. Drying herbs reduces their volatile components, and certain drying techniques may allow for greater volatile compound preservation than others. One of the biggest sectors in the world, the food business, has seen significant transformation in recent years, moving toward novel and healthier products. Because plants have historically been utilized as food preservatives, the plant extract business has infiltrated the food sector [7].
Drying temperature and duration are frequently linked to the loss of volatile molecules. Nazari et al. [8] examined how drying affected thyme’s fragrance components. The research findings showed that a total of 68 compounds were found in thyme, and over 100 components were quantitatively defined. Drying at 60 °C resulted in a notable decrease in the quantity of extracted volatiles, which was typically caused by the loss of non-oxygenated monoterpenes [8]. According to other research, thyme that was oven-dried at 60 °C had the highest levels of headspace (HS) volatiles. According to Yilmaz et al. [9], the levels in thyme oven-dried at 60 °C were 19.4 times higher than those of thyme oven-dried at 30 °C and 4.2 times higher than those of fresh thyme, clearly showing the effect of drying temperature on the overall quality of thyme [9].
Thyme is rich in bioactive compounds and antioxidant properties, but there are very few studies on drying and quality analysis of its volatile components. It is necessary to preserve these components by adequate drying to reduce post-harvest losses. The proximate, functional, color, and bioactive compounds of herbs are extremely temperature-sensitive, making it crucial to select the right drying technique and temperature. Color is an important characteristic to determine the quality of dried herbs, as it is closely related to consumer preference. The bioactive components and functional properties of medicinal herbs are significantly affected by drying temperature. Due to thermal breakdown, high temperatures usually influence the quality and number of bioactive compounds [10]. The duration of drying and drying technique become the most crucial elements that might enhance the essential components’ quality under such storage conditions. The present study was conducted to identify the adequate drying technique and temperature combination for retaining the maximum amount of bioactive compounds, nutritive value, and functional properties. Thus, the drying behavior of thyme was studied at 35, 40, and 45 °C using three dryers, viz. a tray dryer, recirculating tray dryer, and vacuum dryer, and the changes were estimated in terms of quality parameters. This facilitates the proper selection and end-use of dried thyme in food and other industries. Further, the dried thyme was powdered and its techno-functional properties were assessed to determine its suitability for the development of new products.
2. Material and Methods
Thyme (Thymus vulgaris) was procured from Organic Agricultural Institute Farm, Amity University Uttar Pradesh, Noida, India, and stored at 4 ± 1 °C until further processing.
2.1. Drying Techniques
Samples were kept at 5 mm thickness and dried using a tray dryer (M/s Universal Engineering, Vadodara, India) at an air velocity of 1.5 m/s, recirculating tray dryer (M/s BPTL, Kolkata, India) with a constant air flow rate of 1.5 m/s in the co-current direction, and vacuum dryer (M/s Pooja Scientific, Ghaziabad, India) at a vacuum pressure of 500 mm Hg. The drying temperature in this study was set at 35 °C, 40 °C, and 45 °C. The weight loss of the sample was calculated on an hourly basis for each drying technique.
2.2. Proximate Analysis
The proximate composition of fresh and dried thyme powder was determined according to AOAC (2000) methods. The fresh and dried herbs were analyzed for moisture content, ash content, total protein, crude fiber, carbohydrate, and fat content. Triplicate samples were analyzed for each parameter [11].
2.3. Ascorbic Acid
Thyme powder was analyzed for ascorbic acid as follows: 1 g of sample was ground with a pestle and mortar using 20 mL of metaphosphoric acid–acetic acid solution (40 mL of acetic acid and 15 g of metaphosphoric acid were dissolved in 450 mL of distilled water) and was filtered. The ascorbic acid content was calculated by titrating the standard ascorbic acid (0.2 mg/mL) with dye. The ascorbic acid content was calculated using these formulas:
2.4. Total Chlorophyll Content
Chlorophyll is an antioxidant and chemopreventive phytochemical which exhibits prooxidant activity when exposed to light or heat. It is necessary to identify the changes occurring in chlorophyll during drying to quantify the effect on phytochemical properties. The pigments (chlorophyll a and b) were determined and quantified using the procedure proposed by Ghosh et al. [12], using 1 g of sample analyzed at 663 and 645 nm wavelengths with the help of a UV-vis spectrophotometer using acetone and n-hexane (4:6) as a blank [12].
where A663 and A645 are the absorbances at 663 and 645 nm, respectively.
2.5. Bioactive Compound Analysis of Thyme
2.5.1. Preparation of Extract
A hammer mill (M/s Sanco India Pvt Ltd., Delhi, India) was used to grind the dried thyme from all three selected drying techniques into powder and 100 g of defatted sample was used for determination [13].
2.5.2. Total Phenolic Content of Thyme
Gallic acid was used as a standard in the Folin–Ciocalteu technique to calculate the total phenolics, the absorbance at 730 nm was calculated, and the total phenolic content was reported as milligrams of gallic acid equivalent per gram (mg GAEg−1) [14].
2.5.3. Total Flavonoids Content of Thyme
The flavonoids content was estimated following the method proposed by Xu et al. [14]; 1 g of puree sample was homogenized with 10 mL of methanol and 0.5 mL of supernatant was diluted with 1.5 mL of methanol. Absorbance was determined at 415 nm with the help of a UV-vis spectrophotometer [14].
2.5.4. Total Tocopherol Content
By using α-tocopherol as a reference, total tocopherols were calculated using Philip’s approach. After shaking the mixture for ten seconds, the absorbance at 520 nm was measured using ethanol as a blank [15].
2.5.5. Tannin Content
Tannic acid was used as the standard in the Pearson technique, which was slightly modified to determine the tannin concentration, and absorbance at 760 nm was measured in comparison with the reagent blank [16].
2.5.6. 2,2′ Diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Assay
Using the DPPH free-radical-scavenging technique, the extracts’ antioxidant activity was assessed. The absorbance at 517 nm was measured as the reaction steadily approached the plateau, or the time at steady state [17].
2.6. Functional Properties of Powder
Thyme powder’s techno-functional qualities are crucial as they have a direct impact on how they are used in the food and other sectors. These characteristics ensure that the thyme powder maintains its nutritional and bioactive advantages while also retaining its best efficiency throughout processing and end-use. The dried thyme powder characterization included complementary approaches to evaluate the physical, colloidal, and functional property changes of thyme after drying. All analyses of the powder’s properties were performed in triplicate, unless stated otherwise.
2.6.1. Volume Expansion Ratio
The volume expansion ratio of the rehydrated thyme powder was determined using the volume displacement method. Before rehydration, 5 g of dried thyme was poured into a 50 mL graduated cylinder and 20 mL of distilled water was then added [18]. The volume expansion ratio of the rehydrated thyme can be calculated by:
where Vr is the volume of the rehydrated thyme (mL) and Vi is the volume of the dried thyme before rehydration (mL).
2.6.2. Swelling Power (SP)
The swelling powers of the dried thyme samples were estimated using the method suggested by Lai et al. [19]; estimation was performed by preparing a slurry of 9 mL of water and 0.5 g of dried sample. The prepared slurry was then kept in a water bath at 70 °C for 30 min and further processed [19].
2.6.3. Dispersibility
Dispersibility was determined using the method of Nisha et al. [20]. The sample mixture was prepared by adding 10 mg of sample to 100 mL of distilled water in a graduated cylinder; the mixture was mixed vigorously and was allowed to stand for 3 h. The volume of the settled particles was measured and was deducted from 100, and the difference was expressed as the dispersibility percentage [20].
2.6.4. Emulsion Capacity
The emulsion capacity was determined using Kumar et al.’s [21] method. A dried powdered 5 g sample was combined with 25 mL of refined oil, homogenized, and centrifuged at 1500× g for 5 min. The emulsion capacity was calculated using the overall layer of emulsion [21].
2.6.5. Foam Capacity
Measurements of foaming capacity (FC) were conducted by the method suggested by Darniadi et al. [22]. Samples were weighed and 2 g of sample was added to 50 mL of distilled water and mixed, and the volume was determined. Foam was created by blending the mixture at 160 g for 5 min using a Warring Blender Commercial model HGBTWT in Torrington, CT, USA. The foam volume was measured, and foam suspension was poured into a graduated cylinder [22].
2.6.6. Foam Stability
The method recommended by Darniadi et al. [22] was used to determine the foam’s stability. An amount of 100 mL of distilled water was used to flog 1 g of the dried thyme, and the volume of the solution was measured. The resultant suspension was poured into a 250 mL measuring cylinder, agitated for 5 min at 1600 rpm, and the volume was recorded [22]. The foam capacity was calculated using the following formula.
2.7. Color Analysis
A handheld colorimeter (HunterLab MiniScan XE Plus, Reston, VA, USA) was used to measure the color of the thyme samples. A standard white and black tile was used to calibrate the colorimeter prior to measurement. L* (lightness), a* (green to red), b* (blue to yellow), hue angle, and chroma values were among the factors that were noted.
2.8. Statistical Analysis
All the experimental readings were taken in triplicate and were analyzed for significant differences among the mean values using Duncan’s multiple range test using IBM SPSS Statistics 25.
3. Result and Discussion
3.1. Proximate Analysis
The proximate composition of freshly harvested and dried thyme at different temperatures using different dryers is shown in Figure 1. The moisture content of fresh thyme was 51.6 ± 0.04, and it was reduced to 5% by drying using different temperatures and techniques. A high moisture content leads to the breakdown of bioactive compounds, like phenolic acids and flavonoids. Maintaining the moisture content to 5% helps in reducing the probability of losses due to high moisture content. The fat, ash, and carbohydrate contents of freshly harvested thyme were 1.95 ± 0.04, 11.45 ± 0.05, and 23.9 ± 0.04 (g/100 g). The changes in the values of these components were observed, as depicted in Figure 1. The ash content observed in the findings indicated that the quality of thyme was not compromised. Thyme is rich in phenolic and flavonoid contents, hence resulting in a lower ash content because of the lack of interference of the ash content with bioactive compounds. The lower fat content after drying indicated that bioactive antioxidants will not be degraded by the oxidation of unsaturated fatty acids present in fat [23]. The data depicted that the values of dried thyme were significantly decreased by 1.67%, 11.47, and 22.58% at p ≤ 0.05 when compared with fresh thyme. However, the fat content of thyme as observed in this study was lower. According to Kassegn et al. [23], the fat content in the thyme herb grown in the highlands of southern Tigray was also found to be in the same range [23]. The crude fiber and crude protein content were found to be high in thyme dried using VDT 13.97 ± 0.08 and 5.1 ± 0.09 (g/100 g), respectively, when compared with TDT and RTDT dried thyme at temperatures of 35 °C, 40 °C, and 45 °C. The values were significantly decreased by 16.19% and 9.89% at p ≤ 0.05.
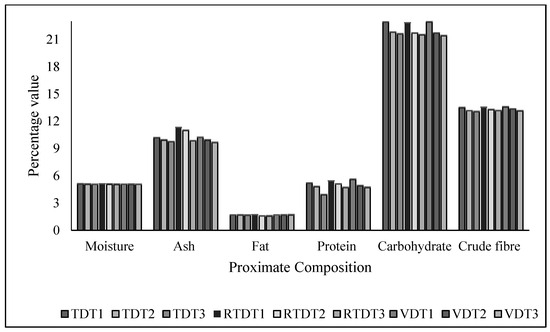
Figure 1.
Proximate composition of fresh and dried thyme using tray dryer, recirculating tray dryer, and vacuum dryer, where TDT1 = Tray drying at 35 °C; TDT2 = Tray drying at 40 °C; TDT3 = Tray drying at 45 °C; RTDT1 = Recirculating Tray drying at 35 °C; RTDT2 = Recirculating Tray drying at 40 °C; RTDT3 = Recirculating Tray drying at 45 °C; VDT1 = Vacuum drying at 35 °C; VDT2 = Vacuum drying at 40 °C; and VDT3 = Vacuum drying at 45 °C.
3.2. Ascorbic Acid
The ascorbic acid content in freshly harvested thyme was 161 mg/100 g; the drying of thyme resulted in a significant reduction in the ascorbic acid content. VDT1, VDT2, and VDT3 vacuum-dried samples contained 85, 72, and 65 mg/100 g, respectively. The ascorbic concentration decreased more in TDT and RTDT samples (68, 52, 48, 76, 58, and 56 mg/100 g for TDT1, TDT2, TDT3, RTDT1, RTDT2, and RTDT3, respectively). Ascorbic acid was significantly affected by the temperature and drier used to dry the thyme. The ascorbic acid levels decreased during the drying of the thyme. Vacuum drying resulted in the best retention among the driers because of its oxygen restriction combined with low-temperature drying. It has been shown that ascorbic acid is lost throughout the preservation process, particularly as the temperature rises. Research has shown in the literature that blanching vegetables in hot water and then drying them results in a loss of vitamin C [24]. Ascorbic acid is susceptible to degradation by heat, light, and air. Dried herbs are often exposed to heat and air, which may reduce their ascorbic acid content. Additionally, some drying methods, such as sun-drying, expose the herbs to light, which accelerates the breakdown of ascorbic acid even further.
3.3. Total Chlorophyll Content
Drying of thyme resulted in a significant loss of total chlorophyll, with RTDT2 showing the highest loss of total chlorophyll relative to fresh thyme (63.61 mg/g) and RTDT1 showing the highest retention of total chlorophyll. The VDT and TDT samples also showed reductions in chlorophyll content of 54.63, 48.76, 51.76, and 45.62 mg/g for VDT1, VDT3, TDT1, and TDT3, respectively. Bahceci et al. [25] conducted studies and found that pigment is more susceptible to damage, and that heat exposure speeds up the breakdown of chlorophyll-containing organelles, causing the compound to leak into the cell [25]. During drying, the water content is drawn out of their tissues, and because chlorophyll molecules are extremely sensitive to changes in their structure, they are destroyed when their structure is compromised by water loss. The oxidation of herbs is caused when they are exposed to the air under drying circumstances. This leads to oxidative stress as the action damages and reduces the chlorophyll molecules. The enzymatic activity of herbs during drying is initiated and enzymes interact with the chlorophyll molecules. The breakdown of chlorophyll molecules is also caused by the physical damage that plant tissues sustain during the drying process [25].
3.4. Bioactive Compounds of Thyme
3.4.1. Estimation of Total Phenolic Content
Thyme extracts were prepared using methanol as a solvent and the results were found to be 8.97 mg GAEg−1 for freshly harvested thyme, followed by thyme dried at 45 °C using VDT3, which was found to be 8.04 mg GAEg−1. The total phenolics content of thyme dried at 35 °C and 40 °C for all three techniques was found to be lower compared with that of samples dried at 45 °C (Table 1). The higher values for the total phenolic content were found to be 7.57, 7.51, and 8.04 mg GAEg−1 for TDT3, RTDT3, and VDT3, respectively. The thyme dried at 40 °C under all the mentioned drying techniques exhibited slight decreases in phenolic contents of 6.94, 6.18, and 6.97 mg GAEg−1 for TDT2, RTDT2, and VDT2 respectively. Drying at 35 °C resulted in lower phenolic contents of 4.65, 4.38, and 5.87 mg GAEg−1 for TDT1, RTDT1, and VDT1, respectively. The higher percentage of total phenols obtained by the vacuum drying process compared with recirculatory tray drying and tray drying process was due to the more effective extraction of insoluble phenolic components, such as condensed tannins and phenolic acids attached to cell wall polysaccharides or proteins. Phenolic–sugar glycosidic linkages can be broken with heat during drying operations, resulting in the development of phenolic aglycons that react better with the Folin–Ciocalteu reagent, resulting in higher total phenolic values at higher drying temperatures [26]. Using the Duncan test between drying temperature levels, the total phenolic content values were found to be significant (p > 0.05).

Table 1.
Changes in bioactive compound contents of freshly harvested thyme and dried thyme.
3.4.2. Estimation of Flavonoids Content
Freshly harvested thyme extracts were found to have a flavonoid content of 2.54 mg CAEg−1. The drying of thyme showed changes in the flavonoid content as the VDT1 sample at 35 °C exhibited the highest value of 2.31, followed by RTDT1 (2.16 mg CAEg−1) and TDT1 (2.03 mg CAEg−1). A decrease in the flavonoid content was observed when increasing the temperature to 40 °C and 45 °C. The flavonoid contents at 40 °C were 1.97, 1.87, and 1.98 mg CAEg−1 for TDT2, RTDT2, and VDT2, respectively, and 1.09, 1.06, and 1.76 mg CAEg−1 for TDT3, RTDT3, and VDT3, respectively. After the leaves are removed from the parent plant, mesophyll cells persist and are under stress for a longer period. Mesophyll cells survive and continue to perform complicated metabolic activities, such as turning specific intermediate molecules into flavonoids, boosting flavonoid accumulation [27]. Drying for a specific time also affects the intensity of the changes occurring in thyme and other similar herbs. However, because the VDT procedure was carried out at low pressure and low temperature, mesophyll cell metabolism was stable, and no noticeable substance changes were seen in the flavonoid content. As a result, the flavonoid levels in thyme were stable. However, high temperatures may decrease the action of flavonoids, as observed between the results. Furthermore, drying at moderate temperatures below 50 °C, such as room temperature, helps to retain flavonoids’ biological activity [28]. The flavonoid content differences determined using Duncan’s test between drying techniques were non-significant (p > 0.05).
3.4.3. Estimation of Total Tocopherol Content
Tocopherols are methylated phenols, many of which have vitamin E activity, which have the potential to resist oxidative stress. The methanol extract of freshly harvested thyme had a maximum tocopherol content of 7.78 mg/g. The drying of thyme showed several changes in samples; the VDT1 samples were found to have 6.41 mg/g, VDT2 samples were found to have 4.86 mg/g, and VDT3 samples had 4.07 mg/g. Similarly, for tray drying and recirculating tray drying, it was found to be 5.72, 3.16, and 1.96 mg/g for TDT1, TDT2, and TDT3 respectively, and 5.98, 4.65, and 3.37 mg/g for RTDT1, RTDT2, and RTDT3, respectively, which indicated that there was no significant effect of temperature on the loss of the tocopherol content in this sample. A significant difference in the tocopherol content was not observed because it is heat-stable, but the duration of heat exposure of herbs affects the overall tocopherol content, which keeps on decreasing as the time of drying increases. After completion of drying, the contents in the TDT samples were substantially lower (p < 0.05) than the RTDT and VDT samples. The VDT samples exhibited a higher tocopherol content because the drying time was lowest for this technique. Laoretani et al. [29] conducted studies on the effect of drying on canola for oil extraction and found that tocopherol content loss increased with an increase in temperature [29]. However, studies also conducted on ginger by Chumroenphat et al. [30] indicated that, under a typical near-to-ambient temperature, moisture flows from leaves to the air, mostly through a relatively thin layer known as the drying zone. It is also observed that, throughout the drying phase, the leaf layer, which serves as the air outlet layer, remains moist, waiting for the drying front to approach, which helps in determining the effective drying by the dryer used for the study [30].
3.4.4. Estimation of Tannins in Thyme
The analysis of tannin content revealed that the methanol extract of thyme contained the highest amount of tannins, at 0.65 mg TAEg−1. The TDT1, RTDT1, and VDT1 samples contained 0.17, 0.32, and 0.28 mg TAEg−1, respectively, and 0.09, 0.18, and 0.15 mg TAEg−1 for TDT2, RTDT2, and VDT2 respectively. The lowest amount of tannin was found in the TDT3 sample at 0.07 mg TAEg−1, followed by the RTDT3 and VDT3 samples, at 0.08 mg TAEg−1. The tannin levels did not show any significant change during the drying techniques, as suggested in the statistical analysis in Table 1 below. As drying leads to the breakdown of tannins and a decrease in their concentration due to the disruption of their complex molecular structure, tannins are better preserved at lower drying temperatures [31]. Techniques such as vacuum-drying or shade-drying are more effective at minimizing tannin loss compared with traditional hot-air drying methods. These findings show that the drying technique used for drying samples plays a key role in tannin inactivation. The absence of oxygen (heating under vacuum) had little protective impact on tannin inactivation in herbs and leaves, indicating that oxygen or metal ions play a minor part in the inactivation process [32]. Statistical analysis of the tannin content values between the drying techniques indicated that they were non-significant (p > 0.05).
3.4.5. Evaluation of Antioxidant Activity
Methanol extract of samples was prepared for determining the antioxidant activity of freshly harvested and dried thyme. The freshly harvested thyme sample had an IC50 value of 15.67 µg/mL. The results in Figure 2 showed that VDT3 extract had the lowest IC50 value of 18.47 µg/mL among the dried thyme samples, which was followed by TDT3 and RTDT3 extract, at 21.23 µg/mL and 33.6523 µg/mL, which were the samples dried at 45 °C. The samples dried at 40 °C using a tray dryer, recirculating tray dryer, and vacuum dryer exhibited extract IC50 values of 42.65 µg/mL, 46.98 µg/mL, and 36.89 µg/mL respectively. Similarly, the thyme dried at 35 °C using a tray dryer, recirculating tray dryer, and vacuum dryer exhibited extract values of 49.76 µg/mL, 50.76 µg/mL, and 47.97 µg/mL, respectively. The difference in the value indicates that the vacuum-dried samples had a weak inhibitory concentration, resulting in stronger antioxidant properties, followed by the tray-dried and recirculating tray-dried samples.
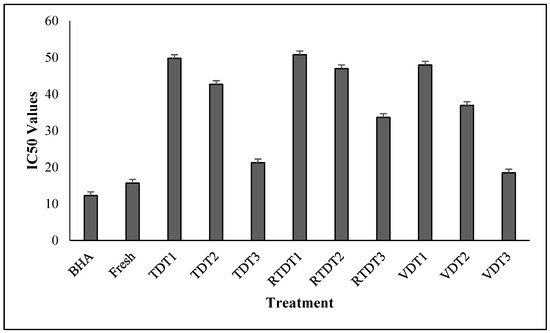
Figure 2.
IC50 values of fresh and dried thyme using tray dryer, recirculating tray dryer, and vacuum dryer. Where: Fresh = Freshly harvested thyme; TDT1 = Tray drying at 35 °C; TDT2 = Tray drying at 40 °C; TDT3 = Tray drying at 45 °C; RTDT1 = Recirculating Tray drying at 35 °C; RTDT2 = Recirculating Tray drying at 40 °C; RTDT3 = Recirculating Tray drying at 45 °C; VDT1 = Vacuum drying at 35 °C; VDT2 = Vacuum drying at 40 °C; and VDT3 = Vacuum drying at 45 °C.
The results clearly indicate a positive correlation between the phenolic composition and antioxidant activity, as the higher free radical scavenging activity of VDT samples was due to the higher phenolic and flavonoid contents. The higher total antioxidant activity in VDT3 samples compared with TDT and RTDT was due to the release of antioxidant nutrients by the thermal destruction of cell walls and subcellular parts, formation of antioxidants by the thermal chemical reaction, and suppression of antioxidant oxidation by the thermal inactivation of oxidative enzymes. Lim and Murtijaya [33] conducted a study on the drying of Phyllanthus anarus and concluded that oven-drying at 50 °C indicated a decrease in antioxidant activity that resulted in induced enzymatic oxidation of natural antioxidants [33]. High temperatures above 50 °C in the treatment of herbs causes a loss in antioxidant activity and deactivates enzymes, along with the degradation of phytochemicals. The changes occurring in a sample after drying can also help in identifying the heat-stable antioxidants present in the sample. The stability of carotenoids results in the obtained antioxidant activity [34].
3.5. Powder Properties of Dried Thyme
The powder properties of thyme were evaluated (Figure 3) to determine its efficiency in usage in different products in culinary flavorings, which include soups, stew, marinates, baked goods, salad dressing, seasonings, beverage infusions, sauces, gravies, and health-supportive additives.
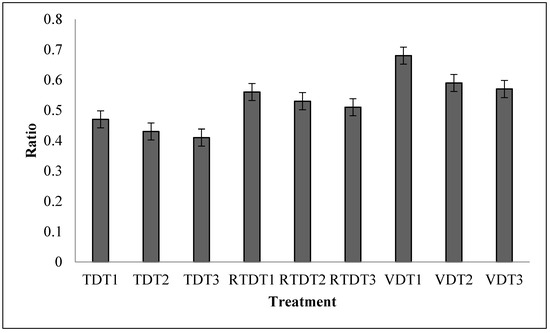
Figure 3.
Volume expansion ratio of dried thyme powder using tray dryer, recirculating tray dryer, and vacuum dryer. Where: TDT1 = Tray drying at 35 °C; TDT2 = Tray drying at 40 °C; TDT3 = Tray drying at 45 °C; RTDT1 = Recirculating Tray drying at 35 °C; RTDT2 = Recirculating Tray drying at 40 °C; RTDT3 = Recirculating Tray drying at 45 °C; VDT1 = Vacuum drying at 35 °C; VDT2 = Vacuum drying at 40 °C; and VDT3 = Vacuum drying at 45 °C.
3.5.1. Volume Expansion Ratio
The volume expansion ratio of the rehydrated thyme powder ranged from 0.428 to 0.633. These results demonstrate that drying under various conditions had no influence on the volume expansion ratio of the rehydrated thyme powder, as demonstrated by the statistical analysis. Figure 3 shows the dried thyme’s volume expansion ratio following a 12 min rehydration in hot water. The findings are in line with the results of Prasert and Suwannaporn [35], who demonstrated that amylose-rich compounds contribute to the volume expansion ratio of rehydrated powder, which should be 1.5–3 times after rehydration volume expansion [35]. However, for thyme, the absence of amylose-rich compounds results in a lower volume expansion ratio of dried thyme powder.
3.5.2. Swelling Power (SP)
The swelling power values for the dried thyme powder were 0.25% for TDT1, and the swelling power was found to increase as the drying temperature increased. An increase in values indicates that the lattice is holding water molecules and results in the swelling of the powder sample. A similar trend was observed in RTDT samples, with a slightly higher value of 0.26% for VDT1 and 0.23% for VDT3. Jin et al. [36] concluded in their study that the swelling power shows how much water a particular molecule absorbs, while solubility shows how much leaching occurs as starch granules swell. They also act as an indicator for the degree of interaction within starch chains, for both amorphous and crystalline structures, determining the swelling power and solubility values [36]. Wang et al. [37] concluded that a significant factor influencing the colloidal characteristics of materials based on starch is its swelling power. Swelling power is affected by a number of factors, such as the source of the starch, granule size, the ratio of amylose to amylopectin, and the processing conditions. Starch granule swelling causes the starch dispersion’s viscosity to rise [37]. The absence of starch in thyme resulted in lower swelling power of thyme powder.
3.5.3. Dispersibility
The dispersibility range was observed to be increasing for both RTDT and VDT samples, which implies that the energy required in the dispersion of thyme powder will keep on decreasing as the drying temperature is increased. The dispersibility of the colloidal system influences its rheological properties, such as flow patterns and viscosity. TDT1 had 27.94%, which increased to a very substantial amount to 28.94% for TDT3 because of the finer lattice of the powdered sample, while RTDT1 had 25.76%, which did not increase much, and that for RTDT3 was estimated to be 29.32%. The properties indicate how the particles break down and disperse throughout the mixture. Samples with finer particles dispersed more readily in the solution, producing a powder that might be utilized to make products that need less energy to disperse the particles requiring high dispersibility. Good dispersibility properties result in a uniform dispersion of the colloidal particles in the medium [38]. Dispersibility is important for colloidal particles in chemical processes because it can alter the rate of reaction. High dispersibility is often associated with an enhanced reaction rate due to the higher rate of contact between the reactants. Furthermore, low dispersibility is the source of non-Newtonian behavior in colloidal systems in which the viscosity of the system depends on the applied shear rate [39].
3.5.4. Emulsion Capacity
The variables that influence and determine a sample’s capacity to create an emulsion include its chemical composition, concentration of active compounds, powder particle size, and processing conditions. Herbs contain naturally occurring emulsifiers including proteins, polysaccharides, and phospholipids that assist in maintaining the stability of the emulsions. The amphiphilic properties of saponins, found in thyme, aid in emulsification. Furthermore, thyme’s essential oils, especially thymol and carvacrol, help to stabilize emulsions. These substances can help emulsions to develop by lowering the surface tension between the water and oil phases. This is why the emulsion capacity for RTDT1 was 52.72%, rising to 54.32% for RTDT2, and 56.53% for RTDT3. VDT had a comparable emulsion capacity. The VDT1 emulsion capacity was 52.94%, while that of VDT3 was 50.27%. Due to the presence of natural emulsifiers, several plants, including aloe vera, chamomile, and lavender, are well recognized for their high emulsion capacity capabilities. The particle size distribution in the emulsion’s dispersed phase is influenced by the emulsion capacity. Smaller and more uniform droplets result from a higher emulsion capacity, which may have an impact on the emulsion’s texture and appearance [40].
3.5.5. Foam Capacity
Thyme has several foaming components, like timosaponin, dioscin and its dervatives, hederangenin glycosides, oleanotic acid glycosides, and ursolic acid glycosides. Proteins also contribute to foam since thyme is not that rich in protein; saponins are the major component in foam formation. For TDT, RTDT, and VDT samples, the foam capacity exhibited comparable behavior; variations in the value were consistent for the mentioned drying techniques, with VDT samples exhibiting higher foam capacity due to the better retention of bioactive components like tannins. Tannins were lower in TDT drying because the drying process leads to the loss of bioactive compound tannins responsible for foam formation, whereas RTDT has good retention of bioactive compound tannins, leading to higher foam formation. RTDT1 was found to have 35.56% FC, which increased significantly to 37.65% for RTDT3, while the samples were found to have 33.51% FC for VDT1, which was significantly higher than that for the RTDT1, and it increased to 35.75% for VDT3.
3.5.6. Foam Stability
Thyme has components responsible for foam stability, including phenolic acids, thymol, carvacrol, and rosamaric acid, which interact with plant proteins and polysaccharides, helping in stabilizing the foam and preventing it from disintegrating or collapsing by forming a robust film around the bubbles. Foam stability was found to be 1.93 for RTDT1, increasing in a uniform manner with an increase in temperature treatment, and it was 2.32 for RTDT3. TDT samples had a higher foam capacity, but the changes in foam stability did not show any significant changes; regarding the VDT samples, it was found to be 1.76 for VDT1, increasing to 2.71 with an increase in temperature for VDT3. Figure 4 shows the changes in the values observed with changes in drying temperature and technique. Due to the presence of natural saponins, which are natural surfactants that may form and stabilize foam, several herbs, including fenugreek, saponaria, and quillaja, are renowned for their high foam stability capabilities [41].
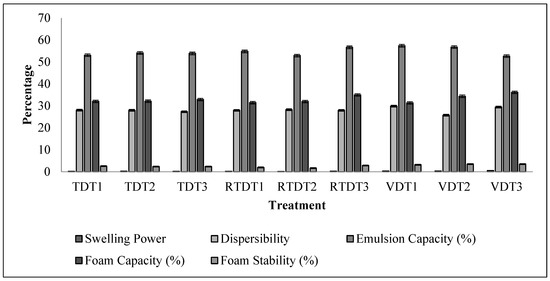
Figure 4.
Techno-functional properties of thyme powder dried using tray dryer, recirculating tray dryer, and vacuum dryer. Where: TDT1 = Tray drying at 35 °C; TDT2 = Tray drying at 40 °C; TDT3 = Tray drying at 45 °C; RTDT1 = Recirculating Tray drying at 35 °C; RTDT2 = Recirculating Tray drying at 40 °C; RTDT3 = Recirculating Tray drying at 45 °C; VDT1 = Vacuum drying at 35 °C; VDT2 = Vacuum drying at 40 °C; and VDT3 = Vacuum drying at 45 °C.
3.6. Color Analysis
The color of dried powder is an important sensory quality from the consumer’s perspective. A bright-green color for dried leaves is preferred by consumers over dark or off-green colors. The color changes of thyme leaves during drying are shown in Table 2. The CIE color scale indicates three values: L (white to black), a (red to green), and b (yellow to blue). The color values of freshly harvested thyme were 3.73, −2.70, and 3.46, respectively, for L, a, and b. The “L” value decreased with increasing temperature and exposure time, which shows that the lightness decreased during drying. The bright-green color of the dried samples that decreased with increasing drying conditions can be seen in Table 2. The highest and lowest “L” values were 5.94 and 3.67, respectively, while the highest and lowest negative “a” values were 2.44 and 1.67, respectively. Conversely, color value “b” decreased with temperature and time. The color component variations in the samples were caused by the degree of degradation of the color components and multiple interaction sites that were slightly affected by the drying technique used for the drying of the thyme [42].

Table 2.
Color analysis of dried thyme using tray dryer, recirculating tray dryer, and vacuum dryer.
4. Conclusions
This study aimed to identify the nutritive components, bioactive compounds, and techno-functional properties of thyme. Three different drying techniques were used to preserve the quality of thyme: tray drying, recirculating tray drying, and vacuum drying at 35 °C, 40 °C, and 45 °C. This research evaluated the total chlorophyll content, ascorbic acid content, bioactive compounds, volume expansion ratio, swelling power, dispersibility, emulsion capacity, foam capacity, stability of foam, color, and proximate composition of dried thyme. The results obtained clearly indicated several differences which indicated that thyme dried by different techniques could be utilized for specific types of products. Vacuum drying at 40 °C resulted in better retention of bioactive compounds and color profile, which makes it a good fit for the development of functional foods, dietary supplements, extruded snacks, and incorporation in breakfast cereals. Meanwhile, vacuum drying at 45 °C could be utilized for the formulation of herbal tea, herbal supplements, tablets, spice mix, and energy drinks because of its retention of nutritive components. Similarly recirculating tray and tray drying of thyme at 45 °C can aid in value-added bakery products like cookies, salad dressings, flavored ice creams, butter, and creamy sauces because of recirculating tray-dried thyme having good emulsion and foam capacity. The development of thyme-incorporated soup mixes, snacks, RTE premixes, salad, and dairy products can be performed owing to the swelling capacity and dispersibility of thyme dried using a tray dryer at 45 °C. This study’s findings indicated that drying technique and temperatures have an impact on the characteristics of thyme, but the outcomes helped in the selection of product-specific drying techniques.
Author Contributions
N.R.—Methodology and Original draft writing; N.S.—Investigation, visualization, writing—review and editing; A.M.M.—Conceptualization, formal analysis, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Soleimani, M.; Arzani, A.; Arzani, V.; Roberts, T.H. Phenolic compounds and antimicrobial properties of mint and thyme. J. Herbal Med. 2022, 36, 100604. [Google Scholar] [CrossRef]
- Dong, Y.; Wei, Z.; Yang, R.; Zhang, Y.; Sun, M.; Bai, H.; Shi, L. Chemical compositions of essential oil extracted from eight thyme species and potential biological functions. Plants 2023, 12, 4164. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, A.; Purkiewicz, A.; Jakuć, P.; Wiśniewski, P.; Sawicki, T.; Chajęcka-Wierzchowska, W.; Tańska, M. Effectiveness of various solvent-produced thyme (Thymus vulgaris) extracts in inhibiting the growth of Listeria monocytogenes in frozen vegetables. NFS J. 2022, 29, 26–34. [Google Scholar] [CrossRef]
- Iftikhar, T.; Majeed, H.; Zahra, S.S.; Waheed, M.; Niaz, M.; Bano, N. Thyme. In Essentials of Medicinal and Aromatic Crops; Springer International Publishing: Cham, Switzerland, 2023; pp. 399–429. [Google Scholar]
- Etri, K.; Pluhár, Z. Exploring Chemical Variability in the Essential Oils of the Thymus Genus. Plants 2024, 13, 1375. [Google Scholar] [CrossRef]
- Xing, Y.; Lei, H.; Wang, J.; Wang, Y.; Wang, J.; Xu, H. Effects of different drying methods on the total phenolic, rosmarinic acid and essential oil of purple perilla leaves. J. Ess. Oil Bear. Plants 2017, 20, 1594–1606. [Google Scholar] [CrossRef]
- Bharadvaja, N. Aromatic plants: A multifaceted asset. Braz. J. Bot. 2023, 46, 241–254. [Google Scholar]
- Nazari, D.; Badi, H.N.; Mehrafarin, A.; Taj-abadi, F.; Soltanipour, M. Expression of the changes in essential oil components of Shirazi thyme (Zataria multiflora Boiss.) as affected by various drying methods. Ind. Crops Prod. 2024, 220, 119222. [Google Scholar] [CrossRef]
- Yilmaz, A.; Alibas, I.; Asik, B.B. The effect of drying methods on the color, chlorophyll, total phenolic, flavonoids, and macro and micronutrients of thyme plant. J. Food Process. Preserv. 2021, 45, e15915. [Google Scholar] [CrossRef]
- Jayasuriya, H.; Pathare, P.B.; Al-Attabi, Z.; Al-Hamdani, A. Drying kinetics and quality analysis of coriander leaves dried in an indirect, stand-alone solar dryer. Processes 2023, 11, 1596. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists Inc.: Arlington, VA, USA, 2000. [Google Scholar]
- Ghosh, P.; Das, P.; Mukherjee, R.; Banik, S.; Karmakar, S.; Chatterjee, S. Extraction and quantification of pigments from Indian traditional medicinal plants: A comparative study between tree, shrub, and herb. Int. J. Pharm. Sci. Res. 2018, 9, 3052–3059. [Google Scholar]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern methods of pre-treatment of plant material for the extraction of bioactive compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.B.; Chen, G.L.; Guo, M.Q. Antioxidant and anti-inflammatory activities of the crude extracts of Moringa oleifera from Kenya and their correlations with flavonoids. Antioxidants 2019, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Mwamatope, B.; Tembo, D.; Chikowe, I.; Kampira, E.; Nyirenda, C. Total phenolic contents and antioxidant activity of Senna singueana, Melia azedarach, Moringa oleifera and Lannea discolor herbal plants. Sci. Afr. 2020, 9, e00481. [Google Scholar] [CrossRef]
- Lahare, R.P.; Yadav, H.S.; Bisen, Y.K.; Dashahre, A.K. Estimation of total phenol, flavonoid, tannin and alkaloid content in different extracts of Catharanthus roseus from Durg district, Chhattisgarh, India. Sch. Bull. 2021, 7, 1–6. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Boluda-Aguilar, M.; Taboada-Rodrıguez, A.; Lopez Gomez, A.; Marın-Iniesta, F.; Barbosa-Canovas, G.V. Quick Cooking Rice by High Hydrostatic Pressure Processing. LWT Food Sci. Technol. 2013, 51, 196–204. [Google Scholar] [CrossRef]
- Lai, K.M.; Cheng, Y.Y.; Tsai, T.H. Integrated LC-MS/MS analytical systems and physical inspection for the analysis of a botanical herbal preparation. Molecules 2015, 20, 10641–10656. [Google Scholar] [CrossRef]
- Nisha, N.; Singh, R.; Sharma, N.; Mohite, A.M. Studies on Different Drying Techniques to Utilized it for Powder Properties for Dried Dill (Anethum graveolens). Bull. Transilv. Univ. Brasov. Ser. II For. Wood Ind. Agric. Food Eng. 2024, 17, 189–202. [Google Scholar] [CrossRef]
- Kumar, V.; Gupta, A.K.; Naik, B.; Makroo, H.A. Effect of high γ-irradiation dosage on physico-chemical, functional and emulsion properties of almond gum powder. Int. J. Bio. Macromol. 2023, 235, 123898. [Google Scholar] [CrossRef]
- Darniadi, S.; Ho, P.; Murray, B.S. Comparison of blueberry powder produced via foam-mat freeze-drying versus spray-drying: Evaluation of foam and powder properties. J. Sci. Food Agric. 2018, 98, 2002–2010. [Google Scholar] [CrossRef]
- Kassegn, H.H.; Mekelle, E.P. Determination Proximate Composition of the Wild Abyssinian Thyme Herb (Thyme schimperi L.) Grown in High Lands of Southern Tigray, North Ethiopia. Food Sci. Qual. Manag. 2016, 50, 1–3. [Google Scholar]
- Murcia, M.A.; López-Ayerra, B.; Martinez-Tomé, M.; Vera, A.M.; García-Carmona, F. Evolution of ascorbic acid and peroxidase during industrial processing of broccoli. J. Sci. Food Agric. 2000, 80, 1882–1886. [Google Scholar] [CrossRef]
- Bahçeci, K.S.; Serpen, A.; Gökmen, V.; Acar, J. Study of lipoxygenase and peroxidase as indicator enzymes in green beans: Change of enzyme activity, ascorbic acid and chlorophylls during frozen storage. J. Food Eng. 2005, 66, 187–192. [Google Scholar] [CrossRef]
- De Oliveira, A.M.F.; Pinheiro, L.S.; Pereira, C.K.S.; Matias, W.N.; Gomes, R.A.; Chaves, O.S.; de Assis, T.S. Total phenolic content and antioxidant activity of some Malvaceae family species. Antioxidants 2012, 1, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Periche, A.; Castelló, M.L.; Heredia, A.; Escriche, I. Effect of different drying methods on the phenolic, flavonoid and volatile compounds of Stevia rebaudiana leaves. Flavour Fragr. J. 2016, 31, 173–177. [Google Scholar] [CrossRef]
- Mohd Zainol, M.K.; Abdul-Hamid, A.; Abu Bakar, F.; Pak Dek, S. Effect of different drying methods on the degradation of selected flavonoids in Centella asiatica. Int. Food Res. J. 2009, 16, 531–537. [Google Scholar]
- Laoretani, D.; Fernández, M.; Crapiste, G.; Nolasco, S. Effect of drying operating conditions on canola oil tocopherol content. Antioxidants 2014, 3, 190–199. [Google Scholar] [CrossRef]
- Aggarwal, S.; Mohite, A.M.; Sharma, N. The maturity and ripeness phenomenon with regard to the physiology of fruits and vegetables: A review. Bull. Transilv. Univ. Brasov. Ser. II For. Wood Ind. Agric. Food Eng. 2018, 11, 77–88. [Google Scholar]
- Julkunen-Tiitto, R.; Sorsa, S. Testing the effects of drying methods on willow flavonoids, tannins, and salicylates. J. Chem. Ecol. 2001, 27, 779–789. [Google Scholar] [CrossRef]
- Khodaie, L.; Sharma, A.; Shah, P.J.; Surana, V. The relationship between the cold and dry nature of herbs and their tannin content: Bridging traditional knowledge and modern-day science. J. Res. Pharm. 2023, 27, p2487. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Murtijaya, J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Sci. Tech. 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Abhiram, G.; Briyangari, A.; Eeswaran, R. Drying of Gymnema sylvestre Using Far-Infrared Radiation: Antioxidant Activity and Optimization of Drying Conditions. AgriEngineering 2023, 5, 611–622. [Google Scholar] [CrossRef]
- Prasert, W.; Suwannaporn, P. Optimization of Instant Jasmine Rice Process and Its Physicochemical Properties. J. Food Eng. 2009, 95, 54–61. [Google Scholar] [CrossRef]
- Fapetu, A.P.; Karigidi, K.O.; Akintimehin, E.S.; Olawuwo, T.; Adetuyi, F.O. Effect of partial substitution of wheat flour with Moringa oleifera leaf powder on physical, nutritional, antioxidant and antidiabetic properties of cookies. Bull. Natl. Res. Cent. 2022, 46, 53. [Google Scholar] [CrossRef]
- Wang, R.; Liu, J.; Zhang, J.; Li, G.; Ding, S. Effect of thermal blanching on the inactivation kinetics of polyphenol oxidase and peroxidase in lily bulb, and the functional properties of its flours. J. Food Meas. Charact. 2023, 17, 615–626. [Google Scholar] [CrossRef]
- Agbo, C.; Jakpa, W.; Sarkodie, B.; Boakye, A.; Fu, S. A review on the mechanism of pigment dispersion. J. Dispers. Sci. Technol. 2018, 39, 874–889. [Google Scholar] [CrossRef]
- Helgeson, M.E. Colloidal behavior of nanoemulsions: Interactions, structure, and rheology. Curr. Opin. Colloid Interface Sci. 2016, 25, 39–50. [Google Scholar] [CrossRef]
- Chettri, S.; Rizwana, R.; Mohite, A.M. Selected Physicochemical and Functional Properties of Extracted Soybean Starch. Bull. Transilv. Univ. Brasov. Ser. II For. Wood Ind. Agric. Food Eng. 2022, 15, 139–150. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Phosanam, A.; Stockmann, R. Perspectives on saponins: Food functionality and applications. Int. J. Mol. Sci. 2023, 24, 13538. [Google Scholar] [CrossRef]
- Ramírez-Fajardo, A.F.; Martín-Vizcaíno, C.; Rodríguez-García, I.; Guil-Guerrero, J.L. Vegetables Treated before Drying with Natural Antioxidants plus UV-C Improve Colour and Bioactive Compounds. AgriEngineering 2024, 6, 3635–3651. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).