Reducing Ammonia Emissions from Digested Animal Manure: Effectiveness of Acidification, Open Disc Injection, and Fertigation in Mediterranean Cereal Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Determinations
2.1.1. Soil
2.1.2. Ammonia Emissions
2.1.3. Harvest
2.2. Data and Statistical Analysis
3. Results
3.1. Digestate Application
3.2. Yield and Soil Mineral Nitrogen
3.3. Ammonia Emissions
4. Discussion
4.1. Crop Response
4.2. Ammonia Emissions
4.3. Advantages and Limitations of the Application Systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EUROSTAT. Pig Population—Annual Data. Available online: https://ec.europa.eu/eurostat/databrowser/view/apro_mt_lspig/default/table?lang=en&category=agr.apro.apro_anip.apro_mt.apro_mt_ls (accessed on 7 May 2025).
- MAPA. Livestock Farming Surveys. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/ganaderia/encuestas-ganaderas/default.aspx (accessed on 7 May 2025).
- Yagüe, M.R.; Quílez, D. Response of maize yield, nitrate leaching, and soil nitrogen to pig slurry combined with mineral nitrogen. J. Environ. Qual. 2010, 39, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aguirre, M.T.; Isidoro, D. Hydrosaline Balance in and Nitrogen Loads from an irrigation district before and after modernization. Agric. Water Manag. 2018, 208, 163–175. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobena, A.; Garnier, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems. A review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef]
- Mateo-Marín, N.; Isla, R.; Quílez, D. Gaseous nitrogen losses from pig slurry fertilisation: Can they be reduced with additives in a wheat crop? Span. J. Agric. Res. 2021, 19, e0302. [Google Scholar] [CrossRef]
- Bosch-Serra, À.D.; Yagüe, M.R.; Teira-Esmatges, M.R. Ammonia emissions from different fertilizing strategies in Mediterranean rainfed winter cereals. Atmos. Environ. 2014, 84, 204–212. [Google Scholar] [CrossRef]
- Ti, C.; Xia, L.; Chang, S.X.; Yan, X. Potential for mitigating global agricultural ammonia emission: A meta-analysis. Environ. Pollut. 2019, 245, 141–148. [Google Scholar] [CrossRef]
- European Environmental Agency. European Union Emission Inventory Report 1990 2022—Under the UNECE Convention on Long Range Transboundary Air Pollution (Air Convention); Report 08/2024; European Environment Agency: Copenhagen, Denmark, 2024; pp. 47–67. [Google Scholar]
- MITECO. Informative Inventory Report, Submission to the Secretariat of the Geneva Convention and EMEP Programme Reporting to the European Commission Under Directive (EU) 2016/2284; Ministerio Para la Transición Ecológica y el Reto Demográfico: Madrid, Spain, 2024; pp. 87–89.
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. J. Environ. Manag. 2022, 323, 116285. [Google Scholar] [CrossRef]
- European Environmet Agency. Air Quality in Europe—2020 Report. EEA Report No 09/2020; European Environment Agency: Copenhagen, Denmark, 2020; pp. 6–8. [Google Scholar]
- MAPAMA. Guía de las Mejores Técnicas Disponibles para Reducir el Impacto Ambiental de la Ganadería; Ministerio de Agricultura Pesca, Alimentación y Medio Ambiente: Madrid, Spain, 2017; pp. 1–137.
- Pan, B.; Lam, S.K.; Mosier, A.; Luo, Y.; Chen, D. Ammonia volatilization from synthetic fertilizers and its mitigation strategies: A global synthesis. Agric. Ecosyst. Environ. 2016, 232, 283–289. [Google Scholar] [CrossRef]
- Hurtado, J.; Velázquez, E.; Lassaletta, L.; Guardia, G.; Aguilera, E.; Sanz-Cobena, A. Drivers of ammonia volatilization in Mediterranean climate cropping systems. Environ. Pollut. 2024, 341, 122814. [Google Scholar] [CrossRef]
- Søgaard, H.T.; Sommer, S.G.; Hutchings, N.J.; Huijsmans, J.F.M.; Bussink, D.W.; Nicholson, F. Ammonia volatilization from field-applied animal slurry—The ALFAM model. Atmos. Environ. 2002, 36, 3309–3319. [Google Scholar] [CrossRef]
- Sommer, S.G.; Génermont, S.; Cellier, P.; Hutchings, N.J.; Olesen, J.E.; Morvan, T. Processes controlling ammonia emission from livestock slurry in the field. Eur. J. Agron. 2003, 19, 465–486. [Google Scholar] [CrossRef]
- Hafner, S.D.; Pacholski, A.; Bittman, S.; Carozzi, M.; Chantigny, M.; Génermont, S.; Häni, C.; Hansen, M.N.; Huijsmans, J.; Kupper, T.; et al. A flexible semi-empirical model for estimating ammonia volatilization from field-applied slurry. Atmos. Environ. 2019, 199, 474–484. [Google Scholar] [CrossRef]
- Hafner, S.D.; Pedersen, J.; Fuß, R.; Kamp, J.N.; Dalby, F.R.; Amon, B.; Pacholski, A.; Adamsen, A.P.S.; Sommer, S.G. Improved tools for estimation of ammonia emission from field-applied animal slurry: Refinement of the ALFAM2 model and database. Atmos. Environ. 2025, 340, 120910. [Google Scholar] [CrossRef]
- Silva, A.A.; Carvalho, M.; Coutinho, J.; Vasconcelos, E.; Fangueiro, D. Dairy slurry application to stubble-covered soil: A study on sustainable alternatives to minimize gaseous emissions. Agriculture 2022, 12, 1021. [Google Scholar] [CrossRef]
- Pedersen, J.; Nyord, T. Effect of low-dose acidification of slurry digestate on ammonia emissions after field application. Atmos. Environ. 2023, 17, 100205. [Google Scholar] [CrossRef]
- Andersson, K.; Delin, S.; Pedersen, J.; Hafner, S.D.; Nyord, T. Ammonia emissions from untreated, separated and digested cattle slurry—effects of slurry type and application strategy on a Swedish clay soil. Biosyst. Eng. 2023, 226, 194–208. [Google Scholar] [CrossRef]
- Langley-Randall, J.; Jones, D.L.; Cotton, J.; Williams, J.R.; Chadwick, D.R. Slurry acidification is as effective as slurry injection at reducing ammonia emissions without increasing N2O emissions: A short-term mesocosm study. Geoderma Reg. 2024, 37, e00791. [Google Scholar] [CrossRef]
- Ellersiek, N.C.T.; Olfs, H.-W. An incubation system for the simulation of ammonia emissions from soil surface-applied slurry: Effect of pH and acid type. Agronomy 2024, 14, 1078. [Google Scholar] [CrossRef]
- Keskinen, R.; Termonen, M.; Salo, T.; Luostarinen, S.; Räty, M. Slurry acidification outperformed injection as an ammonia emission-reducing technique in boreal grass cultivation. Nutr. Cycl. Agroecosyst. 2022, 122, 139–156. [Google Scholar] [CrossRef]
- Pedersen, J.; Andersson, K.; Feilberg, A.; Delin, S.; Hafner, S.; Nyord, T. Effect of exposed surface area on ammonia emissions from untreated, separated, and digested cattle manure. Biosyst. Eng. 2021, 202, 66–78. [Google Scholar] [CrossRef]
- Fangueiro, D.; Hjorth, M.; Gioelli, F. Acidification of animal slurry—A review. J. Environ. Manag. 2015, 149, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, D.; Surgy, S.; Fraga, I.; Cabral, F.; Coutinho, J. Band application of treated cattle slurry as an alternative to slurry injection: Implications for gaseous emissions, soil quality, and plant growth. Agric. Ecosyst. Environ. 2015, 211, 102–111. [Google Scholar] [CrossRef]
- Sommer, S.G.; Jensen, L.S.; Clausen, S.B.; SØGaard, H.T. Ammonia volatilization from surface-applied livestock slurry as affected by slurry composition and slurry infiltration depth. J. Agric. Sci. 2006, 144, 229–235. [Google Scholar] [CrossRef]
- Pedersen, J.; Nyord, T.; Feilberg, A.; Labouriau, R.; Hunt, D.; Bittman, S. Effect of reduced exposed surface area and enhanced infiltration on ammonia emission from untreated and separated cattle slurry. Biosyst. Eng. 2021, 211, 141–151. [Google Scholar] [CrossRef]
- Pedersen, J.; Hafner, S.D. Ammonia emissions after field application of anaerobically digested animal slurry: Literature review and perspectives. Agric. Ecosyst. Environ. 2023, 357, 108697. [Google Scholar] [CrossRef]
- Møller, H.B.; Sørensen, P.; Olesen, J.E.; Petersen, S.O.; Nyord, T.; Sommer, S.G. Agricultural biogas production—Climate and environmental impacts. Sustainability 2022, 14, 1849. [Google Scholar] [CrossRef]
- Efosa, N.; Häni, C.; Kupper, T.; Krause, H.-M.; Six, J.; Bünemann, E.K. Ammonia emissions after trailing hose application of digestates and cattle slurry. Nutr. Cycl. Agroecosyst. 2025, 130, 419–426. [Google Scholar] [CrossRef]
- Herrero, E.; Quílez, D.; Daudén, A.; Salvador, R.; Guillén, M.; Abió, D.; Crespo, A.; Gea, R. Fertigation with pig slurry in demonstration fields in Aragon. In Ammonia Emission Reduction in Mediterranean Agriculture with Innovative Slurry Fertigation Techniques; Quilez, D., Herrero, E., Provolo, G., Eds.; Centro de Investigación y Tecnología Agroalimentaria de Aragón: Zaragoza, Spain, 2021; pp. 58–75. [Google Scholar]
- Ellersiek, N.C.T.; Olfs, H.-W. Lessons learnt from the use of passive samplers to measure ammonia emissions in multi-plot experiments. Plant Soil Environ. 2024, 70, 760–771. [Google Scholar] [CrossRef]
- Kamp, J.N.; Hafner, S.D.; Huijsmans, J.; van Boheemen, K.; Götze, H.; Pacholski, A.; Pedersen, J. Comparison of two micrometeorological and three enclosure methods for measuring ammonia emission after slurry application in two field experiments. Agric. For. Meteorol. 2024, 354, 110077. [Google Scholar] [CrossRef]
- Nyameasem, J.K.; Zutz, M.; Kluß, C.; Huf, M.T.; Essich, C.; Buchen-Tschiskale, C.; Ruser, R.; Flessa, H.; Olfs, H.-W.; Taube, F.; et al. Impact of cattle slurry application methods on ammonia losses and grassland nitrogen use efficiency. Environ. Pollut. 2022, 315, 120302. [Google Scholar] [CrossRef]
- Loyon, L.; Guiziou, F. Ammonia volatilization from different pig slurries applied on wheat stubble using different land spreading techniques under French conditions. Agric. Ecosyst. Environ. 2019, 280, 114–117. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic forms. In Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A., Ed.; Amercian Society of Agronomy Inc.: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar] [CrossRef]
- Flesch, T.K.; Wilson, J.D.; Harper, L.A.; Crenna, B.P.; Sharpe, R.R. Deducing ground-to-air emissions from observed trace gas concentrations: A field trial. J. Appl. Meteorol. 2004, 43, 487–502. [Google Scholar] [CrossRef]
- Loubet, B.; Génermont, S.; Ferrara, R.; Bedos, C.; Decuq, C.; Personne, E.; Fanucci, O.; Durand, B.; Rana, G.; Cellier, P. An inverse model to estimate ammonia emissions from fields. Eur. J. Soil Sci. 2010, 61, 793–805. [Google Scholar] [CrossRef]
- Herrero, E.; Sanz-Cobena, A.; Guido, V.; Guillén, M.; Dauden, A.; Rodríguez, R.; Provolo, G.; Quílez, D. Towards robust on-site ammonia emission measuring techniques based on inverse dispersion modeling. Agric. For. Meteorol. 2021, 307, 108517. [Google Scholar] [CrossRef]
- International VERA Secretariat. Test Protocol for Measurement of Gaseous Emissions from Land Applied Manure; Version 1; (9 December 2019); International VERA Secretariat: Delft, The Netherlands, 2009. [Google Scholar]
- Tang, Y.S.; Cape, J.N.; Sutton, M.A. Development and types of passive samplers for monitoring atmospheric NO2 and NH3 concentrations. Sci. World J. 2001, 1, 513–529. [Google Scholar] [CrossRef]
- Searle, P.L. The berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen: A review. Analyst 1984, 109, 549–568. [Google Scholar] [CrossRef]
- Mateo-Marín, N.; Isla, R.; Guillén, M.; Quílez, D. Agronomic and environmental implications of substituting pig slurry for synthetic nitrogen in Mediterranean wheat systems. Agronomy 2020, 10, 1498. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Fisheries and Food. Encuesta Sobre Superficies y Rendimientos Cultivos (ESYRCE). Available online: https://www.mapa.gob.es/es/estadistica/temas/estadistica-digital/powerbi-esyrce.aspx (accessed on 8 May 2025).
- Quilez, D.; Guillén, M.; Vallés, M.; Daudén, A.; Moreno-García, B. New insights into fertilisation with animal manure for annual double-cropping systems in nitrate-vulnerable zones of Northeastern Spain. Agronomy 2025, 15, 142. [Google Scholar] [CrossRef]
- Yagüe, M.R.; Iguácel, F.; Orús, F. Fertilización con purín: Resultados agronómicos en doble cultivo anual de cebada-maíz y efecto residual en cebada (2006–2012). Inf. Técnicas Gob. Aragón 2013, 244, 1–16. Available online: https://digital.csic.es/bitstream/10261/86478/1/Yag%c3%bceMR_InfTec_2013.pdf (accessed on 14 May 2025).
- Oenema, O.; Brentrup, F.; Lammel, J.; Bascou, P.; Billen, G.; Dobermann, A.; Erisman, J.W.; Garnett, T.; Hammel, M.; Haniotis, T.; et al. Nitrogen Use Efficiency (NUE)—An Indicator for the Utilization of Nitrogen in Agriculture and Food Systems; Wageningen University: Wageningen, The Netherlands, 2015. [Google Scholar]
- Bash, J.O.; Walker, J.T.; Katul, G.G.; Jones, M.R.; Nemitz, E.; Robarge, W.P. Estimation of in-canopy ammonia sources and sinks in a fertilized Zea mays field. Environ. Sci. Technol. 2010, 44, 1683–1689. [Google Scholar] [CrossRef]
- Bussink, D.W.; Huijsmans, J.F.M.; Ketelaars, J.J.M.H. Ammonia volatilization from nitric-acid-treated cattle slurry surface applied to grassland. Neth. J. Agric. Sci. 1994, 42, 293–309. [Google Scholar] [CrossRef]
- Lee, S.B.; Park, S.H.; Lee, B.R.; Kim, T.H. Acidification and biochar effect on ammonia emission and nitrogen use efficiency of pig slurry in the vegetative growth of maize (Zea mays L.). J. Korean Soc. Grassl. Forage Sci. 2022, 42, 47–53. [Google Scholar] [CrossRef]
- Stevens, R.J.; Laughlin, R.J.; Frost, J.P. Effects of separation, dilution, washing and acidification on ammonia volatilization from surface-applied cattle slurry. J. Agric. Sci. 1992, 119, 383–389. [Google Scholar] [CrossRef]
- Nyord, T.; Hafner, S.D.; Adamsen, A.P.; Sommer, S.G. Ammonia Evaporation from Acidified Slurry When Applied with a Drag Hose. In Academic Note that Serves as a Response to the Order Correlation Between the Addition of Acid Amounts to Different Types of Slurry, Achieved pH and Ammonia Reduction (Sammenhæng Mellem Tilsætning af Syremængder til Forskellige Gylletyper, Opnået pH og Ammoniakreduktion); Journal 2020-0188079; DCA, Aarhus Universitet: Tjele, Denmark, 2021; Available online: https://www.ft.dk/samling/20201/almdel/MOF/bilag/448/2360704.pdf (accessed on 15 October 2025).
- Misselbrook, T.H.; Smith, K.A.; Johnson, R.A.; Pain, B.F. SE—Structures and environment: Slurry application techniques to reduce ammonia emissions: Results of some UK field-scale experiments. Biosyst. Eng. 2002, 81, 313–321. [Google Scholar] [CrossRef]
- Webb, J.; Pain, B.; Bittman, S.; Morgan, J. The impacts of manure application methods on emissions of ammonia, nitrous oxide and on crop response—A review. Agric. Ecosyst. Environ. 2010, 137, 39–46. [Google Scholar] [CrossRef]
- Wagner, C.; Nyord, T.; Vestergaard, A.V.; Hafner, S.D.; Pacholski, A.S. Acidification effects on in situ ammonia emissions and cereal yields depending on slurry type and application method. Agriculture 2021, 11, 1053. [Google Scholar] [CrossRef]
- Bittman, S.; Dedina, M.; Howard, C.M.; Oenema, O.; Sutton, M.A. Options for Ammonia Mitigation: Guidance from the UNECE Task Force on Reactive Nitrogen; Centre for Ecology and Hydrology: Edinburgh, UK, 2014. [Google Scholar]
- Finzi, A.; Guido, V.; Riva, E.; Ferrari, O.; Quilez, D.; Herrero, E.; Provolo, G. Performance and sizing of filtration equipment to replace mineral fertilizer with digestate in drip and sprinkler fertigation. J. Clean. Prod. 2021, 317, 128431. [Google Scholar] [CrossRef]
- Dinuccio, E.; Gioelli, F.; Balsari, P.; Dorno, N. Ammonia losses from the storage and application of raw and chemo-mechanically separated slurry. Agric. Ecosyst. Environ. 2012, 153, 16–23. [Google Scholar] [CrossRef]
- Gioelli, F.; Dinuccio, E.; Balsari, P. Residual biogas potential from the storage tanks of non-separated digestate and digested liquid fraction. Bioresour. Technol. 2011, 102, 10248–10251. [Google Scholar] [CrossRef]
- Bhandral, R.; Bittman, S.; Kowalenko, G.; Buckley, K.; Chantigny, M.H.; Hunt, D.E.; Bounaix, F.; Friesen, A. Enhancing soil infiltration reduces gaseous emissions and improves N uptake from applied dairy slurry. J. Environ. Qual. 2009, 38, 1372–1382. [Google Scholar] [CrossRef]
- Lombardi, B.; Orden, L.; Varela, P.; Garay, M.; Iocoli, G.A.; Montenegro, A.; Sáez-Tovar, J.; Bustamante, M.Á.; Juliarena, M.P.; Moral, R. Is dairy effluent an alternative for maize crop fertigation in semiarid regions? An approach to agronomic and environmental effects. Animals 2022, 12, 2025. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Qi, X.; Shi, C.; Yang, S.; Wu, Y. Tomato comprehensive quality evaluation and irrigation mode optimization with biogas slurry based on the combined evaluation model. Agronomy 2022, 12, 1391. [Google Scholar] [CrossRef]
- Capra, F.; Ardenti, F.; Abalos, D.; Lommi, M.; Pochintesta, D.; Ganugi, P.; Perego, A.; Tabaglio, V.; Fiorini, A. Drip fertigation with slurry as a promising tool to reduce nitrogen losses under organic maize. Sci. Rep. 2025, 15, 16890. [Google Scholar] [CrossRef]
- Gamble, J.D.; Feyereisen, G.W.; Papiernik, S.K.; Wente, C.D.; Baker, J.M. Summer fertigation of dairy slurry reduces soil nitrate concentrations and subsurface drainage nitrate losses compared to fall injection. Front. Sustain. Food Syst. 2018, 2, 15. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Liang, X.; Wang, J.; Qiu, X.; Wang, C. Replacing chemical fertilizers with biogas slurry is an environment friendly strategy to reduce the risk of soil nitrogen leaching: Evidence from the HYDRUS model simulation. Agric. Ecosyst. Environ. 2024, 369, 109043. [Google Scholar] [CrossRef]
- Provolo, G.; Guido, V.; Guidetti, A.; Finzi, A.; Herrero, E.; Quílez, D. Fertigation with digestate in demonstration fields in Lombardy (Italy). In Ammonia Emission Reduction in Mediterranean Agriculture with Innovative Slurry Fertigation Techniques; Quilez, D., Herrero, E., Provolo, G., Eds.; Centro de Investigación y Tecnología Agroalimentaria de Aragón: Zaragoza, Spain, 2021; pp. 76–91. [Google Scholar]
- Ricco, C.R.; Finzi, A.; Guido, V.; Riva, E.; Ferrari, O.; Provolo, G. Evaluation of ammonia emissions from filtration of digestate used for fertigation. J. Agric. Eng. 2021, 52, 1187. [Google Scholar] [CrossRef]
- Baldé, H.; VanderZaag, A.C.; Burtt, S.D.; Wagner-Riddle, C.; Evans, L.; Gordon, R.; Desjardins, R.L.; MacDonald, J.D. Ammonia emissions from liquid manure storages are affected by anaerobic digestion and solid-liquid separation. Agric. For. Meteorol. 2018, 258, 80–88. [Google Scholar] [CrossRef]
- Daudén, A.; Provolo, G.; Guidetti, A.; Gea, R.; Quílez, D.; Mallén, E.H. Economic analysis of fertigation in different scenarios. In Ammonia Emission Reduction in Mediterranean Agriculture with Innovative Slurry Fertigation Techniques; Quilez, D., Herrero, E., Provolo, G., Eds.; Centro de Investigación y Tecnología Agroalimentaria de Aragón: Zaragoza, Spain, 2021; pp. 106–119. [Google Scholar]
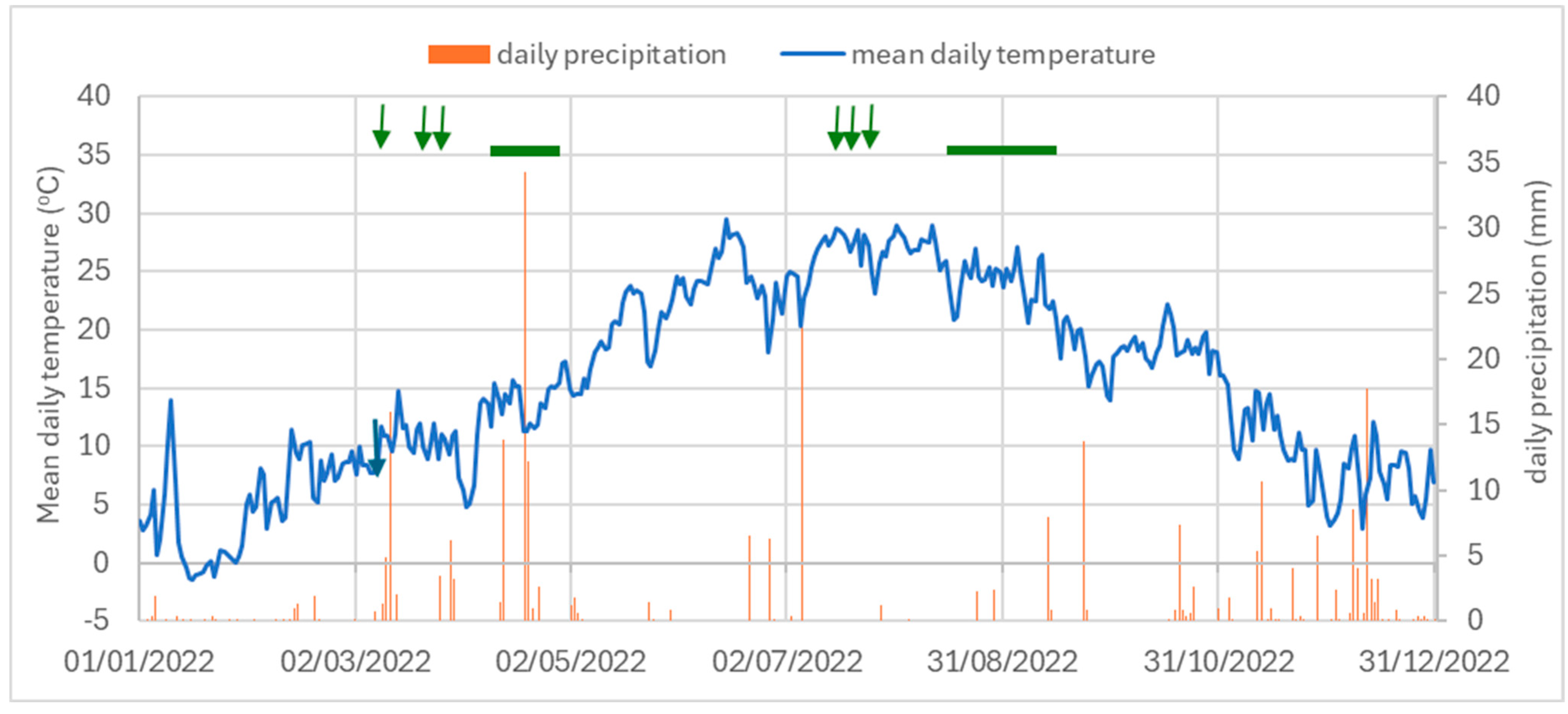
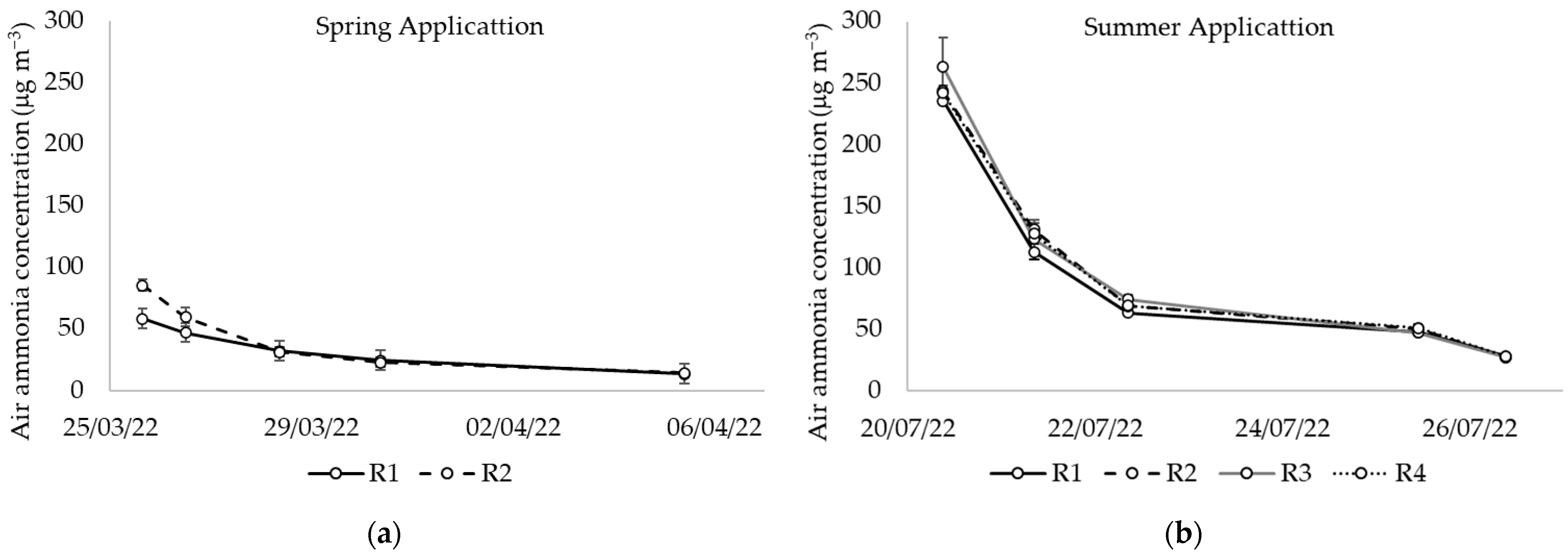
| Spring Application (Wheat) | Summer Application (Maize) | |||||
|---|---|---|---|---|---|---|
| Area ha | Date | NH3 Control Period | Area ha | Date | NH3 Control Period | |
| Digestate | ||||||
| Reference | 0.71 | 24/03 | 24/03–05/04 | 0.85 | 19/07 | 19/07–27/07 |
| Open Discs | 0.86 | 22/03 | 22/03–30/03 | 0.95 | 18/07 | 18/07–26/07 |
| Acidification | 0.60 | 10/03 | 10/03–22/03 | 0.73 | 25/07 | 25/07–31/07 |
| Fertigation | 6.27 | 11/04–29/04 | 8/04–3/05 | 6.27 | 17/08–16/09 | 17/08–21/09 |
| Urea | ||||||
| Reference | 0.85 | 31/08 | 31/08–13/09 | |||
| Acidification + Open discs | 3.47 | 31/08 | 31/08–13/09 | |||
| Concentration | N Rate | |||||
|---|---|---|---|---|---|---|
| Area m2 | Digestate Rate m3 ha−1 | TKN kg m−3 | TAN kg m−3 | TKN kg ha−1 | TAN kg ha−1 | |
| Spring (wheat) | ||||||
| Reference | 0.71 | 71.6 | 2.78 (±0.01) | 1.35 (±0.01) | 199.0 (±0.4) | 96.2 (±0.4) |
| Open Discs | 0.86 | 73.6 | 2.70 (±0.07) | 1.35 (±0.01) | 198.8 (±5.2) | 99.2 (±0.6) |
| Acidification | 0.60 | 52.9 | 1.98 (±0.06) | 1.30 (±0.01) | 104.8 (±3.2) | 68.8 (±0.0) |
| Fertigation | 6.27 | 29.8 | 1.36 (±0.02) | 1.08 (±0.03) | 41.7 (±0.6) | 31.3 (±0.8) |
| Summer (Maize) | ||||||
| Reference | 0.85 | 73.9 | 1.71 (±0.06) | 1.18 (±0.06) | 126.1 (±4.7) | 87.4 (±4.4) |
| Open Discs | 0.95 | 66.4 | 1.59 (±0.15) | 1.21 (±0.02) | 105.4 (±10.2) | 80.4 (±1.4) |
| Acidification | 0.73 | 54.7 | 1.16 (±0.03) | 1.04 (±0.03) | 63.5 (±1.6) | 56.9 (±1.6) |
| Fertigation | 6.27 | 108.8 | 1.35 (±0.10) | 1.08 (±0.02) | 142.0 (±10.7) | 118.8 (±2.3) |
| Grain Yield (kg ha−1) | Grain N Concentration (%) | N in Grain (kg N ha−1) | N Uptake (kg N ha−1) | NUE | |
|---|---|---|---|---|---|
| Wheat | |||||
| Reference | 4853.3 (±533.2) | 1.94 (±0.11) | 85.4 (±8.2) | 167.6 (±18.0) | 0.84 a |
| Open Discs | 5530.7 (±850.2) | 1.59 (±0.05) | 81.4 (±14.7) | 168.8 (±30.4) | 0.84 a |
| Acidification | 5870.6 (±679.6) | 1.85 (±0.22) | 100.3 (±17.3) | 220.3 (±49.6) | 2.10 b |
| Fertigation | 6660.4 (±341.2) | 1.92 (±0.05) | 117.5 (±7.5) | 206.4 (±17.1) | 2.03 b |
| p 1 | ns | ns | ns | ns | 0.000 |
| Maize | |||||
| Reference | 6021.6 (±205.3) a | 1.35 (±0.07) | 74.4 (±2.7) a | 143.3 (±8.6) | 0.50 a |
| Open Discs | 6505.4 (±340.2) a | 1.39 (±0.01) | 82.3 (±2.9) ab | 160.0 (±4.1) | 0.60 ab |
| Acidification | 7387.9 (±283.5) b | 1.36 (±0.02) | 92.2 (±4.6) b | 159.5 (±5.8) | 0.71 b |
| Fertigation | 6707.3 (±345.2) ab | 1.39 (±0.01) | 85.2 (±4.1) ab | 161.7 (±4.4) | 1.14 c |
| p 1 | 0.046 | ns | 0.037 | ns | 0.006 |
| Nmin (0–30 cm) 4 July 2022 (kg N ha−1) | Nmin (0–90 cm) 14 November 2022 (kg N ha−1) | |
|---|---|---|
| Reference | 26.5 a | 96.4 |
| Open Discs | 22.8 a | 103.8 |
| Acidification | 18.6 a | 165.1 |
| Fertigation | 43.7 b | 65.6 |
| p 1 | 0.0005 | ns |
| TKN | TAN | NH3–N Emissions | Reduction vs. Reference 1 | |||
|---|---|---|---|---|---|---|
| (kg N ha−1) | (kg N ha−1) | (kg N ha−1) 2 | % TKN | % TAN 2 | % | |
| Reference | 199.0 | 96.0 | 13.7 a | 6.9% | 14.3% a | - |
| Open Discs | 198.8 | 99.4 | 8.4 ab | 4.2% | 8.5% ab | 40.5% |
| Acidification | 97.7 | 64.2 | 1.9 b | 1.9% | 2.9% b | 79.4% |
| Fertigation | 101.7 | 76.3 | 5.0 b | 4.9% | 6.6% b | 53.8% |
| N Total | NH4+–N Rate | NH3–N Emissions | Reduction vs. Reference 1 | |||
|---|---|---|---|---|---|---|
| (kg N ha−1) | (kg N ha−1) | (kg N ha−1) 2 | % N Total | % TAN 2 | % | |
| Reference | 126.1 | 87.4 | 26.4 a | 20.9% | 30.2% a | - |
| Open Discs | 105.4 | 80.4 | 5.8 b | 5.5% | 7.2% b | 76.2% |
| Acidification | 63.5 | 56.9 | 1.4 b | 2.1% | 2.4% b | 92.1% |
| N Total (kg N ha−1) | NH3–N Emission | Reduction vs. Reference 1 | |||||
|---|---|---|---|---|---|---|---|
| Basal | Side Dressing | Basal kg N ha−1 | Side Dressing (kg N ha−1) | Total (kg N ha−1) | % TAN 2 | % | |
| Reference | 126.1 | 160 | 26.4 | 21.4 | 47.7 | 19.3% a | - |
| Open Discs | 105.4 | 160 | 5.8 | 21.4 | 27.1 | 11.2% b | 41.5% |
| Acidification | 63.5 | 160 | 1.4 | 21.4 | 22.7 | 10.5% b | 45.7% |
| Fertigation | - | 142 | - | 5.5 | 5.5 | 4.7% c | 75.9% |
| Method | Advantages | Limitations |
|---|---|---|
| Acidification | The most efficient in reducing digestate emissions in spring topdressing and summer basal application | Difficulty in acidifying during application. Complex acidification handling. Custom application equipment requiring high investment. |
| Disc Injection | Efficient in reducing ammonia emissions both in spring topdressing and summer basal application | Requires more mechanical traction than trailing hose application. Difficult to apply on wet or stony soils. |
| Fertigation | Highly efficient in reducing emissions from fertilization more important in summer crops. It is very simple if fields are located near a farm/anaerobic digestion facility. Increases nitrogen use efficiency and allows for a reduction in N dose. | Requires separation of slurry and investment in both a separator and injection pump. Difficulty applying in spring if it is rainy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quilez, D.; Balcells, M.; Herrero, E. Reducing Ammonia Emissions from Digested Animal Manure: Effectiveness of Acidification, Open Disc Injection, and Fertigation in Mediterranean Cereal Systems. AgriEngineering 2025, 7, 352. https://doi.org/10.3390/agriengineering7100352
Quilez D, Balcells M, Herrero E. Reducing Ammonia Emissions from Digested Animal Manure: Effectiveness of Acidification, Open Disc Injection, and Fertigation in Mediterranean Cereal Systems. AgriEngineering. 2025; 7(10):352. https://doi.org/10.3390/agriengineering7100352
Chicago/Turabian StyleQuilez, Dolores, Maria Balcells, and Eva Herrero. 2025. "Reducing Ammonia Emissions from Digested Animal Manure: Effectiveness of Acidification, Open Disc Injection, and Fertigation in Mediterranean Cereal Systems" AgriEngineering 7, no. 10: 352. https://doi.org/10.3390/agriengineering7100352
APA StyleQuilez, D., Balcells, M., & Herrero, E. (2025). Reducing Ammonia Emissions from Digested Animal Manure: Effectiveness of Acidification, Open Disc Injection, and Fertigation in Mediterranean Cereal Systems. AgriEngineering, 7(10), 352. https://doi.org/10.3390/agriengineering7100352






