Abstract
The efficient degradation of lignin from agricultural by-products is a critical step in the development of sustainable bioprocessing technologies for waste valorisation. Enzymatic degradation of kraft lignin performed with lignin peroxidase (LiP), manganese peroxidase (MnP), and laccase (Lac) was investigated. A response surface methodology (RSM) based on a Box–Behnken Design (BBD) was employed in order to optimise key process parameters including enzyme concentration, lignin concentration, pH, incubation temperature, and activator concentration. The surface plots were used to determine the best conditions for each enzyme in order to better degrade kraft lignin. Therefore, LiP needed a stronger acidic environment and moderate temperature, MnP needed an almost neutral pH and moderate temperature, and Lac needed a neutral pH and higher temperature. This work contributes to the development of smart agricultural waste management practices by combining enzymatic treatments with statistical modelling for process optimisation. This study provides a framework for lignin degradation that can be used as a starting point for diverse lignocellulosic by-product fragmentation, thus supporting a circular bioeconomy initiative in accordance with today’s trends. The optimised enzymatic parameters could help enhance efficiency, enable process standardisation across feedstocks, and support economically and environmentally sustainable industrial-scale lignin valorisation in integrated biorefineries.
1. Introduction
The world’s annual production of lignocellulose biomass is estimated to be around 182 billion tons, though only a fraction, approximately 8.2 billion tons, is currently used in different applications. The vast availability of plant-based biomass indicates a significant opportunity for conversion into a diverse range of bioproducts across many sectors. Advances in lignocellulose biorefineries are instrumental in developing sustainable energy, bioplastics, biocomposites, and other products derived from this renewable resource [1]. In order to effectively convert lignocellulosic biomass into valuable products, several pretreatments are required, particularly those that facilitate the separation of its constituent components for efficient fermentation processes. While numerous existing methods rely on the use of toxic substances, there remains a critical need for the development and implementation of environmentally friendly alternatives. These environmentally friendly approaches are vital for reducing the ecological impact associated with biomass valorisation and ensuring the sustainability of its derived products [2].
Although there are many reports regarding lignocellulosic biomass valorisation [3,4], the efficiency is still low due mainly to the complexity and recalcitrance of the biomass, which comprises cellulose, hemicellulose, and lignin. The main components are interspersed, and therefore, their individual degradation is not possible due to the lack of access of specific enzymes to their preferred substrate. Amongst them, lignin is the hardest component to depolymerise mainly due to its structure being a cross-linked aromatic polymer that is resistant to chemical or biological degradation [5,6,7].
Lignin is characterised as an aromatic polymer featuring a complex three-dimensional network structure comprising three fundamental phenylpropanolic monomers: p-coumaryl, coniferyl, and sinapyl alcohols. These monolignols are integrated into the lignin macromolecule through guaicyl (G), syringil (S), and p-hydroxyphenyl (H) units, connected by various linkages such as β-O-4, β- β, β-5, α-O-4, 4-O-5, etc. [8].
Kraft lignin is the primary by-product of the kraft pulping process, in which lignocellulose biomass is treated with sodium hydroxide and sodium sulphide to solubilise lignin and liberate cellulose fibres for paper. During this process, the native lignin structure undergoes significant chemical modifications, including cleavage of β-O-4 linkages and incorporation of sulphur-containing groups, resulting in a heterogenous and condensed polymer. Most kraft lignin is currently used for energy recovery, but its abundance and aromatic nature make it a promising feedstock for producing value-added chemicals and materials. Prior to bioconversion, kraft lignin often requires pretreatment to improve solubility and accessibility to enzymes or catalysts [9].
The enzymes involved in the degradation of lignin are mainly oxidative enzymes such as laccase (Lac), lignin peroxidase (LiP), manganese peroxidase (MnP), and versatile peroxidase. Other enzymes either support oxidative reactions (aryl-alcohol oxidase, glyoxal oxidase, etc.) or are accessory enzymes that facilitate breaking the bonds between lignin polysaccharides (esterases, lipases, β-etherases, etc.) [10,11]. Among the major ligninolytic enzymes, LiP catalyses the oxidative cleavage of non-phenolic lignin units through a high redox potential mechanism, MnP oxidises Mn2+ to Mn3+, which then mediates the degradation of phenolic lignin structures, and Lac (multicopper oxidases) oxidises phenolic substrates by reducing molecular oxygen to water. While these enzymes share the capacity to depolymerise lignin, their catalytic specificities, substrate preferences, and operational stabilities differ considerably. In this study, the specific enzymes used were laccase from Trametes versicolor, lignin peroxidase (fungal origin), and manganese peroxidase from white-rot fungus Phanerochaete chrysosporium, and the results obtained in this research are specific to these enzymes. It should be emphasised that the findings cannot be directly generalised to other members of the same enzyme class, as variations in activity and stability among homologs may lead to different degradation outcomes. Therefore, direct extrapolation to other homologs within the same enzyme families may not be appropriate.
Enzymatic lignin degradation presents an environmentally friendly pathway, operating under mild temperature and pH conditions. This approach significantly reduces the need for harsh chemicals, thereby minimising the generation of toxic by-products. A key benefit of using this method in lignocellulose degradation is its high substrate specificity, which allows for the selective cleavage of lignin bonds without compromising the integrity of polysaccharides. Furthermore, the enhanced biocompatibility of enzymes facilitates their integration with microbial consortia for synergistic bioconversion processes. Enzymes are also amenable to genetic or protein engineering, enabling improvements in catalytic performance and substrate range. Their use leads to a reduced formation of fermentation inhibitors, which, in turn, enhances downstream biofuel yields and supports scalability and industrial integration through immobilisation techniques and compatibility with biorefinery operations [12].
This study aims to optimise the enzymatic degradation of kraft lignin through response surface methodology (RSM) for sustainable management of lignocellulosic by-products. The research seeks to enhance the efficiency of kraft lignin depolymerisation by establishing optimal parameters for each enzyme involved in its degradation: LiP, MnP, and Lac.
2. Materials and Methods
2.1. Materials and Chemicals
Plant materials (oat straw, birch sawdust) were provided by the Faculty of Biotechnology collection. Enzymes were as follows: laccase from Trametes versicolor (Sigma-Aldrich, Buchs, Switzerland), lignin peroxidase (Sigma-Aldrich, Buchs, Switzerland), and manganese peroxidase from white-rot fungus Phanerochaete chrysosporium (Sigma-Aldrich, Buchs, Switzerland). Analytical-grade substances were as follows: sodium carbonate (Adrachim, Bucharest, Romania), Folin–Ciocâlteu reagent (Merck KGaA, Darmstadt, Germany), lignin (Sigma-Aldrich, Buchs, Switzerland), and HPLC-grade o-phosphoric acid (Sigma-Aldrich, Buchs, Switzerland). Standards were as follows: gallic acid (Sigma-Aldrich, Buchs, Switzerland), chlorogenic acid (Sigma-Aldrich, Buchs, Switzerland), caffeic acid (Sigma-Aldrich, Buchs, Switzerland), syringic acid (Sigma-Aldrich, Buchs, Switzerland), 4-coumaric acid (Sigma-Aldrich, Buchs, Switzerland), and ferulic acid (Sigma-Aldrich, Buchs, Switzerland).
2.2. Experimental Protocol
The experimental protocol involved the optimisation of kraft lignin degradation conditions with RSM based on a Box–Behnken Design (BBD) to evaluate the interactive effects of pH, temperature, concentration of kraft lignin, concentration of enzyme, and stimulating agent concentration on the activities of Lac, LiP, and MnP. Total phenolic content (TPC) determined for samples at every 24 h for a 144 h period was used as a response variable to assess degradation efficiency under various experimental conditions. Following optimisation with RSM–BBD, the predicted optimal parameters were validated in a confirmatory experiment, performed in three independent biological replicates. In addition, oat straw and birch sawdust were also used as substrates besides kraft lignin. Degradation efficiency was evaluated through TPC, HPLC quantification of phenolic compounds, UV–Vis spectrophotometry, and FT-IR spectroscopy to characterise structural modifications and phenolic compound release.
2.3. Optimisation with Response Surface Methodology—Experimental Design
RSM was employed to establish the optimal parameters for each enzyme’s activity (Lac, LiP, and MnP) upon degrading kraft lignin. Box–Behnken Design was chosen as the experimental model, which was composed of 46 design points and 6 centre-point replicates. The chosen independent variables were pH, temperature, concentration of kraft lignin, concentration of enzyme, and concentration of stimulating agent, specific for each enzyme, as presented in Table 1. The factor ranges chosen for BBD were defined based on enzyme-specific preliminary assays that mapped activity and stability across pH, temperature, and stimulating agents and the reported ranges used by recent, closely related studies in the literature [13,14,15,16]. These objective criteria ensure the BBD ranges target the responsive portion of the factor space while avoiding physically irrelevant or damaging conditions.

Table 1.
Experimental design—range and levels for independent variables.
The objective of the research was to determine the highest concentration of total phenolics in the samples analysed at each 24 h for 144 h in order to provide a comprehensive profile of kraft lignin degradation over an extended period, capturing both early and late stages of enzymatic activity. The samples were filtered and centrifuged with Whatman no. 1 filter paper before further analysis. The samples collected before enzyme addition (0 h) were considered as controls. The samples subjected to HPLC, UV–Vis, and FT-IR measurements corresponded to the 144 h incubation time point.
2.4. Total Phenolic Content (TPC)
The content of total phenolics was determined using a modified Folin–Ciocâlteu method, where diluted samples were mixed with 0.5 mL Folin–Ciocâlteu reagent and allowed to react in the dark for 8 min. After the addition of 7.5% sodium carbonate solution and distilled water, the samples were incubated for an additional 2 h in the dark. The absorbance was measured at 765 nm with a UV–Vis spectrophotometer (SP-UV 1000 DLAB, Beijing, China), and gallic acid (0.005–0.5 mg/mL) was used for obtaining a standard curve. For all TPC measurements, the values at t = 0 h obtained with the Folin–Ciocâlteu assay were subtracted from all subsequent time points to serve as a baseline. The results were expressed as milligrams gallic acid equivalent (mg GAE) per mL sample [17].
2.5. HPLC Quantification of Phenolic Compounds
High-performance liquid chromatography (HPLC) analysis was conducted using a Waters 2695 Alliance system, which included a quaternary pump, an autosampler, and a UV/Vis detector (Waters 2487). The separation was performed using reverse-phase chromatography with a 5 µm SunFire Column (3.9 × 150 mm). A binary elution gradient was employed [18], consisting of 0.5% o-phosphoric acid in water (A) and acetonitrile (B), with the following gradient profile: an initial condition of 10/90, transitioning to 75/35 at 25 min, then to 10/90 at 40 min, returning to 90/10 at 45 min, and held at 90/10 for 10 min. The column was maintained at a controlled temperature of 40 °C, while the samples were kept at 20 °C. The chromatograms were acquired at 280 nm and 300 nm wavelengths. Prior to injection (10 µL), the samples were diluted accordingly and filtered through 0.22 µm nylon syringe filters. The phenolic compounds were identified by comparing their retention times with those of injected standard solutions, with specific retention times noted for gallic acid (4.3 min), chlorogenic acid (9.8 min), caffeic acid (12.1 min), syringic acid (12.9 min), p-coumaric acid (17.3 min), rutin (19.3 min), ferulic acid (19.7 min), and quercetin (24.4 min). The quantification of the analytes was performed by processing the peak’s areas using calibration curves generated for each specific standard.
2.6. UV–Vis Analysis
UV–Vis spectra were analysed for each sample using a spectrophotometric reading range of 200–900 nm with a UV–Vis spectrophotometer (Thermo Helios Alpha UV/Vis Spectrophotometer, Waltham, MA, USA; VISIONlite software version 4.0).
2.7. FT-IR Analysis
Kraft lignin and plant materials (oat straw and birch sawdust) were subjected to FT-IR analysis (Shimadzu IRTracer-100, Kyoto, Japan; LabSolutions IR software version 2.27) in order to determine the structural modification that had occurred. The samples were scanned in the 400–4000 cm−1 region, with a cumulative 64 scans and 4 cm−1 resolution. After the scan, the specific bands corresponding to different functional groups were analysed in both the control and samples.
2.8. Statistical Analysis
The data regarding optimisation of enzymatic degradation with RSM–BBD were processed with Stat-Ease 360 software. The quantitative analysis was conducted in triplicate. The results were represented as means and standard deviations. Following the experimental design of the model, its adequacy was assessed through diagnostics checks and the analysis of plots associated with the estimated response surface model. This evaluation involved verifying the randomness and normality of the residuals, examining Cook’s distance to identify any excessively influential points, and analysing leverage values to detect outliers. Furthermore, the model’s significance was determined by its p-value, using a significance level of 0.05, and the amount of variance explained by the model was assessed using the determination coefficient (R2).
3. Results
The first objective of this research was to optimise the activity of three enzymes (LiP, MnP, and Lac) on degrading kraft lignin. Five parameters were chosen: pH, temperature, kraft lignin concentration, enzyme concentration, and concentration of stimulating agent. The efficacy of each variant was estimated based upon the TPC (mg GAE/mL) obtained. In order to enhance the effectiveness of kraft lignin depolymerisation, RSM was used, with the experiments based on a BBD. After the optimisation, the second objective was to use the predicted parameters established through the model for each enzyme and test their activity on kraft lignin (for confirmation of the model) but also for degrading lignocellulose by-products such as oat straw and birch sawdust. The substrate degradation was evaluated through the total phenolic content obtained and several individual phenolics, UV–Vis spectra, and also FT-IR analysis.
3.1. Optimisation of Kraft Lignin Degradation with Response Surface Methodology
The efficiency of lignocellulose degradation and the stability of the enzymatic process can be significantly improved by optimising the operating parameters for LiP, MnP, and Lac, thereby reducing processing time, enzyme consumption, and environmental impact.
3.1.1. Kraft Lignin Degradation with Lignin Peroxidase
LiP is a heme-containing enzyme that initiates oxidative cleavage of non-phenolic lignin units, and its high redox potential makes it a suitable enzyme for degrading complex aromatic compounds in lignocellulosic by-products. Through the RSM–BBD approach, the response of LiP to multiple process variables was monitored over six time points in order to determine the most favourable conditions for kraft lignin degradation, as estimated with TPC evaluation. The 3D response surface plots showing the interaction between pH and temperature (as these factors are pivotal in enzyme activity) provide insight into the optimal conditions for maximising kraft lignin degradation with LiP. At 24 h, the 3D surface shows only mild elevation in TPC, with the plot largely in cooler tones and low curvature (Figure 1). Minor synergistic effects were observed between enzyme concentration and pH, though not statistically strong. Therefore, early-stage oxidative degradation is detectable but not yet efficient.
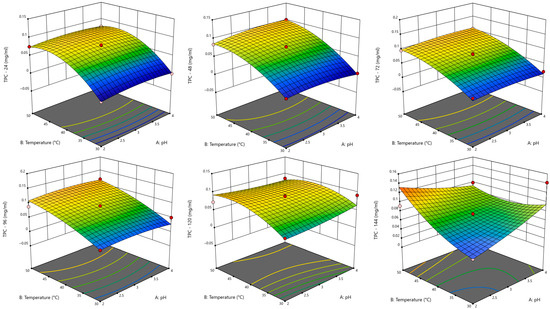
Figure 1.
Response surface plots for TPC for LiP as a function of pH and temperature at every 24 h for 144 h period.
At 48 h, the surface shows increased curvature, with more pronounced gradient shifts toward warmer tones, indicating enhanced TPC levels, as LiP becomes more catalytically effective. At 72 h, a distinct peak emerges with a strong gradient curvature, which could suggest high oxidative degradation. The response surface indicates a clear interaction between enzyme and activator, enhancing kraft lignin breakdown. At 96 h, degradation efficiency is sustained but not significantly improved. This phase could suggest reaching a reaction equilibrium, where kraft lignin availability or active LiP is limiting further conversion. At 120 h, the response surface shows early signs of decline, especially at high enzyme or kraft lignin concentrations, possibly due to oxidative stress or accumulations of by-products that could inhibit enzyme activity. At 144 h, response surface suggests decreasing efficiency, falling at extreme variables.
The results (Table S1) indicated that the significant terms for each model were X2 (temperature), X3 (kraft lignin concentration), and X22; the model with superior statistical performance was the one with the response TPC at 72 h (Figure 2), as it had the highest R2 (0.9792) as well as the lowest standard deviation of residuals (0.007) and coefficient of variation (10.43%).
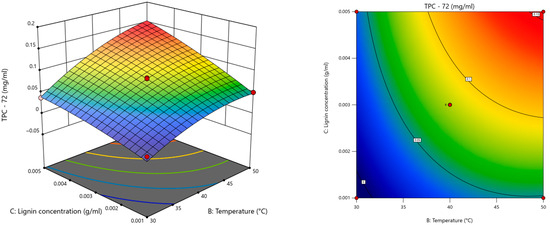
Figure 2.
Response surface plot for TPC for LiP as a function of temperature and kraft lignin concentration at 72 h.
After the overall statistical analysis, it was possible to establish the predicted optimal factors for kraft lignin degradation with LiP: pH = 2.6, temperature = 49.57 °C, kraft lignin concentration = 0.005 g/mL, veratryl alcohol concentration = 0.7807 M, and LiP concentration = 0.0094 U/mL.
3.1.2. Kraft Lignin Degradation with Manganese Peroxidase
MnP plays a pivotal role in lignin degradation by oxidising Mn (II) to Mn (III), which acts as a diffusible mediator, initiating cleavage of phenolic structures. The current study explored MnP performance under varied environmental and chemical conditions across a 144 h incubation. The 3D response surface plots (Figure 3) showing the interaction between pH and temperature provide insight into the optimal conditions for maximising kraft lignin degradation with MnP. At 24 h, the response surface is predominantly low with minimal curvature, suggesting minor elevations in TPC, as MnP shows limited initial action and takes longer to express catalytic effectiveness.
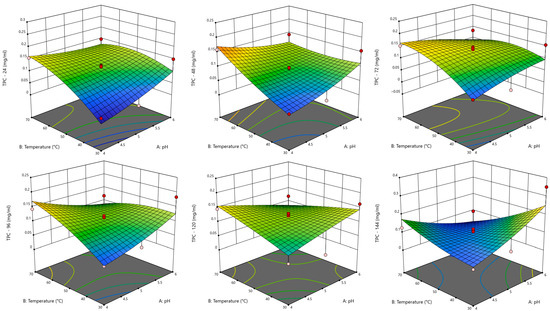
Figure 3.
Response surface plots for TPC for MnP as a function of pH and temperature at every 24 h for 144 h period.
At 48 h, the surface begins to show an increased response, with warmer regions and a slight appearance of curvature, as MnP enters a more active phase, possibly due to improved substrate accessibility and cofactor availability. At 72 h, distinct peaks and a sharper curvature appear, as TPC reaches noticeably higher levels. This could suggest optimal catalytic conditions for MnP, with a strong synergy between pH, temperature, and activator that enhances cleavage of kraft lignin bonds. At 96 h, the surface begins to plateau, with some persistence of peak zones but no substantial increase in TPC. At 120 h, a minor decline or surface flattening at higher kraft lignin or enzyme concentrations is observed. At 144 h, the surface shows decreased elevations, indicating that MnP is most effective at 72–96 h, after which degradation becomes less efficient.
The results (Table S1) indicated that the significant terms for each model were X2 (temperature) and X3 (kraft lignin concentration); the model with superior statistical performance was the one with the response TPC at 48 h (Figure 4), as it had the highest R2 (0.9589) as well as the lowest standard deviation of residuals (0.021) and coefficient of variation (19.68%).
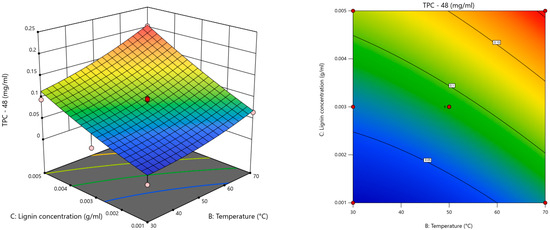
Figure 4.
Response surface plot for TPC for MnP as a function of temperature and kraft lignin concentration at 48 h.
After the statistical analysis, it was possible to establish the predicted optimal factors for kraft lignin degradation with MnP: pH = 6.0, temperature = 50.13 °C, kraft lignin concentration = 0.005 g/mL, MnSO4 concentration = 0.05 mM, and MnP concentration = 0.0041 U/mL.
3.1.3. Kraft Lignin Degradation with Laccase
Lac is a multicopper oxidase capable of oxidising phenolic and non-phenolic lignin subunits; its application in lignocellulosic biomass degradation is gaining prominence due to its mild operating conditions and environmentally friendly nature. The 3D response surface plots showing the interaction between pH and temperature (as these factors are pivotal in enzyme activity) provide insight into the optimal conditions for maximising Lac-catalysed kraft lignin degradation. At 24 h, the response surface (Figure 5) shows a modest increase in TPC, with relatively flat trends and no intense curvature or red zones, indicating limited degradation. The model showed mild responsiveness to individual factors, indicating early-stage enzyme activity. As such, Lac activity has initiated degradation, but optimal conditions are not yet achieved.
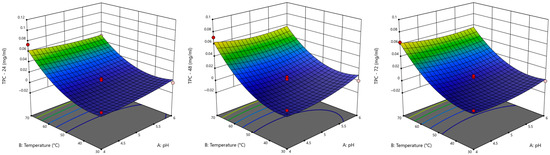
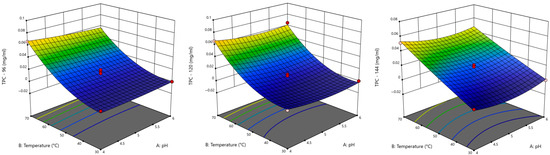
Figure 5.
Response surface plots for TPC for Lac as a function of pH and temperature at every 24 h for 144 h period.
At 48 h and 72 h, TPC shows a slight increase, with the response surface displaying warmer colours and moderate curvature, suggesting a strengthening between enzyme–substrate interactions; a higher enzyme concentration improves degradation. At 96 h, a visible peak appears in the plot, possibly approaching red tones, suggesting that this stage might be the optimal for Lac activity, where the balance of all factors facilitates efficient kraft lignin breakdown. Continued incubation does not yield significant improvement; the response surfaces for 120 and 144 h remain flat, with no notable changes.
The overall statistical analysis (Table S1) indicated that the significant terms for each model were X2 (temperature), X3 (kraft lignin concentration), and X22, and the model with superior statistical performance was the one with the response TPC at 96 h (Figure 6), as it had the highest R2 (0.9665) as well as the lowest standard deviation of residuals (0.0073) and coefficient of variation (17.01%).
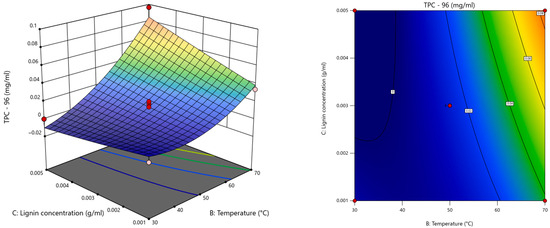
Figure 6.
Response surface and contour plots for TPC for Lac as a function of temperature and kraft lignin concentration at 96 h.
Following the statistical analysis, it was possible to establish the predicted optimal factors for kraft lignin degradation with Lac: pH = 5.8, temperature = 69.98 °C, kraft lignin concentration = 0.005 g/mL, CuSO4 concentration = 0.05 mM, and Lac concentration = 0.3949 U/mL.
The predictive performance of the enzymatic models was evaluated across multiple time points (Table S2). The model for LiP treatment of KL demonstrated high accuracy, as reflected by a low RMSE (~0.012) and a minimal positive bias (+0.004), indicating strong agreement with the observed data. The model for KL degradation with MnP exhibited a slight systematic under-prediction, with an RMSE of ~0.033 and a negative bias of −0.018, but the deviations remained within biologically acceptable limits. The model for Lac showed slightly lower precision, with an RMSE of ~0.015 and a small negative bias (~0.003), suggesting minor discrepancies from the observed values, especially at earlier time points. Overall, while the models differ in precision and systematic bias, they provide broadly reasonable predictions and are suitable for representing the enzymatic responses under the experimental conditions studied.
3.2. Degradation of Kraft Lignin and Lignocellulosic By-Products Using Optimised Parameters
3.2.1. Quantification of Phenolic Compounds
Total Phenolic Content (TPC)
The degradation of lignocellulosic by-products and industrial lignin substrates with ligninolytic enzymes was monitored through total phenolic content measurements at every 24 h over a 144 h period. Phenolic release into the medium serves as an indicator of lignin depolymerisation, since phenolic moieties are primary oxidation products resulting from the cleavage of ether and C-C bonds in the lignin polymer. Each enzyme demonstrated distinct dynamics, which reflect differences in enzymatic selectivity, redox potential, and substrate accessibility.
In the case of oat straw degradation with LiP (OS-LiP), the total phenolics content increased gradually from 0.045 mg/mL at 24 h to 0.059 mg/mL at 144 h (Figure 7). The trend showed a slight plateau at 72–96 h, suggesting a transient equilibrium between phenolics released and possible secondary oxidation and polymerisation reactions, a phenomenon frequently observed during oxidative lignin breakdown.
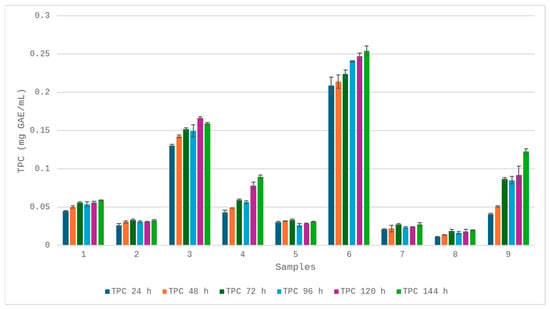
Figure 7.
TPC content of samples: 1-OS-LiP, 2-BS-Lip, 3-KL-LiP, 4-OS-Mnp, 5-BS-MnP, 6-KL-MnP, 7-OS-Lac, 8-BS-Lac, and 9-KL-Lac.
Lip activity on birch sawdust (BS-LiP), a hardwood substrate with a more condensed and syringil-rich lignin structure, yielded a modest increase in TPC from 0.027 mg/mL (Figure 7) to 0.031 mg/mL over 144 h. The relatively flat profile suggests a limited enzyme accessibility, likely due to the recalcitrance of intact lignin in birch and the absence of pretreatment to disrupt its complex matrix.
Kraft lignin treatment with LiP (KL-LiP) resulted in the most pronounced phenolic release among all the substrates treated with LiP, with values ranging from 0.131 to 0.167 mg/mL (Figure 7). This trend is characteristic of the high reactivity of kraft lignin, which is rich in phenolic end groups due to prior chemical depolymerisation during pulping. The subsequent decline in phenolic content after 120 h likely reflects either enzymatic consumption of released phenolics or secondary condensation reactions.
MnP exhibited a slightly different kinetic profile due to its reliance on Mn (II) oxidation to Mn (III), which acts as a diffusible oxidant targeting phenolic units. When applied to oat straw (OS-MnP), MnP led to a continuous increase in TPC from 0.045 to 0.091 mg/mL (Figure 7), a nearly two-fold rise over the 144 h period. This progressive release of phenolics could suggests that Mn (III) chelates were effective in diffusing into the plant matrix and oxidising accessible phenolic groups over time.
In contrast, birch sawdust treated with MnP (BS-MnP) showed only a modest increase in phenolic content, peaking at 0.033 mg/mL (Figure 7) at 72 h and stabilising thereafter. The lower activity mirrors the trend observed with LiP treatment, reinforcing the recalcitrance of hardwood lignin to enzymatic depolymerisation.
The degradation of kraft lignin with MnP (KL-MnP) led to the highest TPC values across all the variants, with a content of 0.259 mg/mL (Figure 7), registered at 144 h. This steady accumulation indicates continuous solubilisation of phenolic moieties from kraft lignin, which is compatible with MnP’s catalytic mechanism.
Unlike peroxidases, Lac has a lower redox potential and is generally limited to the oxidation of free phenolic units. In the case of oat straw (OS-Lac), Lac produced only a slight increase in TPC from 0.021 to 0.029 mg/mL (Figure 7). The trend was characterised by minor fluctuations rather than a clear upward trajectory. This low activity reflects the limited accessibility of oxidisable groups within the complex lignin matrix and the absence of redox mediators, which are typically required to extend Lac activity beyond phenolics.
Birch sawdust treated with Lac (BS-Lac) showed even lower TPC values, reaching only 0.019 mg/mL at 144 h (Figure 7). This minimal increase reinforces the enzyme’s inefficacy against hardwood lignin without the aid of mediators or pretreatment.
On the other hand, the use of Lac on kraft lignin (KL-Lac) resulted in a more substantial increase in phenolic content, rising from 0.041 to 0.125 mg/mL (Figure 7). This pattern underscores the fact that processed lignins, particularly kraft lignin, contain abundant phenolic hydroxyl groups that are readily oxidised by Lac, even in the absence of mediators.
Phenolic Profile
LiP treatment of oat straw resulted in the release of a broad array of phenolic acids, notably chlorogenic acid, caffeic acid, syringic acid, p-coumaric acid, and ferulic acid (Table 2). The most abundant phenolic compounds were rutin and quercetin, flavonoids often linked to the hydrolysis of ester bonds between lignin and hemicellulose. The significant presence of p-coumaric and syringic acids suggests that LiP effectively cleaves ether and C-C bonds in both guaiacyl and syringyl units of grass-type lignin.

Table 2.
Phenolic profile of substrates (oat straw, birch sawdust, kraft lignin) degraded with LiP, MnP, and Lac.
In the degradation of birch sawdust with LiP, the overall release of phenolic acids by LiP was substantially lower. Only caffeic acid, syringic acid, ferulic acid, and quercetin were detected, while the others were absent (Table 2). This limited profile likely reflects the more condensed and less accessible lignin network in hardwoods.
LiP exhibited its most pronounced degradative activity on kraft lignin, liberating high concentrations of caffeic acid, p-coumaric acid, and ferulic acid (Table 2). This extensive breakdown confirms LiP’s ability to oxidise both phenolic and non-phenolic structures in kraft lignin. The dominance of p-coumaric acid suggests LiP preferentially cleaves ether bonds linking this unit in processed lignin samples.
MnP displayed significant degradative activity on oat straw, releasing high levels of p-coumaric and ferulic acids, alongside moderate quantities of chlorogenic, caffeic, and syringic acid (Table 2). The substantial release of hydroxycinnamic acids suggests that MnP efficiently oxidises phenolic side chains via Mn (III) chelates. Additionally, the high rutin concentration, alongside the low quercetin level, may reflect cleavage of ester linkages between lignin–carbohydrate complexes, which is known to be enhanced by MnP in herbaceous lignocellulose.
In birch sawdust degradation, MnP was less effective overall, though it still produced caffeic acid and modest levels of ferulic and quercetin (Table 2). The detection of gallic acid may indicate deeper oxidative breakdown of syringil-derived lignin moieties, which are more prevalent in hardwoods. The minimal presence of p-coumaric further underscores the limited accessibility and lower hydroxycinnamic acid content in birch lignin.
Surprisingly, kraft lignin degradation with MnP yielded the highest caffeic acid content of all the other variants. This was accompanied by moderate levels of ferulic acid and low amounts of syringic and p-coumaric acids (Table 2). The high amount of caffeic acid may point to preferential demethylation and cleavage of guaiacyl units under MnP catalysis. These results suggest that MnP is particularly effective in selectively oxidising guaicyl-type kraft lignin fragments.
Lac treatment of oat straw generated a comparatively low amount of phenolics, with chlorogenic and caffeic acids being the most abundant. Low levels of syringic, ferulic, and p-coumaric acids were detected (Table 2). This limited production of phenolics reflects Lac’s dependency on free phenolic moieties and its lower redox potential. Without mediators, Lac’s activity on complex lignin is, therefore, constrained to surface-accessible phenolics.
In birch sawdust degradation, Lac generated moderate levels of caffeic acid, and minimal amounts of gallic acid, ferulic acid, and quercetin were also found (Table 2). The presence of gallic acid suggests oxidation of syringil-rich units specific to hardwood lignin.
When applied to kraft lignin, Lac yielded moderate levels of caffeic and syringic acids (Table 2). Similarly to degradation of birch sawdust, in this case, we observed the presence of ferulic and gallic acid, which confirms Lac’s ability to attack syringil-derived compounds. The modest activity confirms that Lac alone, without mediators, is only marginally effective on processed lignin.
3.2.2. UV–Vis Analysis
The UV–Vis spectra of the samples (supernatants collected every 24 h) were measured and compared to their controls. The spectra analysis in the range 200–900 nm revealed a somewhat typical pattern in the substrates’ enzymatic degradation.
The spectrum of degradation of oat straw with LiP revealed a prominent absorption peak near 290–310 nm, indicative of aromatic ring structures, such as those found in lignin-derived phenolics (Figure S1). There is also a slight shoulder near 310–330 nm that could be attributed to methoxylated aromatic compounds (syringyl units from lignin), suggesting partial oxidation or depolymerisation. Similar to oat straw, the degradation of birch sawdust with LiP (Figure S1) shows a peak around 280 nm, with slightly greater intensity. This suggests a higher release or exposure of phenolic groups [19], possibly due to more accessible lignin structures in hardwood (birch sawdust) compared to agricultural waste (oat straw). The spectrum of kraft lignin degradation (Figure S1) displays a strong and broad peak centred at approximately 280 nm, with an extended tail toward 400 nm, consistent with a high concentration of conjugated phenolics and breakdown products [19]. The intensity is significantly higher than the natural lignocellulosic substrates, which is expected due to kraft lignin’s processed nature and higher phenolic content.
The spectrum of oat straw degradation with MnP (Figure S2) indicates higher oxidative activity compared to LiP. The high peak observed at 296 nm could indicate the formation of phenolic compounds (vanillic acid, syringic acid, hydroxycinnamic acids, aldehydes, or quinones). A weak shoulder at 360–370 nm may suggest some oxidised lignin fragments [20]. Regarding birch sawdust degradation (Figure S2), the spectrum displays a better-defined absorption around 280–290 nm, reflecting phenolic products formed. The lack of defined shoulders suggests less efficient depolymerisation or production of fewer intermediates. The activity is likely limited by manganese ion diffusion and availability in the matrix. The kraft lignin degradation with MnP spectrum (Figure S2) shows a pronounced peak at 280 nm and a gradual tailing toward 350–370 nm. Compared to oat straw and birch sawdust, this suggests a more effective activity, most likely because the simple, less recalcitrant kraft lignin allows better substrate access for enzymatic degradation and facilitates Mn (III)-mediated oxidation.
The oat straw degradation with Lac spectrum (Figure S3) shows a moderate peak at around 280 nm, consistent with the oxidation of phenolic compounds. The presence of extended absorbance beyond 320 nm suggests the possibility of production of quinones or conjugated oligomers [20]. Lac may be less efficient than LiP and MnP due to low redox potential or substrate inaccessibility. Birch sawdust degradation (Figure S3) produces a dull peak at 280–285 nm. This could suggest slightly higher reactivity or lignin accessibility compared to oat straw with MnP, perhaps owing to structural differences in lignin composition between hardwood and agricultural waste. The kraft lignin degradation with Lac spectrum (Figure S3) shows a broad absorbance in the 280–360 nm range. This could suggest the formation of various oxidation products, including quinones and aromatic aldehydes [20]. The profile could suggest that Lac has a higher affinity for kraft lignin compared with oat straw and birch sawdust.
3.2.3. FT-IR Analysis
FT-IR analysis was carried out for lignocellulosic substrates before and after enzymatic treatment. Before treatment (Figure S4), oat straw exhibits a complex profile due to the presence of lignin, cellulose, and hemicellulose, with typical cellulose/hemicellulose signals: ~1050–1160 cm−1 (C-O-C and C-O stretching), ~1730 cm−1 (C=O stretching of acetyl and uronic ester groups in hemicellulose), and ~1600 and 1510 cm−1 (aromatic C=C in lignin) [21,22,23]. After LiP and MnP treatment, we observed a suppression of lignin peaks in the aromatic region and loss of ester-related peaks (~1725 cm−1), indicating breakdown of lignin and oxidation of aliphatic side chains. The persistent band near 1230 cm−1 further suggests partial cleavage of ether linkages under MnP and LiP treatments. Lac shows mild oxidative modifications, especially near 1260–1220 cm−1 (associated with the syringil ring and C-O stretching), suggestive of selective surface oxidation.
In birch sawdust degradation (Figure S5), untreated birch displays strong signals typical for hardwood lignin and polysaccharides: ~1730 cm−1 (carbonyl stretching), ~1595 and 1510 cm−1 (aromatic skeletal vibrations), ~1260 cm−1 (guaiacyl units), ~1050 and 1160 cm−1 (specific for cellulose and hemicellulose), and ~1325 and 1120 cm−1 (syringil units) [21,22,23]. LiP yielded a marked reduction in aromatic and ester peaks in sediment, while MnP had a similar but lower impact. Consistent attenuation of bands in the 1600–1500 cm−1 region and the appearance of new carbonyl absorption (~1715–1735 cm−1) could indicate cleavage of aryl-ether linkages and formation of quinones/carboxylic groups. Lac showed mild delignification, possibly favouring oxidative coupling or partial depolymerisation without extensive cleavage of lignin structures.
Untreated kraft lignin sediment (Figure S6) shows strong characteristic absorption bands in the aromatic skeletal vibration region at ~1595 cm−1 and ~1510 cm−1 (C=C stretching in phenyl rings), ~1460 cm−1 and ~1425 cm−1 (C-H deformation in methyl and methylene groups of kraft lignin), and ~1260 cm−1 and ~1125 cm−1 (C-O stretching in syringil and guaiacyl units) [23]. LiP treatment leads to substantial reduction in the intensity of aromatic ring vibrations, particularly at ~1510 cm−1, suggesting extensive oxidative cleavage of aromatic structures [21]. The broadening and shift of the band near ~1700 cm−1 indicate the formation of carbonyl-containing degradation products, including aldehydes and carboxylic acids [21]. MnP activity demonstrated similar trends. Lac showed moderate degradation, primarily a decreased intensity in the 1510 cm−1 band and broadening around 1230 cm−1. These observations indicate selective oxidation of phenolic hydroxyls, consistent with the known mechanism of Lac, which lacks the capacity to cleave non-phenolic β-O-4 bonds.
4. Discussion
The efficient degradation of lignin from lignocellulosic agricultural residues represents a major challenge in the field of biomass valorisation due to the complex and recalcitrant nature of lignin’s heterogeneous polymeric structure. In this study, the applications of oxidative enzymes—LiP, MnP, and Lac—were explored as a green and targeted strategy for kraft lignin depolymerisation. These enzymes, derived from white-rot fungi, have demonstrated substantial potential (especially LiP and MnP) in facilitating the selective breakdown of kraft lignin into lower molecular weight phenolics, thereby improving accessibility of cellulose and hemicellulose to other degradative enzymes [22,24].
The use of RSM–BBD enabled the systematic investigation of the interactive effects of multiple process parameters, including enzyme loading, kraft lignin concentration, pH, incubation temperature, and stimulating agent concentration. The constructed second-order polynomial models and associated contour plots revealed significant synergistic interactions, particularly between pH and temperature. Therefore, Lac exhibits optimal activity between 72 and 96 h, with maximum TPC observed at pH 5.5–6.0 and 65–70 °C, under high enzyme concentrations and moderate to high kraft lignin content. After 96 h, degradation efficiency stagnates, most likely due to substrate depletion or enzyme inactivation. The response surfaces also indicate strong interactive effects between pH and temperature, underscoring the need for balanced conditions to sustain enzyme activity over time. Therefore, Lac demonstrates low kraft lignin breakdown within these experimental conditions.
LiP achieved peak activity after 72–96 h of incubation, with optimal parameters observed at pH 2.5–3.0 and temperature 45–50 °C. Beyond 96 h, a decline in performance was observed, likely due to enzyme instability or oxidative stress. These findings highlight the importance of maintaining balanced oxidant levels to prevent enzyme deactivation while maximising catalytic output. Overall, LiP demonstrated a high capacity for kraft lignin degradation.
MnP exhibited a delayed but significant increase in TPC, peaking at 72–96 h, under an observed pH of 4.5–5 and temperature 45–50 °C. The response surface showed strong curvature, reflecting a high dependency on optimal cofactor presence and controlled pH. After the optimal phase, the efficiency declined, suggesting potential enzyme degradation or inhibition from accumulated oxidation by-products. The results highlight the enzyme’s dependency on a well-regulated environment for sustained catalytic activity and reaffirm MnP’s contribution to kraft lignin bioconversion.
The results of the RSM–BBD analysis emphasised that the optimal conditions differ between enzymes, with LiP requiring stronger acidic conditions and a higher concentration of veratryl alcohol as a stimulating agent, while Lac performed best at higher incubation temperatures and slight acidic conditions. After selecting the best optimal parameters for each enzyme, their activities were tested on three substrates: oat straw, birch sawdust, and kraft lignin. It is important to note that the present study investigates specific commercial enzyme preparations, and the optimised parameters identified here may not be directly transferable to other enzymes of the same class. Future studies could explore the combined use of LiP, MnP, and Lac under optimised conditions to investigate potential synergistic effects on lignin degradation, reflecting the multi-enzyme nature of this process in natural systems.
The enzymatic degradation of each substrate was monitored based on their phenolic release over a 144 h period (TPC and HPLC analysis) and UV–Vis and FT-IR spectral analysis. The phenolic release patterns observed over 144 h highlight the complex interplay between enzyme class, substrate structure, and accessibility. Kraft lignin degradation consistently produced the highest total phenolics for all the enzymatic treatments, owing to its pre-fragmented and phenolic-rich nature. Among the enzymes, MnP and LiP demonstrated superior degradative capacities compared to Lac, particularly on unprocessed lignocellulosic by-products. However, Lac exhibited notable performance when applied to kraft lignin, reaffirming its selective efficacy in the presence of accessible phenolic substrates.
The enzymatic degradation of lignocellulose substrates and kraft lignin revealed distinct differences in product profiles across the action of the three enzymes. LiP and MnP demonstrated broad-spectrum activity and released high concentrations of structurally diverse phenolic acids, particularly from kraft lignin and oat straw. MnP excelled at producing caffeic, ferulic, and p-coumaric acids, while LiP released high amounts of syringic acid under specific conditions. In contrast, Lac showed the most limited capacity for kraft lignin depolymerisation, reflecting its inability to cleave non-phenolic structures and lack of redox mediators in the system. The comparative phenolic acid profiles align well with known substrate preferences and catalytic mechanisms described in prior ligninolytic enzyme studies [25], reinforcing the importance of enzyme choice and substrate type in lignin bioconversion applications. It should be noted that the TPC measured by the Folin–Ciocâlteu assay was generally higher than the sum of the phenolic monomers quantified by HPLC. This discrepancy reflects the different detection principles: while HPLC captures only individual phenolics for which standards are available, the TPC assay also responds to phenolic oligomers and other reducing species generated during enzymatic lignin degradation. In addition, the signal obtained in the Folin–Ciocâlteu assay is highly dependent on the specific phenol measured, which further accounts for the differences observed between HPLC and TPC measurements. The two methods, therefore, provide complementary perspectives, with HPLC offering compound-specific resolution and TPC giving an overall measure of total phenolics released.
Analysing all the UV–Vis spectra, LiP shows the most intense and defined peaks on all substrates, implying stronger depolymerisation. MnP exhibits limited bust visible activity, especially on oat straw and kraft lignin, and Lac has moderate activity compared to peroxidases but still presents a significant activity especially on kraft lignin. These trends align with known mechanisms for the enzymes, as LiP operates through direct oxidation of non-phenolic lignin structures with high redox potential, MnP requires Mn (II) oxidation and diffuses through chelates, and Lac prefers phenolic substrates and benefits from mediators [Higuchi].
The FT-IR spectral analysis of the substrates confirms that LiP and MnP are the most effective enzymes in degrading lignin components in all the substrates, as indicated by strong attenuation of aromatic peaks, an increase in carbonyl features, and broader spectral changes. Lac demonstrated limited substrate degradation, primarily targeting phenolic hydroxyls without extensive disruption to the aromatic core.
In other studies regarding ligninolytic enzymes acting on wheat straw, MnP exhibited the most pronounced lignin-degrading activity, outperforming LiP and Lac. This highlights MnP’s superior role in facilitating selective ligninolysis relative to other enzymes evaluated [26,27]. Other studies indicated that LiP and MnP exhibited stronger predicted binding affinities and more extensive interaction networks with lignin model compounds compared to laccase. The results suggest that LiP and MnP are likely the most effective enzymes for catalysing lignin degradation, particularly given their capacity to target non-phenolic and hydrophobic regions of the polymer. The predominance of hydrophobic interactions in these enzyme–substrate complexes further underscores the mechanistic basis for their superior ligninolytic potential, providing valuable insight for the rational design and engineering of enzymes aimed at enhanced lignin depolymerisation [28].
While enzymatic lignocellulose degradation with RSM has been reported in previous studies [29,30], this research contributes with two advances: enzyme-specific optimisation that separates catalytic drivers (pH, temperature, activators) from matrix drivers, enabling transferable settings across substrates, and combined validation on agricultural residues (oat straw and birch sawdust). The optimised parameters identified in this study not only maximise enzymatic efficiency but also highlight the potential for process standardisation across different feedstock, a key consideration for industrial scalability. From an economic perspective, enzymatic treatments offer lower energy and chemical input compared to conventional lignin processing methods, suggesting improved cost-effectiveness for bio-based product streams. However, large-scale application will require integration into continuous or semi-continuous systems, optimisation of enzyme production and recycling, and assessment of substrate handling and downstream processing costs. The findings also support the development of integrated biorefineries where lignin-rich by-products can be enzymatically converted into high-value chemicals. By bridging laboratory-scale optimisation with industrial-scale considerations, this approach aligns with circular bioeconomy goals, promoting sustainable biomass utilisation while minimising waste and environmental impact.
5. Conclusions
This study demonstrated that kraft lignin’s degradation can be efficient with enzymes such as lignin peroxidase, manganese peroxidase, and laccase in specific conditions. By integrating response surface methodology based on a Box–Behnken Design, it was possible to optimise enzymatic degradation of kraft lignin: LiP needs a stronger acidic environment (pH = 2.6) and moderate temperature (~50 °C), MnP needs an almost neutral pH (6.0) and moderate temperature (~50 °C), and Lac needs a neutral pH (5.8) and higher temperature (~70 °C). The optimal parameters (pH, temperature, substrate concentration, enzyme concentration, and activator concentration) were further used for creating the best experimental conditions for degrading oat straw and birch sawdust. This study provides a robust framework tailored for enzymatic strategies regarding valorisation of lignocellulosic by-products. The optimised enzymatic parameters could enhance efficiency, enable process standardisation across feedstocks, and support economically and environmentally sustainable industrial-scale lignin valorisation in integrated biorefineries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriengineering7100314/s1, Table S1: Comparative model fit statistics for LiP, MnP, and Lac at 24 h intervals, Table S2: Comparative model fit statistics for LiP, MnP, and Lac at 24 h intervals, Figure S1: LiP activity in degradation of oat straw, birch sawdust, and lignin, Figure S2: MnP activity in degradation of oat straw, birch sawdust, and lignin, Figure S3: Lac activity in degradation of oat straw, birch sawdust, and lignin, Figure S4: FT-IR spectrum of enzymatic degradation of oat straw: before treatment, after treatment with LiP, MnP, and Lac, Figure S5: FT-IR spectrum of enzymatic degradation of birch sawdust: before treatment, after treatment with LiP, MnP, and Lac, and Figure S6: FT-IR spectrum of enzymatic degradation of lignin: before treatment, after treatment with LiP, MnP, and Lac.
Author Contributions
Conceptualisation, A.P., A.B., and F.I.-R.; methodology, A.P., F.I.-R., and A.B.; software, A.P.; validation A.P. and A.B.; formal analysis, A.P.; investigation, A.P. and A.B.; resources, A.P. and F.I.-R.; data curation, A.P.; writing—original draft preparation, A.P. and A.B.; writing—review and editing, A.P. and F.I.-R.; visualisation, A.P.; supervision, F.I.-R.; project administration, A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| RSM | Response surface methodology |
| BBD | Box–Behnken Design |
| LiP | Lignin peroxidase |
| MnP | Manganese peroxidase |
| Lac | Laccase |
| TPC | Total phenolic content |
| GAE | Gallic acid equivalent |
| HPLC | High-performance liquid chromatography |
| OS | Oat straw |
| BS | Birch sawdust |
| KL | Kraft lignin |
References
- Mujtaba, M.; Fraceto, L.F.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; de Medeiros, G.A.; do Espirito Santo Pereira, A.; Donnini Mancini, S.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Haq, I.U.; Qaisar, K.; Nawaz, A.; Akram, F.; Mukhtar, H.; Xu, Y.; Mumtaz, M.W.; Rashid, U.; Ghani, W.A.W.A.K.; Choong, T.S.Y. Advances in valorization of lignocellulosic biomass towards energy generation. Catalysts 2021, 11, 309. [Google Scholar] [CrossRef]
- Thuppahige, R.T.W.; Barner, L.; Shahbazi, M.; Fraga, G.; Moghaddam, L. A comprehensive review of sustainable valorisation of lignocellulosic biomass and plastic waste into biofuels and chemicals via co-liquefaction. Waste Manag. 2025, 202, 114827. [Google Scholar] [CrossRef] [PubMed]
- Shaba, E.Y.; Jiya, M.J.; Andrew, A.; Salihu, A.M.; Mamma, E.; Anyanwu, S.K.; Mathew, J.T.; Inobeme, A.; Yisa, B.N.; Saba, J.J. Biomass Valorisation: A Sustainable Approach Towards Carbon Neutrality and Circular Economy. In Biomass Valorization: A Sustainable Approach Towards Carbon Neutrality and Circular Economy; Springer Nature: Singapore, 2025; pp. 99–122. [Google Scholar] [CrossRef]
- Grgas, D.; Rukavina, M.; Bešlo, D.; Štefanac, T.; Crnek, V.; Šikić, T.; Habuda-Stanić, M.; Landeka Dragičević, T. The bacterial degradation of lignin—A review. Water 2023, 15, 1272. [Google Scholar] [CrossRef]
- Gan, M.J.; Niu, Y.Q.; Qu, X.J.; Zhou, C.H. Lignin to value-added chemicals and advanced materials: Extraction, degradation, and functionalization. Green Chem. 2022, 24, 7705–7750. [Google Scholar] [CrossRef]
- Wang, H.M.; Yuan, T.Q.; Song, G.Y.; Sun, R.C. Advanced and versatile lignin-derived biodegradable composite film materials toward a sustainable world. Green Chem. 2021, 23, 3790–3817. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, B.; Chen, H.; Wu, W.; Wu, S.; Jin, Y.; Xiao, H. Recent advances in understanding the effects of lignin structural characteristics on enzymatic hydrolysis. Biotechnol. Biofuels 2021, 14, 205. [Google Scholar] [CrossRef]
- Gellerstedt, G.; Henriksson, G. Lignins: Major sources, structure and properties. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2008; pp. 201–224. [Google Scholar]
- Gałązka, A.; Jankiewicz, U.; Orzechowski, S. The role of ligninolytic enzymes in sustainable agriculture: Applications and challenges. Agronomy 2025, 15, 451. [Google Scholar] [CrossRef]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef]
- Singh, R.; Jain, R.; Soni, P.; de Los Santos-Villalobos, S.; Chattaraj, S.; Roy, D.; Mitra, D.; Gaur, A. Graphing the green route: Enzymatic hydrolysis in sustainable decomposition. Curr. Res. Microb. Sci. 2024, 7, 100281. [Google Scholar] [CrossRef] [PubMed]
- Gaur, N.; Narasimhulu, K. Biochemical and kinetic characterization of laccase and manganese peroxidase from novel Klebsiella pneumoniae strains and their application in bioethanol production. Rsc Adv. 2018, 8, 15044–15055. [Google Scholar] [CrossRef] [PubMed]
- Buzzo, B.B.; Lima, N.S.M.; Pereira, P.A.M.; Gomes-Pepe, E.S.; Sartini, C.C.F.; Lemos, E.G.D.M. Lignin degradation by a novel thermophilic and alkaline yellow laccase from Chitinophaga sp. Microbiol. Spectr. 2024, 12, e04013-23. [Google Scholar] [CrossRef]
- Minhas, W.R.; Bashir, S.; Zhang, C.; Raza, A. Optimized production of laccase from Pseudomonas stutzeri and its biodegradation of lignin in biomass. Folia Microbiol. 2024, 1–8. [Google Scholar] [CrossRef]
- Verma, M.; Ekka, A.; Mohapatra, T.; Ghosh, P. Optimization of kraft lignin decolorization and degradation by bacterial strain Bacillus velezensis using response surface methodology. J. Environ. Chem. Eng. 2020, 8, 104270. [Google Scholar] [CrossRef]
- Arlet, A.C.I.; Tociu, M.; Balanuca, B.; Israel-Roming, F. Extraction and evaluation of total phenolics content from red corn bran. Sci. Bull. Ser. F. Biotechnol. 2023, 27, 60–67. [Google Scholar]
- Arlet, A.C.I.; Balanuca, B.; Tociu, M.; Ott, C.; Constantin, L.; Popa, A.; Israel-Roming, F. Influence of membrane separation technique upon the phenolic content of red corn bran extract. Sci. Bull. Ser. F. Biotechnol. 2024, 28, 101–109. [Google Scholar]
- Higuchi, T. Microbial degradation of lignin: Role of lignin peroxidase, manganese peroxidase, and laccase. Proc. Jpn. Acad. Ser. B 2004, 80, 204–214. [Google Scholar] [CrossRef]
- Tribulová, T.E.; Kacik, F.; Evtuguin, D.M.; Cabalová, I. Assessment of chromophores in chemically treated and aged wood by uv-vis diffuse reflectance spectroscopy. Cellul. Chem. Technol. 2016, 50, 659–667. [Google Scholar]
- Martin, E.; Agnihotri, S.; Audonnet, F.; Record, E.; Dubessay, P.; Taherzadeh, M.J.; Michaud, P. From Corncob By-Product to Functional Lignins: Comparative Analysis of Alkaline and Organosolv Extraction Followed by Laccase Treatment. Biomolecules 2025, 15, 1226. [Google Scholar] [CrossRef]
- Deng, Z.; Xia, A.; Liao, Q.; Zhu, X.; Huang, Y.; Fu, Q. Laccase pretreatment of wheat straw: Effects of the physicochemical characteristics and the kinetics of enzymatic hydrolysis. Biotechnol. Biofuels 2019, 12, 159. [Google Scholar] [CrossRef]
- Sammons, R.J.; Harper, D.P.; Labbé, N.; Bozell, J.J.; Elder, T.; Rials, T.G. Characterization of organosolv lignins using thermal and FT-IR spectroscopic analysis. BioResources 2013, 8, 2752–2767. [Google Scholar] [CrossRef]
- Cook, C.; Francocci, F.; Cervone, F.; Bellincampi, D.; Bolwell, P.G.; Ferrari, S.; Devoto, A. Combination of pretreatment with white rot fungi and modification of primary and secondary cell walls improves saccharification. BioEnergy Res. 2015, 8, 175–186. [Google Scholar] [CrossRef]
- Huerta, E.R.; Muddasar, M.; Collins, M.N. Enzymatic hydrolysis lignin and kraft lignin from birch wood: A source of functional bio-based materials. Wood Sci. Technol. 2024, 58, 423–440. [Google Scholar] [CrossRef]
- Arora, D.S.; Chander, M.; Gill, P.K. Involvement of lignin peroxidase, manganese peroxidase and laccase in degradation and selective ligninolysis of wheat straw. Int. Biodeterior. Biodegrad. 2002, 50, 115–120. [Google Scholar] [CrossRef]
- MacDonald, J.; Goacher, R.E.; Abou-Zaid, M.; Master, E.R. Comparative analysis of lignin peroxidase and manganese peroxidase activity on coniferous and deciduous wood using ToF-SIMS. Appl. Microbiol. Biotechnol. 2016, 100, 8013–8020. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zeng, G.; Tan, Z.; Jiang, M.; Li, H.; Liu, L.; Zhu, Y.; Yu, Z.; Wei, Z.; Liu, Y.; et al. Understanding lignin-degrading reactions of ligninolytic enzymes: Binding affinity and interactional profile. PLoS ONE 2011, 6, e25647. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, Q.; Asgher, M.; Sheikh, M.A.; Nawaz, H. Optimization of ligninolytic enzymes production through response surface methodology. Bioresources 2013, 8, 944–968. [Google Scholar] [CrossRef]
- Rath, S.; Paul, M.; Behera, H.K.; Thatoi, H. Response surface methodology mediated optimization of Lignin peroxidase from Bacillus mycoides isolated from Simlipal Biosphere Reserve, Odisha, India. J. Genet. Eng. Biotechnol. 2022, 20, 2. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).