Light Stress Detection in Ficus elastica with Hyperspectral Indices
Abstract
1. Introduction
- (i)
- Limiting light absorption. In order to avoid absorption of the excessive light energy, chloroplasts move towards the anticlinal cell wall [8]. In addition, excessive absorption may be limited by increasing the biosynthesis of the colored phenolic compounds (anthocyanins and carotenoids) acting as optical filters and light reflectors [9].
- (ii)
- Thylakoid cyclic electron transport pathways and independent electron sink from the non-cyclic electron transport chain. The danger of damage to photosystems I and II is maximized when the electron sink from the electron transport chain is impeded due to low Calvin–Benson cycle activity and hence a low NADP+ pool. In this case, there is a high probability of electron transfer from the excited state of P680* to O2 to form superoxide O2*−. There are processes that enable an independent (additional) electron sink from ETC or that exclude its necessity: photorespiration, rerouting of reducing power to the mitochondria, cyclic electron transport around photosystems I and II, the Mehler cycle, and PTOX activity [10,11,12,13]. All these processes are known to provide photoprotection.
- (iii)
- Repairing damaged PSII. The main damage of PSII is related to the D1 protein. A significant part of the thylakoidal ATP hydrolysisis is aimed at restoring the damaged D1 protein pools [14].
2. Materials and Methods
2.1. Object of Study
2.2. Induction of Light Stress
Temperature Control during Light Stress Induction
2.3. Hyperspectral Imaging
2.3.1. Schematic of the Experiment
2.3.2. Assessment of the After-Effects of LL and EL Exposure Using Hyperspectral Imaging
2.3.3. Hyperspectral Data Preprocessing
2.4. Calculation of Vegetation Indices
2.5. Measuring Maximal Quantum Yields of Photosystem II (Fv/Fm) and Photosynthetic Photon Flux Density (PPFD)
3. Results
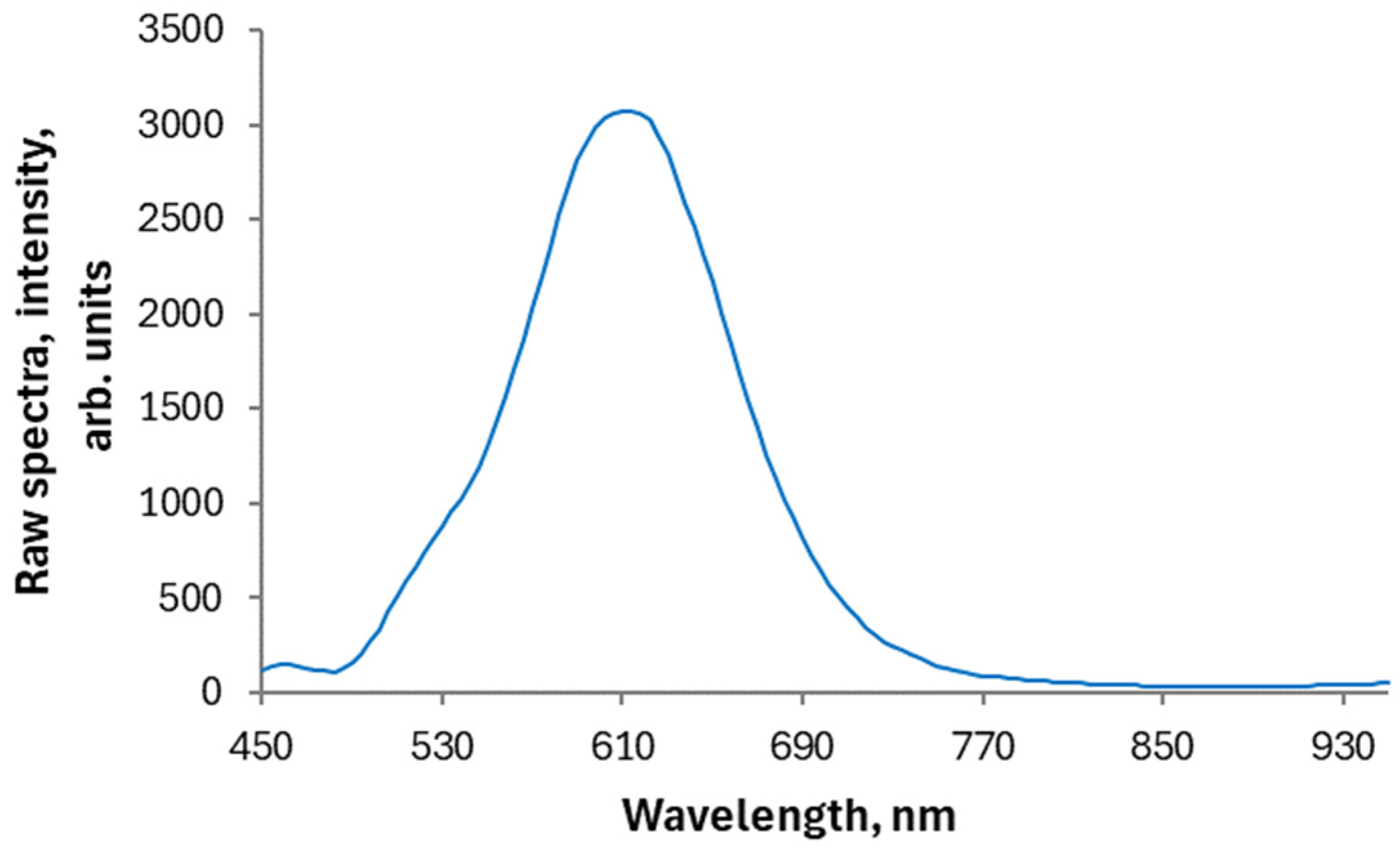
3.1. Character of Changes in the F. elastica Spectral Profiles Induced by the EL Exposure
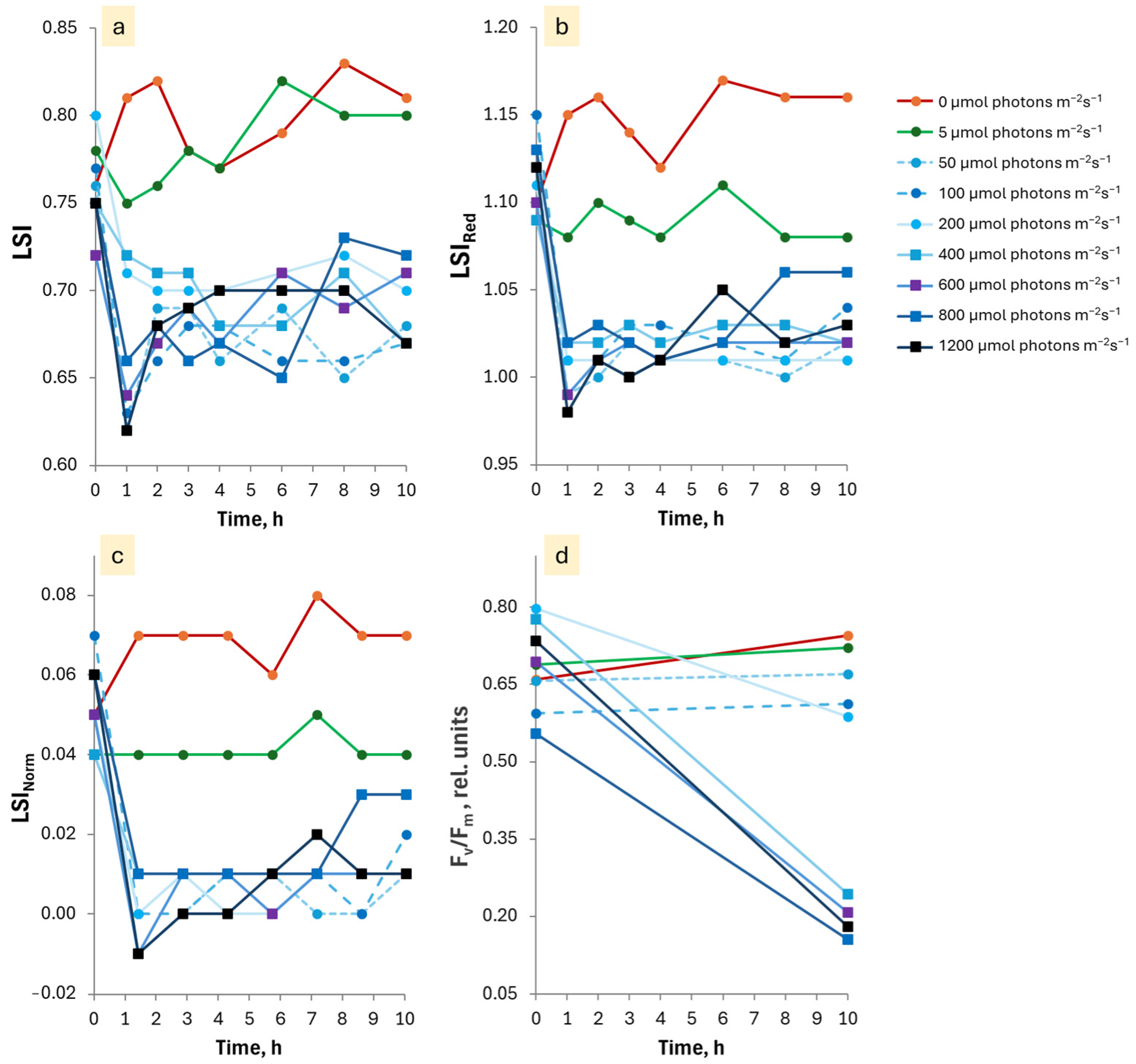
3.2. Dependence of the EL After-Effects on PPFD and Duration of Exposure
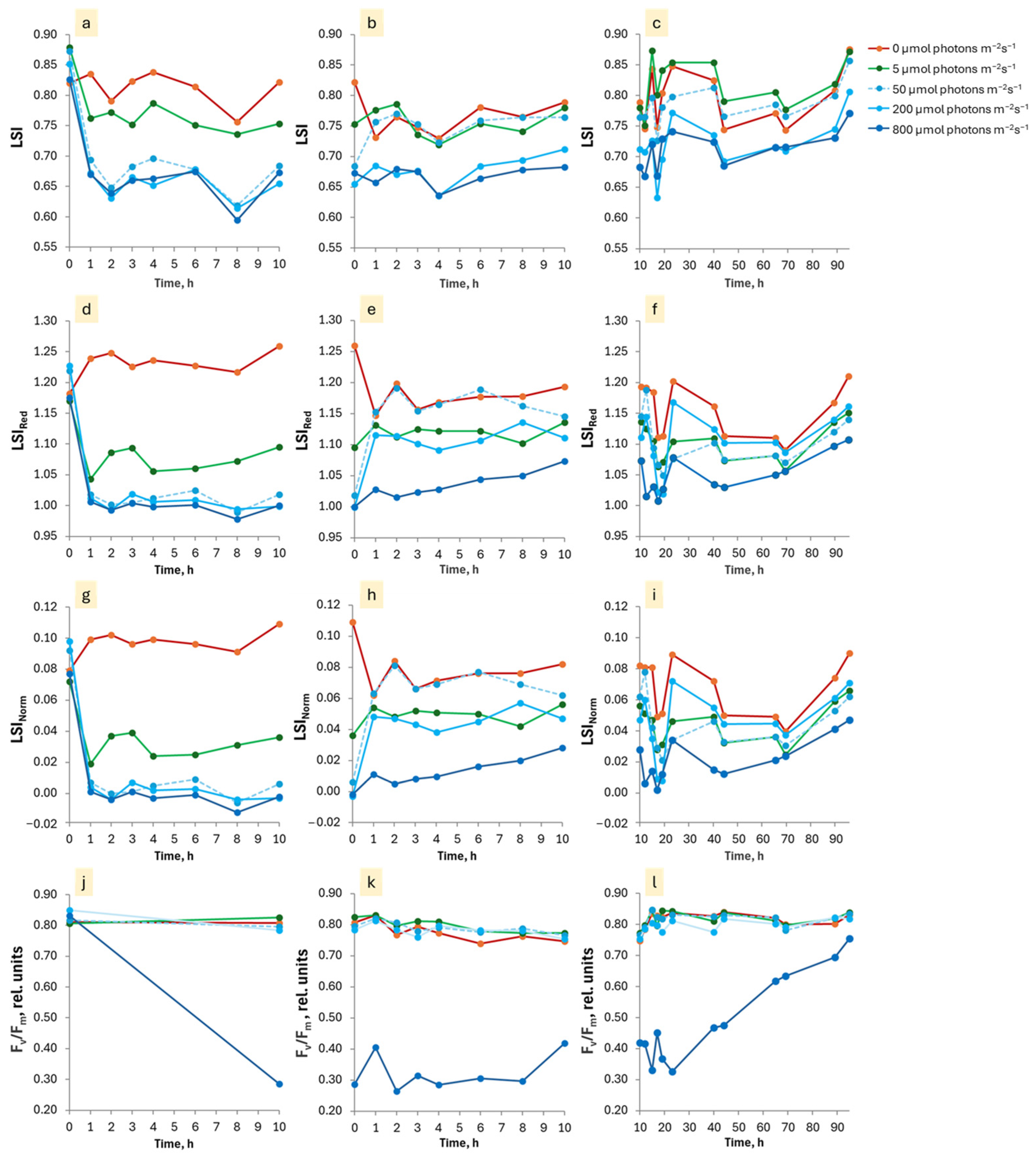
3.3. Duration of the Persistence of the EL and LL After-Effects
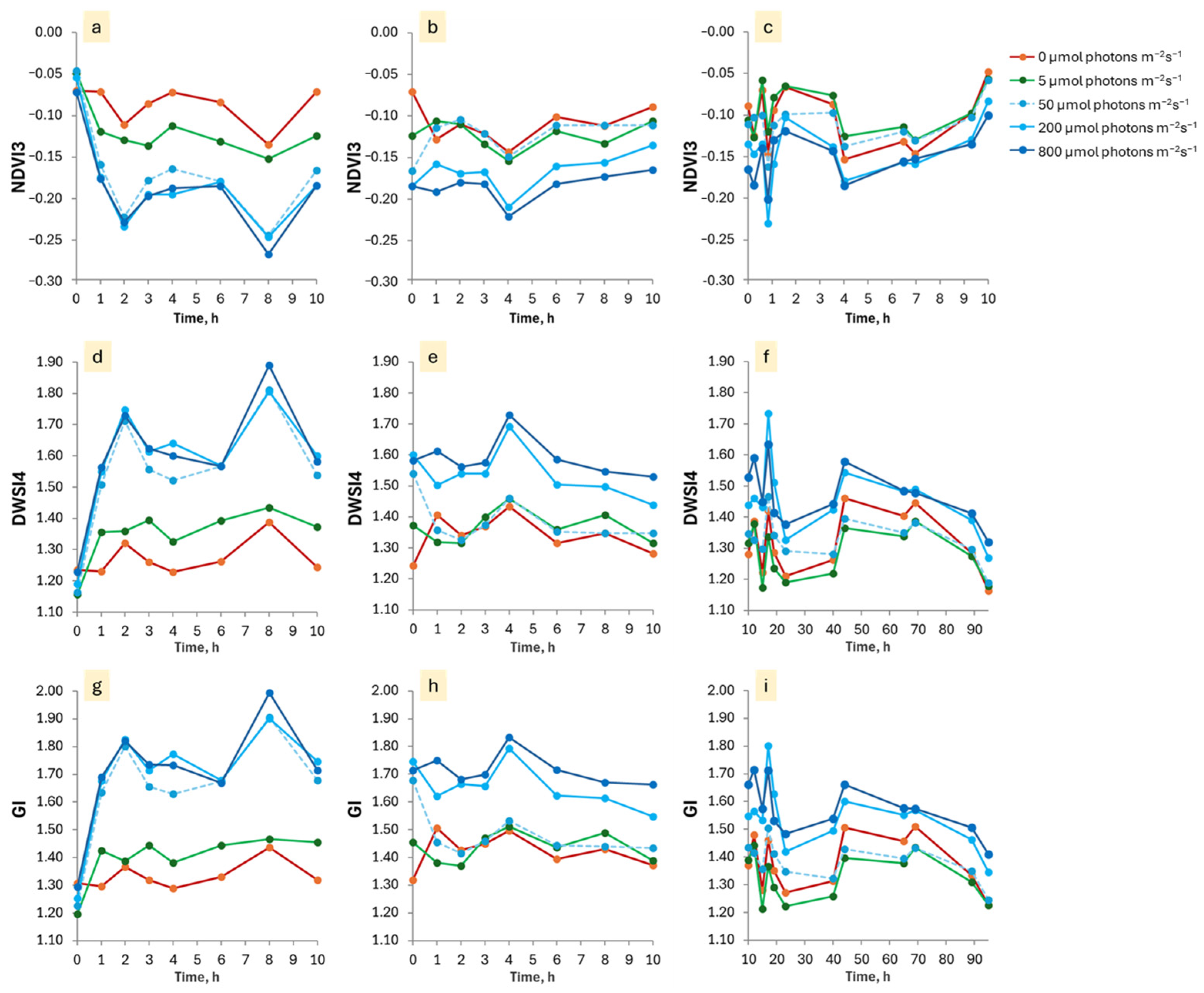
3.4. Comparison of the Response of LSI, LSIRed and LSINorm to Light Stress with Other VIs
3.4.1. Results of Correlation and Regression Analysis
3.4.2. Results of Analysis of Variance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Zhao, Y.; Zhu, J.-K. Thriving under Stress: How Plants Balance Growth and the Stress Response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Modi, P.; Dave, A.; Vijapura, A.; Patel, D.; Patel, M. Effect of Abiotic Stress on Crops. In Sustainable Crop Production; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants response to light stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Roach, T.; Krieger-Liszkay, A. Regulation of photosynthetic electron transport and photoinhibition. Curr. Protein Pept. Sci. 2014, 15, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Zhang, M.; Ming, Y.; Wang, H.-B.; Jin, H.-L. Strategies for adaptation to high light in plants. aBIOTECH 2024. [Google Scholar] [CrossRef]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Baránková, B.; Lazár, D.; Nauš, J. Analysis of the effect of chloroplast arrangement on optical properties of green tobacco leaves. Remote Sens. Environ. 2016, 174, 181–196. [Google Scholar] [CrossRef]
- Araguirang, G.E.; Richter, A.S. Activation of anthocyanin biosynthesis in high light–what is the initial signal? New Phytol. 2022, 236, 2037–2043. [Google Scholar] [CrossRef]
- Nawrocki, W.J.; Tourasse, N.J.; Taly, A.; Rappaport, F.; Wollman, F.-A. The plastid terminal oxidase: Its elusive function points to multiple contributions to plastid physiology. Annu. Rev. Plant Biol. 2015, 66, 49–74. [Google Scholar] [CrossRef]
- Curien, G.; Flori, S.; Villanova, V.; Magneschi, L.; Giustini, C.; Forti, G.; Matringe, M.; Petroutsos, D.; Kuntz, M.; Finazzi, G. The water to water cycles in microalgae. Plant Cell Physiol. 2016, 57, 1354–1363. [Google Scholar] [CrossRef]
- Rochaix, J.-D. Regulation of photosynthetic electron transport. Biochim. Biophys. Acta 2011, 1807, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, V.; DRajput, V.; Kumar Singh, R.; Guo, Y.; Kosolapov, A.; Usova, E.; Varduny, T.; Chalenko, E.; Yadronova, O.; Dmitriev, P.; et al. Chlorophyll fluorometry in evaluating photosynthetic performance: Key limitations, possibilities, perspectives and alternatives. Physiol. Mol. Biol. Plants 2022, 28, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Nishiyama, Y. ATP is a driving force in the repair of photosystem II during photoinhibition. Plant Cell Environ. 2018, 41, 285–299. [Google Scholar] [CrossRef]
- Zeng, Y.; Hao, D.; Huete, A.; Dechant, B.; Berry, J.; Chen, J.M.; Joiner, J.; Frankenberg, C.; Bond-Lamberty, B.; Ryu, Y.; et al. Optical vegetation indices for monitoring terrestrial ecosystems globally. Nat. Rev. Earth Environ. 2022, 3, 477–493. [Google Scholar] [CrossRef]
- Xue, J.; Baofeng, S. Significant Remote Sensing Vegetation Indices: A Review of Developments and Applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Wong, C.Y.S. Plant optics: Underlying mechanisms in remotely sensed signals for phenotyping applications. AoB Plants 2023, 15, plad039. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Lešić, V.; Pajač Živković, I.; Lemić, D. Detection and Evaluation of Environmental Stress in Winter Wheat Using Remote and Proximal Sensing Methods and Vegetation Indices—A Review. Diversity 2023, 15, 481. [Google Scholar] [CrossRef]
- Zhu, K.; Sun, Z.; Zhao, F.; Yang, T.; Tian, Z.; Lai, J.; Zhu, W.; Long, B. Relating Hyperspectral Vegetation Indices with Soil Salinity at Different Depths for the Diagnosis of Winter Wheat Salt Stress. Remote Sens. 2021, 13, 250. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Sensitivity of spectral vegetation indices for monitoring water stress in tomato plants. Comput. Electron. Agric. 2019, 163, 104860. [Google Scholar] [CrossRef]
- Sanches, I.D.A.; Filho, C.R.S.; Kokaly, R.F. Spectroscopic remote sensing of plant stress at leaf and canopy levels using the chlorophyll 680nm absorption feature with continuum removal. ISPRS J. Photogramm. Remote Sens. 2014, 97, 111–122. [Google Scholar] [CrossRef]
- Gamon, J.; Huemmrich, K.; Wong, C.; Ensminger, I.; Garrity, S.; Hollinger, D.; Noormets, A.; Penuelas, J. A remotely sensed pigment index reveals photosynthetic phenology in evergreen conifers. Proc. Natl. Acad. Sci. USA 2016, 113, 201606162. [Google Scholar] [CrossRef]
- Gamon, J.; Serrano, L.; Surfus, J. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Davison, P.; Hunter, C.; Horton, P. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zygielbaum, A.I.; Arkebauer, T.J.; Walter-Shea, E.A.; Solovchenko, A. Stress detection in vegetation based on remotely sensed light absorption coefficient. Int. J. Remote Sens. 2024, 45, 259–277. [Google Scholar] [CrossRef]
- Dmitriev, P.A.; Kozlovsky, B.L.; Dmitrieva, A.A.; Lysenko, V.S.; Chokheli, V.A.; Varduni, T.V. Indication of Light Stress in Ficus elastica Using Hyperspectral Imaging. AgriEngineering 2023, 5, 2253–2265. [Google Scholar] [CrossRef]
- Cun, Z.; Xu, X.Z.; Zhang, J.Y.; Shuang, S.P.; Wu, H.M.; An, T.X.; Chen, J.W. Responses of photosystem to long-term light stress in a typically shade-tolerant species Panax notoginseng. Front. Plant Sci. 2023, 13, 1095726. [Google Scholar] [CrossRef] [PubMed]
- Kothari, S.; Montgomery, R.A.; Cavender-Bares, J. Physiological responses to light explain competition and facilitation in a tree diversity experiment. J. Ecol. 2021, 109, 2000–2018. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Ntagkas, N.; Siebenkäs, A.; Mäenpää, M.; Matsubara, S.; Pons, T. A meta-analysis of plant responses to light intensity for 70 traits ranging from molecules to whole plant performance. New Phytol. 2019, 223, 1073–1105. [Google Scholar] [CrossRef]
- Dmitriev, P.A.; Kozlovsky, B.L.; Dmitrieva, A.A.; Varduni, T.V. Classification of invasive tree species based on the seasonal dynamics of the spectral characteristics of their leaves. Earth Sci. Inform. 2023, 16, 3729–3743. [Google Scholar] [CrossRef]
- Galieni, A.; D’Ascenzo, N.; Stagnari, F.; Pagnani, G.; Xie, Q.; Pisante, M. Past and Future of Plant Stress Detection: An Overview from Remote Sensing to Positron Emission Tomography. Front. Plant Sci. 2021, 11, 609155. [Google Scholar] [CrossRef] [PubMed]
- Zubler, A.V.; Yoon, J.Y. Proximal Methods for Plant Stress Detection Using Optical Sensors and Machine Learning. Biosensors 2020, 10, 193. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Ikeura, H.; Phongchanmaixay, S.; Khanthavong, P. Canopy hyperspectral sensing of paddy fields at the booting stage and PLS regression can assess grain yield. Remote Sens. 2018, 10, 1249. [Google Scholar] [CrossRef]
- Reis Pereira, M.; Verrelst, J.; Tosin, R.; Rivera Caicedo, J.P.; Tavares, F.; Neves dos Santos, F.; Cunha, M. Plant Disease Diagnosis Based on Hyperspectral Sensing: Comparative Analysis of Parametric Spectral Vegetation Indices and Nonparametric Gaussian Process Classification Approaches. Agronomy 2024, 14, 493. [Google Scholar] [CrossRef]
- Prechsl, U.E.; Mejia-Aguilar, A.; Cullinan, C.B. In vivo spectroscopy and machine learning for the early detection and classification of different stresses in apple trees. Sci. Rep. 2023, 13, 15857. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef]
- Datt, B. Remote Sensing of Chlorophyll a, Chlorophyll b, Chlorophyll a+b, and Total Carotenoid Content in Eucalyptus Leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Apan, A.; Held, A.; Phinn, S.; Markley, J. Detecting sugarcane “orange rust” disease using EO-1 Hyperion hyperspectral imagery. Int. J. Remote Sens. 2004, 25, 489–498. [Google Scholar] [CrossRef]
- Smith, R.; Adams, J.; Stephens, D.; Hick, P. Forecasting wheat yield in a mediterranean-type environment from the NOAA satellite. Aust. J. Agric. Res. 1995, 46, 113–125. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Laisk, A.; Oja, V.; Eichelmann, H.; Luca Dall’Osto, L. Action spectra of photosystems II and I and quantum yield of photo-synthesis in leaves in State 1. Biochim. Biophys. Acta 2014, 1837, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Schmiege, S.C.; Griffin, K.L.; Boelman, N.T.; Vierling, L.A.; Bruner, S.G.; Min, E.; Maguire, A.J.; Jensen, J.; Eitel, J.U.H. Vertical gradients in photosynthetic physiology diverge at the latitudinal range extremes of white spruce. Plant Cell Environ. 2022, 46, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Kohzuma, K.; Tamaki, M.; Hikosaka, K. Corrected photochemical reflectance index (PRI) is an effective tool for detecting environmental stresses in agricultural crops under light conditions. J. Plant Res. 2021, 134, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Boochs, F.; Kupfer, G.; Dockter, K.; Kuhbauch, W. Shape of the red edge as vitality indicator for plants. Int. J. Remote Sens. 1990, 11, 1741–1753. [Google Scholar] [CrossRef]
- Kim, M.; Daughtry, C.; Chappelle, E.; McMurtrey, J.; Walthall, C. The use of high spectral resolution bands for estimating absorbed photosynthetically active radiation (Apar). In Proceedings of the Sixth Symposium on Physical Measurements and Signatures in Remote Sensing, Val d’Isere, France, 17–24 January 1994; Volume 17, pp. 299–306. [Google Scholar]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Pushnik, J.C.; Dobrowski, S.; Ustin, S.L. Steadystate chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red-edge effects. Remote Sens. Environ. 2003, 84, 283–294. [Google Scholar] [CrossRef]
- Gitelson, A.; Gritz, Y.; Merzlyak, M. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Oppelt, N.; Mauser, W. Hyperspectral monitoring of physiological parameters of wheat during a vegetation period using AVIS data. Int. J. Remote Sens. 2004, 25, 145–159. [Google Scholar] [CrossRef]
- Datt, B. Visible/near infrared reflectance and chlorophyll content in Eucalyptus leaves. Int. J. Remote Sens. 1999, 20, 2741–2759. [Google Scholar] [CrossRef]
- Maire, G.; Francois, C.; Dufrene, E. Towards universal broad leaf chlorophyll indices using PROSPECT simulated database and hyperspectral reflectance measurements. Remote Sens. Environ. 2004, 89, 1–28. [Google Scholar] [CrossRef]
- Penuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance indices associated with physiological-changes in nitrogen-limited and water-limited sun ower leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Huete, A.; Liu, H.Q.; Batchily, K.; van Leeuwen, W. A comparison of vegetation indices over a global set of TM images for EOS–MODIS. Remote Sens. Environ. 1997, 59, 440–451. [Google Scholar] [CrossRef]
- Gitelson, A.; Buschmann, C.; Lichtenthaler, H. The chlorophyll fluorescence ratio F735/F700 as an accurate measure of the chlorophyll content in plants—Experiments with autumn chestnut and maple leaves. Remote Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Maccioni, A.; Agati, G.; Mazzinghi, P. New vegetation indices for remote measurement of chlorophylls based on leaf directional reflectance spectra. J. Photochem. Photobiol. B Biol. 2001, 61, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Daughtry, C.; Walthall, C.; Kim, M.; Brown de Colstoun, E.; McMurtrey, J.E., III. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Hernandez-Clemente, R.; Navarro-Cerrillo, R.M.; Suarez, L.; Morales, F.; Zarco-Tejada, P.J. Assessing structural effects on PRI for stress detection in conifer forests. Remote Sens. Environ. 2011, 115, 2360–2375. [Google Scholar] [CrossRef]
- Qi, J.; Chehbouni, A.; Huete, A.; Kerr, Y.; Sorooshian, S. A modified soil adjusted vegetation index. Remote Sens. Environ. 1994, 48, 119–126. [Google Scholar] [CrossRef]
- Chen, J.M. Evaluation of vegetation indices and a modified simple ratio for boreal applications. Can. J. Remote Sens. 1996, 22, 229–242. [Google Scholar] [CrossRef]
- Dash, J.P.; Curran, P.J. The MERIS terrestrial chlorophyll index. Int. J. Remote Sens. 2004, 25, 5403–5413. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative estimation of chlorophyll-a using reflectance spectra: Experiments with autumn chestnut and maple leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Gandia, S.; Fernandez, G.; Garcia, J.; Moreno, J. Retrieval of vegetation biophysical variables from CHRIS/PROBA data in the SPARC campaign. In Proceedings of the ESA SP-578, 2nd CHRIS/Proba Workshop, ESA/ESRIN, Frascati, Italy, 28–30 April 2004; pp. 40–48. [Google Scholar]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Chappelle, E.W.; Kim, M.S.; McMurtrey, J.E. Ratio analysis of reflectance spectra (rars)—An algorithm for the remote estimation of the concentrations of chlorophyll-a, chlorophyll-b, and carotenoids in soybean leaves. Remote Sens. Environ. 1992, 39, 239–247. [Google Scholar] [CrossRef]
- Gamon, J.; Penuelas, J.P.; Field, C. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Gonzalez-Dugo, V.; Williams, L.E.; Suarez, L.; Berni, J.A.J.; Goldhamer, D.; Fereres, E. A PRI-based water stress index combining structural and chlorophyll effects: Assessment using diurnal narrow-band airborne imagery and the CWSI thermal index. Remote Sens. Environ. 2013, 138, 38–50. [Google Scholar] [CrossRef]
- Garrity, S.R.; Eitel, J.U.; Vierling, L.A. Disentangling the relationships between plant pigments and the photochemical reflectance index reveals a new approach for remote estimation of carotenoid content. Remote Sens. Environ. 2011, 115, 628–635. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Blackburn, G.A. Quantifying chlorophylls and caroteniods at leaf and canopy scales: An evaluation of some hyperspectral approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Roujean, J.L.; Breon, F.M. Estimating par absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Guyot, G.; Baret, F. Utilisation de la haute resolution spectrale pour suivre l’etat des couverts vegetaux. Spectr. Signat. Objects Remote Sens. 1988, 287, 279–286. [Google Scholar]
- Huete, A. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Vincini, M.; Frazzi, E.; D’Alessio, P. Angular dependence of maize and sugar beet VIs from directional CHRIS/PROBA data. In Proceedings of the 4th ESA CHRIS PROBA Workshop, Frascati, Italy, 19–21 September 2006; Volume 1, pp. 19–21. [Google Scholar]
- Jordan, C.F. Derivation of leaf-area index from quality of light on forest floor. Ecology 1969, 50, 663–678. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- McMurtrey, J.E.; Chappelle, E.W.; Kim, M.S.; Meisinger, J.J.; Corp, L.A. Distinguishing nitrogen-fertilization levels in-field corn (Zea mays L) with actively induced fluorescence and passive reflectance measurements. Remote Sens. Environ. 1994, 47, 36–44. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.J.; Miller, J.R. Land cover mapping at BOREAS using red edge spectral parameters from CASI imagery. J. Geophys. Res.-Atmos. 1999, 104, 27921–27933. [Google Scholar]
- Hernandez-Clemente, R.; Navarro-Cerrillo, R.M.; Zarco-Tejada, P.J. Carotenoid content estimation in a heterogeneous conifer forest using narrowband indices and PROSPECT + DART simulations. Remote Sens. Environ. 2012, 127, 298–315. [Google Scholar] [CrossRef]
- Elvidge, C.D.; Chen, Z.K. Comparison of broad-band and narrow-band red and near-infrared vegetation indices. Remote Sens. Environ. 1995, 54, 38–48. [Google Scholar] [CrossRef]
- Filella, I.; Penuelas, J. The red edge position and shape as indicators of plant chlorophyll content, biomass, and hydric status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.; Perry, E.M.; Akhmedov, B. A visible band index for remote sensing leaf chlorophyll content at the canopy scale. Int. J. Appl. Earth Obs. Geoinf. 2013, 21, 103–112. [Google Scholar] [CrossRef]
- Broge, N.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2000, 76, 156–172. [Google Scholar] [CrossRef]
- Vogelmann, J.E.; Rock, B.N.; Moss, D.M. Red edge spectral measurements from sugar maple leaves. Int. J. Remote Sens. 1993, 14, 1563–1575. [Google Scholar] [CrossRef]



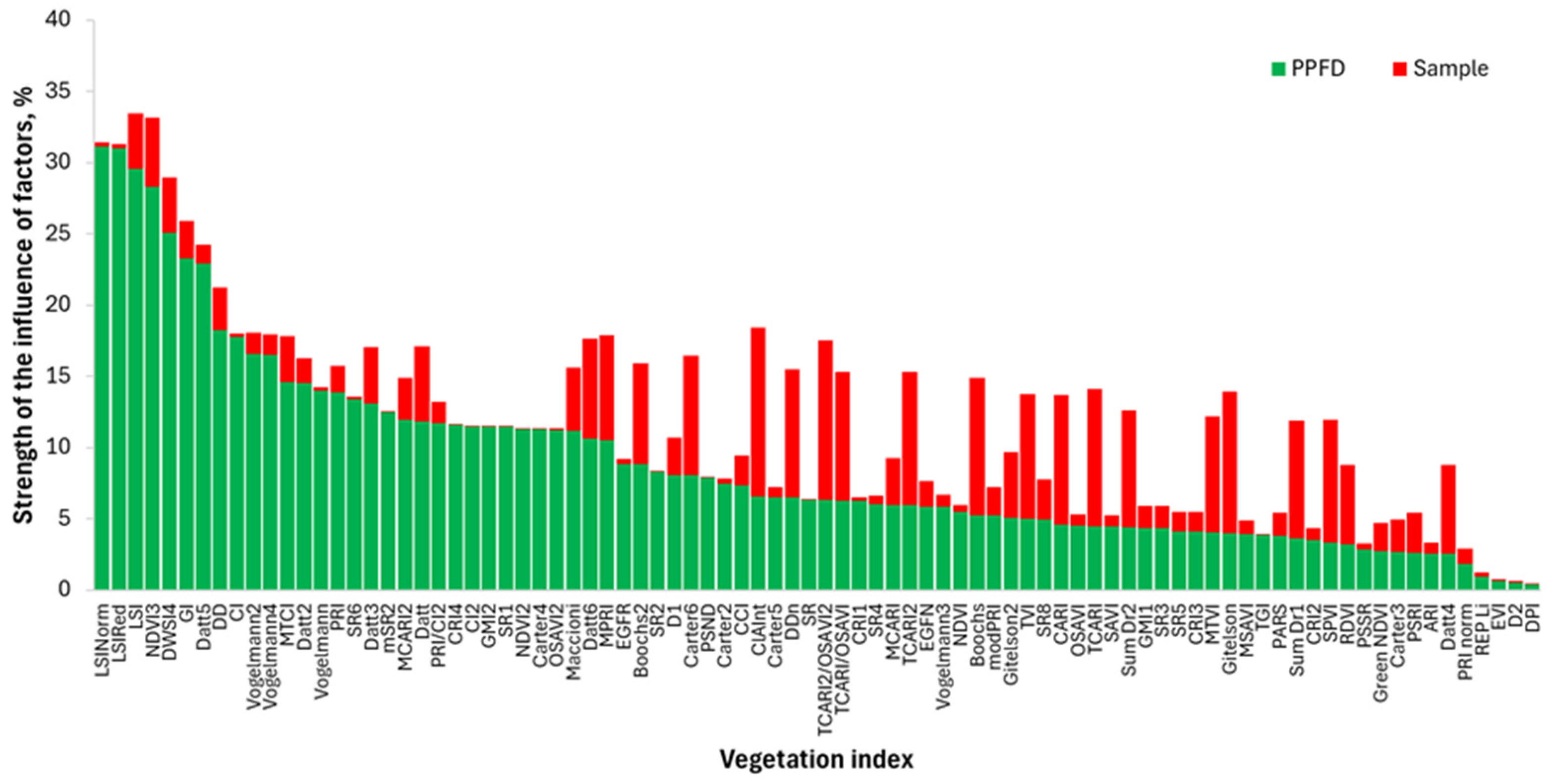
| Measurement Variant | PPFD, μmol Photons m−2s−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 50 | 100 | 200 | 400 | 600 | 800 | 1200 | |
| No ventilation | 25.0 | 25.0 | 26.9 | 27.3 | 28.0 | 28.8 | 29.2 | 29.9 | 31.7 |
| Ventilated | NA | NA | 25.0 | 25.5 | 27.0 | 27.2 | 27.3 | 27.5 | 27.8 |
| Date of Experiment | PPFD, μmol Photons m−2s−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 50 | 100 | 200 | 400 | 600 | 800 | 1200 | |
| 26 January 2024 | X | X | X | X | |||||
| 2 February 2024 | X | X | X | X | |||||
| 8 February 2024 | X | X | X | X | |||||
| 9 February 2024 | X | X | X | X | |||||
| 16 February 2024 | X | X | X | X | |||||
| LSI | ||||
| Factor | Df | Sum Sq | Mean Sq | F value |
| PPFD | 8 | 6.217 | 0.777 | 135.500 |
| Sample | 2 | 0.818 | 0.409 | 71.300 |
| PPFD & Sample | 16 | 1.165 | 0.073 | 12.700 |
| Residuals | 2236 | 12.820 | 0.006 | – |
| LSINorm | ||||
| Factor | Df | Sum Sq | Mean Sq | F value |
| PPFD | 8 | 1.088 | 0.136 | 133.651 |
| Sample | 2 | 0.011 | 0.006 | 5.595 |
| PPFD & Sample | 16 | 0.121 | 0.008 | 7.444 |
| Residuals | 2236 | 2.275 | 0.001 | – |
| LSIRed | ||||
| Factor | Df | Sum Sq | Mean Sq | F value |
| PPFD | 8 | 5.226 | 0.653 | 132.893 |
| Sample | 2 | 0.049 | 0.024 | 4.951 |
| PPFD & Sample | 16 | 0.584 | 0.037 | 7.426 |
| Residuals | 2236 | 10.991 | 0.005 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitriev, P.A.; Kozlovsky, B.L.; Dmitrieva, A.A.; Varduni, T.V.; Lysenko, V.S. Light Stress Detection in Ficus elastica with Hyperspectral Indices. AgriEngineering 2024, 6, 3297-3311. https://doi.org/10.3390/agriengineering6030188
Dmitriev PA, Kozlovsky BL, Dmitrieva AA, Varduni TV, Lysenko VS. Light Stress Detection in Ficus elastica with Hyperspectral Indices. AgriEngineering. 2024; 6(3):3297-3311. https://doi.org/10.3390/agriengineering6030188
Chicago/Turabian StyleDmitriev, Pavel A., Boris L. Kozlovsky, Anastasiya A. Dmitrieva, Tatyana V. Varduni, and Vladimir S. Lysenko. 2024. "Light Stress Detection in Ficus elastica with Hyperspectral Indices" AgriEngineering 6, no. 3: 3297-3311. https://doi.org/10.3390/agriengineering6030188
APA StyleDmitriev, P. A., Kozlovsky, B. L., Dmitrieva, A. A., Varduni, T. V., & Lysenko, V. S. (2024). Light Stress Detection in Ficus elastica with Hyperspectral Indices. AgriEngineering, 6(3), 3297-3311. https://doi.org/10.3390/agriengineering6030188






