Use of Conyza canadensis L. Extracts as Biostimulant in Cyclamen persicum Mill.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location
2.2. Obtaining and Growing Cyclamen
2.3. Cultivation and Obtaining C. canadensis Extract
2.4. SPAD Index Measures
2.5. Biomass Measurements and Number of Leaves
2.6. Determination of Gas Exchange Rates
2.7. LC-MS/MS and GC-MS Analysis
2.8. Statistical Analysis
3. Results and Discussion
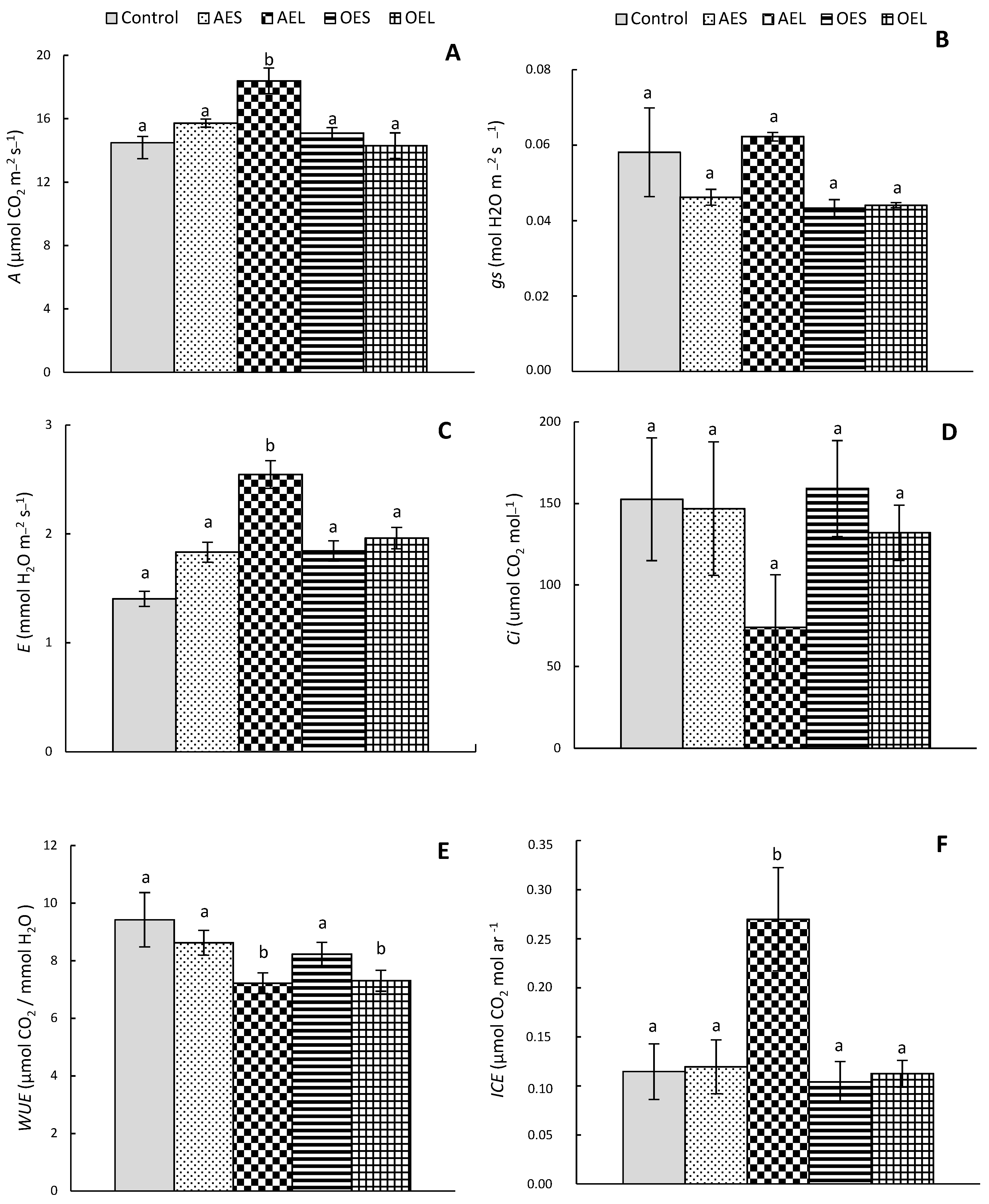
3.1. Gas Exchange Parameters
3.2. SPAD Index Assessment
3.3. Biomass and Leaf Quantity Measurements
3.4. Identification of Bioactive Compounds
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cozzolino, E.; Giordano, M.; Fiorentino, N.; El-Nakhel, C.; Pannico, A.; Di Mola, I.; Mori, M.; Kyriacou, M.; Colla, G.; Rouphael, Y. Appraisal of biodegradable mulching films and vegetal-derived biostimulant application as eco-sustainable practices for enhancing lettuce crop performance and nutritive value. Agronomy 2020, 10, 427. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 511937. [Google Scholar] [CrossRef]
- Godlewska, K.; Ronga, D.; Michalak, I. Plant extracts-importance in sustainable agriculture. Ital. J. Agron. 2021, 16, 1851. [Google Scholar] [CrossRef]
- Bogatek, R.; Gniazdowska, A. ROS and phytohormones in plant-plant allelopathic interaction. Plant Signal. Behav. 2007, 2, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef]
- Farooq, M.; Rizwan, M.; Nawaz, A.; Rehman, A.; Ahmad, R. Application of natural plant extracts improves the tolerance against combined terminal heat and drought stresses in bread wheat. J. Agron. Crop Sci. 2017, 203, 528–538. [Google Scholar] [CrossRef]
- Dipak Kumar, H.; Aloke, P. Role of biostimulant formulations in crop production: An overview. Int. J. Agric. Sci. Vet. Med. 2020, 8, 38–46. [Google Scholar]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture Through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Castiglione, A.M.; Mannino, G.; Contartese, V.; Bertea, C.M.; Ertani, A. Microbial biostimulants as response to modern agriculture needs: Composition, role and application of these innovative products. Plants 2021, 10, 1533. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants application: A low input cropping management tool for sustainable farming of vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhihui, C. Allium Sativum extract as a biopesticide affecting pepper blight. Int. J. Veg. Sci. 2009, 15, 13–23. [Google Scholar] [CrossRef]
- Roy, S.; Mukhopadhyay, A.; Gurusubramanian, G. Field efficacy of a biopesticide prepared from clerodendrum viscosum vent. (Verbenaceae) against two major tea pests in the sub himalayan tea plantation of North Bengal, India. J. Pest Sci. 2010, 83, 371–377. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Tinti, A.; Nardi, S. Biological activity of vegetal extracts containing phenols on plant metabolism. Molecules 2016, 21, 205. [Google Scholar] [CrossRef]
- Ali, M.; Cheng, Z.H.; Hayat, S.; Ahmad, H.; Ghani, M.I.; Liu, T. Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L.). J. Integr. Agric. 2019, 18, 1001–1013. [Google Scholar] [CrossRef]
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef]
- Saa, S.; Del Rio, A.O.; Castro, S.; Brown, P.H. Foliar application of microbial and plant based biostimulants increases growth and potassium uptake in almond (Prunus dulcis [Mill.] D. A. Webb). Front. Plant Sci. 2015, 6, 87. [Google Scholar] [CrossRef] [PubMed]
- Del-Toro-Sánchez, C.L.; Rodríguez-Félix, F.; Cinco-Moroyoqui, F.J.; Juárez, J.; Ruiz-Cruz, S.; Wong-Corral, F.J.; Borboa-Flores, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; Tapia-Hernández, J.A. Recovery of phytochemical from three safflower (Carthamus tinctorius L.) by-products: 20, protective effect of human erythrocytes and profile by UPLC-DAD-MS. J. Food Process. Preserv. 2021, 45, 3. [Google Scholar] [CrossRef]
- Ali, E.F.; Hassan, F.A.S.; Elgimabi, M. Improving the growth, yield and volatile oil content of Pelargonium graveolens L. herit by foliar application with moringa leaf extract through motivating physiological and biochemical parameters. S. Afr. J. Bot. 2018, 119, 383–389. [Google Scholar] [CrossRef]
- Nawaz, M.; Wang, X.; Saleem, M.H.; Khan, M.H.U.; Afzal, J.; Fiaz, S.; Ali, S.; Ishaq, H.; Khan, A.H.; Rehman, N.; et al. Deciphering Plantago Ovata Forsk leaf extract mediated distinct germination, growth and physio-biochemical improvements under water stress in maize (Zea mays L.) at Early Growth Stage. Agronomy 2021, 11, 1404. [Google Scholar] [CrossRef]
- Balal, R.M.; Shahid, M.A.; Javaid, M.M.; Anjum, M.A.; Ali, H.H.; Mattson, N.S.; Garcia-Sanchez, F. Foliar Treatment with Lolium perenne (Poaceae) leaf extract alleviates salinity and nickel-induced growth inhibition in pea. Rev. Bras. Bot. 2016, 39, 453–463. [Google Scholar] [CrossRef]
- Figueiredo, F.R.A.; Nóbrega, J.S.; de Fátima, R.T.; Ferreira, J.T.A.; da Silva Leal, M.P.; Melo, M.F.; Dias, T.J.; de Albuquerque, M.B. Impact of biostimulant and saline water on cape gooseberry (Physalis peruviana L.) in Brazil. Physiol. Mol. Biol. Plants 2021, 27, 2141–2150. [Google Scholar] [CrossRef]
- Lefi, E.; Badri, M.; Hamed, S.B.; Talbi, S.; Mnafgui, W.; Ludidi, N.; Chaieb, M. Influence of brown seaweed (Ecklonia maxima) extract on the morpho-physiological parameters of melon, cucumber, and tomato plants. Agronomy 2023, 13, 2745. [Google Scholar] [CrossRef]
- Rouphael, Y.; De Micco, V.; Arena, C.; Raimondi, G.; Colla, G.; De Pascale, S. Effect of Ecklonia maxima seaweed extract on yield, mineral composition, gas exchange, and leaf anatomy of zucchini squash grown under saline conditions. J. Appl. Phycol. 2017, 29, 459–470. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-based biostimulants influence the agronomical, physiological, and qualitative responses of baby rocket leaves under diverse nitrogen conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Goncalves, P.; Copeland, E.; Qi, S.S.; Dai, Z.C.; Li, G.L.; Wang, C.Y.; Du, D.L.; Thomas, T. Invasion by the weed Conyza canadensis alters soil nutrient supply and shifts microbiota structure. Soil Biol. Biochem. 2020, 143, 107739. [Google Scholar] [CrossRef]
- Ruiz, M.R.; Mangolin, C.A.; Oliveira Júnior, R.S.; Mendes, R.R.; Takano, H.K.; Eisele, T.G.; Machado, M.F.P.S. Mechanisms that may lead to high genetic divergence and to the invasive success of tall fleabane (Conyza sumatrensis; Asteraceae). Weed Sci. 2022, 70, 64–78. [Google Scholar] [CrossRef]
- Sahil, L.D.; Florentine, S.; Chauhan, B.S. Germination ecology of hairy fleabane (Conyza bonariensis) and its implications for weed management. Weed Sci. 2020, 68, 411–417. [Google Scholar] [CrossRef]
- Huang, H.; Ye, R.; Qi, M.; Li, X.; Miller, D.R.; Stewart, C.N.; Dubois, D.W.; Wang, J. Wind-mediated horseweed (Conyza canadensis) gene flow: Pollen emission, dispersion, and deposition. Ecol. Evol. 2015, 5, 2646–2658. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhang, Z.H. Aqueous tissue extracts of inhibit the germination and shoot growth of three native herbs with no autotoxic effects. Planta Daninha 2013, 31, 805–811. [Google Scholar] [CrossRef]
- Bhowmik, P.C. Horseweed (Conyza canadensis) seed production, emergence, and distribution in no-tillage and conventional-tillage corn (Zea mays). Agron. Trends Agric. Sci 1993, 1, 67–71. [Google Scholar]
- Shields, E.J.; Dauer, J.T.; VanGessel, M.J.; Neumann, G. Horseweed (Conyza canadensis) seed collected in the planetary boundary layer. Weed Sci. 2006, 54, 1063–1067. [Google Scholar] [CrossRef]
- Peralta, A.C.; Soriano, G.; Zorrilla, J.G.; Masi, M.; Cimmino, A.; Fernández-Aparicio, M. Characterization of Conyza bonariensis allelochemicals against broomrape weeds. Molecules 2022, 27, 7421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, Z.; Zhong, S.; Yu, Y.; Xu, Z.; Du, D.; Wang, C. Nitrogen influence to the independent invasion and the co-invasion of Solidago canadensis and Conyza canadensis via intensified allelopathy. Sustainability 2022, 14, 11970. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Soriano, G.; Masi, M.; Carretero, P.; Vilariño-Rodríguez, S.; Cimmino, A. (4Z)-lachnophyllum lactone, an acetylenic furanone from Conyza bonariensis, identified for the first time with allelopathic activity against Cuscuta campestris. Agriculture 2022, 12, 790. [Google Scholar] [CrossRef]
- Zhang, H.; Rutherford, S.; Qi, S.; Huang, P.; Dai, Z.; Du, D. Transcriptome profiling of Arabidopsis thaliana roots in response to allelopathic effects of Conyza canadensis. Ecotoxicology 2022, 31, 53–63. [Google Scholar] [CrossRef]
- Queiroz, S.C.N.; Cantrell, C.L.; Duke, S.O.; Wedge, D.E.; Nandula, V.K.; Moraes, R.M.; Cerdeira, A.L. Bioassay-directed isolation and identification of phytotoxic and fungitoxic acetylenes from Conyza canadensis. J. Agric. Food Chem. 2012, 60, 5893–5898. [Google Scholar] [CrossRef]
- Marque, J.M.; Kobori, R.F.; Kato, S.M. Ocorrência de Colletotrichum gloeosporioides em ciclamem cultivado em vaso sob estufa no Estado de São Paulo. Fitopatol. Bras. 2006, 31, 323. [Google Scholar] [CrossRef]
- Neves, M.F.; Pinto, M.J.A. Mapeamento e Quantificação da Cadeia de Flores e Plantas Ornamentais Do Brasil; OCESP: São Paulo, Brazil, 2015; 132p, Available online: https://www.ibraflor.com.br (accessed on 1 January 2024).
- R CORE TEAM. R Foundation for Statistical Computing: Vienna, Austria, 2020. Available online: https://www.r-project.org (accessed on 3 February 2024).
- Jones, H. Whats is water use efficiency. In Water Use Efficiency in Plant Biology; Bacon, M., Ed.; Blackwell Publishing: Oxford, UK, 2004; pp. 27–41. [Google Scholar]
- Ahmad, A.; Blasco, B.; Martos, V. Combating salinity through natural plant extracts based biostimulants: A review. Front. Plant Sci. 2022, 13, 862034. [Google Scholar] [CrossRef] [PubMed]
- Castronuovo, D.; Comegna, A.; Belviso, C.; Satriani, A.; Lovelli, S. Zeolite and Ascophyllum nodosum-based biostimulant effects on spinach gas exchange and growth. Agriculture 2023, 13, 754. [Google Scholar] [CrossRef]
- Luiz Piati, G.; Ferreira de Lima, S.; Lustosa Sobrinho, R.; dos Santos, O.F.; Vendruscolo, E.P.; Jacinto de Oliveira, J.; do Nascimento de Araújo, T.A.; Mubarak Alwutayd, K.; Finatto, T.; AbdElgawad, H. Biostimulants in corn cultivation as a means to alleviate the impacts of irregular water regimes induced by climate change. Plants 2023, 12, 2569. [Google Scholar] [CrossRef] [PubMed]
- Mutlu-Durak, H.; Arikan, Y.; Kutman, B.Y. Willow (Salix babylonica) extracts can act as biostimulants for enhancing salinity tolerance of maize grown in soilless culture. Plants 2023, 12, 856. [Google Scholar] [CrossRef] [PubMed]
- Sardar, H.; Nisar, A.; Anjum, M.A.; Naz, S.; Ejaz, S.; Ali, S.; Ahmad, R. Foliar spray of moringa leaf extract improves growth and concentration of pigment, minerals and stevioside in stevia (Stevia rebaudiana Bertoni). Ind. Crops Prod. 2021, 166, 113485. [Google Scholar] [CrossRef]
- Abd El-Mageed, T.A.; Semida, W.M.; Rady, M.M. Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agric. Water Manag. 2017, 193, 46–54. [Google Scholar] [CrossRef]
- Mona, M.A. The potential of moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. sativa) Plants. Int. J. Plant Physiol. Biochem. 2013, 5, 42–49. [Google Scholar] [CrossRef]
- Lazaroto, C.A.; Fleck, N.G.; Vidal, R.A. Biologia e ecofisiologia de buva (Conyza bonariensis e Conyza canadensis). Ciência Rural 2008, 38, 852–860. [Google Scholar] [CrossRef]
- Albrecht, A.J.P.; Pereira, V.G.C.; Souza, C.N.Z.D.; Zobiole, L.H.S.; Albrecht, L.P.; Adegas, F.S. Multiple resistance of Conyza sumatrensis to three mechanisms of action of herbicides. Acta Scientiarum. Agron. 2020, 42, e42485. [Google Scholar] [CrossRef]
- Da Silva, Í.A.; Campelo, L.H.D.B.P.; de Fátima Padilha, M.D.R.; Shinohara, N.K.S. Mecanismos de resistência das plantas alimentícias não convencionais (PANC) e benefícios para a saúde humana. An. Da Acad. Pernambucana De Ciência Agronômica 2018, 15, 77–91. Available online: https://www.journals.ufrpe.br/index.php/apca/article/view/1950 (accessed on 1 January 2024).
- Franzener, G.; da Silva Martinez-Franzener, A.; Stangarlin, J.R.; Czepak, M.P.; Schwan-Estrada, K.R.F.; Cruz, M.E.S. Atividades antibacteriana, antifúngica e indutora de fitoalexinas de hidrolatos de plantas medicinais. Semin. Ciências Agrárias 2007, 28, 29–38. [Google Scholar] [CrossRef][Green Version]
- Chen, K.; Khan, R.A.A.; Cao, W.; Ling, M. Sustainable and ecofriendly approach of managing soil born bacterium Ralstonia solanacearum (Smith) using dried powder of Conyza canadensis. Pathogens 2020, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Tanveer, M.U.; Liaqat, M.; Dole, J.M. Comparison of corm soaks with preharvest foliar application of moringa leaf extract for improving growth and yield of cut Freesia hybrida. Sci. Hortic. 2019, 254, 21–25. [Google Scholar] [CrossRef]
- Akram, N.A.; Saleem, M.H.; Shafiq, S.; Naz, H.; Farid-ul-Haq, M.; Ali, B.; Shafiq, F.; Iqbal, M.; Jaremko, M.; Qureshi, K.A. Phytoextracts as Crop Biostimulants and Natural Protective Agents—A Critical Review. Sustainability 2022, 14, 14498. [Google Scholar] [CrossRef]
- Barhoumi, L.M.; Shakya, A.K.; Al-Fawares, O.L.; Al-Jaber, H.I. Conyza canadensis from Jordan: Phytochemical Profiling, Antioxidant, and Antimicrobial Activity Evaluation. Molecules 2024, 29, 2403. [Google Scholar] [CrossRef]
- Shaukat, S.S.; Munir, N.; Siddiqui, I.A. Allelopathic responses of Conyza canadensis (L.) Cronquist: A cosmopolitan weed. Asian J. Plant Sci. 2003, 2, 1034–1039. [Google Scholar] [CrossRef]
- Vo, Q.V.; Bay, M.V.; Nam, P.C.; Quang, D.T.; Flavel, M.; Hoa, N.T.; Mechler, A. Theoretical and experimental studies of the antioxidant and antinitrosant activity of syringic acid. J. Org. Chem. 2020, 85, 15514–15520. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.; Küçükboyacı, N.; Demirci, B. Chemical composition and antimicrobial activity of the essential oil of Conyza canadensis (L.) Cronquist from Turkey. J. Essent. Oil Res. 2017, 29, 336–343. [Google Scholar] [CrossRef]
- Lo, Y.-C.; Rensi, S.E.; Torng, W.; Altman, R.B. Machine learning in chemoinformatics and drug discovery. Drug Discov. Today 2018, 23, 1538–1546. [Google Scholar] [CrossRef]
- Antonova, G.F.; Varaksina, T.N.; Zheleznichenko, T.V.; Stasova, V.V. Changes in phenolic acids during maturation and lignification of Scots pine xylem. Russ. J. Dev. Biol. 2012, 43, 199–208. [Google Scholar] [CrossRef]
- Hassoon, A.S.; Abduljabbar, I.A. Review on the role of salicylic acid in plants. In Sustainable Crop Production; Hasanuzzaman, M., Filho, M.C.M.T., Fujita, M., Nogueira, T.A.R., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic acid in root growth and development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef] [PubMed]
- Steenackers, W.; El Houari, I.; Baekelandt, A.; Witvrouw, K.; Dhondt, S.; Leroux, O.; Gonzalez, N.; Corneillie, S.; Cesarino, I.; Inzé, D.; et al. cis-Cinnamic acid is a natural plant growth-promoting compound. J. Exp. Bot. 2019, 70, 6293–6304. [Google Scholar] [CrossRef] [PubMed]
- Laoué, J.; Fernandez, C.; Ormeño, E. Plant flavonoids in Mediterranean species: A focus on flavonols as protective metabolites under climate stress. Plants 2022, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Kaneko, T.; Sato, S.; Saeki, K. Hijacking of leguminous nodulation signaling by the rhizobial type III secretion system. Proc. Natl. Acad. Sci. USA 2013, 110, 17131–17136. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, R.; Li, M.; Dang, H.; Solaiman, Z.M. Molecular signal communication during arbuscular mycorrhizal formation induces significant transcriptional reprogramming of wheat (Triticum aestivum) roots. Ann. Bot. 2019, 124, 1109–1119. [Google Scholar] [CrossRef]
- Keunen, E.; Peshev, D.; Vangronsveld, J.; Van Den Ende, W.; Cuypers, A. Plant sugars are crucial players in the oxidative challenge during abiotic stress: Extending the traditional concept. Plant Cell Environ. 2013, 36, 1242–1255. [Google Scholar] [CrossRef] [PubMed]



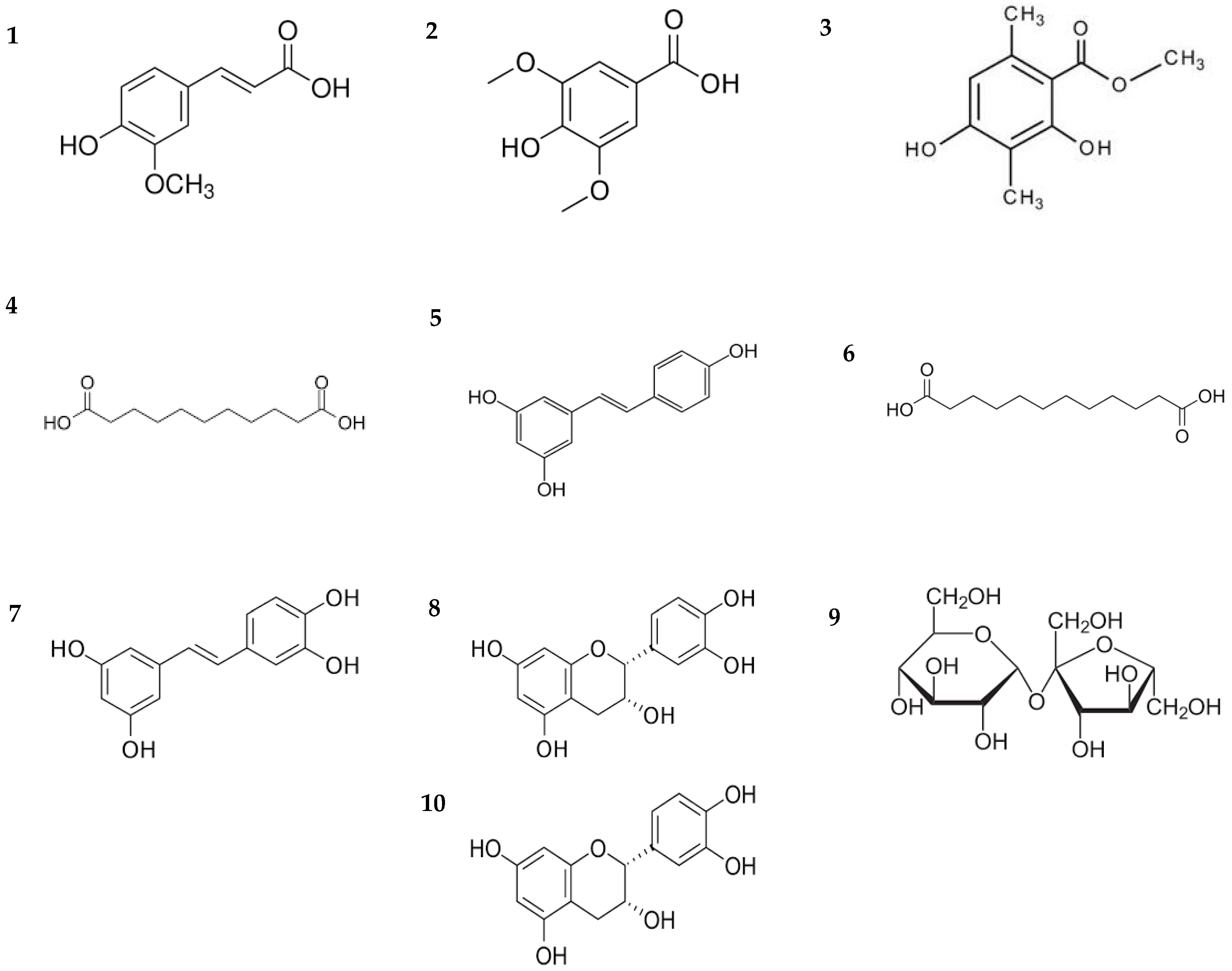
| Entry | m/z Experimental | m/z Calculated | Error (ppm) | iFit | Retention Time | Proposed Formula | Compound Class | Compound |
|---|---|---|---|---|---|---|---|---|
| 1 | 193.0494 | 193.0501 | −3.6 | 0 | 6.26 | C10H10O4 | Phenolic | Ferulic acid a,b |
| 2 | 199.0613 | 199.0606 | 3.5 | 0 | 1.14 | C9H10O5 | Phenolic | Syringic acid a |
| 3 | 209.0789 | 209.0814 | −12 | 0 | 9.00 | C11H14O4 | Phenolic | Ethyl 2,4-dihydroxy-5,6-dimethylbenzoate a |
| 4 | 215.1287 | 215.1283 | 1.9 | 0 | 14.46 | C11H20O4 | Carboxilic acid | Undecanedioic acid a |
| 5 | 229.0861 | 229.0865 | −1.7 | 0 | 9.04 | C14H12O3 | Phenolic | Resveratrol a |
| 6 | 229.1436 | 229.1440 | −1.7 | 0.2 | 16.10 | C12H22O4 | Carboxilic acid | Dodecanedioic acid a |
| 7 | 245.0798 | 245.0814 | −6.5 | 0.9 | 14.10 | C14H12O4 | Phenolic | trans-piacetannol a |
| 8 | 291.0864 | 291.0869 | −1.7 | 0.6 | 6.20 | C15H14O6 | Phenolic (flavonoid) | Epicathechin a |
| 9 | 341.1108 | 341.1084 | 7 | 0 | 13.99 | C12H22O11 | Disaccharide | Sucrose a |
| 10 | 577.1339 | 577.1346 | −1.2 | 0.7 | 10.20 | C30H24O12 | Phenolic (flavonoid) | Procianidin a |
| 11 | 607.1677 | 607.1663 | 2.3 | 0.1 | 6.30 | C28H32O15 | Phenolic (glycoside flavonoid) | Sorbifolin 6-O-α-rhamnopyranosyl(1′′′→6′′)-β-glucopyranoside a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, E.R.; May, A.; Procópio, S.O.; Assalin, M.R.; Quevedo, H.D.; Binhardi, N.; Queiroz, S.C.N. Use of Conyza canadensis L. Extracts as Biostimulant in Cyclamen persicum Mill. AgriEngineering 2024, 6, 2926-2940. https://doi.org/10.3390/agriengineering6030168
Batista ER, May A, Procópio SO, Assalin MR, Quevedo HD, Binhardi N, Queiroz SCN. Use of Conyza canadensis L. Extracts as Biostimulant in Cyclamen persicum Mill. AgriEngineering. 2024; 6(3):2926-2940. https://doi.org/10.3390/agriengineering6030168
Chicago/Turabian StyleBatista, Eunice R., Andre May, Sergio O. Procópio, Marcia R. Assalin, Helio D. Quevedo, Nicole Binhardi, and Sonia C. N. Queiroz. 2024. "Use of Conyza canadensis L. Extracts as Biostimulant in Cyclamen persicum Mill." AgriEngineering 6, no. 3: 2926-2940. https://doi.org/10.3390/agriengineering6030168
APA StyleBatista, E. R., May, A., Procópio, S. O., Assalin, M. R., Quevedo, H. D., Binhardi, N., & Queiroz, S. C. N. (2024). Use of Conyza canadensis L. Extracts as Biostimulant in Cyclamen persicum Mill. AgriEngineering, 6(3), 2926-2940. https://doi.org/10.3390/agriengineering6030168







