Trichoderma Production and Encapsulation Methods for Agricultural Applications
Abstract
1. Introduction
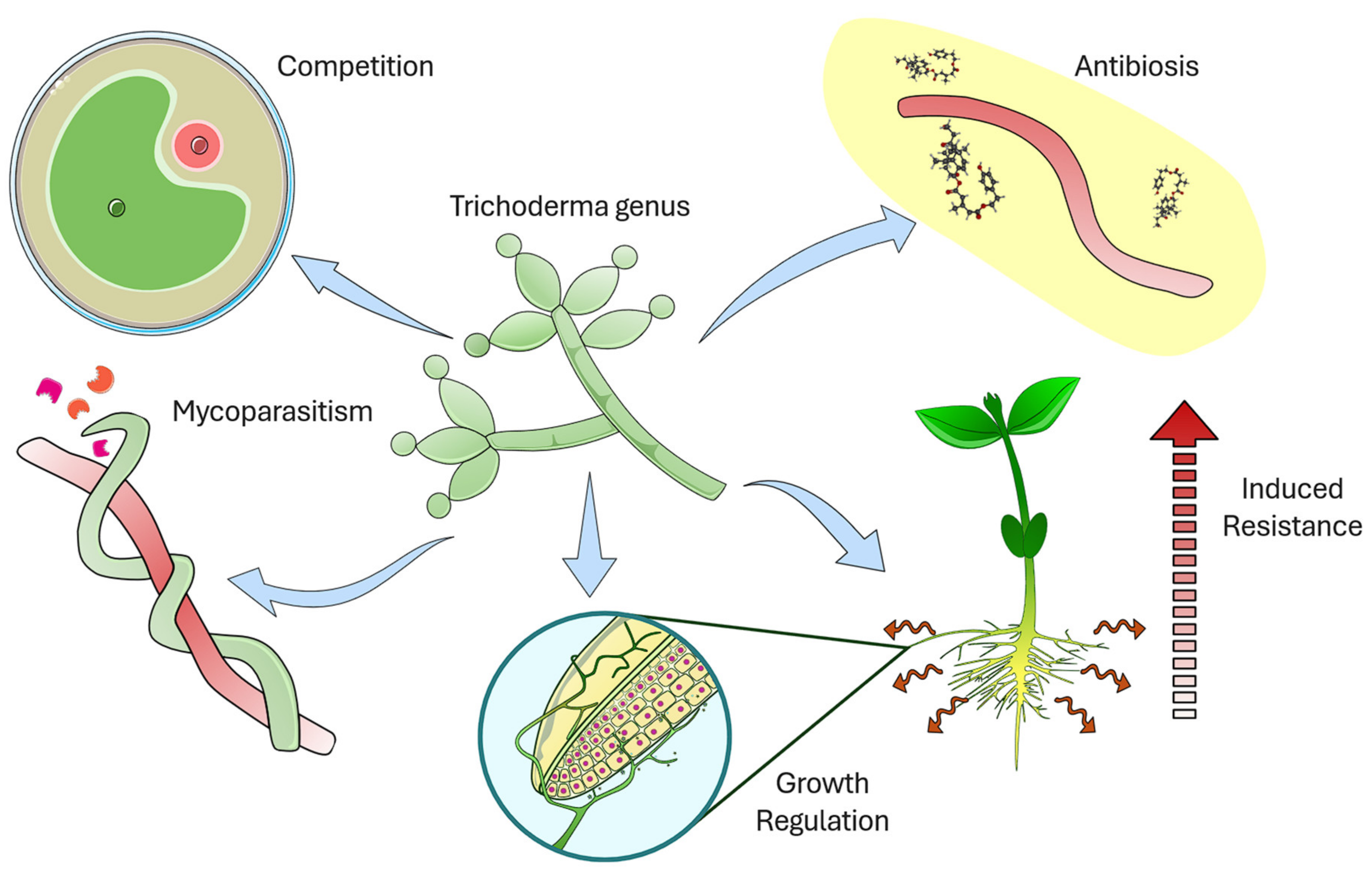
2. Trichoderma as a Biocontrol Agent
3. Agricultural Product Formulations of Trichoderma sp.
- Environmental: biotic (interactions with the resident microbiome, alterations of the original effect depending on the crop) and abiotic (variations in soil properties, interactions with other agricultural components).
- Practical: social aspects (added value and perspective from consumers), accessibility (BCA’s limited versatility, adaptability, or shelf-life; knowledge of manipulation and application by farmers), and regulations (lack of protocols, guides, laws, or regulations)
3.1. Liquid Formulations
3.1.1. Oil Dispersions
3.1.2. Concentrated Suspensions
3.2. Solid Formulations
3.2.1. Soluble Powders
3.2.2. Granulated Compounds
3.2.3. Microencapsulation
4. Integration with Emerging Technologies
4.1. Spore Encapsulation Methods
- Chemical processes: including techniques such as “suspension, dispersion, and emulsion” and “polycondensation”.
- Physicochemical processes: “coacervation”, “Layer-by-Layer (L-B-L)”, “ionic gelation”, and “supercritical microencapsulation with CO2”.
- Physical-mechanical processes: “spray-drying”, “spraying with multiple nozzles”, “fluid bed coating”, “centrifugal techniques”, “vacuum encapsulation”, and “electrostatic encapsulation”.
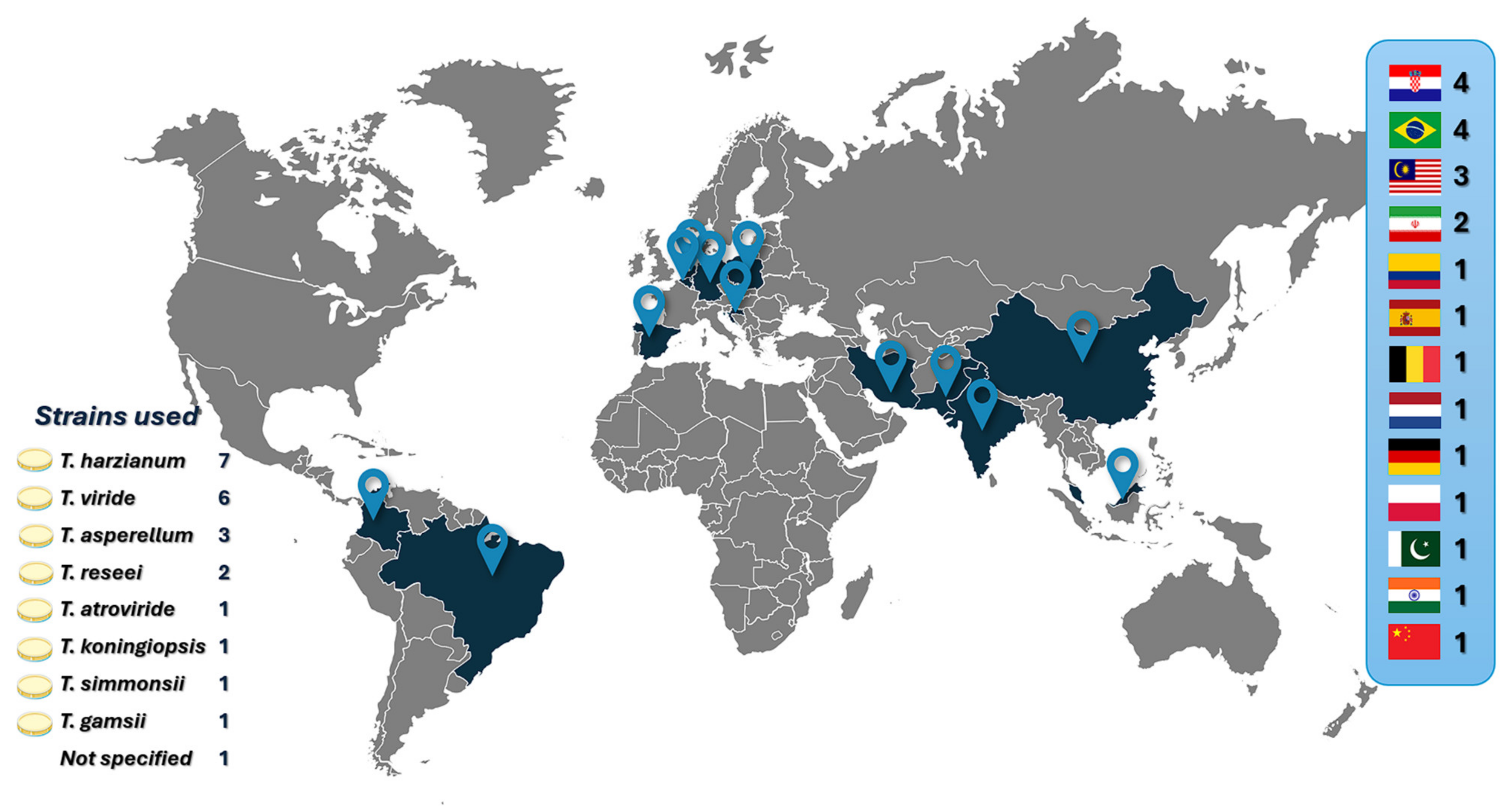
4.2. Reported Trichoderma Microencapsulation Models
- T. harzianum: Known for inducing systemic resistance in plants through the production of enzymes and metabolites that stimulate plant immune responses and enhance growth. It is also effective in environments where chemical fungicides are ineffective due to its ability to efficiently colonize plant roots and persist in the soil [124].
- T. viride: Produces extracellular enzymes, such as cellulases and chitinases, that degrade the cell walls of plant pathogens, inhibiting their growth and recycling nutrients in the soil. This enhances soil fertility and makes it an effective biocontrol agent against root rot and other soil-borne diseases [125].
- T. asperellum: Exhibits biocontrol activity by competing for space and nutrients, suppressing the growth of fungi. It promotes plant and root growth, enhancing nutrient uptake and improving the soil microenvironment [123].
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsegaye, Z.; Assefa, F.; Genene, T.; Tenkegna, T.; Gizaw, B.; Abatenh, E. Concept, Principle and Application of Biological Control and Their Role in Sustainable Plant Diseases Management Strategies. Int. J. Res. Stud. Biosci. 2018, 6, 18–34. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and Its Protective Role against Biotic Stress Impacts on Plants. Biomolecules 2019, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Atreya, K. Pesticide Use in Agriculture: The Philosophy, Complexities and Opportunities. Sci. Res. Essays 2012, 7, 2168–2173. [Google Scholar] [CrossRef]
- Ghormade, V.; Deshpande, M.V.; Paknikar, K.M. Perspectives for Nano-Biotechnology Enabled Protection and Nutrition of Plants. Biotechnol. Adv. 2011, 29, 792–803. [Google Scholar] [CrossRef] [PubMed]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P. Screening for Novel Biocontrol Agents Applicable in Plant Disease Management—A Review. Biol. Control 2020, 144, 104240. [Google Scholar] [CrossRef]
- Filizola, P.R.B.; Luna, M.A.C.; de Souza, A.F.; Coelho, I.L.; Laranjeira, D.; Campos-Takaki, G.M. Biodiversity and Phylogeny of novel Trichoderma Isolates from Mangrove Sediments and Potential of Biocontrol against Fusarium Strains. Microb. Cell Factories 2019, 18, 89. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Guzmán, K.; Torres-León, C.; Saldaña-Mendoza, S.; Martinez-Medina, G.; Tranier, M.; Roussos, S.; De la Cruz-Quiroz, R.; Parra-Saldívar, R.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R.; et al. Biocontrol Agents as Strategy of Agro-Ecosystem Management to Restitution of Productive Soils for Food Production. In Phytobiont and Ecosystem Restitution; Kumar, V., Kumar, M., Prasad, R., Eds.; Springer: Singapore, 2018; pp. 185–213. ISBN 9789811311871. [Google Scholar]
- Al-Ani, L.K.T. Trichoderma: Beneficial Role in Sustainable Agriculture by Plant Disease Management. In Plant Microbiome: Stress Response; Egamberdieva, D., Ahmad, P., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2018; pp. 105–126. ISBN 978-981-10-5514-0. [Google Scholar]
- Sellitto, V.M.; Zara, S.; Fracchetti, F.; Capozzi, V.; Nardi, T. Microbial Biocontrol as an Alternative to Synthetic Fungicides: Boundaries between Pre- and Postharvest Applications on Vegetables and Fruits. Fermentation 2021, 7, 60. [Google Scholar] [CrossRef]
- Dai, Z.; Ahmed, W.; Yang, J.; Yao, X.; Zhang, J.; Wei, L.; Ji, G. Seed Coat Treatment by Plant-Growth-Promoting Rhizobacteria Lysobacter Antibioticus 13–6 Enhances Maize Yield and Changes Rhizosphere Bacterial Communities. Biol. Fertil. Soils 2023, 59, 317–331. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, J.; Ahmed, W.; Wan, X.; Wei, L.; Ji, G. Exploiting the Antibacterial Mechanism of Phenazine Substances from Lysobacter Antibioticus 13-6 against Xanthomonas oryzae pv. Oryzicola. J. Microbiol. 2022, 60, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Qiao, J.; Wang, R.; Lu, J.; Wang, Z.; Li, P.; Zhang, L.; Ali, Q.; Khan, A.R.; Gao, X.; et al. The Role of Pyoluteorin from Pseudomonas protegens Pf-5 in Suppressing the Growth and Pathogenicity of Pantoea ananatis on Maize. Int. J. Mol. Sci. 2022, 23, 6431. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Dai, Z.; Zhang, J.; Li, S.; Ahmed, A.; Munir, S.; Liu, Q.; Tan, Y.; Ji, G.; Zhao, Z. Plant-Microbe Interaction: Mining the Impact of Native Bacillus amyloliquefaciens WS-10 on Tobacco Bacterial Wilt Disease and Rhizosphere Microbial Communities. Microbiol. Spectr. 2022, 10, e0147122. [Google Scholar] [CrossRef] [PubMed]
- Maksimov, I.; Blagova, D.; Veselova, S.; Sorokan, A.; Burkhanova, G.; Cherepanova, E.; Sarvarova, E.; Rumyantsev, S.; Alekseev, V.Y.; Khayrullin, R. Recombinant Bacillus subtilis 26DCryChS Line with Gene Btcry1ia Encoding Cry1Ia Toxin from Bacillus thuringiensis Promotes Integrated Wheat Defense against Pathogen Stagonospora nodorum Berk. and Greenbug schizaphis Graminum Rond. Biol. Control 2020, 144, 104242. [Google Scholar] [CrossRef]
- Wang, M.; Geng, L.; Sun, X.; Shu, C.; Song, F.; Zhang, J. Screening of Bacillus thuringiensis Strains to Identify New Potential Biocontrol Agents against Sclerotinia sclerotiorum and Plutella xylostella in Brassica campestris L. Biol. Control 2020, 145, 104262. [Google Scholar] [CrossRef]
- Liang, Z.; Ali, Q.; Wang, Y.; Mu, G.; Kan, X.; Ren, Y.; Manghwar, H.; Gu, Q.; Wu, H.; Gao, X. Toxicity of Bacillus thuringiensis Strains Derived from the Novel Crystal Protein Cry31Aa with High Nematicidal Activity against Rice Parasitic Nematode Aphelenchoides besseyi. Int. J. Mol. Sci. 2022, 23, 8189. [Google Scholar] [CrossRef]
- Al Raish, S.M.; Saeed, E.E.; Alyafei, D.M.; El-Tarabily, K.A.; AbuQamar, S.F. Evaluation of Streptomycete Actinobacterial Isolates as Biocontrol Agents against Royal Poinciana Stem Canker Disease Caused by the Fungal Pathogen Neoscytalidium Dimidiatum. Biol. Control 2021, 164, 104783. [Google Scholar] [CrossRef]
- Shao, Z.; Schenk, P.M.; Dart, P. Phyllosphere bacterial strains Rhizobium b1 and Bacillus subtilis b2 Control Tomato Leaf Diseases Caused by Pseudomonas syringae pv. tomato and Alternaria solani. J. Appl. Microbiol. 2023, 134, lxad139. [Google Scholar] [CrossRef]
- Iqbal, M.; Jamshaid, M.; Zahid, M.A.; Andreasson, E.; Vetukuri, R.R.; Stenberg, J.A. Biological Control of Strawberry Crown Rot, Root Rot and Grey Mould by the Beneficial Fungus Aureobasidium pullulans. Bio. Control 2021, 66, 535–545. [Google Scholar] [CrossRef]
- Mota, S.F.; Pádua, P.F.; Ferreira, A.N.; Gomes, L.d.B.W.; Dias, M.A.; Souza, E.A.; Pereira, O.L.; Cardoso, P.G. Biological Control of Common Bean Diseases Using Endophytic Induratia spp. Biol. Control 2021, 159, 104629. [Google Scholar] [CrossRef]
- Sinno, M.; Ranesi, M.; Di Lelio, I.; Iacomino, G.; Becchimanzi, A.; Barra, E.; Molisso, D.; Pennacchio, F.; Digilio, M.C.; Vitale, S.; et al. Selection of Endophytic Beauveria bassiana as a Dual Biocontrol Agent of Tomato Pathogens and Pests. Pathogens 2021, 10, 1242. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, J.; Feng, Q.; Chu, L.; Tan, Z.; Ji, X.; Jin, P. Insecticidal Effect of the Entomopathogenic Fungus Lecanicillium araneicola HK-1 in Aphis craccivora (Hemiptera: Aphididae). Insects 2023, 14, 860. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.A.A.; Najeeb, S.; Mao, Z.; Ling, J.; Yang, Y.; Li, Y.; Xie, B. Bioactive Secondary Metabolites from Trichoderma spp. against Phytopathogenic Bacteria and Root-Knot Nematode. Microorganisms 2020, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Gutiérrez, C.; Arroyave, C.; Llugany, M.; Poschenrieder, C.; Martos, S.; Peláez, C. Trichoderma asperellum as a Preventive and Curative Agent to Control Fusarium wilt in Stevia rebaudiana. Biol. Control 2021, 155, 104537. [Google Scholar] [CrossRef]
- Degani, O.; Dor, S. Trichoderma Biological Control to Protect Sensitive Maize Hybrids against Late Wilt Disease in the Field. J. Fungi 2021, 7, 315. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; Haga, S.; Suzuki, S. Direct antagonistic Activity of Chitinase Produced by Trichoderma sp. SANA20 as Biological Control Agent for Grey Mould Caused by Botrytis cinerea. Cogent Biol. 2020, 6, 1747903. [Google Scholar] [CrossRef]
- Rivera-Méndez, W.; Obregón, M.; Morán-Diez, M.E.; Hermosa, R.; Monte, E. Trichoderma asperellum Biocontrol Activity and Induction of Systemic Defenses against Sclerotium Cepivorum in Onion Plants under Tropical Climate Conditions. Biol. Control 2020, 141, 104145. [Google Scholar] [CrossRef]
- Jones, E.E.; Rabeendran, N.; Stewart, A. Biocontrol of Sclerotinia Sclerotiorum Infection of Cabbage by Coniothyrium Minitans and trichoderma spp. Biocontrol Sci. Technol. 2014, 24, 1363–1382. [Google Scholar] [CrossRef]
- Meddad-Hamza, A.; Benzina, F.; Meddad, C.; Hamza, N.; Reghmit, A.; Ziane, H.; Ksentini, H. Biological Control of Arbuscular Mycorrhizal Fungi and Trichoderma Harzianum against Fusarium Oxysporum and Verticillium Dahliae Induced Wilt in Tomato Plants. Egypt. J. Biol. Pest Control. 2023, 33, 91. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Celaya, A.L.; Ortiz-García, M.; Vernon-Carter, E.J.; Jauregui-Rincón, J.; Galindo, E.; Serrano-Carreón, L. Spray-Drying Microencapsulation of Trichoderma Harzianum Conidias in Carbohydrate Polymers Matrices. Carbohydr. Polym. 2012, 88, 1141–1148. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based Products and their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Singh, A.; Shukla, N.; Kabadwal, B.; Tewari, A.; Kumar, J. Review on Plant-Trichoderma-Pathogen Interaction. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2382–2397. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, R.; Ni, M.; Yu, J.; Li, Y.; Yu, C.; Dou, K.; Ren, J.; Chen, J. Identification of a novel Fungus, Trichoderma asperellum GDFS1009, and Comprehensive Evaluation of Its Biocontrol Efficacy. PLoS ONE 2017, 12, e0179957. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, M.K.; Nehra, M. Biotechnological Applications of Trichoderma Species for Environmental and Food Security. In Plant Biotechnology: Recent Advancements and Developments; Gahlawat, S.K., Salar, R.K., Siwach, P., Duhan, J.S., Kumar, S., Kaur, P., Eds.; Springer: Singapore, 2017; pp. 125–156. ISBN 978-981-10-4732-9. [Google Scholar]
- Hermosa, R.; Rubio, M.B. The Contribution of Trichoderma to Balancing the Costs of Plant Growth and Defense. Int. Microbiol. 2013, 16, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-T.; Hwang, S.-G.; Huang, Y.-M.; Huang, C.-H. Effects of Trichoderma asperellum on Nutrient Uptake and Fusarium Wilt of Tomato. Crop Prot. 2018, 110, 275–282. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Yu, J.; Saravanakumar, K.; Chen, J. Antagonistic and Biocontrol Potential of Trichoderma asperellum ZJSX5003 against the Maize Stalk Rot Pathogen Fusarium graminearum. Indian J. Microbiol. 2016, 56, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Leal, Y.; Lamz-Piedra, A.; Martínez-Coca, B. Antagonismo in Vitro de Aislamientos de Trichoderma asperellum Samuels, Lieckfeldt & Nirenberg Frente a Sclerotium Rolfsii Sacc. Rev. Protección Veg. 2017, 32. [Google Scholar]
- Baiyee, B.; Ito, S.-I.; Sunpapao, A. Trichoderma asperellum T1 Mediated Antifungal Activity and Induced Defense Response against Leaf Spot Fungi in Lettuce (Lactuca sativa L.). Physiol. Mol. Plant Pathol. 2019, 106, 96–101. [Google Scholar] [CrossRef]
- Konappa, N.; Dhamodaran, N.; Shanbhag, S.S.; Sampangi, M.A.; Krishnamurthy, S.; Arakere, U.C.; Chowdappa, S.; Jogaiah, S. Chapter 18—Trichoderma: A Potential Biopesticide for Sustainable Management of Wilt Disease of Crops. In Biopesticides; Rakshit, A., Meena, V.S., Abhilash, P.C., Sarma, B.K., Singh, H.B., Fraceto, L., Parihar, M., Singh, A.K., Eds.; Advances in Bio-Inoculant Science; Woodhead Publishing: Cambridge, UK, 2022; pp. 261–275. ISBN 978-0-12-823355-9. [Google Scholar]
- Oszako, T.; Voitka, D.; Stocki, M.; Stocka, N.; Nowakowska, J.A.; Linkiewicz, A.; Hsiang, T.; Belbahri, L.; Berezovska, D.; Malewski, T. Trichoderma asperellum Efficiently Protects Quercus Robur Leaves against Erysiphe Alphitoides. Eur. J. Plant Pathol. 2021, 159, 295–308. [Google Scholar] [CrossRef]
- González-Marquetti, I.; Infante-Martínez, D.; Arias-Vargas, Y.; Gorrita-Ramírez, S.; Hernández-García, T.; de la Noval-Pons, B.M.; Martínez-Coca, B.; Peteira, B.; González-Marquetti, I.; Infante-Martínez, D.; et al. Efecto de Trichoderma aasperellum Sa-muels, Lieckfeldt & Nirenberg Sobre Indicadores de Crecimiento y Desarrollo de Phaseolus vulgaris L. Cultivar BAT-304. Rev. Prot. Veg. 2019, 34, 1–10. [Google Scholar]
- Scudeletti, D.; Crusciol, C.A.C.; Bossolani, J.W.; Moretti, L.G.; Momesso, L.; Servaz Tubaña, B.; de Castro, S.G.Q.; De Oliveira, E.F.; Hungria, M. Trichoderma asperellum Inoculation as a Tool for Attenuating Drought Stress in Sugarcane. Front. Plant Sci. 2021, 12, 645542. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-Y.; Kong, F.-D.; Yang, L.; Ma, Q.-Y.; Xie, Q.-Y.; Yu, J.; Chen, P.-W.; Zhou, L.-M.; Wu, Y.-G.; Dai, H.-F.; et al. Phenethoxy Derivatives with Anti-Inflammatory Activities from the Betelnut Endophytic Trichoderma Asperellum G10. J. Nat. Prod. 2022, 85, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Gómez Alvarez, M.; Grijalba, E.; Santos, A.; Barrera, F.M.; Cotes, A. Capitulo 12: Desarrollo y Escalamientode Bioplaguicidas; AGROSAVIA: Mosquera, Colombia, 2019; pp. 632–682. ISBN 978-958-740-254-4. [Google Scholar]
- Ravensberg, W.J. A Roadmap to the Successful Development and Commercialization of Microbial Pest Control Products for Control of Arthropods; Springer: Dordrecht, The Netherlands, 2011; ISBN 978-94-007-0436-7. [Google Scholar]
- Ibáñez, A.; Garrido-Chamorro, S.; Vasco-Cárdenas, M.F.; Barreiro, C. From Lab to Field: Biofertilizers in the 21st Century. Horticulturae 2023, 9, 1306. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, M.; Manokaran, R.; Shanmugam, V. Status of Trichoderma Research in India: A Review. Indian Phytopethol. 2014, 67, 1–19. [Google Scholar]
- Van Long, N.N.; Vasseur, V.; Coroller, L.; Dantigny, P.; Le Panse, S.; Weill, A.; Mounier, J.; Rigalma, K. Temperature, Water Activity and pH during Conidia Production Affect the Physiological State and Germination Time of Penicillium Species. Int. J. Food Microbiol. 2017, 241, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ramanujam, B.; Prasad, R.D.; Sriram, S.; Rangeswaran, R. Mass Production, Formulation, Quality Control and Delivery of Trichoderma for Plant Disease Management. J. Plant Prot. Sci. 2010, 2, 1–8. [Google Scholar]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Antagonistic Fungi, Trichoderma spp.: Panoply of Biological Control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Domingues, M.V.P.F.; de Moura, K.E.; Salomão, D.; Elias, L.M.; Patricio, F.R.A. Effect of Temperature on Mycelial Growth of Trichoderma, Sclerotinia Minor and S. sclerotiorum, as well as on Mycoparasitism. Summa Phytopathol. 2016, 42, 222–227. [Google Scholar] [CrossRef]
- Bahadur, A.; Dutta, P. Trichoderma spp.: Their Impact in Crops Diseases Management; IntechOpen: London, UK, 2022; ISBN 978-1-80355-355-9. [Google Scholar]
- Martinez, Y.; Ribera, J.; Schwarze, F.W.M.R.; De France, K. Biotechnological Development of Trichoderma-Based Formulations for Biological Control. Appl. Microbiol. Biotechnol. 2023, 107, 5595–5612. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Kato, H.; Kumakura, K.; Ishibashi, E.; Nagayama, K. Properties and Biological Control Activities of Aerial and Submerged Spores in Trichoderma Asperellum SKT-1. J. Pestic. Sci. 2006, 31, 375–379. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, L.; Wang, J.; Guo, L.; Huang, J. Synergistic Effect between Trichoderma virens and Bacillus velezensis on the Control of Tomato Bacterial Wilt Disease. Horticulturae 2021, 7, 439. [Google Scholar] [CrossRef]
- Sentosa, F.B.; Sutarman; Nurmalasari, I.R. The Effect of Trichoderma and Onion Extract on the Success of Grafting in Mango Seedlings. IOP Conf. Ser. Earth Environ. Sci. 2021, 819, 012008. [Google Scholar] [CrossRef]
- Gullino, M.L.; Albajes, R.; Nicot, P.C. (Eds.) Integrated Pest and Disease Management in Greenhouse Crops; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-22303-8. [Google Scholar]
- Ons, L.; Bylemans, D.; Thevissen, K.; Cammue, B.P. Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms 2020, 8, 1930. [Google Scholar] [CrossRef] [PubMed]
- Dey, A. Liquid Biofertilizers and Their Applications: An Overview. In Environmental and Agricultural Microbiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 275–292. ISBN 978-1-119-52589-9. [Google Scholar]
- Singh, D.; Thapa, S.; Geat; Mehriya, M.; Rajawat, M.V. Biofertilizers: Mechanisms and Application; Elsevier, Woodhead Publishing: Delhi, India, 2021; pp. 151–166. ISBN 978-0-12-821667-5. [Google Scholar]
- Faria, M.; Martins, I.; Souza, D.A.; Mascarin, G.M.; Lopes, R.B. Susceptibility of the Biocontrol Fungi Metarhizium Anisopliae and Trichoderma asperellum (Ascomycota: Hypocreales) to Imbibitional Damage is Driven by Conidial Vigor. Biol. Control 2017, 107, 87–94. [Google Scholar] [CrossRef]
- Melin, P.; Håkansson, S.; Schnürer, J. Optimisation and Comparison of Liquid and Dry Formulations of the Biocontrol Yeast Pichia Anomala J121. Appl. Microbiol. Biotechnol. 2007, 73, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.F.; Lopes, E.A.; Moraes, A.R.F.; Soares, M.S.; Visôtto, L.E.; Oliveira, C.R.; Valente, V.M.M. Formulation of Botanicals for the Control of Plant-Pathogens: A Review. Crop Prot. 2018, 110, 135–140. [Google Scholar] [CrossRef]
- Teixidó, N.; Usall, J.; Torres, R. Insight into a Successful Development of Biocontrol Agents: Production, Formulation, Packaging, and Shelf Life as Key Aspects. Horticulturae 2022, 8, 305. [Google Scholar] [CrossRef]
- Cumagun, C.J.R. Advances in Formulation of Trichoderma for Biocontrol. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 527–531. ISBN 978-0-444-59576-8. [Google Scholar]
- Rhodes, D.J. Formulation of Biological Control Agents. In Exploitation of Microorganisms; Jones, D.G., Ed.; Springer: Dordrecht, The Netherlands, 1993; pp. 411–439. ISBN 978-94-011-1532-2. [Google Scholar]
- Oancea, F.; Raut, I.; Şesan, T.E.; Cornea, P.C. Dry Flowable Formulation of Biostimulants Trichoderma Strains. Agric. Agric. Sci. Procedia 2016, 10, 494–502. [Google Scholar] [CrossRef][Green Version]
- Meng, X.; Yu, J.; Yu, M.; Yin, X.; Liu, Y. Dry Flowable Formulations of Antagonistic Bacillus Subtilis Strain T429 by Spray Drying to Control Rice Blast Disease. Biol. Control 2015, 85, 46–51. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as Biostimulant: Exploiting the Multilevel Properties of a Plant Beneficial Fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Vemmer, M.; Patel, A.V. Review of Encapsulation Methods Suitable for Microbial Biological Control Agents. Biol. Control 2013, 67, 380–389. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Benita, S. Microencapsulation: Methods and Industrial Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-1-4200-2799-0. [Google Scholar]
- Vinceković, M.; Jalšenjak, N.; Topolovec-Pintarić, S.; Đermić, E.; Bujan, M.; Jurić, S. Encapsulation of Biological and Chemical Agents for Plant Nutrition and Protection: Chitosan/Alginate Microcapsules Loaded with Copper Cations and Trichoderma viride. J. Agric. Food Chem. 2016, 64, 8073–8083. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-Box Model-Based Gelation of Alginate and Pectin: A Review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Ekanayake, S.A.; Godakumbura, P.I. Synthesis of a Dual-Functional Nanofertilizer by Embedding ZnO and CuO Nanoparticles on an Alginate-Based Hydrogel. ACS Omega 2021, 6, 26262–26272. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, P.G.; Mani, S. Material and Environmental Properties of Natural Polymers and Their Composites for Packaging Applications—A Review. Polymers 2022, 14, 4033. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Soroush, F.; Varma, R.S. Nano/Microencapsulation of Plant Biocontrol Agents by Chitosan, Alginate, and Other Important Biopolymers as a Novel Strategy for Alleviating Plant Biotic Stresses. Int. J. Biol. Macromol. 2022, 222, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Hennecke, D. What Can We Learn from Biodegradation of Natural Polymers for Regulation? Environ. Sci. Eur. 2023, 35, 50. [Google Scholar] [CrossRef]
- de Oliveira, J.L. 1—Nano-Biopesticides: Present Concepts and Future Perspectives in Integrated Pest Management. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Jogaiah, S., Singh, H.B., Fraceto, L.F., de Lima, R., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2021; pp. 1–27. ISBN 978-0-12-820092-6. [Google Scholar]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; El-Mageed, T.A.A.; Negm, S.H.; et al. Plant Growth-Promoting Microorganisms as Biocontrol Agents of Plant Diseases: Mechanisms, Challenges and Future Perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Alagumalai, A.; Balaji, D.; Song, H. Bio-Based Agricultural Products: A Sustainable Alternative to Agrochemicals for Promoting a Circular Economy. RSC Sustain. 2023, 1, 746–762. [Google Scholar] [CrossRef]
- Barratt, B.I.P.; Moran, V.C.; Bigler, F.; van Lenteren, J.C. The statUS of Biological Control and Recommendations for Improving Uptake for the Future. BioControl 2018, 63, 155–167. [Google Scholar] [CrossRef]
- de Oliveira, J.L.; Campos, E.V.R.; Fraceto, L.F. Recent Developments and Challenges for Nanoscale Formulation of Botanical Pesticides for Use in Sustainable Agriculture. J. Agric. Food Chem. 2018, 66, 8898–8913. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K. Functional Coatings and Microencapsulation: A General Perspective; Wiley: Hoboken, NJ, USA, 2006; pp. 1–28. [Google Scholar]
- Batista, D.P.C.; de Oliveira, I.N.; Ribeiro, A.R.B.; Fonseca, E.J.S.; Santos-Magalhães, N.S.; de Sena-Filho, J.G.; Teodoro, A.V.; Grillo, L.A.M.; de Almeida, R.S.; Dornelas, C.B. Encapsulation and release of Beauveria bassiana from alginate–bentonite nanocomposite. RSC Adv. 2017, 7, 26468–26477. [Google Scholar] [CrossRef]
- Locatelli, G.O.; dos Santos, G.F.; Botelho, P.S.; Finkler, C.L.L.; Bueno, L.A. Development of Trichoderma sp. Formulations in Encapsulated Granules (CG) and Evaluation of Conidia Shelf-Life. Biol. Control 2018, 117, 21–29. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.; Brar, S.; Surampalli, R.; Prévost, D. Bio-Encapsulation of Microbial Cells for Targeted Agricultural Delivery. Crit. Rev. Biotechnol. 2011, 31, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Lalaymia, I.; Cranenbrouck, S.; Draye, X.; Declerck, S. Preservation at Ultra-Low Temperature of In Vitro Cultured Arbuscular Mycorrhizal Fungi Via Encapsulation–Drying. Fungal Biol. 2012, 116, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.R.d.O.; Locatelli, G.O.; Barbosa, R.d.M.; Lobo Junior, M.; Moura Mascarin, G.; Lamenha Luna Finkler, C. Preparation, characterization and cell viability of encapsulated Trichoderma asperellum in alginate beads. J. Microencapsul. 2020, 37, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Mancera-López, M.E.; Izquierdo-Estévez, W.F.; Escalante-Sánchez, A.; Ibarra, J.E.; Barrera-Cortés, J. Encapsulation of Trichoderma Harzianum Conidia As a Method of Conidia Preservation at Room Temperature and Propagation in Submerged Culture. Biocontrol Sci. Technol. 2019, 29, 107–130. [Google Scholar] [CrossRef]
- Maruyama, C.R.; Bilesky-José, N.; de Lima, R.; Fraceto, L.F. Encapsulation of Trichoderma harzianum Preserves Enzymatic Activity and Enhances the Potential for Biological Control. Front. Bioeng. Biotechnol. 2020, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Jurić, S.; Jurić, M.; Jambrak, A.R.; Vinceković, M. Tailoring Alginate/Chitosan Microparticles Loaded with Chemical and Biological Agents for Agricultural Application and Production of Value-Added Foods. Appl. Sci. 2021, 11, 4061. [Google Scholar] [CrossRef]
- Solak, A.O.; Dyankova, S.M. Composite Films from Sodium Alginate and High Methoxyl Pectin—Physicochemical Properties and Biodegradation in Soil. Ecol. Balk. 2014, 6, 25–34. [Google Scholar]
- Adzmi, F.; Meon, S.; Musa, M.H.; Yusuf, N.A. Preparation, Characterisation and Viability of Encapsulated Trichoderma harzianum UPM40 in Alginate-Montmorillonite Clay. J. Microencapsul. 2012, 29, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Anis, M.; Zaki, M.J.; Dawar, S. Development of a Na-Alginate-Based Bioformulation and Its Use in the Management of Charcoal Rot of Sunflower (Helianthus annuus L.). Pak. J. Bot. 2012, 44, 1167–1170. [Google Scholar]
- Ramos, L.D.; Chavez, S.; Rodríguez-Aguilera, J.C.; Smet, F. Developing a Novel Method for the Screening of Fungal Germinated Spores Using Hydrogel Microencapsulation and Large Particle Flow Cytometry. COPAS™ QUICK TECH NOTES, Spain, 2013. Available online: https://www.unionbio.com/documents/QTN020__Screening_libraries_of_fungal_spores_FINAL.pdf (accessed on 20 April 2024).
- Topolovec-Pintarić, S.; Žutić, I.; Đermić, E. Enhanced Growth of Cabbage and Red Beet by Trichoderma Viride. Acta Agric. Slov. 2013, 101, 87–92. [Google Scholar] [CrossRef]
- Szczech, M.; Maciorowski, R. Microencapsulation Technique with Organic Additives for Biocontrol Agents. J. Hortic. Res. 2016, 24, 111–122. [Google Scholar] [CrossRef]
- Jurić, S.; Đermić, E.; Topolovec-Pintarić, S.; Bedek, M.; Vinceković, M. Physicochemical Properties and Release Characteristics of Calcium Alginate Microspheres Loaded with Trichoderma Viride Spores. J. Integr. Agric. 2019, 18, 2534–2548. [Google Scholar] [CrossRef]
- Jurić, S.; Šegota, S.; Vinceković, M. Influence of Surface Morphology and Structure of Alginate Microparticles on the Bioactive Agents Release Behavior. Carbohydr. Polym. 2019, 218, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Anuar, I.M.; Sulong, K.K.; Ghani, H.A.; Wahab, M. Alginate Encapsulation of Trichoderma Harzianum against Brown Spot Disease on Rice (Oryzae sativa) In Vivo Assays. Food Res. 2020, 4, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Adzmi, F. Development of Alginate-Montmorillonite-Starch with Encapsulated Trichoderma harzianum and Evaluation of Conidia Shelf Life. Int. J. Agric. Biol. 2021, 26, 87–96. [Google Scholar] [CrossRef]
- Braga, A.B.A.C.; Costa, C.J.M.; Ribeiro, E.J.; Zotarelli, M.F.; Santos, L.D. Evaluation of the Microencapsulation Process of Conidia of Trichoderma Asperellum by Spray Drying. Braz. J. Microbiol. 2022, 53, 1871–1880. [Google Scholar] [CrossRef]
- Brondi, M.; Florencio, C.; Mattoso, L.; Ribeiro, C.; Farinas, C. Encapsulation of Trichoderma Harzianum with Nanocellulose/Carboxymethyl Cellulose Nanocomposite. Carbohydr. Polym. 2022, 295, 119876. [Google Scholar] [CrossRef] [PubMed]
- Shahiri Tabarestani, M. Evaluation of Antifungal Effect of Biodegradable Nano Encapsulated Extract of Trichoderma harzianum. J. Iran. Plant Prot. Res. 2022, 36, 183–195. [Google Scholar] [CrossRef]
- Qi, Q.; Fan, C.; Wu, H.; Sun, L.; Cao, C. Preparation of Trichoderma asperellum Microcapsules and Biocontrol of Cucumber Powdery Mildew. Microbiol. Spectr. 2023, 11, e0508422. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, V.; Maruthi, P.; Devi, G.U.; Rajanikant, P.; Kannan, C. Encapsulation of Native Strains of Bioagents Trichoderma asperellum and Pseudomonas putida Using Different Biopolymers. Int. J. Plant Soil Sci. 2023, 35, 521–528. [Google Scholar] [CrossRef]
- Cruz-Barrera, M.; Izquierdo-García, L.F.; Gómez-Marroquín, M.; Santos-Díaz, A.; Uribe-Gutiérrez, L.; Moreno-Velandia, C.A. Hydrogel Capsules as New Delivery System for Trichoderma Koningiopsis Th003 to Control Rhizoctonia Solani in Rice (Oryza sativa). World J. Microbiol. Biotechnol. 2024, 40, 108. [Google Scholar] [CrossRef] [PubMed]
- Lotfalinezhad, E.; Taheri, A.; Razavi, S.E.; Sanei, S.J. Preparation and Assessment of Alginate-Microencapsulated Trichoderma harzianum for Controlling Sclerotinia Sclerotiorum and Rhizoctonia Solani on Tomato. Int. J. Biol. Macromol. 2024, 259, 129278. [Google Scholar] [CrossRef] [PubMed]
- Løvschall, K.B.; Velasquez, S.T.R.; Kowalska, B.; Ptaszek, M.; Jarecka, A.; Szczech, M.; Wurm, F.R. Enhancing Stability and Efficacy of Trichoderma Bio-Control Agents Through Layer-by-Layer Encapsulation for Sustainable Plant Protection. Adv. Sustain. Syst. 2024, 2300409, 2366–7486. [Google Scholar] [CrossRef]
- Velázquez-Gutiérrez, S.K.; Alpizar-Reyes, E.; Cruz-Olivares, J.; Barrera-Pichardo, J.F.; Rodríguez-Huezo, M.E.; Pérez-Alonso, C. Ionic Gelation Encapsulation of Sesame Oil with Sodium Alginate-Nopal Mucilage Blends: Encapsulation Efficiency and Oxidative Stability. Rev. Mex. Ing. Quimica 2020, 19, 349–362. [Google Scholar] [CrossRef]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Rhein-Knudsen, N.; Ale, M.T.; Ajalloueian, F.; Meyer, A.S. Characterization of Alginates from Ghanaian Brown Seaweeds: Sargassum spp. and Padina spp. Food Hydrocoll. 2017, 71, 236–244. [Google Scholar] [CrossRef]
- Zazzali, I.; Calvo, T.R.A.; Ruíz-Henestrosa, V.M.P.; Santagapita, P.R.; Perullini, M. Effects of pH, Extrusion Tip Size and Storage Protocol on the Structural Properties of Ca(II)-alginate Beads. Carbohydr. Polym. 2019, 206, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Benko, B.; Haramija, F.; Vinceković, M.; Toth, N.; Uher, S.F.; Žutić, I. Lettuce Yield and Nutritive Value as Affected by a Biopolymer Microparticles Application. Acta Hortic. 2021, 1320, 181–188. [Google Scholar] [CrossRef]
- Mikula, K.; Izydorczyk, G.; Skrzypczak, D.; Mironiuk, M.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Controlled Release Micronutrient Fertilizers for Precision Agriculture—A Review. Sci. Total Environ. 2020, 712, 136365. [Google Scholar] [CrossRef] [PubMed]
- Global Market Insights Inc. Biocontrol Agents Market Size, Share & Report, 2024–2032. 2024. Available online: https://www.gminsights.com/industry-analysis/biocontrol-agents-market (accessed on 10 June 2024).
- S&P Global Inc. Biological Control Agents Strategic Report 2022. IHS Markit®: Biological Control Agents. Biopesticides and Related BCAs. 2022. Available online: https://commodityinsights.spglobal.com/biological_control_agents-2022.html (accessed on 10 June 2024).
- Yao, X.; Guo, H.; Zhang, K.; Zhao, M.; Ruan, J.; Chen, J. Trichoderma and Its Role in Biological Control of Plant Fungal and Nematode Disease. Front. Microbiol. 2023, 14, 1160551. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Hakim, S.; Alshammari, W.; Alshammari, N.; Salih, Z.; Sm, H.; Sulieman, A.M. Capability of Trichoderma Viride to Produce Cellulolytic and Pectolytic Enzymes How to Cite. Adv. Life Sci. B 2023, 10, 491–496. [Google Scholar]
- Ghazanfar, M.; Raza, M.; Raza, W.; Qamar, M. Trichoderma as potential biocontrol agent, its exploitation in agriculture: A review. Plant Prot. 2018, 2, 109–135. [Google Scholar]

| BCA Group | BCA Species | Pathogen Group | Pathogen Species | Disease | Crop(s) | Reference |
|---|---|---|---|---|---|---|
| Aerobic Gram-negative antagonistic bacterium | Pseudomonas protegens | Aerobic Gram-negative endophytic bacterium | Pantoea ananatis | Maize white spot | Zea mays | [12] |
| Aerobic facultative Gram-negative bacterium | Lysobacter antibioticus | Obligate aerobic Gram-negative bacterium | Xanthomonas oryzae pv. oryzicola | Bacterial leaf streak | Oryza sativa L. | [11] |
| Aerobic Gram-positive antagonistic bacterium | Bacillus amyloliquefaciens | Aerobic Gram-negative endophytic bacterium | Ralstonia solanacearum | Tobacco bacterial wilt | Nicotiana tabacum Linne | [13] |
| Aerobic Gram-positive antagonistic bacterium | Bacillus subtilis | Epiphytic fungus Insect | Stagonospora nodorum Berk. Schizaphis graminum Rond. (pest) | Glume blotch | T. aestivum | [14] |
| Aerobic Gram-positive antagonistic bacterium | Bacillus thuringiensis | Endophytic mycoparasitic fungus Insect | Sclerotinia sclerotiorum Plutella xylostella (pest) | Sclerotiniose | Brassica campestris L. | [15] |
| Aerobic Gram-positive antagonistic bacterium | Bacillus thuringiensis | Nematode | Aphelenchoides besseyi | Rice white tip | Oryza sativa L. | [16] |
| Aerobic Gram-positive antagonistic bacterium | Streptomyces antibioticus | Endophytic fungus | Neoscytalidium dimidiatum | Stem canker | Delonix regia | [17] |
| Aerobic chemoorganotropic Gram-negative symbiotic bacterium Aerobic Gram-positive antagonistic bacterium | Rhizobium sp. Bacillus subtilis | Aerobic Gram-negative endophytic bacterium Epiphytic fungus | Pseudomonas syringae Alternaria solani | Leaf diseases | Solanum lycopersicum L. | [18] |
| Endophytic fungus | Aureobasidium pullulans | Endophytic oomycete Endophytic mycoparasitic antagonistic fungus | Phytophthora cactorum Botrytis cinerea Pers | Crown rot Root rot Grey mold | Fragaria × ananassa duch. | [19] |
| Endophytic fungus | Induratia spp. | Endophytic fungus Endophytic mycoparasitic fungus Endophytic fungus | Colletotrichum lindemuthianum Sclerotinia sclerotiorum Pseudocercospora griseola | Anthracnose White mold Angular leaf spot | Phaseolus vulgaris L. | [20] |
| Entomopathogenic fungus | Beauveria bassiana | Endophytic fungus Endophytic mycoparasitic antagonistic fungus Insect | Alternaria alternata Botrytis cinerea Macrosiphum euphorbiae (pest) | Spots/Rot on fruits | Solanum lycopersicum L. | [21] |
| Entomopathogenic fungus | Lecanicillium araneicola | Insect | Aphis craccivora (pest) | Viral vector of barley yellow dwarf virus, papaya ring spot virus, and watermelon mosaic virus | Fruits and cereals | [22] |
| Mycoparasitic antagonistic/nematicide fungus | Trichoderma spp. | Aerobic Gram-negative endophytic bacterium Obligate aerobic Gram-negative bacterium Nematode | Ralstonia solanacearum Xanthomonas campestris Meloidogyne incognita (pest) | Bacterial leaf spot Bacterial wilt Root-Knot nematode | Solanum lycopersicum L. | [23] |
| Mycoparasitic antagonistic fungus | Trichoderma asperellum | Endophytic fungus | Fusarium oxysporum | Fusarium wilt | Stevia rebaudiana | [24] |
| Mycoparasitic antagonistic fungus Mycoparasitic antagonistic fungus | Trichoderma longibrachiatum Trichoderma asperelloides | Endophytic fungus | Magnaporthiopsis maydis | Late wilt | Zea mays L. | [25] |
| Mycoparasitic antagonistic fungus | Trichoderma sp. | Mycoparasitic antagonistic fungus | Botrytis cinerea | Grey mold | Prunus mume | [26] |
| Mycoparasitic antagonistic fungus | Trichoderma asperellum | Epiphytic fungus | Sclerotium cepivorum | White rot | Allium cepa L. | [27] |
| Mycoparasitic antagonistic fungus | Coniothyrium minitans Trichoderma spp. | Endophytic fungus | Sclerotinia sclerotiorum | Head rot | Brassica oleracea var. oleracea | [28] |
| Mycoparasitic antagonistic fungi Mycorrizae consortium | Trichoderma harzianum Claroideoglomus claroideum Claroideoglomus etunicatum Funneliformis geosporum Funneliformis mosseae Glomus micro-aggregatum Rhizophagus intraradices | Endophytic fungus Endophytic fungus | Fusarium oxysporum Verticillium dahliae | Tomato wilt | Solanum lycopersicon esculentum Mill. | [29] |
| Type of Particle | Synthesis Method | Materials Used | Microorganism | State of the Inoculum | Applications | Country | Reference |
|---|---|---|---|---|---|---|---|
| Beads | Extrusion/ionic gelation | Montmorillonite, sodium alginate, glycerol, CaCl2 | T. harzianum (UPM40) | Conidial suspension grown on PDA medium | Agricultural applications as a delivery system for the biocontrol agent | Malaysia | [98] |
| Seed coating | Adhesion and drying | Sodium alginate | T. viride, T. resei | Not mentioned | Management of charcoal rot in sunflowers (Helianthus Annuus L.) | Pakistan | [99] |
| Microparticles | Ionic gelation | Sodium alginate | T. reseei | Not mentioned | Selección de esporas/mejoramiento genético. | Spain, Belgium | [100] |
| Beads | Droplets/ionic gelation | Sodium alginate, talc powder calcium gluconate | T. viride | Liquefied biomass and PDA culture medium | Enhanced growth of cabbage and red beet | Croatia | [101] |
| Microparticles | Water-in-oil emulsification/ionic gelation | Alginate, chitosan, peat, skim milk | T. virens (TRS106) | Conidial suspension grown on malt extract agar medium | Biological control of F. oxysporum wilt in tomatoes | Poland | [102] |
| Microcapsules | Ionic gelation/polyelectrolyte complexation | Sodium alginate, chitosan, copper sulfate pentahydrate | T. viride | Filtered biomass cultured in PDB liquid medium | Plant nutrition and protection | Croatia | [76] |
| Granules | Ionic gelation | Sodium alginate, soluble starch, citric pectin | Trichoderma sp. | Pulverized microorganism obtained from solid-state fermentation | Control biológico | Brazil | [89] |
| Microparticles | Ionic gelation | Sodium alginate, calcium chloride | T. viride | Filtered biomass cultured in PDB liquid medium | Agricultural applications as a delivery system of bioagent | Croatia | [103] |
| Microparticles | Ionic gelation/polyelectrolyte complexation | Sodium alginate, medium molecular weight chitosan, calcium chloride, eosin | T. viride | Filtered biomass cultured in PDB liquid medium | Agricultural applications as a delivery system of bioagent | Croatia | [104] |
| Microparticles | Ionic gelation | Sodium alginate, calcium chloride | T. harzianum | Pulverized microorganism obtained from solid-state fermentation | Biological control of S. sclerotiorum for applications in agriculture | Brazil | [95] |
| Beads | Ionic gelation | Sodium alginate, calcium chloride | T. harzianum | Conidial suspension grown on PDA medium | Biological control of B. oryzaet in rice | Malaysia | [105] |
| Beads | Extrusion/ionic gelation | Sodium alginate, montmorillonite, starch | T. harzianum (UPMC243) | Conidial suspension grown on PDA medium | Evaluation of conidia shelf life | Malaysia | [106] |
| Microcapsules | Spray drying | Maltodextrin DE20 | T. asperellum | Pulverized microorganism obtained from solid-state fermentation | Agricultural applications as a delivery system of bioagent | Brazil | [107] |
| Beads | Ionic gelation | Cellulose nanocrystals (CNCs) and carboxymethyl cellulose (CMC), calcium chloride (CaCl2) | T. harzianum (LQC-99) | Conidial suspension grown on PDA medium | Agricultural applications as a delivery system of bioagent | Brazil | [108] |
| Microcapsules | Ionic gelation | Chitosan, tripolyphosphate | T. harzianum | Extraction of fungi secondary metabolites | Biological control of Macrophomina phaseolina and growth promoter | Iran | [109] |
| Microparticles | Extrusion/ionic gelation | Sodium alginate (SA), calcium chloride (CaCl2) | T. asperellum | Conidial suspension grown on PDA medium | Biocontrol of cucumber powdery mildew | China | [110] |
| Microparticles/microcapsules | Ionic gelation and spray drying | Maltodextrin, sodium alginate, carboxy methyl cellulose (CMC), gum arabic, gelatin, calcium chloride | T. asperellum (TAIK 1) | Not mentioned | Maintaining soil health, promoting plant growth, and reducing disease incidence by creating unfavorable conditions for pathogens | India | [111] |
| Beads | Ionic gelation | Alginate, Amidated pectinpectina amidada, calcium gluconate, plant based biochar, polydextrose | T. koningiopsis (Th003) | Conidial suspension grown on PDA medium | Biological control of sheath blight caused by R. solani in rice. | Colombia | [112] |
| Microparticles | Ionic gelation | Sodium alginate, calcium chloride | T. harzianum (Ah90) | Conidial suspension grown on PDA medium | Biological control of S. sclerotiorum and Rhizoctonia solani in tomatoes | Iran | [113] |
| Microcapsules | Layer-by-layer (LbL) encapsulation | Cationic lignin, lignosulfonate | T. atroviride (TRS14), T. simmonsii (TRS75), T. gamsii TRS123) | Not mentioned | Biological control of Fusarium oxysporum f. sp. lycopersici in tomato and improve spore stability | Germany and Netherlands | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vindas-Reyes, E.; Chacón-Cerdas, R.; Rivera-Méndez, W. Trichoderma Production and Encapsulation Methods for Agricultural Applications. AgriEngineering 2024, 6, 2366-2384. https://doi.org/10.3390/agriengineering6030138
Vindas-Reyes E, Chacón-Cerdas R, Rivera-Méndez W. Trichoderma Production and Encapsulation Methods for Agricultural Applications. AgriEngineering. 2024; 6(3):2366-2384. https://doi.org/10.3390/agriengineering6030138
Chicago/Turabian StyleVindas-Reyes, Erick, Randall Chacón-Cerdas, and William Rivera-Méndez. 2024. "Trichoderma Production and Encapsulation Methods for Agricultural Applications" AgriEngineering 6, no. 3: 2366-2384. https://doi.org/10.3390/agriengineering6030138
APA StyleVindas-Reyes, E., Chacón-Cerdas, R., & Rivera-Méndez, W. (2024). Trichoderma Production and Encapsulation Methods for Agricultural Applications. AgriEngineering, 6(3), 2366-2384. https://doi.org/10.3390/agriengineering6030138






