1. Introduction
Garlic (
Allium sativum L.) is a temperate crop native to Central Asia. Its development is highly influenced by temperature, where the initial phase requires mild temperatures (18–20 °C); the bulbification stage requires low temperatures (10–15 °C), and higher temperatures (20–25 °C) are desirable for the maturation phase. Although a tropical climate characterizes Brazil, the country’s southern regions experience low winter temperatures conducive to garlic cultivation. However, the tropical climate of Brazil does not provide suitable temperatures for garlic cultivation, so additional cultural treatments are necessary [
1].
Management strategies have been adjusted to achieve self-sufficiency in the Brazilian market, such as population density, fertilization types, vegetation cover content, and vernalization, which permitted garlic production in tropical regions [
2,
3]. Although the diversity of techniques applied in garlic cultivation stimulates a more abundant production, several studies have reported an increase in physiological anomalies such as super-sprouting or pseudo-sprouting, also known as secondary growth of the plant (equal to tillering). This factor causes a reduction in the productivity of commercial bulbs [
4].
Secondary growth in garlic is a genetic-physiological anomaly causing early germination of bulbils during the reproductive growth phase and physical changes in bulb formation, the so-called oval bulbs, and, in some cases, bulbs present a rupture of the covering tunic, called smile. The tissues normally differentiate into the reserve, vegetative, and covering leaves, but due to some endogenous factor of the plant, the covering leaves germinate and form additional leaves instead of transforming only into the layer that covers and protects the bulbils, giving the plant a thicket appearance. Tunic and bulbil exposure depreciates the commercial value of the bulb [
2,
3].
Among the main factors reported to stimulate garlic secondary growth are changes in photoperiod [
4,
5], susceptible/sensitive cultivars, temperature, vernalization periods [
2,
6], high doses of nitrogen fertilization [
7,
8,
9], time of irrigation, water stress [
10,
11], and gibberellin levels [
12,
13]. According to Burba, 1983, the vernalization of bulbils stimulates the accumulation of cytokinins and gibberellins, modifying the hormonal balance and causing precocious germination of the bulbil [
14].
The most prevalent technique applied to reduce this anomaly is water stress during the bulbification period, which causes a moderate water deficit in plants aiming to reduce the secondary growth of the plants [
15]. From a physiological point of view, mild water stress stimulates the partial closure of plant stomata, promoting a reduction in water loss without completely interrupting photosynthesis [
16]. However, this technique limits the vegetative vigor of several crops and usually reduces yield [
16]. Cortés et al., 2003, tested the combination of different levels of water stress in a garlic crop and found that the treatments that received a deficit during bulbification achieved higher values of water use efficiency; however, the control treatment, which was not subjected to water stress, reached the highest productivity values [
17].
An alternative method of inhibition of secondary plant growth uses growth inhibitors. Resende et al., 1999, evaluated the influence of cycocel and gibberellic acid on this anomaly, but these treatments were not significantly effective in controlling the physiological disturbance [
18]. According to Barbosa, 2021, different forms of chlormequat chloride (CCC) and paclobutrazol (PBZ) in the initial phase of differentiation of garlic plants showed low efficiency in controlling secondary plant growth. In addition, when the growth inhibitor ethyl-trinexapac (ETP) was applied, there was an observed reduction in physiological disturbances. However, the reduced gas exchange and shoot growth in plants increased the production of non-commercial-grade bulbs [
19].
Pre-harvest germination is a disorder that significantly affects wheat [
20] and rice [
21] crops, among others. Germination begins before harvest when humidity, temperature, and oxygen conditions are conducive to embryo growth; thus, early germination of the grains still occurs in the ears in the field. To reduce early germination of cereals, several researchers, such as Hu et al., 2017, and Darabi et al., 2011, reported that the application of clove oil (
S. aromaticum) in the pre-harvest period significantly reduced the early germination of these cereals [
20,
21]. Hu et al., 2017, found that eugenol-treated seeds had increased ABA levels and decreased the expression of ABA-catabolizing genes [
21]. Another important fact is that the tea plant (
Camellia sinensis L.) can absorb eugenol from the air and convert it into glycosides. This process helps the plant develop an increased tolerance to cold and drought. Eugenol’s effect is also linked to the alteration of ABA homeostasis [
22].
Clove essential oil (
S. aromaticum) is mainly composed of eugenol, a molecule with various functionalities such as toxicity against insects, bacteria, phytopathogenic fungi, soil microbes, nematodes, and weeds [
23]. It delays or inhibits the germination of several plant species and exhibits herbicidal activity [
24,
25].
The CEO has shown inhibitory actions on pre-harvest sprouting in cereals; however, there are no reports on its inhibitory activity on garlic bulb germination or the control of anomalies. Being a plant-derived product and cost-effective, the CEO has the potential to promote garlic production and reduce the use of agrochemicals.
Although traditional agricultural techniques can reduce the growth of secondary plants in garlic, in most cases, they directly impact crop production in terms of quality or productivity. Therefore, the objective of this work was to develop a novel plant growth retardant from the essential oil of clove (CEO) to inhibit the secondary growth of garlic bulbs during the bulbing phase. The goal was to improve the crop’s productivity with better-quality bulbs, using bioinputs to reduce the impact on the environment.
2. Materials and Methods
2.1. Chemical Characterization of Clove Oil
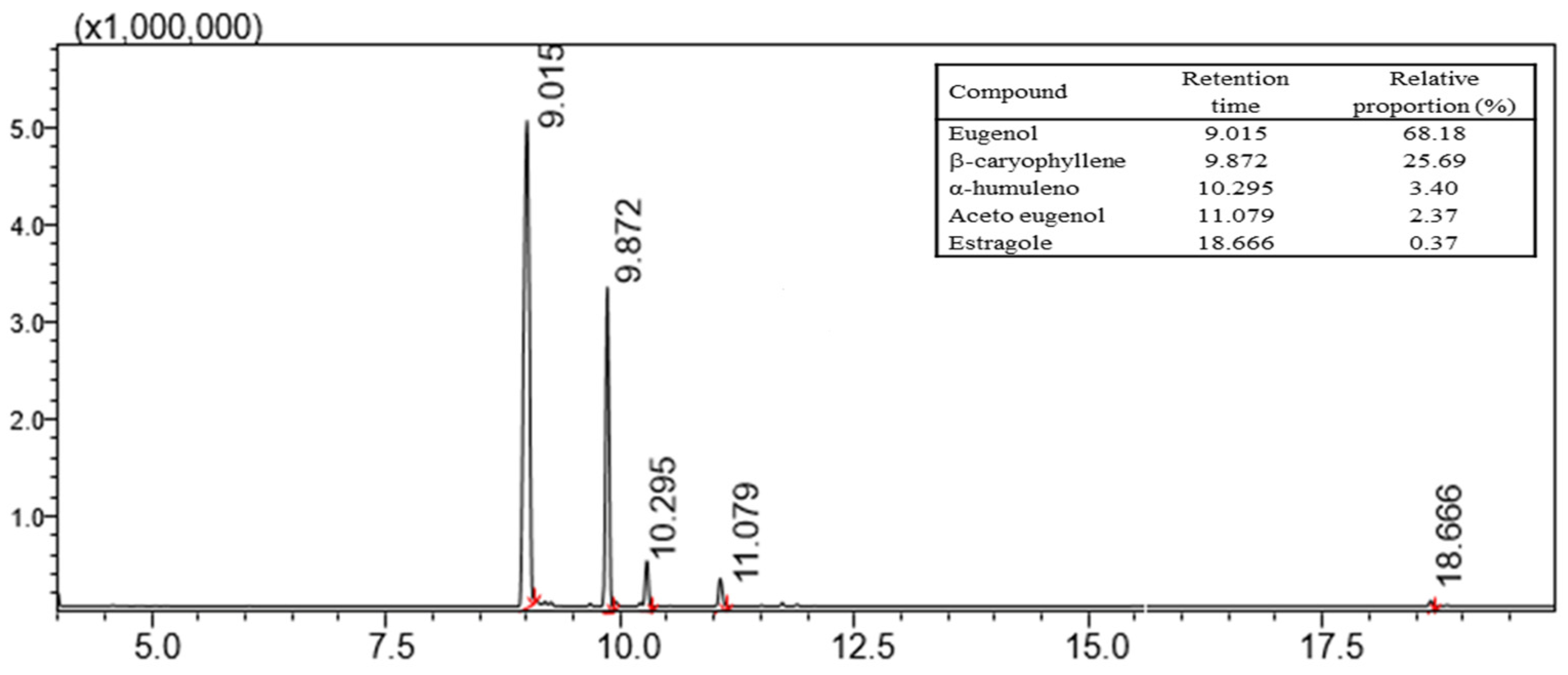
The composition of CEO was determined using a gas chromatograph coupled with a mass spectrometer (GC-MS) (Shimadzu GC-MS QP2010 Ultra) by dissolving 10 mg mL
−1 in dichloromethane. The injection volume was 1 μL with a 40:1 split ratio; the injector temperature was 240 °C. Helium was used as the carrier gas at a flow rate of 1 mL min
−1. The capillary column Rtx-5MS (30 m × 0.25 mm × 0.25 μm) was used with a temperature program starting at 60 °C followed by a ramp rate of 10 °C min
−1 up to 280 °C and a hold time of 3 min for chromatographic separation. The mass spectrometry parameters were as follows: the interface and the ion source temperatures were 240 °C, impact ionization 70 eV, and mass spectrum scanned from 35 to 350 m/z. The solvent cutting time was 3 min [
26,
27,
28]. The CEO components were identified using spectral correspondence with the mass spectral library of the National Institute of Standards and Technology (NIST-2014), and the retention index was calculated using a standard series of linear hydrocarbons (C9–C30) in comparison with data in the literature with columns of similar polarity [
29]. The CEO used in all experiments had the following relative proportions: eugenol, up to 68.18%; β-caryophyllene, 25.69%; α-humulene, 3.40%; eugenol acetate, 2.37%; and 0.37% stragole (
Figure 1).
2.2. Laboratory Experiment
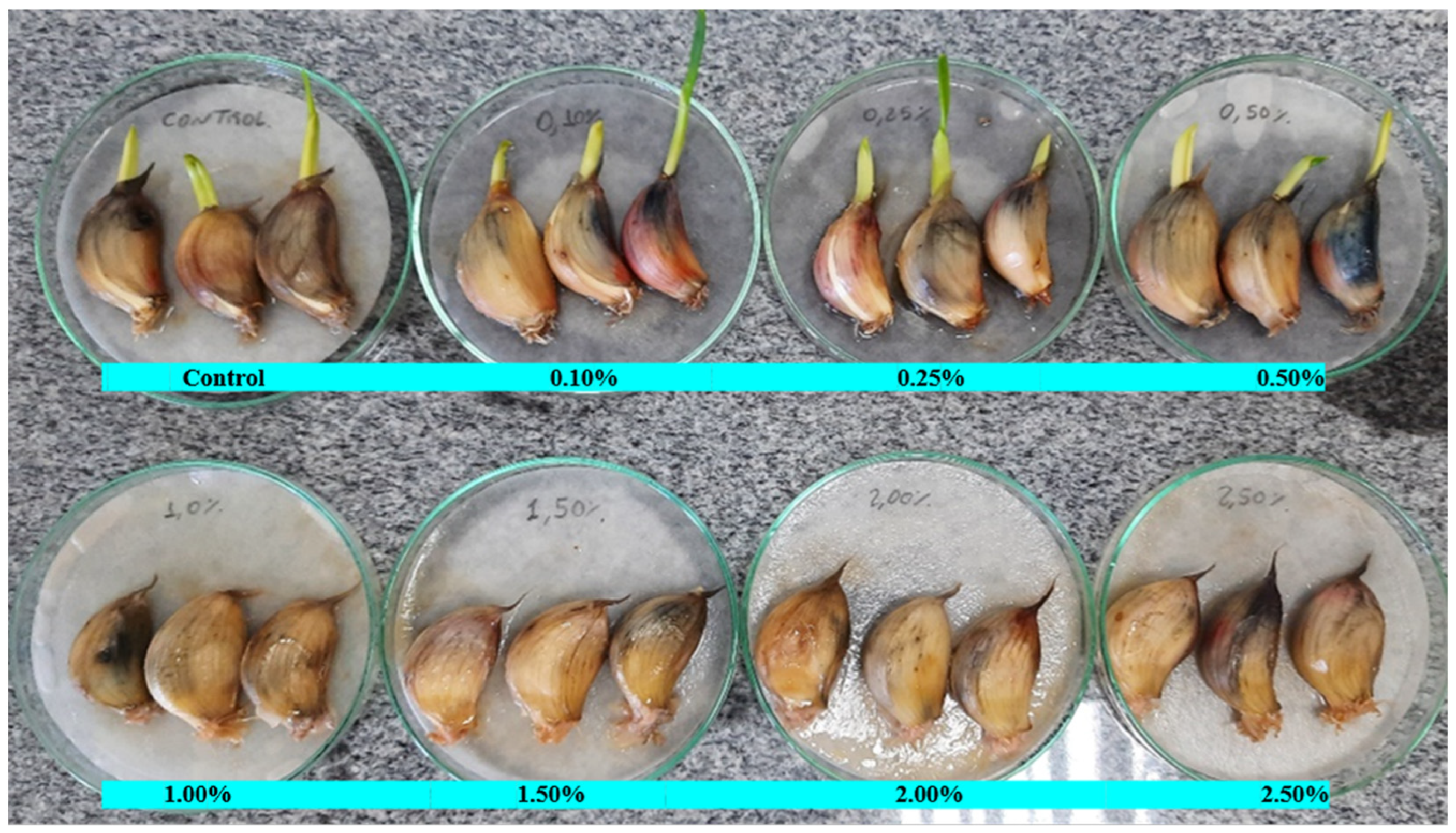
In an in vitro experiment, the inhibitory effect of CEO application on bulbil germination was evaluated. Garlic bulbils of size (16 mm) and weight (5.5 ± 0.5 g) were used, immersed in an emulsion containing water, 0.01% silicon-based surfactant, and clove oil at concentrations from 0 to control, 0.10, 0.25, 0.50, 1.00, 2.00, and 2.50% for 30 min using 10 mL per bulbils seed. Treated bulbils were placed on moistened germinating paper in Petri dishes for 5 days in a growth chamber with a 12 h photoperiod at 25 °C and evaluated for inhibition of germination.
2.3. Field Experiment
The experiments were conducted under field conditions in the region of the municipality of São Gotardo, Brazil (19°26′53″ S, 46°14′35″ W) from April to August in the years 2021 and 2022. The cultural treatments, such as liming and fertilization, were applied according to the production farm’s program (Sekita Agribusiness, Rio Paranaíba, MG, Brazil), and irrigation was carried out using a pivot system.
In 2021, an average rainfall of 90 mm was recorded, with an average temperature of 18.33 °C, and an irrigation volume of 326.80 mm was applied. In 2022, the registered climatic conditions were similar, with an average precipitation of 80 mm, an average temperature of 18.6 °C, and an applied irrigation volume of 432 mm. In both years of the experiment, irrigation in the area was interrupted for 20 days during bulb differentiation. Meanwhile, the experimental Randomized Complete Block Design (RCDB) with CEO application continued to receive manual irrigation, ensuring that the plants did not experience water deficit.
The 2021 experiment used three treatments: doses of 0 (control), 0.2%, and 0.4% of emulsion CEO with a spray volume of 200 L ha−1 in three randomized blocks. Each treatment involved 10 plants (n = 30). The CEO emulsion was sprayed on the plants during the transition period between the vegetative stage (V10) and the reproductive stage (R1, the start of differentiation).
The 2022 experiment was carried out under similar conditions to 2021, differing by adding the water deficit treatment, the number of blocks being equal to five, and the use of 100 plants per treatment in five randomized blocks (RCBD). Each treatment contained 20 plants (n = 100).
Both experiments were evaluated 10 days after harvest for weight, diameter, number of bulbs, and presence of abnormalities (being crooked, over-sprouted, or cracked).
In the 2021 experiment, after spraying, photosynthetic variables, amylolytic activity, and protein content of fresh leaves and developing bulbs were evaluated on days 3 and 7. Metabolites were analyzed 3 days after spraying the plants by using GC-MS.
2.4. Experiment for the Evaluation of the Mode of Action of CEO as a Plant Growth Retardant
2.4.1. Total Soluble Protein (TSP) Quantification
For analysis, leaves and bulbs in the formation stage (n = 5) were macerated with a mortar and pestle using liquid nitrogen. The ground material (200 mg) was weighed in a 2 mL microtube, and 1500 µL of the mix (750 µL 200 mM potassium phosphate buffer, pH 7.8, 15 µL 10 mM ethylenediamine tetraacetic acid (EDTA), 150 µL 200 µL ascorbic acid mM, and 585 µL of ultrapure water) was added and vortexed for 5 min. The mixture was centrifuged at 10,000 rpm for 5 min at 4 °C to obtain the crude extract [
30].
Protein was quantified using the Bradford method, where 10 µL of extract, 40 µL of 1M NaOH, and 2.5 mL of Coomassie blue solution were added into a test tube and homogenized by vortexing; the color that developed was measured at 595 nm using a spectrophotometer, and the protein content was estimated from the values obtained based on the standard albumin curve [
30,
31].
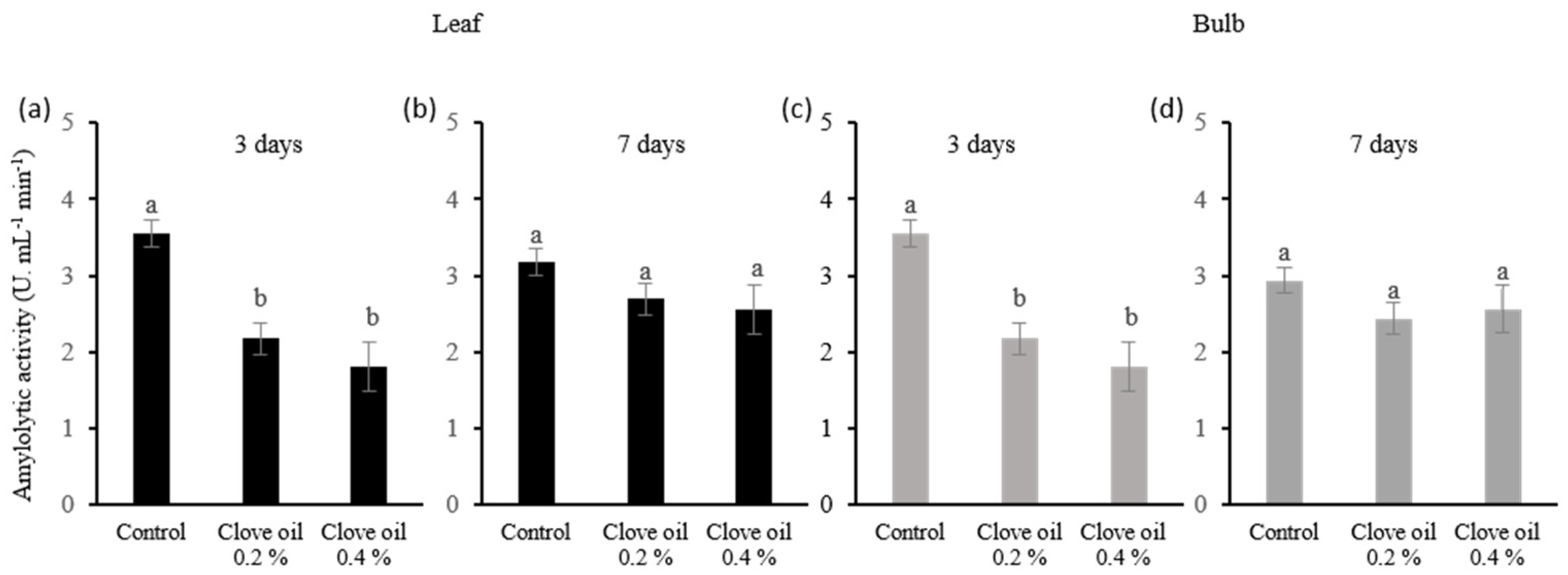
2.4.2. Determination of Amylolytic Activity
The leaves and bulb samples were frozen using liquid nitrogen, ground into a fine powder, homogenized with distilled water, and centrifuged at 5000×
g for 10 min. The supernatants were used as the enzymatic extracts. The amylolytic activity was determined by quantifying the reducing sugars released by the amylase-catalyzed starch hydrolysis reaction. The complex formed by reducing sugar with 3,5-dinitro salicylic acid (DNS) was measured by colorimetry, and the concentration was estimated using the standard glucose curve [
21,
30].
2.4.3. Leaf Gas Exchange Analysis
The gas exchange variables of garlic leaves were measured using a portable gas exchange system (LI-6400XT; LI-COR, Lincoln, NE, USA, with a modulated fluorometer (LCF-40 LI-COR Inc.) under photosynthetically active radiation saturation (PAR) of 1000 μmol m−2 s−1, and the samples were wholly expanded leaves. The analysis was conducted between 9:00 a.m. and 11:00 a.m. The following parameters were measured: A-Photosynthesis rate (µmol CO2 m−2 s−1), Cond-leaf conductance (mol H2O m−2 s−1), Ci-Concentration intercellular CO2 (µmol CO2 mol−1), PhiPS2-Quantum efficiency of photosynthetic electron transport through photosystem II, qP-photochemical extinction coefficient (photosynthetic active radiation 400 to 700 nm), ETR-Photochemical extinguishing efficiency, and Ci/Ca–CO2 internal/CO2 external.
2.4.4. Metabolic Profile Analysis Using GC-MS
At the beginning of the differentiation phase, the plants were sprayed with a 0.2% and 0.4% CEO emulsion and without oil (control). After 3 days of spraying, four replicates per treatment evaluated the metabolic profile of the leaves and tissues covering the developing bulb. Each sample consisted of a mixture of tissues from different plants (n = 4; each sample consisted of three plants). One gram of leaf tissue was homogenized in 9 mL of a water–methanol mixture (80:20) using a Turrax-type disperser for 1 min at 18,000 rpm (Ultra Turrax T25-IKA). Next, 1 mL of the extract was centrifuged at 10,000 rpm. The supernatant (200 µL) was concentrated by nitrogen injection (Concentrator TE019-Tecnal, Männedorf, Switzerland). The dry material was solubilized in 120 µL of methoxylamine hydrochloride solution (20 mgmL
−1 pyridine) and 40 µL of ribitol (2 mgmL
−1) as an internal standard in a closed system for 60 min at 65 °C for the formation of oximes. For silylation reactions, 40 µL of N, O-Bis(trimethylsilyl) trifluoroacetamide (BSTFA) with 1% trimethylsilyl chloride (TMCS) was added and rested for another 60 min at 65 °C, then transferred to 200 µL inserts [
32,
33,
34]. A mix of plants from both treatments was used as the quality control sample (QC).
The extracts were analyzed using a GC-MS (Shimadzu GC-MS QP2010 Ultra) with an AOC-20i auto-injector. A total of 1 μL of the sample was injected at an injector temperature of 240 °C with a split ratio of 1:20. Helium was used as the carrier gas at a flow rate of 1 mLmin
−1, and linear velocity was used as the flow control mode. The capillary column Rtx-5MS (30 m × 0.25 mm × 0.25 μm) was used with isothermal programming for 2 min at 80 °C, with a temperature ramp rate of 5 °C min
−1 to 300 °C, with a hold time of 3 min. The mass spectrometry parameters were ion source temperature, 280 °C; interface, 280 °C; impact ionization, 70 eV; mass spectrum scan from 45–700 m/z. The solvent cut-off time was 3 min [
34,
35].
Data extracted from GC-MS in QGD format were converted to the Analysis Base Framework (ABF) using the converter (AbfConverter, Yokohama City, Kanagawa, Japan) once compatibility with (MS-DIAL4.90, Yokohama City, Kanagawa, Japan) software was recognized. The chromatograms were aligned, deconvoluted, and identified using MS-DIAL 4.90, adopting parameters similar to those described by Leite (2021) for peak detection: smoothing method, linear weighted moving average; smoothing level, three sweeps; average peak width, 20 scans; minimum peak height, amplitude 1000 [
36]. Deconvolution parameters: Sigma window value, 0.5; electron ionization (E.I.) spectral clipping, amplitude, 10. Identification settings: retention index (IR); MSP file, GCMSDB-Public-KovatsRI-VS3 database, metabolites were identified using spectral matching (≥85% similarity) for confirmation using the NIST 2014 mass spectral library (National Institute of Standards and Technology); a mixture of alkanes (C7-30, Sigma-Aldrich, St. Louis, MO, USA) was used for RI tolerance; RT tolerance, 0.5; m/z tolerance, 0.5; EI similarity cut, 85%; cut identification score, 85% [
37]. The identified metabolites were normalized using the internal standard ribitol and then subjected to statistical analyses.
Statistical analyses were performed using Metaboanalyst-5.0, an R-based program explicitly designed for metabolomics to determine the metabolomic profile. Furthermore, the data were adjusted using relative standard deviation, sum normalization, logarithmic transformation (base 10), and automatic scaling. A multivariate statistical approach was also applied by discriminant analysis of partial least squares (PLS–DA) and applying a graphical model of the heat map based on the hierarchical cluster, where the measure of similarity of the compounds by distance measure underwent Euclidean evaluation [
35]. The levels of the metabolites that were detected in the different treatments were compared using analysis of variance (ANOVA) and mean test (Tukey), where a
p-value < 0.05 was considered statistically significant.
Metabolic pathway analysis was performed using MetPA MetaboAnalyst 5.0 to interpret the biological relevance of our findings when analyzing the control treatment and the treatment treated with the highest dose of CEO (0.4%), integrating two approaches to metabolite analysis and enrichment. Via metabolism analysis, the expressed metabolites were identified using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database to interpret the metabolic changes that occurred between the control treatment and the treatment with CEO. For this analysis, the discriminant metabolites highlighted in the PLS–DA models were used.
2.4.5. Statistical Analysis
The experimental data were subjected to an analysis of variance, and means were compared using the
t-test, adopting five blocks and 10 replicates for each treatment. In the figures, different letters indicate means that differ significantly (
p-value < 0.05), bars indicate the standard error of the mean, and data were analyzed using the software RStudio (
https://www.rstudio.com/authors/rstudio-team/) [
38].
4. Discussion
The application of CEO on garlic leaves significantly impacted various metabolic pathways, revealing direct correlations with five pathways related to the plants’ defense system. These findings contribute to a better understanding of the physiological effects of CEO in plants. One affected pathway was fatty acid elongation (1), which is associated with synthesizing the long-chain fatty acids that play essential roles in processes such as cell membrane formation. Additionally, the application of CEO impacted the regulation of specific compound biosynthesis, such as cutin, suberin, and wax (2). These compounds play critical roles in protecting plants against environmental stresses. Another correlation was identified with the biosynthesis of saturated fatty acids (3) and unsaturated fatty acids (4), which showed a positive increase. The synthesis of these fatty acids is commonly associated with the physiological response of plants to abiotic stress conditions.
The evaluation of the metabolites of the treated leaves revealed a significant increase in palmitic and stearic acid levels in response to the application of CEO solutions, indicating damage to the leaf surface, as these are lipid precursors to cutin and suberin. These are insoluble lipid polymers that act as barrier functions in the cell walls of plant tissues, including the epidermis, endodermis, and periderm. This suggests that the plant redirects its metabolic synthesis to restore these tissues and repair possible injuries.
Under adverse conditions, plants initiate alternative metabolic pathways to photorespiration to enhance their efficiency and growth. One alternative is the degradation of amino acids, which can contribute to the energy state of plant cells under certain physiological conditions, such as carbon demand [
39]. The degradation pathway of leucine, isoleucine, and valine (the fifth identified metabolic pathway) leads to the production of acetyl-CoA and CO
2, with one of its intermediate metabolites being methylmalonic acid (methylmalonate), which is approximately 5.8 times more concentrated in the CEO-treated samples, indicating that the amino acid degradation pathway has been altered to meet the demand for acetyl-CoA and CO
2. Additionally, plants treated with CEO showed reduced photosynthetic rates, as well as lower concentrations of citric acid and fructose (decreased by 4.5 and 1.8 times, respectively), which are substrates for the Krebs cycle and the glyoxylate cycle (glyoxylate and dicarboxylate metabolism (6)) in cellular energy production. The complete network of metabolites can be seen in the valine, leucine, and isoleucine degradation map (
https://www.kegg.jp/pathway/map00280+C02170, accessed on 10 September 2022) and the citrate cycle map (
https://www.kegg.jp/pathway/map00020+C00158, accessed on 10 September 2022), which demonstrates that the products of amino acid degradation can be directed towards fatty acid biosynthesis, corroborating with the higher concentrations of palmitic and stearic acids, which had an approximately 5.3- and 3.1-fold higher intensity, respectively, in treatment with 0.4% CEO. The glyoxylate cycle, an alternative pathway to photorespiration in plants, primarily converts two molecules of acetyl-CoA into succinate. However, in the glyoxylate cycle, the substrate isocitrate, instead of undergoing decarboxylation as in the citric acid cycle, is cleaved by isocitrate lyase into succinate and glyoxylate. Subsequently, another molecule of acetyl-CoA condenses with glyoxylate to form malate, which is catalyzed by malate synthase. Then, malate is oxidized to oxaloacetate, continuing the cycle, and the formed succinate enters energy production pathways [
40]. Plants treated with CEO emulsion showed elevated expression for malic acid (malate, 3.03-fold higher intensity), indicating a positive impact on this anaplerotic pathway as it bypasses the two decarboxylation steps present in the citric acid cycle, generating an additional molecule of succinate [
41]. CEO affects the metabolism of garlic plants and their photosynthetic rates. However, the plants seem to activate alternative pathways and quickly metabolize the compounds generated by the stress without causing permanent damage to the crop, thereby not affecting bulb productivity and quality. The stress induced in the plants effectively interferes with the appearance of sprouting shoots, indicating that the exogenous application of CEO can direct the plant to reduce its vegetative phase and the emergence of new leaves, consequently channeling more resources towards bulb production.
The inhibition of secondary growth is challenging for garlic producers in tropical regions. This study has shown that using CEO, an essential oil rich in eugenol (a substance known for its herbicidal and germination inhibitory properties at 0.4%), can effectively regulate plant growth and prevent secondary growth in garlic crops when applied in the differentiation phenological stage. However, it is essential to note that the application of CEO causes changes in leaf function, such as decreased photosynthesis and amylase enzyme activity, with stress being able to reduce secondary growth in garlic, and the plant reestablishes its metabolism 7 days after application. Although ABA dosage was not performed in the experiment, literature data suggest that the reduction in secondary growth must be related to changes in the ABA content in garlic plants after the application of CEO. According to Hu et al., 2017, eugenol can inhibit rice seed germination in the field, increasing the ABA level while decreasing the expression of ABA-catabolizing genes [
21]. Additionally, Zhao et al., 2022, observed that
C. sinensis absorbs eugenol from the air and converts it into glycosides, altering ABA homeostasis and conferring increased tolerance to cold and drought [
22].
The results obtained from CEO, a natural and eco-friendly product, are more promising than the the literature data for ETP, as CEO reduced secondary growth without affecting the production of commercial-grade garlic bulbs [
19].
The practice of water deficit also significantly reduced the presence of secondary growth but decreased the average weight of the bulbs, resulting in a negative impact on final production. Rainfall during the differentiation phenological stage nullifies the effects of the water deficit, justifying the search for complementary techniques for controlling secondary growth in the field of garlic.
5. Conclusions
Utilizing CEO, a naturally occurring compound, as a growth inhibitor for secondary growth in garlic is an ecologically sustainable solution. Applying a 0.4% emulsion of CEO has shown promising results as a plant growth retardant. It effectively curtails secondary growth, producing more high-quality garlic bulbs, and contributing to the producer’s profitability.
Furthermore, this solution is a viable alternative in agricultural scenarios where rainfall during the garlic differentiation phenological stage prevents the use of water deficit practice. Therefore, using CEO as a plant growth retardant in such situations can contribute to the quality of the garlic bulbs.
Clove essential oil is nonpolar, thus requiring optimized formulations and modes of application for effective plant growth retardation.