Abstract
Hemp, commonly known as Cannabis sativa L., is a medicinal plant species of the Cannabaceae family. For the efficient extraction of C. sativa leaves using the conventional stirring process with water as the solvent, three crucial extraction parameters (i.e., extraction duration, liquid–solid ratio, and temperature) were investigated through the response surface methodology (RSM). The concentrations of the extracted bioactive compounds (polyphenols, ascorbic acid, and carotenoids) showed significant variations in the RSM design points, suggesting the importance of finding the optimal extraction conditions in which liquid–solid ratio and extraction temperature were found to have the highest impact. Further analysis was conducted on the optimal extract employing several assays to determine their polyphenol content, total carotenoid content, color evaluation, anti-inflammatory activity, and antioxidant capacity through FRAP, DPPH, and H2O2 assays. A low extraction time (30 min) at 50 °C and a high liquid–solid ratio (50:1) were required for the highest possible yield of polyphenols. The total polyphenol content was determined to be 9.76 mg gallic acid equivalents/g under optimum conditions, with pelargonin being the most abundant polyphenol (1.51 mg/g) in C. sativa extracts. Ascorbic acid was measured at 282.23 μg/g and total carotenoids at 356.98 μg/g. Correlation analyses revealed that anti-inflammatory activity was negatively correlated with specific polyphenols. As determined by DPPH (27.43 μmol ascorbic acid equivalents (AAE)/g), FRAP (49.79 μmol AAE/g), and H2O2 (230.95 μmol AAE/g) assays, the optimized aqueous extract showed a high antioxidant capacity. Furthermore, it demonstrated considerable anti-inflammatory activity at 17.89%, with the potential to increase to 75.12% under particular extraction conditions. Given the high added-value of the aqueous extracts, the results of this study highlight the potential utility of C. sativa leaves as a source of health-improving antioxidant compounds in the pharmaceutical and food industries.
1. Introduction
Herbaceous plants have been utilized by humans for the last 5000 years [1]. Developed and domesticated agriculturally during the Bronze Age as traditional medicine, hemp (Cannabis sativa L.) is a plant of the Cannabaceae family and was introduced to Europe from central Asia [2]. Irrespective of its place of origin, the modern domesticated variety of C. sativa L. is grown extensively around the globe, from Asia to North America, Europe, and Africa [3]. The primary reason is that all aerial parts can be utilized in various applications. From agriculture and phytoremediation to the cosmetic, pharmaceutical, food, and construction sectors, this versatile crop has a low environmental effect while serving several purposes. In terms of valorization, many useful industrial products can be extracted from this adaptable plant, e.g., apart from energy generation, straw fibers have been utilized in the construction and textile industries [4].
Regarding nutrition, C. sativa is notable for the presence of the unique psychotropic compound δ-9-tetrahydrocannabinol. Such cannabinoids and their pharmacological characteristics have attracted great research interest, and it is the main reason why the plant is illicit [5]. In addition, C. sativa seeds provide an excellent source of oil for human consumption. Hempseed oil is generally recognized to be among the few seed oils that have approximately 80% polyunsaturated fatty acids which are suggested as optimal for human diets [6], such as ω-6 acid isomers, γ-linolenic, and α-linolenic acid [7]. Hemp seed oil has shown beneficial effects on humans, such as decreasing hypertension and preventing cardiovascular disease [8]. Terpenes, another group of molecules found in C. sativa, have a vast role in the unique scent of the plant. In addition to their positive biological actions, these volatile compounds, primarily mono- and sesquiterpenes, have anti-inflammatory and antidepressant properties [9,10]. A major sesquiterpene called β-caryophyllene may bind to the type 2 cannabinoid receptor, which could have a synergistic effect on health benefits in conjunction with the other phytochemicals in this plant [11].
Natural antioxidants have recently gained popularity, given their potential to add biological value to food and cosmetics [12,13]. Polyphenols, which are natural antioxidants, have a beneficial effect on the nutritional content, taste, and shelf-life of food products. They can delay lipid oxidation and other radical reactions that lead to food spoilage [14,15]. Polyphenols aid in the protection of plants, namely against detrimental UV radiation, due to their antioxidant and radical scavenging characteristics [16]. These compounds have the potential to slow the development of a wide range of illnesses and ailments, including cardiovascular and neurological disorders, asthma, inflammatory disorders, and cancers [17]. C. sativa is unique among plants in that it includes several types of polyphenols, including flavonoids, phenolic acids, and cannflavins, a kind of prenylated flavone [18]. Antioxidant, anti-inflammatory, neuroprotective, and antiparasitic properties have previously been attributed to these compounds [19,20]. C. sativa possesses several antioxidant compounds which can be beneficial for the wellness of people. With these beneficial characteristics, cannabinoids A and B stand out among the normal flavones found in C. sativa [21]. Under the impact of intense solar radiation and freezing temperatures, cannflavin A was found in significant concentrations in certain C. sativa cultivars.
Polyphenols and other beneficial molecules can be obtained from a plant by extraction. In order to maximize the extraction yield, many extraction methods have been developed and employed. However, in many cases, sophisticated equipment or tailored solvents are needed. As such, the employment of more simple, yet efficient, extraction methods, that are human and environmentally friendly at the same time, is gaining attention [22]. The conventional stirring method is a simple and commonly employed technique for the effective extraction of bioactive compounds [23]. Several factors, including the temperature, solvent, extraction duration, and liquid–solid ratio are known to have a crucial influence on the extraction of polyphenols and other bioactive compounds. For instance, although temperature can promote the extraction of various compounds, some compounds can degrade upon heating at high temperatures [23]. As such, fine-tuning the above parameters can significantly enhance the extraction yield. In order to avoid tedious optimization workflows that may miss optimum parameters, the use of statistical methods is highly suggested. As such, the above parameters can be optimized through the response surface methodology (RSM) to guarantee the highest extraction yield [24]. As such, using an improved stirring procedure with water can be a promising procedure to extract compounds [25].
Although various attempts have been carried out to obtain extracts from C. sativa leaves, in most cases, the use of either organic solvents or mixtures with water is being employed. This results in increased extraction costs and higher environmental impact, while additional steps are needed to eliminate the organic solvent prior to the usage of the extract. Despite the above efforts, there are no detailed studies regarding the optimization of the extraction using only water. In addition, the studies that report the use of water to prepare extracts focus solely on a few compounds (such as polyphenols) without taking into account other compounds with antioxidant properties, such as ascorbic acid and carotenoids. To that end, the study aimed to optimize the extraction parameters of C. sativa leaves with water as a solvent, including the temperature, extraction time, and liquid–solid ratio through RSM. In addition, the bioactive compound concentration (polyphenols, carotenoids, and ascorbic acid) of C. sativa extracts was measured, whereas optimum conditions were revealed through a partial least squares (PLS) model. The influence of these parameters was further explored with color evaluation, the antioxidant activity from three assays, and the anti-inflammatory activity of the extracts. All the above were conducted to determine whether C. sativa leaves could serve as a feasible source of antioxidant compounds, with a particular focus on their potential use in the food and pharmaceutical sectors.
2. Materials and Methods
2.1. Chemicals and Reagents
Gallic acid, ethanol, and the Folin–Ciocalteu reagent were bought from Panreac Co. (Barcelona, Spain). Hydrochloric acid, methanol, L-ascorbic acid, phosphate buffer solution, aluminum chloride, 2,2-diphenyl-1-picrylhydrazyl (DPPH) 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), trichloroacetic acid, albumin, β-carotene analytical standard, and all chemical HPLC standards for the polyphenol determination were obtained from Sigma-Aldrich (Darmstadt, Germany). Anhydrous sodium carbonate was bought from Penta (Prague, Czech Republic). Iron (III) chloride was purchased from Merck (Darmstadt, Germany). Hydrogen peroxide (35% v/v) was purchased from Chemco (Malsch, Germany). Deionized water from a deionizing column was used for all the experiments.
2.2. Hemp Leaf Material
For all experiments, hemp (Cannabis sativa var. Finola) leaves (without flowering and fruiting tops) were donated by CBD Extraction Ι.Κ.Ε. (Farsala, Greece), gathered from the Krokio area in Almiros region (at 39°20′58″ N and 22°75′16″ E, based on Google Earth version 9.185.0.0). The leaves were rinsed extensively with distilled water and dried with paper towels. The sample underwent freeze-drying using a Biobase BK-FD10P freeze-dryer (Jinan, China). The moisture level of the fresh leaves was measured to be 79.12 ± 1.26%. The dried C. sativa leaves were then ground to a fine powder (<400 μm diameter) using a blender. Finally, until further analysis, the powder was preserved at −40 °C.
2.3. Hemp Leaf Extraction
To identify the optimal conditions for the extraction of bioactive compounds from C. sativa leaves, different quantities of the dried powder (0.40, 0.57, and 1 g) were weighted and inserted into screw-capped glass bottles, and 20 mL of water was added to achieve a liquid–solid ratio of 50:1, 35:1, and 20:1, respectively. The mixtures were heated at 20, 50, and 80 °C for 30, 90, or 150 min, under continuous stirring at 500 rpm. After the extraction was completed, the samples were centrifuged for 10 min at 10,000× g in a NEYA 16R centrifuge (Remi Elektrotechnik Ltd., Palghar, India). Finally, the supernatants were stored at −40 °C. All experiments were carried out in various combinations of the examined parameters, the coded levels of which are shown in Table 1.

Table 1.
The actual and coded levels of the independent variables that were used to optimize the process.
2.4. Optimization with Response Surface Methodology (RSM), Experimental Design, and Model Validation
The RSM technique was employed to achieve optimal efficiency in extracting the bioactive compounds and evaluating the antioxidant activity from the C. sativa aqueous extracts. Therefore, the main objective of the design was to effectively maximize the levels of these values. This was accomplished by optimizing the liquid–solid ratio (R, mL/g), extraction time (t, min), and extraction temperature (T, °C). The optimization process was based on an experiment that utilized a Box–Behnken design with a main impact screening arrangement. The experiment consisted of 15 design points, including 3 center points. According to the experimental design, three levels of process variables were created. The overall model significance, as shown by the R2 and p-values, and the significance of the model coefficients, as represented by the equations, were assessed using the analysis of variance (ANOVA) and summary-of-fit tests, with a minimum level of 95% confidence.
In addition, the response variable was predicted as a function of the examined independent factors using a second-order polynomial model, as illustrated in Equation (1):
The predicted response variable is denoted as Yk, while the independent variables are Xi and Xj. The intercept and regression coefficients for the linear, quadratic, and interaction terms of the model are denoted as β0, βi, βii, and βij, respectively.
To determine the greatest peak area and assess the effect of a substantial independent variable on the response, the RSM was applied. The development of three-dimensional surface response graphs was initiated to represent the model equation visually.
Furthermore, the model validation process involved comparing the model’s predictions with the actual outcomes to measure its accuracy. This step was essential to ensure that the model was reliable and could be used for future predictions. The data were divided into three subsets: training, validation, and test. The model parameters were learned from the training subset. The validation subset was used to adjust the model parameters and select a model that can predict well. The test subset was used to measure the performance of the final model. In the case of our study, we used k-fold cross-validation to validate the model’s predictive ability. The statistics of model validation are given in Table S1.
2.5. Bioactive Compound Determination
2.5.1. Total Polyphenol Content (TPC)
An established methodology [26] was applied to determine TPC. Briefly, 0.10 mL of a sample properly diluted extract was mixed with 0.10 mL of Folin–Ciocalteu reagent and after 2 min, 0.80 mL of 5% w/v aqueous sodium carbonate solution was added. The mixture was incubated for 20 min at 40 °C and the absorbance was measured at 740 nm in a Shimadzu UV-1700 PharmaSpec Spectrophotometer (Kyoto, Japan). The total polyphenol concentration (CTP) was calculated from a gallic acid calibration curve. Total polyphenol yield (YTP) was determined as mg gallic acid equivalents (GAE) per g of dry weight (dw) using the following Equation (2):
where the volume of the extraction medium is indicated with V (expressed in L) and the dry weight of the sample is w (expressed in g).
2.5.2. HPLC Quantification of Polyphenolic Compounds
Individual polyphenols were identified and quantified from the sample extracts using High-Performance Liquid Chromatography (HPLC), according to our prior research [27]. A Shimadzu CBM-20A liquid chromatograph and a Shimadzu SPD-M20A diode array detector (DAD) (both purchased by Shimadzu Europa GmbH, Duisburg, Germany) were employed for the analysis of the C. sativa extracts. The separation of the compounds was performed in a Phenomenex Luna C18(2) column from Phenomenex Inc. in Torrance, California, with the temperature at 40 °C (100 Å, 5 μm, 4.6 mm × 250 mm). The mobile phase included 0.5% aqueous formic acid (A) and 0.5% formic acid in acetonitrile/water (3:2) (B). The gradient program had a total of 70 min and was set as follows: initially from 0 to 40% B for 40 min, then to 50% B in 10 min, to 70% B in another 10 min, and finally constant for 10 min. The flow rate of the mobile phase was set at 1 mL/min. The identification of the compounds was made through comparing the absorbance spectrum and retention time to pure standards. The quantification was conducted through calibration curves (0–50 μg/mL), and the results were given in mg/g.
2.5.3. Ascorbic Acid Content (AAC)
Ascorbic acid content (AAC) was evaluated with a previously established method [28]. In an Eppendorf tube, a quantity of 100 μL sample extract along with 500 μL of 10% (v/v) Folin–Ciocalteu reagent was mixed with 900 μL of 10% (w/v) trichloroacetic acid. The absorbance was measured at 760 nm after 10 min. Ascorbic acid was the calibration standard.
2.5.4. Total Carotenoids Content (TCC)
A slightly modified method introduced by Ayour et al. [29] was employed to determine the total carotenoid content (TCC) of the analyzed extracts. In brief, the samples underwent a ten-fold dilution during the preparation process; consequently, the absorbance measurement was recorded at 450 nm. Using a calibration curve from β-carotene, the TCC was quantified in mg of β-carotene equivalents per gram of dried weight.
2.6. Antioxidant Capacity of the Extracts
2.6.1. Ferric-Reducing Antioxidant Power (FRAP) Assay
An established methodology [30] was used for the evaluation of FRAP. In a 2 mL Eppendorf tube, 100 μL of the properly diluted sample was mixed with 100 μL of FeCl3 solution (4 mM in 0.05 M HCl). The mixture was incubated at 37 °C for 30 min. A quantity of 1800 μL of TPTZ solution (1 mM in 0.05 M HCl) was immediately added and the absorbance was measured after 5 min at 620 nm. The ferric-reducing power (PR) was calculated using an ascorbic acid calibration curve (CAA) in 0.05 M HCl with ranging values (50–500 μM). The PR was calculated as μmol of ascorbic acid equivalents (AAE) per gram of dw using Equation (3):
where V represents (in L) the entire volume of the extraction medium and w (in g) represents the dried weight of the material.
2.6.2. DPPH• Antiradical Activity Assay
The extracted polyphenols from the dried material were evaluated for their antiradical activity (AAR) using a slightly modified DPPH• method by Shehata et al. [31]. Briefly, 50 μL of the properly diluted sample was mixed with a quantity of 1950 μL of a 0.1 mM DPPH• solution in methanol, with the solution being kept at room temperature for 30 min in the dark right after. The absorbance was measured right after at 515 nm. Moreover, a blank solution sample was used instead of the sample, including DPPH• solution and methanol, with the absorbance immediately being measured. To calculate the percentage of scavenging, Equation (4) was employed:
The ascorbic acid calibration curve in Equation (5) was used to evaluate the antiradical activity (AAR), which was expressed as μmol AAE per gram of dw:
where V represents (in L) the entire volume of the extraction medium and w (in g) represents the dried weight of the material.
2.6.3. Hydrogen Peroxide (H2O2) Scavenging Assay
A previous method [28] was applied for the H2O2 scavenging assay. A quantity of 400 μL of the properly diluted extract and 600 μL of an H2O2 solution (40 mM, made in phosphate buffer, pH 7.4) was added into an Eppendorf tube. The absorbance was recorded right after 10 min at 230 nm. The scavenging capacity of the H2O2 was expressed as follows:
where the absorbances of the blank solution, the extract solution in the absence of hydrogen peroxide, and the sample are denoted by A0, Ac, and A, respectively.
The concentration of ascorbic acid ranged in the calibration curve (CAA, 50–500 μmol/L in 0.05 M HCl) and the following Equation (7) was used to determine the anti-hydrogen peroxide activity (AAHP) as μmol AAE per g of dw:
where V denotes the volume of the extraction medium (in L), and w is the dry weight of the sample.
2.7. Biological and Physicochemical Parameters of the Extracts
2.7.1. Assessment of In Vitro Anti-Inflammatory Activity
The in vitro evaluation of the anti-inflammatory properties of the C. sativa extracts was conducted using the albumin denaturation assay [32]. Briefly, a mixture containing egg albumin and PBS (pH = 6.4) with a 0.1:1.4 mL ratio (mixture A), respectively, was prepared. Then, 400 μL of the sample extract or standard were mixed with 600 μL of the mixture A in a 1.5 mL Eppendorf tube, and then, the tubes were incubated at 37 °C for 15 min. Afterwards, the mixture was heated at 70 °C for 5 min. The absorbance was then recorded at 660 nm. To determine the inhibition of protein denaturation, the following equation was used:
2.7.2. Color Evaluation
The CIELAB color of the C. sativa extracts was measured by using a prior established method [33] with the use of a colorimeter (Lovibond CAM-System 500, The Tintometer Ltd., Amesbury, UK), where the CIELAB parameters (L*, a*, and b*) measured the aqueous extracts. Three parameters were fundamental to measure the color of the aqueous extracts: The L* value which denotes the lightness of a color, ranging from 0 to 100 (black to white). The a* value specifies the degree of redness (negative values) or greenness (positive values) in a color. Likewise, the b* value measures the extent of yellowness (negative values) or blueness (positive values) in a color. The measure of color intensity is expressed as Cab or C* (chroma or saturation). The hue angle (hab or H) and psychological coordinate chroma (Cab*) were measured by the following equations:
2.8. Statistical Analysis
The statistical analysis related to the response surface methodology, distribution analysis, and model validation were applicable through the JMP® Pro 16 software (SAS, Cary, NC, USA). The extraction procedures were repeated a minimum of twice for each batch of the C. sativa extract, and the quantitative analysis was conducted in triplicate. The results are represented as means and standard deviations. The multivariate correlation analysis (MCA), principal component analysis (PCA), and partial least squares (PLS) were performed through the JMP® Pro 16 software (SAS, Cary, NC, USA).
3. Results and Discussion
The aim of this research was to maximize the extraction of polyphenols from C. sativa. To enhance the effectiveness of recovering the bioactive compounds, several extraction parameters were examined, such as the extraction duration (30–150 min) and the extraction temperature (20–50 °C). To verify the appropriate ratio of liquid–solid and produce the finest results, corresponding experiments were conducted within the range of 20–50 mL/g. Furthermore, to evaluate the impact of certain components and improve the efficiency of water extraction, the response surface methodology (RSM) was employed. The efficacy of the RSM and model adequacy were evaluated by employing the ANOVA and summary-of-fit tests to compare the experimental values with the anticipated values.
3.1. Total Polyphenol Content and Antioxidant Activity of the Extracts
Polyphenolic compounds are among the most widely recognized categories of compounds found in natural products. There is significant potential for the implementation of these compounds in several sectors, including the food and pharmaceutical sectors [34,35]. The measured responses and predictions for the TPC, as well as of the FRAP, DPPH, and H2O2 assays for each prepared extract, are presented in Table 2. It can be noted that the predicted and actual measurements have a low variance. The range of the TPC values for the extracts was between 7.28 and 9.77 mg GAE/g dw. The variation in TPC may be attributed to several parameters of the extraction process, including the liquid–solid ratio, temperature, time, and extraction solvent, as per Koraqi et al. [36]. In a study conducted by Aazza et al. [37], the TPC of the Moroccan C. sativa plant was measured with different extraction solvents (i.e., water, ethanol, methanol, hexane, dichloromethane, ethyl acetate, and chloroform). When water was utilized as a solvent, the TPC was ~8 mg GAE/g dw, whereas ethanol and methanol achieved more than two-fold increased polyphenol recovery. In another study [38] with ethanolic mixtures or water used as extraction solvents, young and mature C. sativa extracts were analyzed. The TPC value from aqueous extracts was comparable to our study and was measured at 8.44 and 6.21 mg/g dw, respectively. Mixtures consisting of 50% ethanol were again found preferable, achieving a two-fold increase in recovered polyphenols. Our objective was to obtain an extract using green and food-grade solvents, with water being a highly cost-effective option. A similar hydroethanolic solution could be employed in a future investigation.

Table 2.
Experimental findings for the three investigated independent variables and the dependent variables’ responses.
Regarding the antioxidant tests, the data displayed a greater variance compared to those of the TPC. For instance, the corresponding range for the FRAP assay was from 35.07 to 49.63 μmol AAE/g, in the DPPH assay was from 12.05 to 27.34 μmol AAE/g revealing a two-fold increase, and in the H2O2 assay was from 98.58 to 238.56 μmol AAE/g, showing almost a two-and-a-half-fold increase. In the previously mentioned study by Aazza [38], the antioxidant capacity was measured at ~140 μmol AAE/g dw, three-fold higher than our results, highlighting this interesting trend. As such, via the optimization process of the extraction, the antioxidant properties of the extracts can significantly be improved.
Table 3 presents the measured amounts of the identified polyphenols with the use of HPLC-DAD. Several types of polyphenols were quantified such as anthocyanins (pelargonin, from 0.41 to 1.53 mg/g dw), phenolic acids (ferulic acid, from 0.07 to 0.17 mg/g dw), and flavonoids (luteolin-7-glucoside, from 0.18 to 0.34 mg/g, and kaempferol-3-glucoside, from 0.18 to 0.44 mg/g dw), with a total sum of 0.84–2.84 mg/g dw. Pelargonin measured concentration was highest in the design point 10, which was previously indicated as the optimum sample, whereas the design points 4 and 12 had the highest concentrations in the other three polyphenols, revealing an interesting trend. In all three optimum design points, the liquid–solid ratio had a major impact on the extraction of polyphenols compared to the other variables, which will be further discussed below.

Table 3.
Coded values of the three independent variables under investigation and the actual concentration of the polyphenolic compounds, represented in mg/g dw.
3.2. Other Bioactive Compounds, and Biological and Physicochemical Determination of Extracts
Carotenoids are pigments present in many plants that have a significant association with color properties. The amount of carotenoids present is an important quality criterion since it directly affects the visual features of the end product [39]. Regarding the content of both total carotenoids and ascorbic acid, along with the anti-inflammatory activity, the results are shown in Table 4 and reveal an interesting trend. The TCC ranged from 283.27 to 371.29 μg CtE/g, whereas AAC ranged from 215.88 to 526.94 μg/g, suggesting almost a two-and-a-half-fold difference between the design points 11 and 7. In a study by Spano et al. [40], similar TCC was observed between seven cultivars of C. sativa which ranged from 106 to 317 μg/g. They used ultrasonication-assisted extraction with methanol as the extraction solvent. It was observed that the impact of the seven cultivars highly affected the TCC. Finally, the anti-inflammatory activity ranged from 7.82 to 75.12%, revealing a ten-fold difference between the design points 2 and 3. The interesting trend lies in the fact that the samples with a high TCC showed a low AAC and low anti-inflammatory activity, and vice versa. For example, the design point 2 sample showed the highest anti-inflammatory activity, but moderate AAC. A possible synergism of polyphenols, carotenoids, ascorbic acid, and other unidentified cannabinoids could explain this trend.

Table 4.
Coded values of the three investigated independent variables and the actual concentration of the total carotenoids, ascorbic acid content, and anti-inflammatory activity.
Figure 1 contains the results of the color analysis. The L* coordinate showed considerable variance among the samples, with the design points 9 and 11 having the lowest values (~32) and the design point 4 (~50) having the highest value. A similar trend regarding these samples was also observed in the C* coordinate. The design point 9 gave the lowest value in the C* coordinate (~16), whereas the design point 4 was found among the highest values (~30). However, these values were considerably low, resulting in non-saturated (achromatic) extracts. In the case of hue, most extracts had values above 60, as the majority of the extracts had a greenish-to-brownish color.
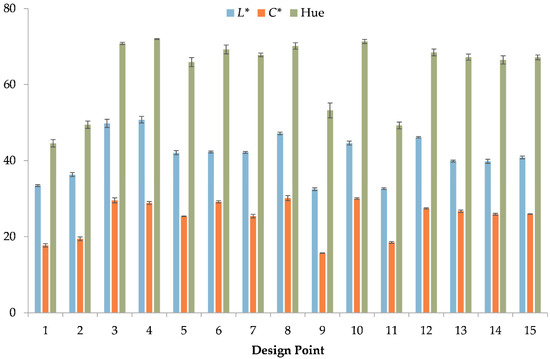
Figure 1.
Color coordinate analysis of the C. sativa extracts.
The developed models are suggested to be well fitting by the second-order polynomial equations (models), statistical parameters, and coefficients (≥0.97) which are shown in Table 5. The desirability functions and plots of the actual response versus the predicted response for each examined parameter are presented in Figures S1–S4, which will be further explained below. Figure 2 presents the three-dimensional response plots for the TPC. Regarding the TPC, it can be concluded that the highest performance was with relatively medium values for the parameters X1 (~50 mL/g) and X2 (~50 °C), as can be seen in Figure 2A. The optimum conditions were ~45 mL/g and ~20 min of extraction in Figure 2B, whereas ~65 °C and ~25 min of extraction was the best combination as could be concluded in Figure 2C. The interpretation of the three-dimensional response plots for the rest of the responses in Figures S5–S7 lies on a similar rationale.

Table 5.
Mathematical models generated through the RSM that were used to optimize the extraction process of C. sativa. The models contained only significant terms.
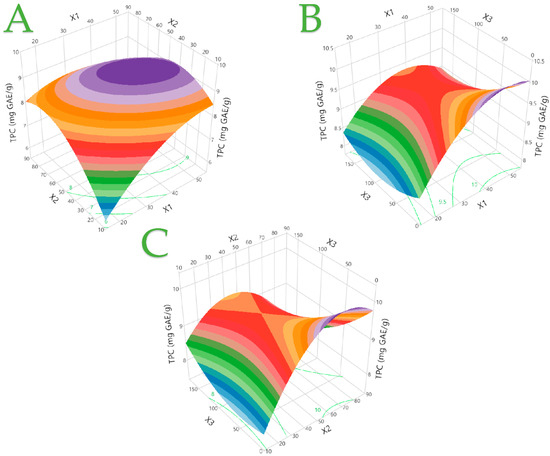
Figure 2.
The optimal extraction of the C. sativa extracts is shown in 3D graphs that show the impact of the process variables considered in the response (total polyphenol content—TPC, mg GAE/g). Plot (A), covariation of X1 and X2; plot (B), covariation of X1 and X3; plot (C), covariation of X2 and X3.
3.3. Optimal Extraction Conditions
Improving efficiency necessitates optimizing the extraction parameters. Diverse bioactive component structures could introduce challenges to the extraction procedure due to variations in solubility and polarity [41]. Moreover, the yield, composition, and antioxidant activity of the extract are significantly impacted by the extraction method and a multitude of processing parameters; thus, it is imperative to optimize this procedure [42]. Over the past decade, there has been a notable emphasis on extraction methods that aim to reduce the utilization of environmentally hazardous and noxious solvents, safeguard human health, and minimize energy consumption. The implementation of environmentally friendly solvents such as water is key for this approach [43]. Despite not being the optimum extraction solvent, water could effectively extract polyphenols across plant tissues [44]. For instance, flavonoid glucosides are more water-soluble compounds than aglycones [45], as was previously observed through HPLC-DAD analysis. The liquid–solid ratio is also a parameter that is often investigated and optimized. As the liquid–solid ratio increases, the amount of compounds obtained also increases, irrespective of the solvent used [46]. Furthermore, it is essential to optimize the extraction duration and temperature to reduce the energy usage caused by the procedure. A thorough evaluation must be conducted to determine the effect of time on extraction, given that previous studies have established the effectiveness of both rapid [47] and prolonged [48] extraction durations. Inevitably, several factors influence the duration of the extraction, one of which is the matrix composition. Moreover, elevated temperatures are known to have an advantageous impact on extraction processes by facilitating increased solubility of solutes and strengthening diffusion coefficients. Despite this, it is necessary to be aware that polyphenolic compounds could decompose beyond a certain threshold, given the fact that polyphenols are thermolabile compounds [49]. The usual temperature range for conventional extraction methods to produce the greatest recovery of polyphenols is typically between 40 and 80 °C [50,51]. It should be noted that differences in polarity and solubility among bioactive components may introduce complexity into the extraction process due to their diverse structures.
To that end, in order to identify the highest anticipated values for the TPC and antioxidant activity (measured by the FRAP, DPPH, and H2O2 assays), the desirability function was employed. The highest values of the assays were accomplished through different extraction conditions. To effectively quantify the TPC from C. sativa at a predicted value of 9.80 mg GAE/g dw, a 30 min extraction was required with a 44:1 liquid–solid ratio at 55 °C. The same duration was necessitated for the maximum predicted response for the antioxidant assays FRAP and DPPH, whereas a significantly low temperature (26 °C) was required for the optimum H2O2 scavenging activity. Further details about the optimal extraction conditions are depicted in Table 6 below.

Table 6.
Maximum predicted responses and optimum extraction conditions for the dependent variables.
3.4. Principal Component Analysis (PCA) and Multivariate Correlation Analysis (MCA)
A PCA was employed to derive more information from the variables and conduct a more thorough data analysis. The focus of this investigation was to examine whether there was a correlation between the TPC and anti-inflammatory activity, antioxidant compounds (ascorbic acid and individual polyphenols), antioxidant assays (i.e., FRAP, DPPH, and H2O2), color coordinates, and carotenoids. The two principal components illustrated in Figure 3 were chosen based on their eigenvalues > 1. These components accounted for a combined 74.00% of the variance. The results indicated whether the parameters exhibited a negative or a positive correlation. For example, the anthocyanin pelargonin has a positive correlation with the TPC and antioxidant assays, as one would anticipate. A key point to highlight, due to its intriguing discovery, was the negative correlation between the ascorbic acid concentration and the variables in question. This is noteworthy since ascorbic acid is a chemical compound of huge biological significance. Furthermore, another unexpected negative correlation that might be observed was the one between the anti-inflammatory and H2O2 scavenging activity, which might occur due to synergism or antagonism between the bioactive compounds.

Figure 3.
Principal component analysis (PCA) for the measured variables.
In addition, an MCA was conducted to further assess the association between the examined variables. The main advantage of this analysis over the previous is its capacity to quantify how much positive or negative correlation the variables have with one another. The color map used in this context employs a color scale that represents correlation values ranging from −1 to 1, as indicated in the following caption. The outcomes of this analysis are shown in Figure 4, where the previously mentioned negative correlation between ascorbic acid and the TPC seems non-significantly high (<0.8). Furthermore, interesting enough were the findings that the anti-inflammatory activity had a negative correlation (>0.6) with individual polyphenols luteolin-7-glucoside, along with the H2O2 scavenging activity. On the contrary, the TCC had a strong positive correlation (>0.8) with pelargonin, and a generally good positive correlation with the TPC and FRAP, but not with DPPH and H2O2 assays.

Figure 4.
Multivariate correlation analysis of measured variables.
3.5. Partial Least Squares (PLS) Analysis
A PLS analysis was conducted to determine the essential extraction parameters (X1, X2, and X3). Figure 5 depicts the application of PLS analysis to generate a correlation loading plot, which graphically displays the extraction conditions of C. sativa. A higher variable significance for the projection (VIP) factor, especially over 0.8, indicates a bigger contribution from this variable. According to the results, X1 and X3 (i.e., liquid–solid ratio and extraction duration) were shown to be the key factors affecting the extraction of the bioactive compounds, demonstrating a much higher relevance compared to the other variables. This trend was also confirmed in the assessment of the H2O2 scavenging activity. As mentioned before, it is advantageous for the mixture to have high values of this ratio and a low extraction time (i.e., 50 mL/g and 40 min) in order to ensure favorable results. Factor X2 (extraction temperature) has little effect on the optimization of the extraction process. It was observed that extracting for more than 45 min had an adverse effect on the extraction process, which has also been reported elsewhere [52], whereas the temperature was found to reach a plateau at 50 °C.
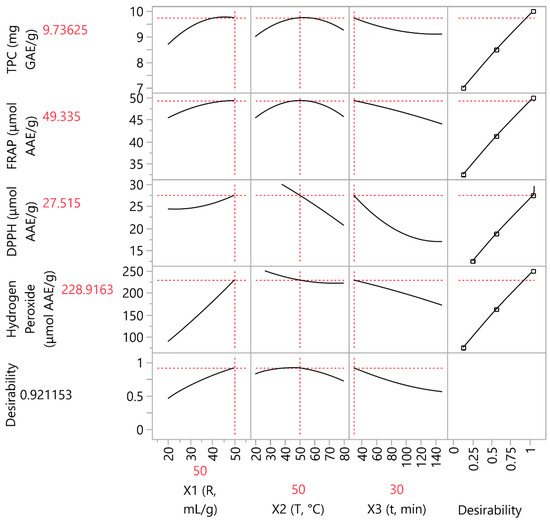
Figure 5.
Partial least squares (PLS) prediction profiler of each examined variable and desirability function with extrapolation control for the optimization of the C. sativa extracts.
The results of the experimental analysis and the values provided by the PLS model are highly correlated (0.9999) and do not deviate from each other (p < 0.0001). Table 7 represents the PLS-predicted values along with the experimental values of the TPC and antioxidant assays, in which the optimum parameters were found to be 50 mL/g, 30 min extraction at 50 °C. Table 8 depicts the values of several parameters (individual antioxidant compounds, and physicochemical and biological properties) in these optimum extraction conditions.

Table 7.
Maximum desirability for all examined variables using the partial least squares (PLS) prediction profiler under the optimal extraction conditions (X1:50, X2:50, X3:30).

Table 8.
Different parameter and polyphenolic compound analyses under optimal extraction conditions (X1:50, X2:50, X3:30).
Following optimization, the TPC showed minor modifications from the initial RSM results, leading to 9.76 mg GAE/g, whereas major polyphenols pelargonin, ferulic acid, luteolin-7-glucoside, and kaempferol-3-glucoside reached values of 1.51, 0.17, 0.35, and 0.45 mg/g, respectively. In a study by Ferrante et al. [53], the TPC of four aqueous extracts of four cultivars of C. sativa were measured from 4.7 to 8.1 mg GAE/g dw using ultrasound-assisted extraction, leading to comparable results with our study. It should be noted that by using the same extraction solvent (i.e., water) but employing a different extraction method which was more simple, we were able to achieve a more effective extraction of polyphenols. This outcome could be attributed to the process of optimizing the extraction conditions. In a similar study, Izzo et al. [54] studied the polyphenolic compounds of four commercial C. sativa inflorescence methanolic extracts. They averaged ~30 mg GAE/g, a value higher than we found. The hemp cultivar and extraction solvent could explain these differences in polyphenol recovery. However, the measured individual non-cannabinoid polyphenols were significantly lower than our values, as they averaged ~0.015 mg/g of luteolin-7-glucoside, ~0.015 mg/g of kaempferol-3-glucoside, and 0.023 mg/g of ferulic acid. Regarding TCC, Irakli et al. [4] investigated the antioxidant properties of seven different industrial cultivars of defatted hemp seeds. The concentration of the total carotenoids (expressed as the sum of β-carotene and zeaxanthin) ranged from 14 to 43 μg/g dw, whereas our optimal extract yielded 356.98 μg CtE/g. Specifically, methanolic hemp seed extract of Finola var. was found to have 29 μg/g of total carotenoids, indicating a significantly lower value compared to hemp leaf extract.
4. Conclusions
The purpose of this study was to examine the best extraction method for bioactive components recovered from C. sativa by thoroughly investigating and optimizing various conditions. Water, a high-polarity environmentally friendly solvent, was used for the experiments. In contrast to the PLS analysis, which revealed which parameters had a major impact on the extraction, the RSM enabled the correct adjustment of the extraction parameters. Reducing the extraction time and increasing the liquid–solid ratio were determined to have a positive impact on the effectiveness. The extraction process was determined to be quite unaffected by the temperature. Overall, a combination of the liquid–solid ratio (50:1), extraction time (30 min), and temperature (50 °C) were found to be the most preferable for maximum polyphenol recovery (9.76 mg GAE/g). On the other hand, the suitable adjustment of the parameters would lead to extracts with a high anti-inflammatory effect (75%). However, a negative correlation between the total individual polyphenols and anti-inflammatory activity was revealed through the use of MCA and PCA. The findings of the study highlight the potential of C. sativa to supply the pharmaceutical and food sectors with bioactive compounds that improve health, owing to the high added-value of the water extracts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriengineering6020075/s1, Table S1 presents the model validation statistics using the k-fold cross-validation technique. Figures S1–S4 comprise the plots that illustrate the comparison between the actual response and the predicted response for each parameter under examination, accompanied by the desirability functions. Figures S5–S7 present the three-dimensional response plots for the remaining responses.
Author Contributions
Conceptualization, V.A., T.C. and S.I.L.; methodology, V.A., T.C. and S.I.L.; software, V.A. and T.C.; validation, V.A., T.C., D.K., I.M. and E.B.; formal analysis, V.A. and T.C.; investigation, V.A. and T.C.; resources, S.I.L.; data curation, V.A., T.C. and S.I.L.; writing—original draft preparation, V.A. and D.K.; writing—review and editing, V.A., T.C., D.K., I.M., E.B. and S.I.L.; visualization, V.A. and T.C.; supervision, S.I.L.; project administration, S.I.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding author.
Acknowledgments
The authors would like to thank the CBD Extraction Ι.Κ.Ε. (Farsala, Greece) for donating hemp (Cannabis sativa var. Finola) leaf material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Karas, J.A.; Wong, L.J.M.; Paulin, O.K.A.; Mazeh, A.C.; Hussein, M.H.; Li, J.; Velkov, T. The Antimicrobial Activity of Cannabinoids. Antibiotics 2020, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- McPartland, J.M.; Guy, G.W.; Hegman, W. Cannabis Is Indigenous to Europe and Cultivation Began during the Copper or Bronze Age: A Probabilistic Synthesis of Fossil Pollen Studies. Veg. Hist. Archaeobotany 2018, 27, 635–648. [Google Scholar] [CrossRef]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The Seed of Industrial Hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Tsaliki, E.; Kalivas, A.; Kleisiaris, F.; Sarrou, E.; Cook, C.M. Effect οf Genotype and Growing Year on the Nutritional, Phytochemical, and Antioxidant Properties of Industrial Hemp (Cannabis sativa L.) Seeds. Antioxidants 2019, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- André, A.; Leupin, M.; Kneubühl, M.; Pedan, V.; Chetschik, I. Evolution of the Polyphenol and Terpene Content, Antioxidant Activity and Plant Morphology of Eight Different Fiber-Type Cultivars of Cannabis sativa L. Cultivated at Three Sowing Densities. Plants 2020, 9, 1740. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Byrne, C.D. Omega-3 Fatty Acids, Hepatic Lipid Metabolism, and Nonalcoholic Fatty Liver Disease. Annu. Rev. Nutr. 2013, 33, 231–248. [Google Scholar] [CrossRef]
- Da Porto, C.; Decorti, D.; Tubaro, F. Fatty Acid Composition and Oxidation Stability of Hemp (Cannabis sativa L.) Seed Oil Extracted by Supercritical Carbon Dioxide. Ind. Crops Prod. 2012, 36, 401–404. [Google Scholar] [CrossRef]
- Kaul, N.; Kreml, R.; Austria, J.A.; Richard, M.N.; Edel, A.L.; Dibrov, E.; Hirono, S.; Zettler, M.E.; Pierce, G.N. A Comparison of Fish Oil, Flaxseed Oil and Hempseed Oil Supplementation on Selected Parameters of Cardiovascular Health in Healthy Volunteers. J. Am. Coll. Nutr. 2008, 27, 51–58. [Google Scholar] [CrossRef]
- Florensa-Zanuy, E.; Garro-Martínez, E.; Adell, A.; Castro, E.; Díaz, Á.; Pazos, Á.; Mac-Dowell, K.S.; Martín-Hernández, D.; Pilar-Cuéllar, F. Cannabidiol Antidepressant-like Effect in the Lipopolysaccharide Model in Mice: Modulation of Inflammatory Pathways. Biochem. Pharmacol. 2021, 185, 114433. [Google Scholar] [CrossRef]
- Vieira, G.; Cavalli, J.; Gonçalves, E.C.D.; Braga, S.F.P.; Ferreira, R.S.; Santos, A.R.S.; Cola, M.; Raposo, N.R.B.; Capasso, R.; Dutra, R.C. Antidepressant-Like Effect of Terpineol in an Inflammatory Model of Depression: Involvement of the Cannabinoid System and D2 Dopamine Receptor. Biomolecules 2020, 10, 792. [Google Scholar] [CrossRef]
- Jha, N.K.; Sharma, C.; Hashiesh, H.M.; Arunachalam, S.; Meeran, M.N.; Javed, H.; Patil, C.R.; Goyal, S.N.; Ojha, S. β-Caryophyllene, A Natural Dietary CB2 Receptor Selective Cannabinoid Can Be a Candidate to Target the Trinity of Infection, Immunity, and Inflammation in COVID-19. Front. Pharmacol. 2021, 12, 590201. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Mousavi Khaneghah, A.; Gavahian, M.; Marszałek, K.; Eş, I.; Munekata, P.E.S.; Ferreira, I.C.F.R.; Barba, F.J. Understanding the Potential Benefits of Thyme and Its Derived Products for Food Industry and Consumer Health: From Extraction of Value-Added Compounds to the Evaluation of Bioaccessibility, Bioavailability, Anti-Inflammatory, and Antimicrobial Activities. Crit. Rev. Food Sci. Nutr. 2019, 59, 2879–2895. [Google Scholar] [CrossRef]
- Sharma, P.; Vishvakarma, R.; Gautam, K.; Vimal, A.; Kumar Gaur, V.; Farooqui, A.; Varjani, S.; Younis, K. Valorization of Citrus Peel Waste for the Sustainable Production of Value-Added Products. Bioresour. Technol. 2022, 351, 127064. [Google Scholar] [CrossRef]
- Koski, A.; Pekkarinen, S.; Hopia, A.; Wähälä, K.; Heinonen, M. Processing of Rapeseed Oil: Effects on Sinapic Acid Derivative Content and Oxidative Stability. Eur. Food Res. Technol. 2003, 217, 110–114. [Google Scholar] [CrossRef]
- Quiles, J.L.; Ramírez-Tortosa, M.C.; Gómez, J.A.; Huertas, J.R.; Mataix, J. Role of Vitamin E and Phenolic Compounds in the Antioxidant Capacity, Measured by ESR, of Virgin Olive, Olive and Sunflower Oils after Frying. Food Chem. 2002, 76, 461–468. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660. [Google Scholar] [CrossRef]
- Rea, K.A.; Casaretto, J.A.; Al-Abdul-Wahid, M.S.; Sukumaran, A.; Geddes-McAlister, J.; Rothstein, S.J.; Akhtar, T.A. Biosynthesis of Cannflavins A and B from Cannabis sativa L. Phytochemistry 2019, 164, 162–171. [Google Scholar] [CrossRef]
- Werz, O.; Seegers, J.; Schaible, A.M.; Weinigel, C.; Barz, D.; Koeberle, A.; Allegrone, G.; Pollastro, F.; Zampieri, L.; Grassi, G.; et al. Cannflavins from Hemp Sprouts, a Novel Cannabinoid-Free Hemp Food Product, Target Microsomal Prostaglandin E2 Synthase-1 and 5-Lipoxygenase. PharmaNutrition 2014, 2, 53–60. [Google Scholar] [CrossRef]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, Bioactivities, and Biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Hourfane, S.; Mechqoq, H.; Bekkali, A.Y.; Rocha, J.M.; El Aouad, N. A Comprehensive Review on Cannabis Sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities. Plants 2023, 12, 1245. [Google Scholar] [CrossRef]
- da Silva, R.F.; Carneiro, C.N.; do C. de Sousa, C.B.; J. V. Gomez, F.; Espino, M.; Boiteux, J.; de los Á. Fernández, M.; Silva, M.F.; de S. Dias, F. Sustainable Extraction Bioactive Compounds Procedures in Medicinal Plants Based on the Principles of Green Analytical Chemistry: A Review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- More, P.R.; Jambrak, A.R.; Arya, S.S. Green, Environment-Friendly and Sustainable Techniques for Extraction of Food Bioactive Compounds and Waste Valorization. Trends Food Sci. Technol. 2022, 128, 296–315. [Google Scholar] [CrossRef]
- Jurinjak Tušek, A.; Šamec, D.; Šalić, A. Modern Techniques for Flavonoid Extraction—To Optimize or Not to Optimize? Appl. Sci. 2022, 12, 11865. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Kalompatsios, D.; Athanasiadis, V.; Palaiogiannis, D.; Lalas, S.I.; Makris, D.P. Valorization of Waste Orange Peels: Aqueous Antioxidant Polyphenol Extraction as Affected by Organic Acid Addition. Beverages 2022, 8, 71. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Makrygiannis, I.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. An Investigation into Crithmum Maritimum L. Leaves as a Source of Antioxidant Polyphenols. Compounds 2023, 3, 532–551. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Bozinou, E.; Lalas, S.I. Response Surface Optimization for the Enhancement of the Extraction of Bioactive Compounds from Citrus limon Peel. Antioxidants 2023, 12, 1605. [Google Scholar] [CrossRef]
- Ayour, J.; Alahyane, A.; Harrak, H.; Neffa, M.; Taourirte, M.; Benichou, M. Assessment of Nutritional, Technological, and Commercial Apricot Quality Criteria of the Moroccan Cultivar “Maoui” Compared to Introduced Spanish Cultivars “Canino” and “Delpatriarca” towards Suitable Valorization. J. Food Qual. 2021, 2021, e6679128. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Mantiniotou, M.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Investigation of the Polyphenol Recovery of Overripe Banana Peel Extract Utilizing Cloud Point Extraction. Eng 2023, 4, 3026–3038. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction Optimisation Using Water/Glycerol for the Efficient Recovery of Polyphenolic Antioxidants from Two Artemisia Species. Sep. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Kasouni, A.I.; Chatzimitakos, T.G.; Stalikas, C.D.; Trangas, T.; Papoudou-Bai, A.; Troganis, A.N. The Unexplored Wound Healing Activity of Urtica dioica L. Extract: An In Vitro and In Vivo Study. Molecules 2021, 26, 6248. [Google Scholar] [CrossRef] [PubMed]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of Processing Effects on Anthocyanin Content and Colour Modifications of Blueberry (Vaccinium Spp.) Extracts: Comparison between HPLC-DAD and CIELAB Analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, J.S.F.; de Souza, E.L.; Oliveira, J.R.; Gomes, A.C.A.; Kotzebue, L.R.V.; da Silva Agostini, D.L.; de Oliveira, D.L.V.; Mazzetto, S.E.; da Silva, A.L.; Cavalcanti, M.T. Microencapsulation of Sweet Orange Essential Oil (Citrus aurantium Var. Dulcis) by Liophylization Using Maltodextrin and Maltodextrin/Gelatin Mixtures: Preparation, Characterization, Antimicrobial and Antioxidant Activities. Int. J. Biol. Macromol. 2020, 143, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Koraqi, H.; Petkoska, A.T.; Khalid, W.; Sehrish, A.; Ambreen, S.; Lorenzo, J.M. Optimization of the Extraction Conditions of Antioxidant Phenolic Compounds from Strawberry Fruits (Fragaria × ananassa Duch.) Using Response Surface Methodology. Food Anal. Methods 2023, 16, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Aazza, S. Application of Multivariate Optimization for Phenolic Compounds and Antioxidants Extraction from Moroccan Cannabis sativa Waste. J. Chem. 2021, 2021, e9738656. [Google Scholar] [CrossRef]
- Drinić, Z.; Vidović, S.; Vladić, J.; Koren, A.; Kiprovski, B.; Sikora, V. Effect of Extraction Solvent on Total Polyphenols Content and Antioxidant Activity of Cannabis sativa L. Lek. Sirovine 2018, 38, 17–21. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish; Marwal, A. Vital Roles of Carotenoids in Plants and Humans to Deteriorate Stress with Its Structure, Biosynthesis, Metabolic Engineering and Functional Aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Botta, B.; Quaglio, D.; Ghirga, F.; Balducci, S.; Cammarone, S.; Campiglia, E.; Giusti, A.M.; et al. A Multimethodological Characterization of Cannabis sativa L. Inflorescences from Seven Dioecious Cultivars Grown in Italy: The Effect of Different Harvesting Stages. Molecules 2021, 26, 2912. [Google Scholar] [CrossRef]
- Thoo, Y.; Ng, S.Y.; Khoo, M.; Mustapha, W.; Ho, C. A Binary Solvent Extraction System for Phenolic Antioxidants and Its Application to the Estimation of Antioxidant Capacity in Andrographis paniculata Extracts. Int. Food Res. J. 2013, 20, 1103–1111. [Google Scholar]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green Extraction of Bioactive Compounds from Plant Biomass and Their Application in Meat as Natural Antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Hanczné, E.L.; Kapcsándi, V.; Ajtony, Z. Conventional and Nonconventional Extraction Techniques for Optimal Extraction Processes of Rosmarinic Acid from Six Lamiaceae Plants as Determined by HPLC-DAD Measurement. J. Pharm. Biomed. Anal. 2020, 184, 113173. [Google Scholar] [CrossRef]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of Techniques and Solvents on the Antimicrobial and Antioxidant Potential of Extracts from Acacia Dealbata and Olea Europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, O.; Pinho, S.P. Solubility of Flavonoids in Pure Solvents. Ind. Eng. Chem. Res. 2012, 51, 6586–6590. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of Extraction Time, Temperature and Solvent on Concentration and Antioxidant Activity of Grape Marc Phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Oxygen Radical Absorbance Capacities of Grape/Wine Industry Byproducts and Effect of Solvent Type on Extraction of Grape Seed Polyphenols. J. Food Compos. Anal. 2006, 19, 41–48. [Google Scholar] [CrossRef]
- Lapornik, B.; Prošek, M.; Golc Wondra, A. Comparison of Extracts Prepared from Plant By-Products Using Different Solvents and Extraction Time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Ali Redha, A.; Salauddin, M.; Harahap, I.A.; Rupasinghe, H.P.V. Factors Affecting the Extraction of (Poly)Phenols from Natural Resources Using Deep Eutectic Solvents Combined with Ultrasound-Assisted Extraction. Crit. Rev. Anal. Chem. 2023, 1–22. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.N.T.; Tang Nguyen, V.; Van Vuong, Q.; Bowyer, M.C.; Scarlett, C.J. Bioactive Compound Yield and Antioxidant Capacity of Helicteres hirsuta Lour. Stem as Affected by Various Solvents and Drying Methods. J. Food Process. Preserv. 2017, 41, e12879. [Google Scholar] [CrossRef]
- Ferrante, C.; Recinella, L.; Ronci, M.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Di Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple Pharmacognostic Characterization on Hemp Commercial Cultivars: Focus on Inflorescence Water Extract Activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).