The Cultivation of Spirulina maxima in a Medium Supplemented with Leachate for the Production of Biocompounds: Phycocyanin, Carbohydrates, and Biochar
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum Production
2.2. Cultivation of S. maxima in Zarrouk’s Medium Modified with Leachate
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
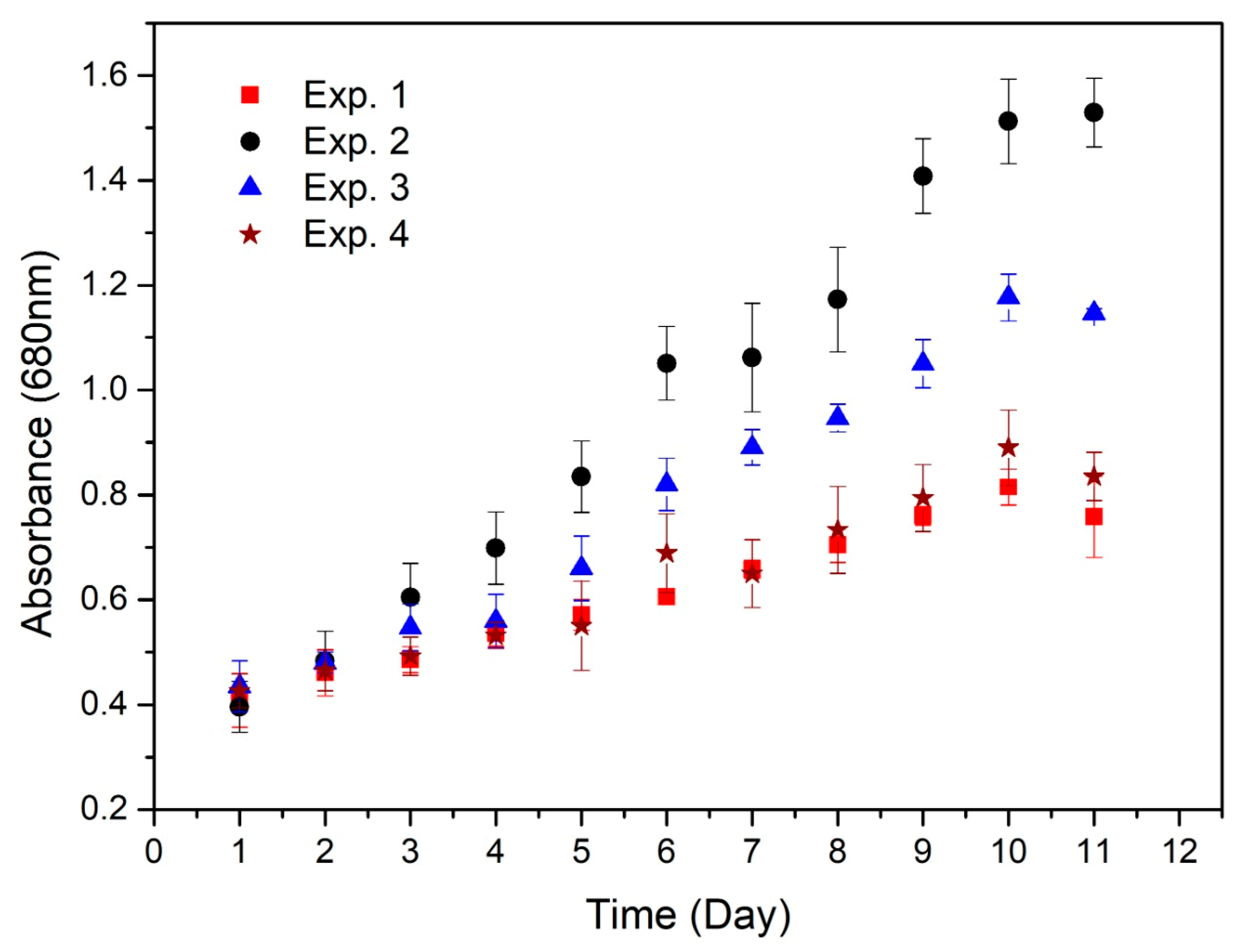
3.1. Development of Spirulina maxima in Medium Supplemented by a Leachate
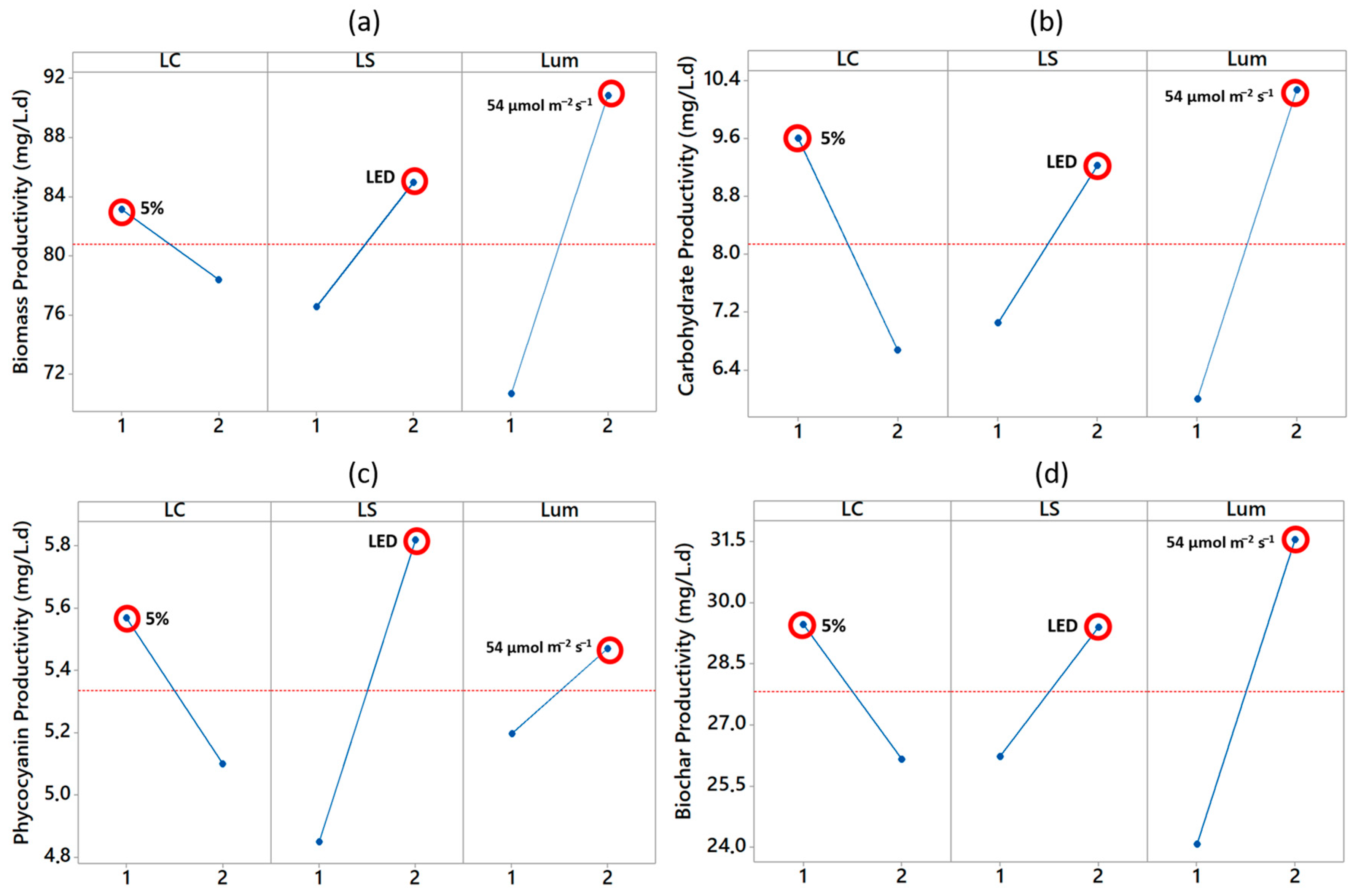
3.2. Factor Analysis of Productivities of Biomass, Carbohydrates, Phycocyanin, and Biochar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paiva, A.L.P.; Silva, D.G.d.F.; Couto, E. Recycling of landfill leachate nutrients from microalgae and potential applications for biomass valorization. J. Environ. Chem. Eng. 2021, 9, 105952. [Google Scholar] [CrossRef]
- Deviram, G.; Mathimani, T.; Anto, S.; Ahamed, T.S.; Ananth, D.A.; Pugazhendhi, A. Applications of microalgal and cyanobacterial biomass on a way to safe, cleaner and a sustainable environment. J. Clean. Prod. 2020, 253, 119770. [Google Scholar] [CrossRef]
- Astolfi, A.L.; Rempel, A.; Cavanhi, V.A.F.; Alves, M.; Deamici, K.M.; Colla, L.M.; Costa, J.A.V. Simultaneous saccharification and fermentation of Spirulina sp. and corn starch for the production of bioethanol and obtaining biopeptides with high antioxidant activity. Bioresour. Technol. 2020, 301, 122698. [Google Scholar] [CrossRef] [PubMed]
- Binda, G.; Spanu, D.; Bettinetti, R.; Magagnin, L.; Pozzi, A.; Dossi, C. Comprehensive comparison of microalgae-derived biochar from different feedstocks: A prospective study for future environmental applications. Algal Res. 2020, 52, 102103. [Google Scholar] [CrossRef]
- Simão, B.L.; Júnior, J.A.S.; Chagas, B.M.; Cardoso, C.R.; Ataíde, C.H. Pyrolysis of Spirulina maxima: Kinetic modeling and selectivity for aromatic hydrocarbons. Algal Res. 2018, 32, 221–232. [Google Scholar] [CrossRef]
- Akmukhanova, N.R.; Leong, Y.K.; Seiilbek, S.N.; Konysbay, A.; Zayadan, B.K.; Sadvakasova, A.K.; Sarsekeyeva, F.K.; Bauenova, M.O.; Bolatkhan, K.; Alharby, H.F.; et al. Eco-friendly biopesticides derived from CO2-Fixing cyanobacteria. Environ. Res. 2023, 239, 117419. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Marín, M.d.C.; López-Lozano, A.; Moreno-Cabezuelo, J.; Díez, J.; García-Fernández, J.M. Mixotrophy in cyanobacteria. Curr. Opin. Microbiol. 2024, 78, 102432. [Google Scholar] [CrossRef] [PubMed]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M. Treatment of Wastewaters by Microalgae and the Potential Applications of the Produced Biomass—A Review. Water 2021, 13, 27. [Google Scholar] [CrossRef]
- Iamtham, S.; Sornchai, P. Biofixation of CO2 from a power plant through large-scale cultivation of Spirulina maxima. S. Afr. J. Bot. 2022, 147, 840–851. [Google Scholar] [CrossRef]
- Ragaza, J.A.; Hossain, S.; Meiler, K.A.; Velasquez, S.F.; Kumar, V. A review on Spirulina: Alternative media for cultivation and nutritive value as an aquafeed. Rev. Aquac. 2018, 12, 2371–2395. [Google Scholar] [CrossRef]
- García-López, D.; Olguín, E.; González-Portela, R.; Sánchez-Galván, G.; De Philippis, R.; Lovitt, R.; Llewellyn, C.; Fuentes-Grünewald, C.; Saldívar, R.P. A novel two-phase bioprocess for the production of Arthrospira (Spirulina) maxima LJGR1 at pilot plant scale during different seasons and for phycocyanin induction under controlled conditions. Bioresour. Technol. 2020, 298, 122548. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.G.; Lombardi, A.T.; Silva, J.S.d.J.; Lemos, P.V.F.; Costa, J.A.V.; de Souza, C.O.; Druzian, J.I.; Chinalia, F.A. Scaling-up production of Spirulina sp. LEB18 grown in aquaculture wastewater. Aquaculture 2021, 544, 737045. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Economou, C.N.; Markou, G.; Nicodemou, A.; Koutinas, M.; Tekerlekopoulou, A.G.; Vayenas, D.V. Cultivation of Arthrospira platensis in Brewery Wastewater. Water 2022, 14, 1547. [Google Scholar] [CrossRef]
- Rempel, A.; Gutkoski, J.P.; Biolchi, G.N.; Biduski, B.; Hoff, R.B.; Perin, M.; Treichel, H.; Colla, L.M. Microalgae growth using treated domestic effluent added to emerging pollutants: Removal mechanism and generation of by products. J. Water Process. Eng. 2023, 55, 11. [Google Scholar] [CrossRef]
- Vernaz, J.E. Fate and Transport of Mercury, Cadmium, Selenium, and Arsenic in the Presence of Growing Spirulina maxima. Ph.D. Thesis, University of Nevada, Reno, NV, USA, 2022. [Google Scholar]
- Andrade, B.B.; Cardoso, L.G.; Assis, D.d.J.; Costa, J.A.V.; Druzian, J.I.; Lima, S.T.D.C. Production and characterization of Spirulina sp. LEB 18 cultured in reused Zarrouk’s medium in a raceway-type bioreactor. Bioresour. Technol. 2019, 284, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, C. Contribution à l´étude d´une Cyanophycée Influence de Divers Facteurs Physiques et Chimiques sur la Croissance et la Photosynthése de Spirulina maxima (Setch et Gardner) Geitler; University of Paris: Paris, France, 1966. [Google Scholar]
- Taguchi, G.; Rafanelli, A.J. Taguchi on Robust Technology Development: Bringing Quality Engineering Upstream. J. Electron. Packag. 1994, 116, 2. [Google Scholar] [CrossRef][Green Version]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Moxley, G.; Zhang, Y.-H.P. More accurate determination of acid-labile carbohydrates in lignocellulose by modified quantitative saccharification. Energy Fuels 2007, 21, 3684–3688. [Google Scholar] [CrossRef]
- Chaiwong, K.; Kiatsiriroat, T.; Vorayos, N.; Thararax, C. Study of bio-oil and bio-char production from algae by slow pyrolysis. Biomass Bioenergy 2013, 56, 600–606. [Google Scholar] [CrossRef]
- Liew, L.W.; Bashir, M.J.; Toh, P.Y.; Alazaiza, M.Y.; Abu Amr, S.S.; Khoo, K.S.; Raksasat, R.; Lim, J.W. Microalgae cultivation in stabilized landfill leachate for simultaneous treatment and biomass production. J. Taiwan Inst. Chem. Eng. 2023, 105068. [Google Scholar] [CrossRef]
- Song, H.; Qian, J.; Fan, L.; Toda, T.; Li, H.; Sekine, M.; Song, P.; Takayama, Y.; Koga, S.; Li, J.; et al. Enhancing biomass yield, nutrient removal, and decolorization from soy sauce wastewater using an algae-fungus consortium. Algal Res. 2022, 68, 102878. [Google Scholar] [CrossRef]
- Gomes, I.G.R.F.; Andrade, F.R.D.N.; Facundo, G.d.M.; Marques, C.H.P.; Albuquerque, L.F.G.; Silva, J.W.A.; Maciel, R.L.; Matias, J.F.N.; Oliveira, E.G.; Costa, F.H.F. Evaluation of phycocyanin production by marine microalgae Arthrospira platensis, grown in fish wastewater. Braz. J. Dev. 2020, 6, 20298–20317. [Google Scholar] [CrossRef]
- Milia, M.; Corrias, F.; Addis, P.; Chini Zitelli, G.; Cicchi, B.; Torzillo, G.; Andreotti, V.; Angioni, A. Influence of Different Light Sources on the Biochemical Composition of Arthrospira spp. Grown in Model Systems. Foods 2022, 11, 399. [Google Scholar] [CrossRef]
- Lima, G.M.; Teixeira, P.C.; Teixeira, C.M.; Filócomo, D.; Lage, C.L. Influence of spectral light quality on the pigment concentrations and biomass productivity of Arthrospira platensis. Algal Res. 2018, 31, 157–166. [Google Scholar] [CrossRef]
- Park, J.; Dinh, T.B. Contrasting effects of monochromatic LED lighting on growth, pigments and photosynthesis in the commercially important cyanobacterium Arthrospira maxima. Bioresour. Technol. 2019, 291, 121846. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.G.; Margarites, A.C.; Reinehr, C.O.; Gonçalves, G.C.; Rodigheri, G.; Costa, J.A.V.; Colla, L.M. Spirulina platensis biomass composition is influenced by the light availability and harvest phase in raceway ponds. Environ. Technol. 2018, 39, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.L.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.-H.; Salleh, M.A.M. Biochar production from microalgae cultivation through pyrolysis as a sustainable carbon sequestration and biorefinery approach. Clean Technol. Environ. Policy 2018, 20, 2047–2055. [Google Scholar] [CrossRef]
- Zhu, H.; Zou, H. Characterization of algae residue biochar and its application in methyl orange wastewater treatment. Water Sci. Technol. 2021, 84, 3716–3725. [Google Scholar] [CrossRef]
| Parameter | Concentration (mg L−1) |
|---|---|
| Al | 4.53 |
| P | 141.13 |
| Si | 3.27 |
| Se | 0.17 |
| Cr | 2.3 |
| Mg | 56.86 |
| Na | 4005.5 |
| K | 2084.5 |
| Zn | 1.2 |
| Ti | 0.8 |
| Fe | 6.16 |
| Ca | 36.76 |
| Sn | 1.14 |
| COD (mg L−1 O2) | 3565.0 |
| pH | 8.78 |
| Codified Factor | Factor | Levels | |
|---|---|---|---|
| 1 | 2 | ||
| A | Leachate concentration (% v/v) | 5 | 10 |
| B | Luminous source (lamp) | Fluorescent | Tubular LED |
| G | Luminosity (µmol m−2 s−1) | 13.5 | 54 |
| Factors | |||
|---|---|---|---|
| Experiment | A | B | C |
| 1 | 1 | 1 | 1 |
| 2 | 1 | 2 | 2 |
| 3 | 2 | 1 | 2 |
| 4 | 2 | 2 | 1 |
| Exp. | Factor | Productivity (mg L−1d−1) | |||||
|---|---|---|---|---|---|---|---|
| LC | LS | Lum | Biomass | Carbohydrates | Phycocyanin | Biochar | |
| 1 | 1 | 1 | 1 | 68.89 ± 2.83 | 6.38 ± 0.19 | 4.95 ± 0.60 | 24.16 ± 1.82 |
| 2 | 1 | 2 | 2 | 97.44 ± 3.20 | 12.82 ± 0.38 | 6.19 ± 1.54 | 34.79 ± 3.62 |
| 3 | 2 | 1 | 2 | 84.30 ± 2.08 | 7.73 ± 0.48 | 4.75 ± 0.08 | 28.32 ± 0.78 |
| 4 | 2 | 2 | 1 | 72.52 ± 2.33 | 5.63 ± 0.22 | 5.45 ± 0.97 | 24.02 ± 0.85 |
| Biomass | Carbohydrate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Factors | QSF | FD | AQSF | F | p | QSF | FD | AQSF | F | p |
| LC | 0.49174 | 1 | 0.49174 | 5.8988 | 0.041283 | 22.64121 | 1 | 22.64121 | 167.9292 | 0.000001 |
| LS | 2.18155 | 1 | 2.18155 | 26.1692 | 0.000912 | 8.25511 | 1 | 8.25511 | 61.2279 | 0.000051 |
| Lum | 14.01257 | 1 | 14.01257 | 168.0908 | 0.000001 | 58.06519 | 1 | 58.06519 | 430.6677 | 0.000000 |
| Residual | 0.66691 | 8 | 0.08336 | 1.07861 | 8 | 0.13483 | ||||
| Phycocyanin | Biochar | |||||||||
| Factors | QSF | FD | AQSF | F | p | QSF | FD | AQSF | F | p |
| LC | 0.919344 | 1 | 0.919344 | 0.438901 | 0.543877 | 1.63075 | 1 | 1.63075 | 4.64895 | 0.097309 |
| LS | 4.367091 | 1 | 4.367091 | 2.084882 | 0.222257 | 1.49474 | 1 | 1.49474 | 4.26120 | 0.107929 |
| Lum | 0.262902 | 1 | 0.262902 | 0.125511 | 0.741019 | 10.52729 | 1 | 10.52729 | 30.01120 | 0.005405 |
| Residual | 8.378589 | 4 | 2.094647 | 1.40311 | 4 | 0.35078 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, W.R.; da Silva, M.L.; Tagliaferro, G.V.; Ferreira, A.L.G.; Guimarães, D.H.P. The Cultivation of Spirulina maxima in a Medium Supplemented with Leachate for the Production of Biocompounds: Phycocyanin, Carbohydrates, and Biochar. AgriEngineering 2024, 6, 1289-1299. https://doi.org/10.3390/agriengineering6020074
dos Santos WR, da Silva ML, Tagliaferro GV, Ferreira ALG, Guimarães DHP. The Cultivation of Spirulina maxima in a Medium Supplemented with Leachate for the Production of Biocompounds: Phycocyanin, Carbohydrates, and Biochar. AgriEngineering. 2024; 6(2):1289-1299. https://doi.org/10.3390/agriengineering6020074
Chicago/Turabian Styledos Santos, Wallyson Ribeiro, Matheus Lopes da Silva, Geronimo Virginio Tagliaferro, Ana Lucia Gabas Ferreira, and Daniela Helena Pelegrine Guimarães. 2024. "The Cultivation of Spirulina maxima in a Medium Supplemented with Leachate for the Production of Biocompounds: Phycocyanin, Carbohydrates, and Biochar" AgriEngineering 6, no. 2: 1289-1299. https://doi.org/10.3390/agriengineering6020074
APA Styledos Santos, W. R., da Silva, M. L., Tagliaferro, G. V., Ferreira, A. L. G., & Guimarães, D. H. P. (2024). The Cultivation of Spirulina maxima in a Medium Supplemented with Leachate for the Production of Biocompounds: Phycocyanin, Carbohydrates, and Biochar. AgriEngineering, 6(2), 1289-1299. https://doi.org/10.3390/agriengineering6020074








