Natural Compounds and Derivates: Alternative Treatments to Reduce Post-Harvest Losses in Fruits
Abstract
1. Introduction
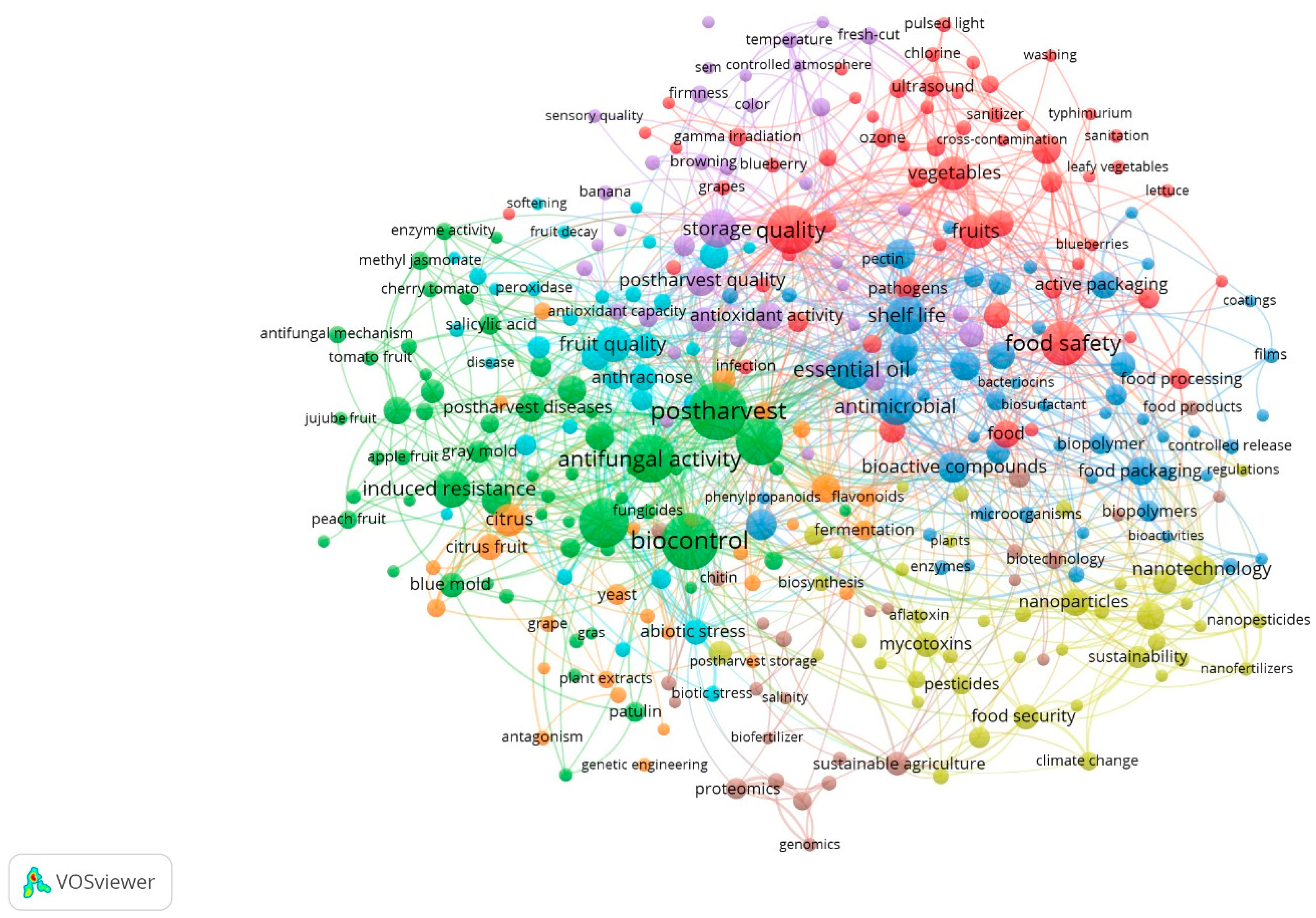
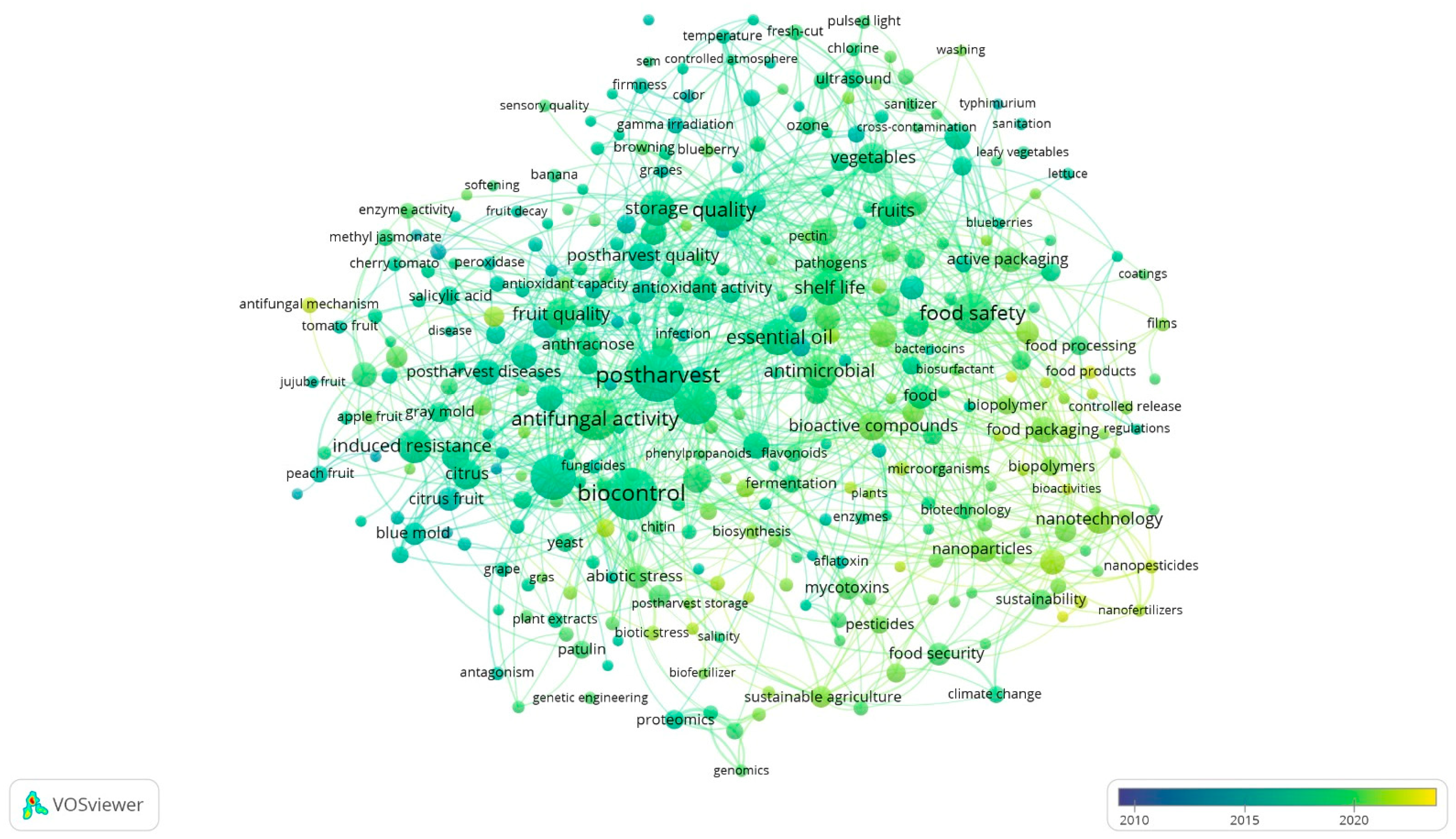
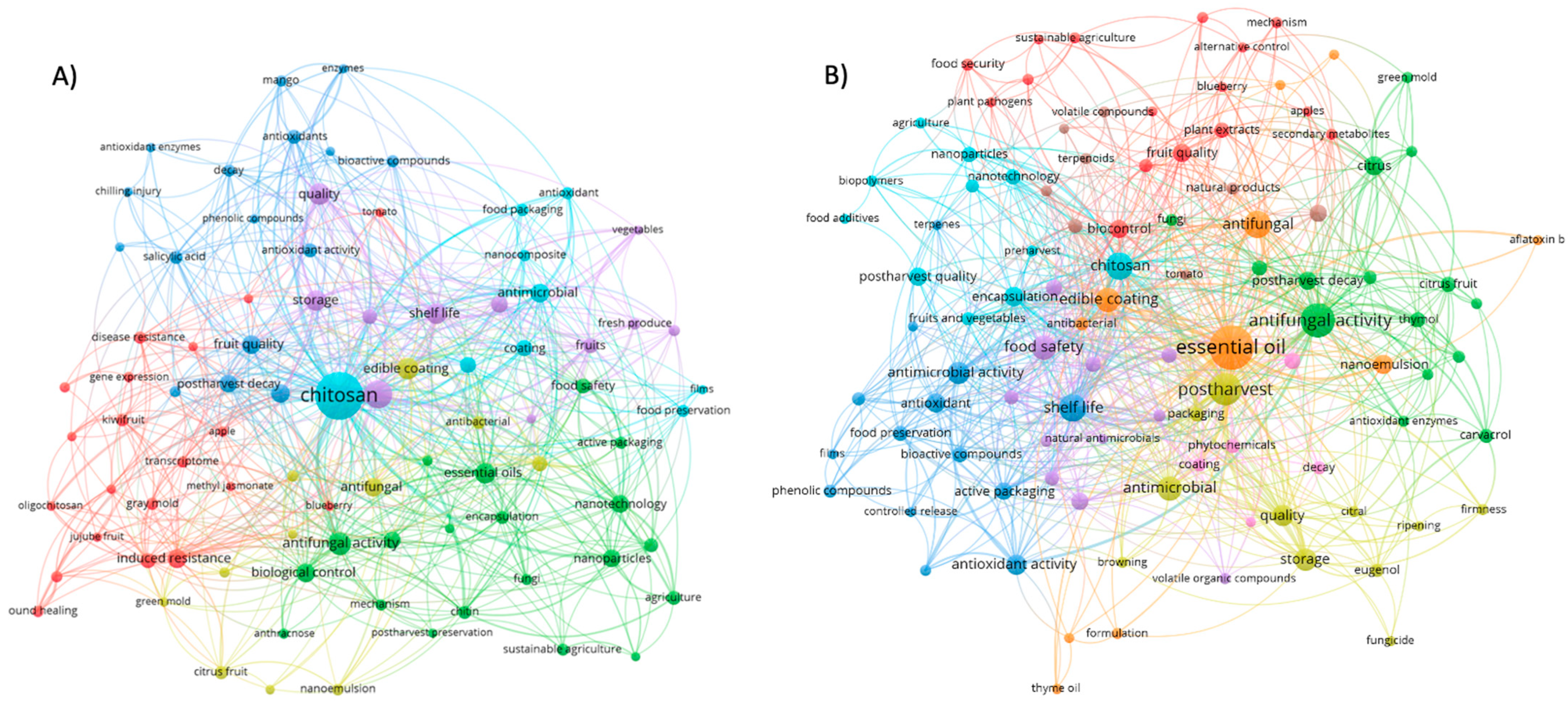
2. Bibliometric Analysis of Alternative Post-Harvest Treatments
2.1. Bibliometric Methodology in Data Collection
2.2. Findings from the Bibliometric Study of Contemporary Alternative Post-Harvest Treatments
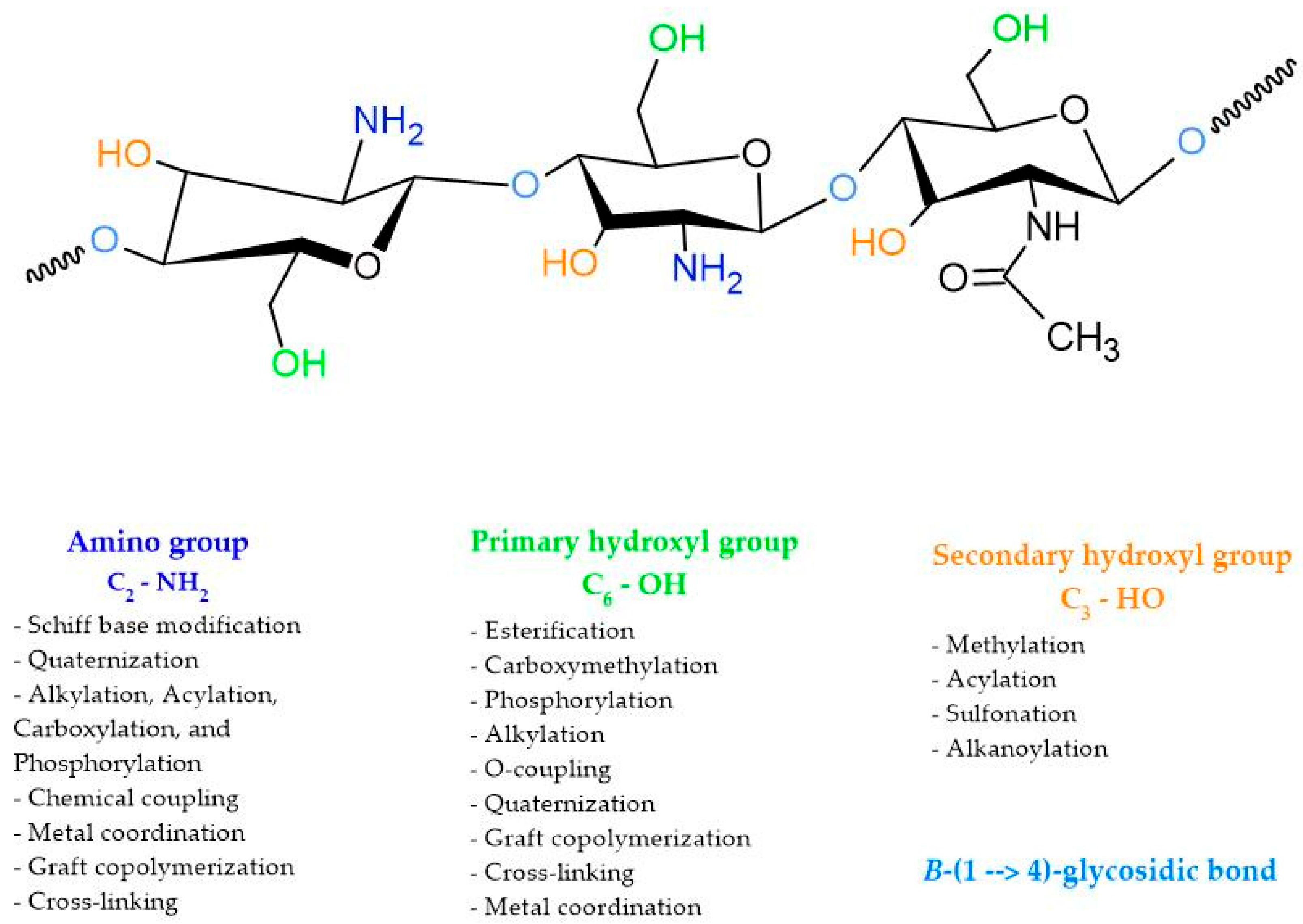
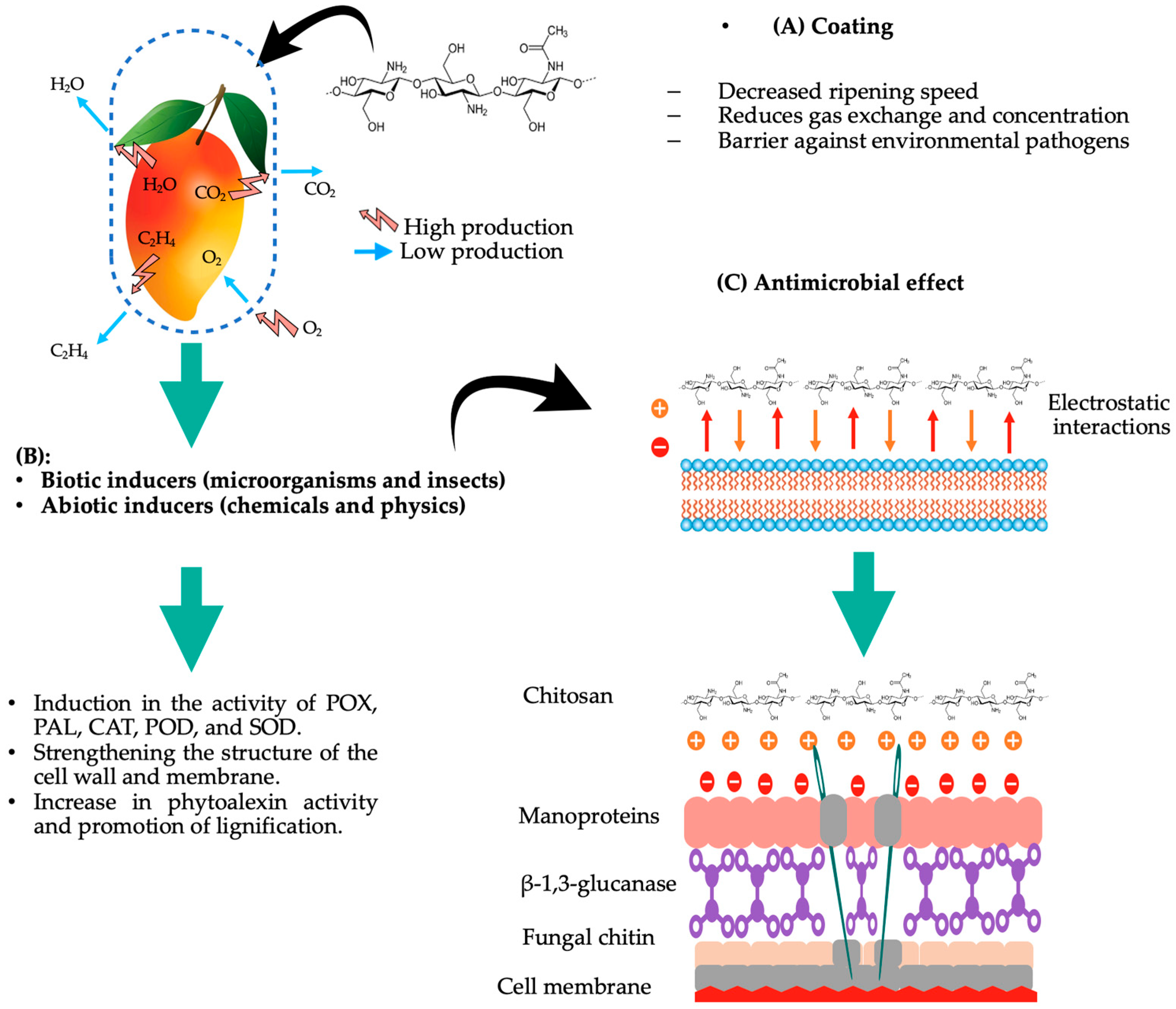
3. Chitosan, the Biopolymer with Multiple Properties
Effect of Chitosan against Phytopathogens in Fruits
4. Aromatic Compounds and Their Effects
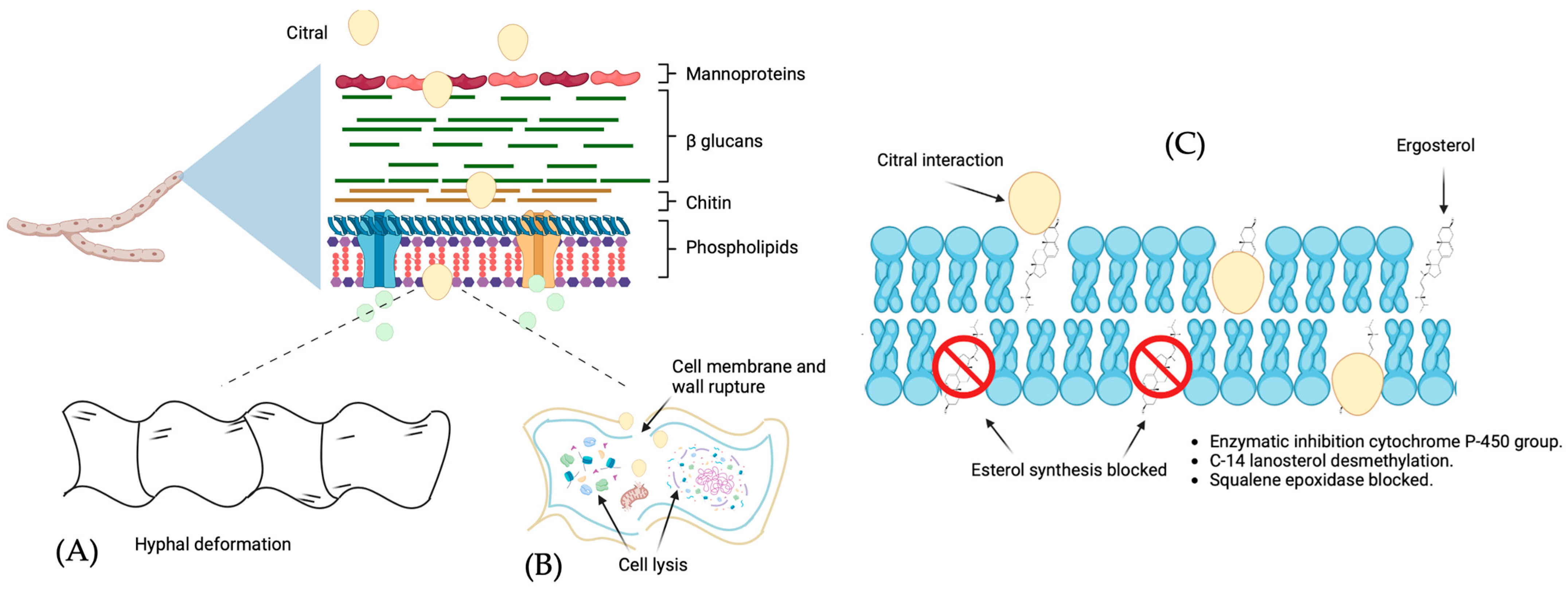
4.1. Citral’s Antifungal Properties and Mechanisms
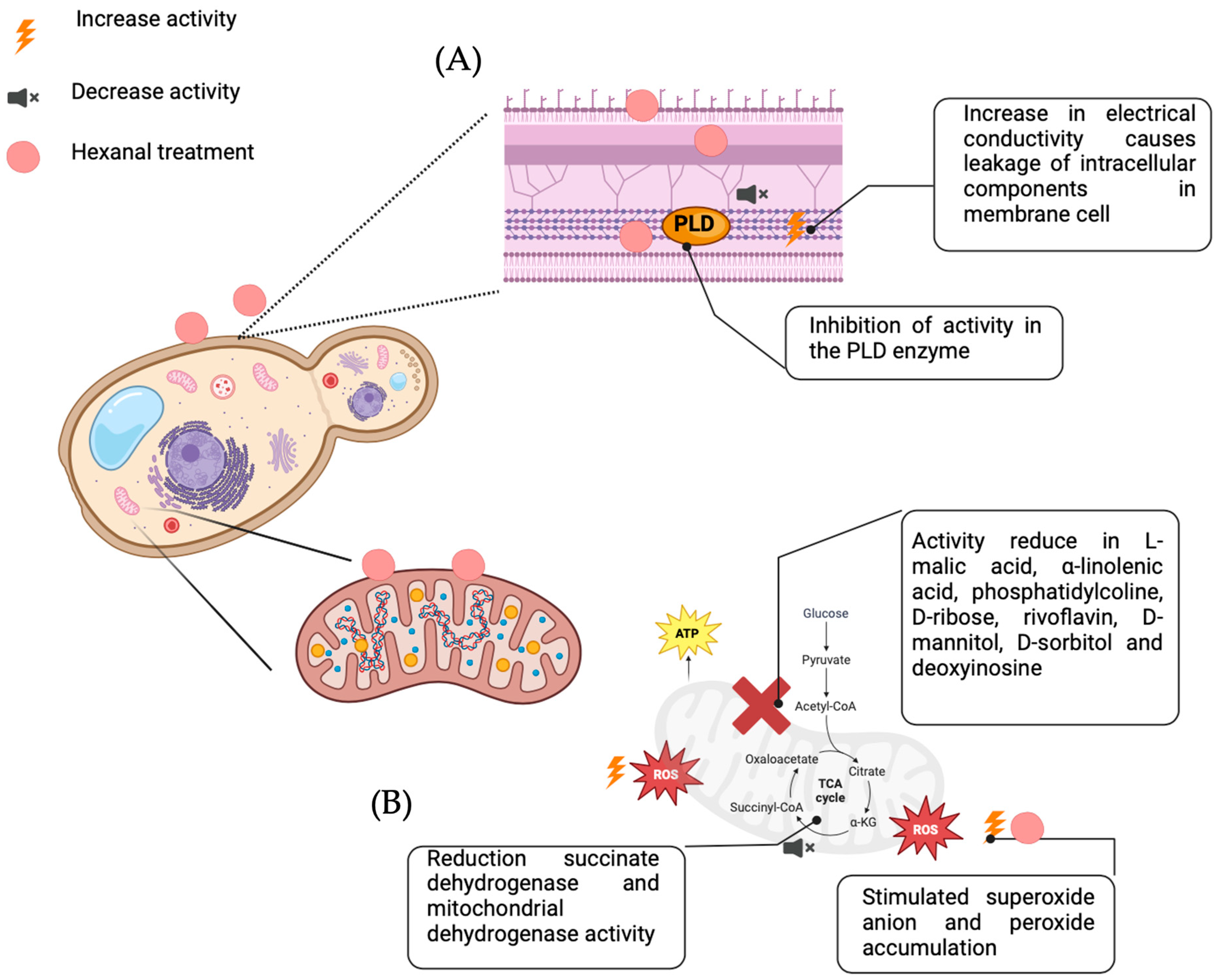
4.2. Hexanal’s Antifungal Properties and Mechanisms
| Application | Concentration | Effects | References |
|---|---|---|---|
| Pre- and post-harvest | 2–3% | Pre-harvest spray application in banana var. Grand Nain improves fruit retention by 12–18 days compared with the control. Post-harvest application decreases peroxidase activity and protein synthesis in the abscission zone, delayed the climacteric peak, and decreased the activity of the enzymes that convert stored carbs to soluble sugars. | [97] |
| Pre-harvest | 800–2000 µM | Hexanal applications to 1600 µM 30 and 15 days before harvesting reduce significantly the incidence of pathogens, pectin methyl-esterase activity, and the respiration rate and delay the activity of phospholipase-D of mango fruits, exhibiting an increase in firmness, total soluble solids, and acidity and acceptable palatability during 28 days at 12 °C in storage. | [98] |
| Post-harvest | 600–1200 ppm | Vapor application at 800 ppm reduces by 75–80% pathogen incidence; on the other hand, it increases peroxidase, polyphenol oxidase, phenylalanine ammonia-lyase, and glucanase activity and phospholipase-D inhibition of the main enzymes in the hydrolysis of phospholipids, thereby increasing the shelf life of fruits and contributing to the phenylpropanoid pathway´s induction of resistance in banana fruits against Colletotrichum gloeosporioides and Lasiodiplodia theobromae. | [92] |
| Post-harvest | 2.24–2.52 mg/mL | Hexanal concentration showed an inhibitory effect on the growth of Escherichia coli. The antimicrobial activity blocking the activity of superoxide dismutase and phospholipase-D inhibition and, combined with heat shock, provoked the overexpression of genes related to fimbria, curli, and biofilm regulation, suggesting that bacteria are induced to stress and are unable to induce biofilm formation in these conditions. | [99] |
| Pre-harvest | 0.02% | An application in apples evidenced that fruit retention and firmness improved, without showing an effect on parameters such as sugar contents and weight. Enzymes that break down the cell wall were less active after being sprayed with hexanal, such as polygalacturonase, glucanase, and gene expression, such expansins. In addition, the authors suggest that hexanal is involved in ethylene biosynthesis, decreasing the expression of four genes related to commercial maturity. | [94] |
| Post-harvest | 0.15, 0.20 y 0.25% | Suppression of cell wall degrading enzymes activity and maintenance of parameters such as firmness, total soluble solids’ content, carotenoids, and antioxidant activity on jujube fruits “Umran”. The antioxidant enzymes activity, such superoxide dismutase and peroxidase, led to a positively active increase in the commercial life of the fruits up to 21 days in cold storage. | [100] |
5. The Ability of Aldehydes and Chitosan to Improve Their Post-Harvest Mechanisms
6. Chitin Derivatives and Commercial Natural Compounds: Their Integration into Post-Harvest Management and Food Sovereignty
7. “Omics” Sciences and Their Participation in the Knowledge of New Defense Mechanisms and Their Interactions
8. Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Portal Fructícola. India Fortalece su Liderazgo en la Producción Mundial de Frutas y Hortalizas y Plantea Nuevos Desafiós. Available online: https://www.portalfruticola.com/noticias/2022/09/07/india-fortalece-su-liderazgo-en-la-produccion-mundial-de-frutas-y-hortalizas-y-plantea-nuevos-desafios-para-la-industria/ (accessed on 26 January 2023).
- Mena-Roa, M. China, el Mayor Productor de Fruta del Mundo. Available online: https://es.statista.com/grafico/30539/paises-con-el-mayor-volumen-de-produccion-de-fruta/ (accessed on 11 August 2023).
- Grand View Research. Fresh Fruit Market Size, Share & Trends Analysis Reports by Product (Apples & Pears, Bananas, Berries & Grapes, Citrus Fruits, Watermelon & Melon, Mangoes & Guava, Pinneapples), by Distribution Channel, by Region and Segment Forecasts 2022–2028; San Francisco. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/fresh-fruits-market-report (accessed on 17 August 2023).
- Secretaria de Agricultura y Desarrollo Rural (SAGARPA). ¿Qué es la Poscosecha y por qué es Importante? Blog. Available online: https://www.gob.mx/agricultura/es/articulos/que-es-la-poscosecha-y-por-que-es-importantePublicacionesRecientes (accessed on 2 October 2023).
- Naciones Unidas. La Agenda 2030 y Los Objetivos de Desarrollo Sostenible: Una Oportunidad Para América Latina y El Caribe; 18; 3; Santiago. 2018. Available online: www.issuu.com/publicacionescepal/stacks (accessed on 14 June 2023).
- FAO. Abordar Los Temas de Género En La Gestión de Plaguicidas; Roma. 2020. Available online: https://www.fao.org/3/cc0356es/cc0356es.pdf (accessed on 15 February 2023).
- Atanasovski, R.; El Desperdicio de Comida, una Oportunidad para Acabar con el Hambre. Naciones Unidas Noticias. Available online: https://news.un.org/es/story/2018/10/1443382 (accessed on 11 February 2023).
- Romero, J.; Albertos, I.; Díez-Méndez, A.; Poveda, J. Control of Postharvest Diseases in Berries through Edible Coatings and Bacterial Probiotics. Sci. Hortic. 2022, 304, 111326. [Google Scholar] [CrossRef]
- Mudaliar, K.; Sharma, V.; Agnihotri, C.; Agnihotri, S.; Deora, A.; Singh, B.P. Microbiological Impact and Control Strategies to Monitor Postharvest Losses in Fruits and Vegetables. In Postharvest Management of Fresh Produce: Recent Advances; Elsevier: Amsterdam, The Netherlands, 2023; pp. 113–147. [Google Scholar] [CrossRef]
- FAO. World Food and Agriculture–Statistical Yearbook 2022; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2022. [Google Scholar]
- Chatterjee, S.; Kuang, Y.; Splivallo, R.; Chatterjee, P.; Karlovsky, P. Interactions among filamentous fungi Aspergillus niger, Fusarium verticillioides and Clonostachys rosea: Fungal biomass, diversity of secreted metabolites and fumonisin production. BMC Microbiol. 2016, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Cordero, F.T.; Casado-Mármol, G.; Arenas-Arenas, F.J. Guía de Gestión Integrada de Enfermedades de Frutales de Hueso; Sevilla. 2016. Available online: https://www.juntadeandalucia.es/agriculturaypesca/ifapa/servifapa/registro-servifapa/8c9d036b-dc0e-4988-86a7-3f831c2d5220 (accessed on 21 September 2023).
- Valle, R.V.; Torres, L.R.R. Foliar Necrosis; New Symptom Associated to Root Rot of Pepper (Capsicum Annum) in Durango and Zacatecas, México. Sci. Fungorum 2018, 46, 47–53. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. Informe de La OMS Señala Que Los Niños Menores de 5 Años Representan Casi Un Tercio de Las Muertes Por Enfermedades de Transmisión Alimentaria; Ginebra. 2015. Available online: https://www.who.int/es/news/item/03-12-2015-who-s-first-ever-global-estimates-of-foodborne-diseases-find-children-under-5-account-for-almost-one-third-of-deaths (accessed on 3 March 2023).
- Shahbaz, M.; Akram, A.; Raja, N.I.; Mukhtar, T.; Mashwani, Z.U.; Mehak, A.; Fatima, N.; Sarwar, S.; Haq, E.U.; Yousaf, T. Green Synthesis and Characterization of Selenium Nanoparticles and Its Application in. Plant Disease Management: A Review. Pak. J. Phytopathol. 2022, 34, 189–202. [Google Scholar] [CrossRef]
- Gupta, T.; Saxena, J. Biogenic Synthesis of Silver Nanoparticles from Aspergillus Oryzae MTCC 3107 against Plant Pathogenic Fungi Sclerotinia Sclerotiorum MTCC 8785. J. Microbiol. Biotechnol. Food Sci. 2023, 12, e9387. [Google Scholar] [CrossRef]
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A Review of Recent Innovative Strategies for Controlling Mycotoxins in Foods. Food Control. 2023, 144. [Google Scholar] [CrossRef]
- Hollomon, D.W. Fungicide Resistance: 40 Years on and Still a Major Problem. In Fungicide Resistance in Plant Pathogens; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. [Google Scholar] [CrossRef]
- Hollomon, D.W. Fungicide resistance: Facing the challenge—A review. Plant Prot. Sci. 2015, 51, 170–176. [Google Scholar] [CrossRef]
- De Miccolis Angelini, R.M.; Pollastro, S.; Faretra, F. Genetics of Fungicide Resistance. In Fungicide Resistance in Plant Pathogens; Springer: Berlin/Heidelberg, Germany, 2015; pp. 13–34. [Google Scholar] [CrossRef]
- Flores-López, M.L.; Cerqueira, M.A.; de Rodríguez, D.J.; Vicente, A.A. Perspectives on Utilization of Edible Coatings and Nano-laminate Coatings for Extension of Postharvest Storage of Fruits and Vegetables. Food Eng. Rev. 2016, 8, 292–305. [Google Scholar] [CrossRef]
- Palou, L. Postharvest Treatments with GRAS Salts to Control Fresh Fruit Decay. Horticulturae 2018, 4, 46. [Google Scholar] [CrossRef]
- López, E.; Aguirre, L.M. La ciencia de la ciencia. Rev. Digit. Univ. 2018, 19, 1–7. [Google Scholar] [CrossRef]
- Devos, P. Research and bibliometrics: A long history. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Romaní, F.; Huamaní, C.; González-Alcalde, G. Estudios Bibliométricos Como Linea de Investigacion En Las Ciencias Biomédicas: Una Aproximación Para El Pregrado. CIMEL Cienc. Investig. Médica Estud. Latinoam. 2011, 16, 52–62. [Google Scholar]
- Jia, C.; Mustafa, H. A Bibliometric Analysis and Review of Nudge Research Using VOSviewer. Behav. Sci. 2023, 13, 19. [Google Scholar] [CrossRef]
- Cáceres-Zambrano, J.; Jiménez-Hernández, C.N.; Barrios, D. Tendencias en Investigación y Desarrollo Tecnológico en la Cadena Productiva de Aguacate (Persea americana L.). Rev. EIA 2022, 19, 3826. [Google Scholar] [CrossRef]
- López-Muñoz, F.; García-Perez, A.; Cárdenas, V.O.; Meramo, S.; Ricardez-Sandoval, L.; González-Delgado, D.; León-Pulido, J.; Mainardi, D. A Bibliometric Study of Chitosan Applications: Insights from Processes. Rev. ION 2023, 36, 59–78. [Google Scholar] [CrossRef]
- Espinosa-Cavazos, K.G.; Sáenz-Galindo, A.; Castañeda-Facio, A.O. Películas de Quitosano Propiedades y Aplicaciones. Afinidad 2020, 77, 203–208. [Google Scholar]
- Sotelo-Boyás, M.E.; Valverde-Aguilar, G.; Plascencia-Jatomea, M.; Correa-Pacheco, Z.N.; Jiménez-Aparicio, A.; Solorza-Feria, J.; Barrera-Necha, L.; Bautista-Baños, S. Characterization of Chitosan Nanoparticles Added with Essential Oils. In Vitro Effect on Pectobacterium Carotovorum. Rev. Mex. Ing. Quim. 2015, 14, 589–599. [Google Scholar]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Nacional, I.P.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.; Corona-Rangel, M. LChitosan: A Versatile Antimicrobial Polysaccharide for Fruit and Vegetables in Postharves—A Review. Rev. Chapingo Ser. Hortic. 2017, XXIII, 103–121. [Google Scholar] [CrossRef]
- Torres-Rodriguez, J.A.; Reyes-Pérez, J.J.; Castellanos, T.; Angulo, C.; Quiñones-Aguilar, E.E.; Hernandez-Montiel, L.G. A Biopolymer with Antimicrobial Properties and Plant Resistance Inducer against Phyto-pathogens: Chitosan. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12231. [Google Scholar] [CrossRef]
- Arias, M.J.L.; López, A.; Vilaseca, M.; Vallès, B.; Prieto, R.; Simó, M.; Valle, J.A.B.; Valle, R.D.C.S.C.; Bezerra, F.M.; Bellalta, J.P. Influence of Chitosan Characteristics in the Microencapsulation of Essential Oils. J. Biomed. Sci. Eng. 2021, 14, 119–129. [Google Scholar] [CrossRef]
- Moreno-Hernández, C.; de Tepic, I.T.; Zambrano-Zaragoza, M.; Velázquez-Estrada, R.; Sánchez-Burgos, J.; Gutiérrez-Martinez, P. Identification of a Colletotrichum Species from Mango Fruit and Its In Vitro Control by GRAS Compounds. Rev. Mex. Ing. Quim. 2022, 21, 1–18. [Google Scholar] [CrossRef]
- Heras-Mozos, R. Envases Activos Basados en el Anclaje Covalente Reversible de Compuestos Antimicrobianos en Quitosano. Ph.D. Thesis, Universitat Politècnica de Valencia, Valencia, Spain, 2022. [Google Scholar]
- Luangapai, F.; Peanparkdee, M.; Iwamoto, S. Biopolymer Films for Food Industries: Properties, Applications, and Future Aspects Based on Chitosan. Rev. Agric. Sci. 2019, 7, 59–67. [Google Scholar] [CrossRef]
- Gutiérrez-Martínez, P.; Ramos-Guerrero, A.; Rodríguez-Pereida, C.; Coronado-Partida, L.D.; Angulo-Parra, J.; González-Estrada, R. Chitosan for Postharvest Disinfection of Fruits and Vegetables. In Postharvest Disinfection of Fruits and Vegetables; Siddiqui, M.W., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 231–241. [Google Scholar]
- Herrera-González, J.A.; Bautista-Baños, S.; Serrano, M.; Romanazzi, G.; Gutiérrez-Martínez, P. Non-Chemical Treatments for the Pre- and Post-Harvest Elicitation of Defense Mechanisms in the Fungi–Avocado Pathosystem. Molecules 2021, 26, 6819. [Google Scholar] [CrossRef] [PubMed]
- Porras, G.; Calvo, M.; Esquivel, M.; Sibaja, M.; Madrigal-Carballo, S. Quitosano N-Acilado Con Cinamaldehído, Un Potencial Bioplaguicida Contra Agentes Patógenos En El Campo Agrícola. Rev. Iberoam. Polímeros 2009, 10, 197–206. [Google Scholar]
- Gutiérrez-Martínez, P.; Ledezma-Morales, A.; Romero-Islas, L.d.C.; Ramos-Guerrero, A.; Romero-Islas, J.; Rodríguez-Pereida, C.; Casas-Junco, P.; Coronado-Partida, L.D.; González-Estrada, R. Antifungal Activity of Chitosan against Postharvest Fungi of Tropical and Subtropical Fruits. In Chitin-Chitosan, Myriad Functionalities in Science and Technology; Dongre, R., Ed.; IntechOpen, Ltd.: London, UK, 2018; pp. 171–199. [Google Scholar] [CrossRef]
- Perdones-Montero, Á. Antifungal Chitosan-Based Films and Coatings Containing Essential Oils for Fruit Applications. Ph.D. Thesis, Universitat Politecnica de Valencia, Valencia, Spain, 2015. [Google Scholar]
- Martínez, P.G.; Bautista-Baños, S.; Berúmen-Varela, G.; Ramos-Guerrero, A.; Hernández-Ibañez, A.M. In Vitro Response of Colletotrichum to Chitosan. Effect on Incidence and Quality on Tropical Fruit. Enzymatic Expression in Mango. Acta Agron. 2017, 66. [Google Scholar] [CrossRef]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Maluin, F.N.; Hussein, M.Z. Chitosan-Based Agronanochemicals as a Sustainable Alternative in Crop Protection. Molecules 2020, 25, 1611. [Google Scholar] [CrossRef]
- Rayón-Díaz, E.; Birke-Biewendt, A.B.; Velázquez-Estrada, R.M.; González-Estrada, R.R.; Ramírez-Vázquez, M.; Rosas-Saito, G.H.; Gutierrez-Martinez, P. Sodium Silicate and Chitosan: An Alternative for the in Vitro Control of Colletotrichum gloeosporioides Isolated from Papaya (Carica papaya L.). Rev. Bio Cienc. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Antony, R.; Arun, T.; Manickam, S.T.D. A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Morales-Chávez, F.; Núñez-Colín, C.; Mariscal-Amaro, L.; Morales-Vargas, A.; Alia-Tejacal, I.; Rafael Rodea-Montero, E.; Grijalva-Verdugo, C.; Veloz-García, R.; Rubén Rodríguez-Núñez, J. Chitosan Coatings with Essential Oils against Colletotrichum gloeosporioides (Penz.) Penz. & Sacc in Annona muricata L. Fruits. Horticulturae 2023, 9, 1–12. [Google Scholar] [CrossRef]
- Fouad, A.W. Chitosan: A Promising Plant Stimulant. Int. J. Agric. Sci. Food Technol. 2023, 9, 98–103. [Google Scholar] [CrossRef]
- Gh, J.P.; Sinclair, B.J.; Perinbarajan, G.K.; Dutta, R.; Shekhawat, R.; Saikia, N.; Chidambaram, R.; Mossa, A.-T. An Overview on Smart and Active Edible Coatings: Safety and Regulations. Eur. Food Res. Technol. 2023, 249, 1935–1952. [Google Scholar] [CrossRef]
- Salgado-Cruz, M.d.l.P.; Salgado-Cruz, J.; García-Hernández, A.B.; Calderón-Domínguez, G.; Gómez-Viquez, H.; Oliver-Espinoza, R.; Fernández-Martínez, M.C.; Yáñez-Fernández, J. Chitosan as a Coating for Biocontrol in Postharvest Products: A Bibliometric Review. Membranes 2021, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Elagamey, E.; Abdellatef, M.A.; Arafat, Y. Proteomic Insights of Chitosan Mediated Inhibition of Fusarium Oxysporum f. Sp. Cucumerinum. J. Proteom. 2022, 260, 104560. [Google Scholar] [CrossRef]
- Álvarez, M.; Andrade, M.J.; Núñez, F.; Rodríguez, M.; Delgado, J. Proteomics as a New-Generation Tool for Studying Moulds Related to Food Safety and Quality. Int. J. Mol. Sci. 2023, 24, 4709. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Vatankhah, M.; Hassanisaadi, M.; Shafiei-Hematabad, Z.; Kennedy, J.F. Advancements in Coating Technologies: Unveiling the Potential of Chitosan for the Preservation of Fruits and Vegetables. Int. J. Biol. Macromol. 2024, 254, 127677. [Google Scholar] [CrossRef]
- Sui, Y.; Ma, Z.; Meng, X. Proteomic Analysis of the Inhibitory Effect of Oligochitosan on the Fungal Pathogen, Botrytis cinerea. J. Sci. Food Agric. 2019, 99, 2622–2628. [Google Scholar] [CrossRef]
- Pervaiz, T.; Jia, H.; Zhang, P.; Salman-Haider, M.; Lu, S.; Wei, X.; Jia, H.; Fang, J. RNA-Seq and Metabolome Revealed Counter Effect of Chitosan against Botrytis Cinerea on Grape Berries. Res. Sq. 2019, 1, 1–33. [Google Scholar] [CrossRef]
- El-Araby, A.; Janati, W.; Ullah, R.; Ercisli, S.; Errachidi, F. Chitosan, Chitosan Derivatives, and Chi-tosan-Based Nanocomposites: Eco-Friendly Materials for Advanced Applications (a Review). Front. Chem. 2023, 11, 1327426. [Google Scholar] [CrossRef] [PubMed]
- Shinde, R.S.; Pawar, A. A Review on Production of Chitosan Nanoparticles from Shrimp Shells. Micro Environ. 2023, 3, 28–35. [Google Scholar]
- Abdalla, G.; Mussagy, C.U.; Brasil, G.S.P.; Scontri, M.; Sasaki, J.C.d.S.; Su, Y.; Bebber, C.; Rocha, R.R.; Abreu, A.P.d.S.; Goncalves, R.P.; et al. Eco-Sustainable Coatings Based on Chitosan, Pectin, and Lemon Essential Oil Nanoemulsion and Their Effect on Strawberry Preservation. Int. J. Biol. Macromol. 2023, 249, 126016. [Google Scholar] [CrossRef]
- Caro-León, F.J.; López-Martínez, L.M.; Lizardi-Mendoza, J.; Argüelles-Monal, W.; Goycoolea-Valencia, F.M.; Carvajal-Millán, E.; López-Franco, Y.L. Preparation Methods of Chitosan Nanoparticles: A Review. BIOtecnia 2019, 21, 13–25. [Google Scholar] [CrossRef]
- Abd-Elkader, D.Y.; Salem, M.Z.M.; Komeil, D.A.; Al-Huqail, A.A.; Ali, H.M.; Salah, A.H.; Akrami, M.; Hassan, H.S. Post-Harvest Enhancing and Botrytis cinerea Control of Strawberry Fruits Using Low Cost and Eco-Friendly Natural Oils. Agronomy 2021, 11, 1246. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, Z.; Leng, J.; Sui, Y.; Jiang, M.; Wisniewski, M.; Liu, J.; Wang, Q. Eco-Friendly Management of Postharvest Fungal Decays in Kiwifruit. Crit. Rev. Food Sci. Nutr. 2021, 62, 8307–8318. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, Y.; Paidari, S.; Baghbaderani, S.A.; Nateghi, L.; Al-Hassan, A.A.; Ariffin, F. Essential oils as natural antimicrobial agents in postharvest treatments of fruits and vegetables: A review. J. Food Meas. Charact. 2021, 16, 507–522. [Google Scholar] [CrossRef]
- Véliz-Jaime, M.Y.; González-Diaz, Y.; Martínez-Despaigne, Y. Technical and Economic Evaluation of the Project to Obtain Essential Oils. Tecnol. Química 2018, 39, 208–220. [Google Scholar]
- Mesa, V.A.M.; Marín, P.; Ocampo, O.; Calle, J.; Monsalve, Z. Fungicidas a Partir de Extractos Vegetales: Una Alternativa En El Manejo Integrado de Hongos Fitopatógenos. Rev. Investig. Agropecu. 2019, 45, 23–30. [Google Scholar]
- Rodríguez-Sauceda, E.N. Natural Antimicrobial Agent Use in the Preservation of Fruits and Vegetables. Ra Ximhai 2011, 7, 153–170. [Google Scholar]
- Salvatore, M.M.; Nicoletti, R.; Andolfi, A. Essential Oils in Citrus Fruit Ripening and Postharvest Quality. Horticulturae 2022, 8, 396. [Google Scholar] [CrossRef]
- Tao, N.; OuYang, Q.; Jia, L. Citral Inhibits Mycelial Growth of Penicillium Italicum by a Membrane Damage Mechanism. Food Control 2014, 41, 116–121. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, Y.; Lu, Z.; Xing, R.; Yao, X.; Jin, Z.; Wang, Y.; Yu, F. Citral-Loaded Chitosan/Carboxymethyl Cellulose Copolymer Hydrogel Microspheres with Improved Antimicrobial Effects for Plant Protection. Int. J. Biol. Macromol. 2020, 164, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Tao, N.; Jia, L. Antifungal Activity of Citral, Octanal and α-Terpineol against Geotrichum Citri-Aurantii. Food Control 2014, 37, 277–283. [Google Scholar] [CrossRef]
- Guo, L.; Li, Y.; Mao, X.; Tao, R.; Tao, B.; Zhou, Z. Antifungal Activity of Polymethoxylated Flavonoids (PMFs)-Loaded Citral Nanoemulsion against Penicillium italicum by Causing Cell Membrane Damage. J. Fungi 2022, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Yoplac, I.; Vargas, L.; Robert, P.; Hidalgo, A. Characterization and Antimicrobial Activity of Microencapsu-lated Citral with Dextrin by Spray Drying. Heliyon 2021, 7, e06737. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Bao, X.; Bové, M.; Rigole, P.; Meng, X.; Su, J.; Coenye, T. Antibiofilm Activities of Borneol-Citral-Loaded Pickering Emulsions against Pseudomonas aeruginosa and Staphylococcus aureus in Physiologically Relevant Chronic Infection Models. Microbiol. Spectr. 2022, 10, e0169622. [Google Scholar] [CrossRef] [PubMed]
- Trigos, Á. Encontrando El Punto Débil de Los Hongos. In El Maravilloso Mundo de los Hongos; Zulueta-Rodríguez, R., Trejo-Aguilar, D., Trigos-Landa, Á.R., Eds.; Universidad Veracruzana: Xalapa, Mexico, 2007; Volume 1, pp. 91–102. [Google Scholar]
- de Menezes, C.P.; Perez, A.L.A.d.L.; de Sousa, J.P.; Pereira, J.A.; Pinheiro, L.D.S.; de Medeiros, M.A.A.; Alves, M.D.S.; Filho, A.A.d.O.; Lima, E.D.O. Investigation on Mechanism of Antifungal Activity of Citral against Cladosporium sphaerospermum. Anal. Biol. 2022, 43–53. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, T.; Yuan, Y.; Yue, T. Overall Quality Properties of Kiwifruit Treated by Cinnamaldehyde and Citral: Microbial, Antioxidant Capacity during Cold Storage. J. Food Sci. 2016, 81, H3043–H3051. [Google Scholar] [CrossRef]
- Shenglong, D.; Jihong, Z.; Shaoyang, C.; Shuang, M.; Li, Z. The Combined Effect of 1-Methylcyclopropene and Citral Suppressed Postharvest Grey Mould of Tomato Fruit by Inhibiting the Growth of Botrytis cinerea. J. Phytopathol. 2019, 167, 123–134. [Google Scholar] [CrossRef]
- Fan, F.; Tao, N.; Jia, L.; He, X. Use of Citral Incorporated in Postharvest Wax of Citrus Fruit as a Botanical Fungicide against Penicillium digitatum. Postharvest Biol. Technol. 2014, 90, 52–55. [Google Scholar] [CrossRef]
- Li, R.-Y.; Wu, X.-M.; Yin, X.-H.; Liang, J.-N.; Li, M. The Natural Product Citral Can Cause Significant Damage to the Hyphal Cell Walls of Magnaporthe grisea. Molecules 2014, 19, 10279–10290. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Jing, G.; Wang, X.; Ouyang, Q.; Jia, L.; Tao, N. Citral Exerts Its Antifungal Activity against Penicil-lium Digitatum by Affecting the Mitochondrial Morphology and Function. Food Chem. 2015, 178, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, Q.; Zhou, A.; Wen, X.; Li, M.; Li, R.; Liao, X.; Xu, T. The Antifungal Effects of Citral on Magnaporthe oryzae Occur via Modulation of Chitin Content as Revealed by RNA-Seq Analysis. J. Fungi 2021, 7, 1023. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Chen, C.; Wan, C.; Chen, M.; Chen, J. Citral Delays Postharvest Senescence of Kiwifruit by Enhancing Antioxidant Capacity under Cold Storage. J. Food Qual. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Duan, B.; Reymick, O.O.; Liu, Z.; Zhou, Y.; Wang, X.; Feng, Z.; Tao, N. Citral Enhances Disease Resistance in Postharvest Citrus Fruit through Inducing Jasmonic Acid Pathway and Accumulating Phenylpropanoid Compounds. Postharvest Biol. Technol. 2024, 207. [Google Scholar] [CrossRef]
- Song, J.; Leepipattanawit, R.; Deng, W.; Beaudry, R.M. Hexanal Vapor Is a Natural, Metabolizable Fungicide: Inhibition of Fungal Activity and Enhancement of Aroma Biosynthesis in Apple Slices. J. Am. Soc. Hortic. Sci. 1996, 121, 937–942. [Google Scholar] [CrossRef]
- Jincy, M.; Djanaguiraman, M.; Jeyakumar, P.; Subramanian, K.; Jayasankar, S.; Paliyath, G. Inhibition of Phospholipase D Enzyme Activity through Hexanal Leads to Delayed Mango (Mangifera Indica L.) Fruit Ripening through Changes in Oxidants and Antioxidant Enzymes Activity. Sci. Hortic. 2017, 218, 316–325. [Google Scholar] [CrossRef]
- Paliyath, G.; Padmanabhan, P. Chapter 4: Preharvest and Postharvest Technologies Based on Hexanal: An Overview. In Postharvest Biology and Nanotechnology, 1st ed.; John Wiley & Sons, Inc.: Pondicherry, India, 2019; pp. 89–101. [Google Scholar] [CrossRef]
- El Kayal, W.; Paliyath, G.; Sullivan, J.A.; Subramanian, J. Phospholipase D Inhibition by Hexanal Is Associ-ated with Calcium Signal Transduction Events in Raspberry. Hortic. Res. 2017, 4, 17042. [Google Scholar] [CrossRef]
- Cheema, A.; Padmanabhan, P.; Amer, A.; Parry, M.J.; Lim, L.-T.; Subramanian, J.; Paliyath, G. Postharvest Hexanal Vapor Treatment Delays Ripening and Enhances Shelf Life of Greenhouse Grown Sweet Bell Pepper (Capsicum Annum L.). Postharvest Biol. Technol. 2018, 136, 80–89. [Google Scholar] [CrossRef]
- Alba-Jiménez, J.E. Estudio de Factores Que Afectan La Resistencia Mecánica de Los Frutos de Guayaba (Psisium Guajava L.) Durante Su Maduración. Doctoral Thesis, Universidad de Querétaro, Querétaro, Mexico, 2018. [Google Scholar]
- Debysingh, N.; Wickham, L.D.; Mohammed, M.; Legall, G.; Paliyath, G.; Subramanian, J. Effects of Pre-and Post-Harvest Treatments with Hexanal Formulations on Time to Ripening and Shelf Life of Papaya (Carica Papaya L.) Fruits. J. Fac. Food Agric. 2018, 95, 36–42. [Google Scholar]
- Dhakshinamoorthy, D.; Sundaresan, S.; Iyadurai, A.; Subramanian, K.S.; Janavi, G.J.; Paliyath, G.; Subramanian, J. Hexanal Vapor Induced Resistance against Major Postharvest Pathogens of Banana (Musa acuminata L.). Plant Pathol. J. 2020, 36, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Arora, N.; Gill, K.B.S.; Sharma, S.; Gill, M. Hexanal Formulation Reduces Rachis Browning and Postharvest Losses in Table Grapes Cv. ‘Flame Seedless’. Sci. Hortic. 2019, 248, 265–273. [Google Scholar] [CrossRef]
- Sriskantharajah, K.; El Kayal, W.; Torkamaneh, D.; Ayyanath, M.M.; Saxena, P.K.; Sullivan, A.J.; Paliyath, G.; Subramanian, J. Transcriptomics of Improved Fruit Retention by Hexanal in ‘Honeycrisp’ Reveals Hormonal Crosstalk and Reduced Cell Wall Degradation in the Fruit Abscission Zone. Int. J. Mol. Sci. 2021, 22, 8830. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, M.J.; Ouko, J.R.; Yumbya, P.M.; Ambuko, J.L.; Owino, W.O.; Subramanian, J. Efficacy of Hexanal Field Spray on the Postharvest Life and Quality of Papaya Fruit (Carica papaya L.) in Kenya. Adv. Agric. 2022, 2022, 1–15. [Google Scholar] [CrossRef]
- Li, S.-F.; Zhang, S.-B.; Lv, Y.-Y.; Zhai, H.-C.; Li, N.; Hu, Y.-S.; Cai, J.-P. Metabolomic Analyses Revealed Multi-faceted Effects of Hexanal on Aspergillus Flavus Growth. Appl. Microbiol. Biotechnol. 2021, 105, 3745–3757. [Google Scholar] [CrossRef] [PubMed]
- Yumbya, P.; Hutchinson, M.; Ambuko, J.; Owino, W. Effect of Hexanal as a Post-harvest Treatment to Extend the Shelf-life of Banana Fruits (Musa acuminata var. Sweet Banana) in Kenya. Int. J. Plant Soil Sci. 2019, 29, 1–16. [Google Scholar] [CrossRef]
- Kaur, K.; Kaur, G.; Brar, J.S. Pre-Harvest Application of Hexanal Formulations for Improving Post-Harvest Life and Quality of Mango (Mangifera Indica L.) Cv. Dashehari. J. Food Sci. Technol. 2020, 57, 4257–4264. [Google Scholar] [CrossRef]
- Caballero-Prado, C.J.; Merino-Mascorro, J.A.; Heredia, N.; Dávila-Aviña, J.; García, S. Eugenol, Citral, and Hexanal, Alone or in Combination with Heat, Affect Viability, Biofilm Formation, and Swarming on Shi-ga-Toxin-Producing Escherichia coli. Food Sci. Biotechnol. 2021, 30, 599–607. [Google Scholar] [CrossRef]
- Sharma, A.; Bons, H.K.; Jawandha, S.K.; Chung, S.W. Effect of Hexanal Treatment on Fruit Qualities and Antioxidant Activities on “Umran” Indian Jujube Fruit during Cold Storage 2. bioRxiv 2023, 1–27. [Google Scholar] [CrossRef]
- Montes-Ramírez, P.; Montaño-Leyva, B.; Blancas-Benitez, F.J.; Bautista-Rosales, P.U.; Ruelas-Hernández, N.D.; Martínez-Robinson, K.; González-Estrada, R.R. Active Films and Coatings Based on Commercial Chi-tosan with Natural Extracts Addition from Coconut By-Products: Physicochemical Characterization and Antifungal Protection on Tomato Fruits. Food Control 2024, 155, 110077. [Google Scholar] [CrossRef]
- Riseh, R.S.; Vatankhah, M.; Hassanisaadi, M.; Varma, R.S. A Review of Chitosan Nanoparticles: Na-ture’s Gift for Transforming Agriculture through Smart and Effective Delivery Mechanisms. Int. J. Biol. Macromol. 2024, 260, 129522. [Google Scholar] [CrossRef]
- Mejía, E.M.T.; Cruz, J.F.G.; Figueredo, C.R.P. Síntesis de una Base De Schiff a Partir de Dopamina y Cinamaldehído. Cienc. Desarro. 2021, 12, 119–128. [Google Scholar] [CrossRef]
- Perentena, L.; González, C.; Celis, B.; Valbuena, A.; Colina, M. Síntesis de Bases de Schiff Derivadas Del Quitosano Por Reacción Con P-Dimetilaminobenzaldehído y 4-Hidroxi-3-Metoxibenzaldehído. Rev. Iber. Polímeros 2015, 16, 1–27. [Google Scholar]
- Agusnar, H.; Wirjosentono, B.; Salim, S.; Rihayat, T.; Fauzi, T. Synthesis and Characterization of Chitosan with Addition of Patchouli Oil to Improve Mechanical Properties Biofilm. J. Phys. Conf. Ser. 2018, 1116, 042001. [Google Scholar] [CrossRef]
- Rosero, A.; Espinoza-Montero, P.J.; Fernández, L. Recubrimiento Comestibles para la Conservación De Frutas y Verduras: Una Revisión. infoANALÍTICA 2020, 8, 150–179. [Google Scholar] [CrossRef]
- Heras-Mozos, R.; Hernández, R.; Gavara, R.; Hernández-Muñoz, P. Dynamic Covalent Chemistry of Imines for the Development of Stimuli-Responsive Chitosan Films as Carriers of Sustainable Antifungal Volatiles. Food Hydrocoll. 2022, 125. [Google Scholar] [CrossRef]
- Giacomini, G.X.; Nachtigal, G.d.F.; Martins, C.R.; Hirdes, A.R.; Valgas, R.A.; dos Santos, A.J.R.W.A. Eco-Friendly Fungicide Based on Chitosan and Pecan Nut Oil: Development and Evaluation in Anthracnose Control. Acta Sci. Biol. Sci. 2023, 45, e62090. [Google Scholar] [CrossRef]
- Marei, G.I.K.; Rabea, E.I.; Badawy, M.E.I. Preparation and Characterizations of Chitosan/Citral Nanoemulsions and Their Antimicrobial Activity. Appl. Food Biotechnol. 2018, 5, 69–78. [Google Scholar]
- Reyes, J.J.; de Quevedo, U.T.E.; Vero, S.; Diaz-Rivera, E.; Lara-Capistran, L.; Noa-Carrazana, J.C.; Hernandez-Montiel, L.G.; Noroeste, C.d.I.B.d.; Veracruzana, U. Application of chlorine dioxide (ClO2) and marine yeasts to control postharvest anthracnose disease in mango (Mangifera indica L.). Rev. Cienc. Investig. Agrar. 2019, 46, 266–275. [Google Scholar] [CrossRef]
- Esquivel-Rodríguez, B. De la Naturaleza, Miles de Productos Benéficos para el ser Humano. Boletín UNAM-DGCS-303. Available online: https://www.dgcs.unam.mx/boletin/bdboletin/2022_303.html (accessed on 4 December 2023).
- González, C.; Valbuena, A.; Celis, B.; Perentena, L.; Colina, M. Degradación Oxidativa de Quitosano Con Peróxido de Hidrógeno. Iber. Polímeros 2015, 16, 43–68. [Google Scholar]
- Portal TecnoAgrícola. BIOREND México. Productoas Agricultura Comercial. Available online: https://www.buscador.portaltecnoagricola.com/vademecum/mex/producto/BIOREND (accessed on 15 September 2023).
- Secretaría de Economía. Cetonas-Alcoholes y Cetonas-Aldehídos: Intercambio Comercial, Compras y Ventas Internacionales, Mercado y Especialización | Data México; Ciudad de México. 2022. Available online: https://www.economia.gob.mx/datamexico/es/profile/product/ketones-and-ketone-alcohols-aldehydes (accessed on 4 December 2023).
- Aguirre-Flores, A. Usos y Aplicaciones de Aldehídos y Cetonas. Eñengi. Available online: https://enengiedublog.wordpress.com/2019/06/13/usos-y-aplicaciones-de-aldehidos-y-cetonas/# (accessed on 13 November 2023).
- Rodríguez-Guzmán, C.A.; Montaño-Leyva, B.; Velázquez-Estrada, R.M.; Sánchez-Burgos, J.A.; García-Magaña, M.D.L.; González-Estrada, R.R.; Gutiérrez-Martínez, P. Estado Actual de Métodos Alternati-vos, de Control de Hongos y Su Efecto En La Calidad Postcosecha de Frutos de Jitomate (Solanum Lycopersicum). TIP Rev. Espec. Cienc. Químico Biológicas 2021, 24, 1–15. [Google Scholar] [CrossRef]
- Socha, C.L.M.; Cuellar, J.C.R. Citral Nanocontainers Applied to Guava Fruits (Psidium Guajava L.) in Postharvesting. Dyna 2020, 87, 267–276. [Google Scholar] [CrossRef]
- Arias, J.A.A.; Trigoso, F.B.M. Soberanía alimentaria y tecnologías sociales: Una experiencia de desarrollo autónomo desde los Andes del Perú. Cienc. Ergo-Sum 2019, 26, 1–12. [Google Scholar] [CrossRef]
- CONAHCYT. Soberanía Alimentaria—Conahcyt. Programas Nacionales Estratégicos. Available online: https://conahcyt.mx/pronaces/pronaces-soberania-alimentaria/ (accessed on 15 October 2023).
- Secretaría de Bienestar. En Busca de una Soberanía Alimentaria. Blog. Available online: https://www.gob.mx/bienestar/articulos/en-busca-de-una-soberania-alimentaria (accessed on 15 October 2023).
- Delgado-Burgoa, F.; Escobar-Vásquez, C.G. Innovación Tecnológica, Soberanía y Seguridad Alimentaria; AGRUCO-CAPTURED; Delgado-Burgoa, F., Escobar-Vásquez, C.G., Eds.; Plural Editores: La Paz, Bolivia, 2009; Volume 1. [Google Scholar]
- Montoya, M.A.; Hernández-Adame, L. Problematización de La Investigación En Nanotecnología Agrícola y Alimentaria En El Marco de La Nueva Política de Ciencia, Tecnología e Innovación En México. Rev. Interdiscip. Nanociencias Nanotecnología 2022, 16, 1e–24e. [Google Scholar] [CrossRef]
- Vargas-Salinas, M. Caracterización Molecular de Begomovirus y Fitoplasmas Asociados a Una Infección Mixta En Calabacita Cucurbita Pepo L. En B.C.S., México. Master’s Thesis, Centro de Investigaciones Biológicas del Noroeste, S.C., La Paz, Mexico, 2017. [Google Scholar]
- Decco Iberica. Frutales de Hueso: Enfermedades en Postcosecha y Soluciones. Poscosecha—Fitosanitarios. Available online: https://poscosecha.com/decco-iberica/frutales-hueso-enfermedades-postcosecha-soluciones (accessed on 5 October 2023).
- Organización Panamericana de la Salud (OPS). Enfermedades Transmitidas por Alimentos. Inocuidad de Alimentos. Available online: https://www.paho.org/es/temas/enfermedades-transmitidas-por-alimentos (accessed on 9 November 2023).
- FAO. Cartilla Tecnológica 8: Producción Segura y Efectiva de Cultivos ¿Qué Son Plagas y Enfermedades? Mejorando la Nutrición a Través de Huertos y Granjas Familiares. Roma. 2023. Available online: https://www.fao.org/3/V5290S/v5290s00.htm#TopOfPage (accessed on 1 January 2023).
- Córdova-Albores, L.C.; Zelaya-Molina, L.X.; Ávila-Alistac, N.; Valenzuela-Ruíz, V.; Cortés-Martínez, N.E.; Parra-Cota, F.I.; Burgos-Canul, Y.Y.; Chávez-Díaz, I.F.; Fajardo-Franco, M.L.; Santos-Villalobos, S.D.L. Omics Sciences Potential on Bioprospecting of Biological Control Microbial Agents: The Case of the Mexican Agro-Biotechnology. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2020, 39, 147–184. [Google Scholar] [CrossRef]
- Ramírez-Navas, J.S.; Jaramillo-López, F.; López-Parra, L.L. Las disciplinas ómicas en la ciencia de los alimentos. Rev. Colomb. Investig. Agroindustriales 2023, 10, 1–22. [Google Scholar] [CrossRef]
- Capote-Maínez, N.; Hernández-Fort, C. Ómicas y Fitopatología; Sociedad Española de Fitopatología: Madrid, Spain, 2021; pp. 1–75. [Google Scholar]
- Rodríguez-Sifuentes, L.; Soto-Cruz, N.Ó. Metabolomics and Its Application to Phytopathology. In Phytopathology in the Omics Era; Rodríguez-Herrera, R., Aguilar, C.N., Simpson-Williamson, J.K., Gutierrez-Sanchez, G., Eds.; Trivandrum: Kerala, India, 2011; Volume 1, pp. 175–190. [Google Scholar]
- Nguyen, T.V.; Alfaro, A.C.; Merien, F. Omics Approaches to Investigate Host–Pathogen Interactions in Mass Mortality Outbreaks of Crassostrea gigas. Rev. Aquac. 2018, 11, 1308–1324. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Lv, C.; Zhang, L.-J.; Guo, X.; Shen, Y.-W.; Nagle, D.G.; Zhou, Y.-D.; Liu, S.-H.; Zhang, W.-D.; Luan, X. Application of Omics- and Multi-Omics-Based Techniques for Natural Product Target Discovery. Biomed. Pharmacother. 2021, 141, 111833. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Singh, P.; Mehrotra, R. Application of Omics Technologies in Tropical and Subtropical Fruit Crops. In Omics in Horticultural Crops; Elsevier: Amsterdam, The Netherlands, 2022; pp. 119–145. [Google Scholar] [CrossRef]
- Sirangelo, T.M.; Rogers, H.J.; Spadafora, N.D. Multi-Omic Approaches to Investigate Molecular Mechanisms in Peach Post-Harvest Ripening. Agriculture 2022, 12, 553. [Google Scholar] [CrossRef]
- Yerasu, S.R.; Reddy, B.R.; Singh, D.P.; Singh, J. Omics Studies for Vegetable Improvement. In Omics in Horticultural Crops; Elsevier: Amsterdam, The Netherlands, 2022; pp. 215–238. [Google Scholar] [CrossRef]
- Licciardello, C.; Perrone, I.; Gambino, G.; Talon, M.; Velasco, R. Editorial: Functional Genomics in Fruit Trees: From ‘Omics to Sustainable Biotechnologies. Front. Plant Sci. 2021, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.S.; Lam, S.D.; Yang, Y.; Naqiuddin, M.; Addis, S.N.K.; Yong, W.T.L.; Luang-In, V.; Sonne, C.; Ma, N.L. Omics Technologies Used in Pesticide Residue Detection and Mitigation in Crop. J. Hazard. Mater. 2021, 420, 126624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhao, P.; Zhang, P.; Su, L.; Jia, H.; Wei, X.; Fang, J.; Jia, H. Integrative Transcriptomics and Metab-olomics Data Exploring the Effect of Chitosan on Postharvest Grape Resistance to Botrytis cinerea. Postharvest Biol. Technol. 2020, 167, 111248. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Martin-Urdiroz, M.; Oses-Ruiz, M.; Were, V.M.; Fricker, M.D.; Littlejohn, G.; Lopez-Llorca, L.V.; Talbot, N.J. Chitosan Inhibits Septin-Mediated Plant Infection by the Rice Blast Fungus Magnaporthe Oryzae in a Protein Kinase C and Nox1 NADPH Oxidase-Dependent Manner. New Phytol. 2021, 230, 1578–1593. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Torres, E.A.; Aguilera-Aguirre, S.; Hernández-Oñate, M.; López-García, U.M.; Vega-Arreguín, J.; Montalvo-González, E.; Ortiz-Basurto, R.I.; Chacón-López, A. Molecular Aspects Revealed by Omics Technologies Related to the Defense System Activation in Fruits in Response to Elicitors: A Review. Horticulturae 2023, 9, 558. [Google Scholar] [CrossRef]
- Almenar, E.; Auras, R.; Rubino, M.; Harte, B. A New Technique to Prevent the Main Post Harvest Diseases in Berries during Storage: Inclusion Complexes β-Cyclodextrin-Hexanal. Int. J. Food Microbiol. 2007, 118, 164–172. [Google Scholar] [CrossRef]
- Utto, W.; Mawson, A.J.; Bronlund, J.E. Hexanal Reduces Infection of Tomatoes by Botrytis Cinerea Whilst Maintaining Quality. Postharvest Biol. Technol. 2008, 47, 434–437. [Google Scholar] [CrossRef]
| Type of Publication | Total of Publications | Percentage of Total Publication (%) |
|---|---|---|
| Research articles | 1657 | 52.89% |
| Book chapters | 823 | 26.27% |
| Review articles | 479 | 15.29% |
| Encyclopedias | 103 | 3.29% |
| Others | 71 | 2.27% |
| Total | 3133 | 100% |
| Assay | Concentration | Effects | References |
|---|---|---|---|
| In vitro | 0–200 µg/mL | Magnaporthe grisea hyphae exposure to 50 ug/mL showed ultrastructural changes in the morphology, and the application of high concentrations led to severe cellular degeneration; the cell walls appeared to be degraded and displayed cellular disorganization. This proposes that citral ruptures the cell wall and penetrates the cell membrane, as has been seen through scanning and transmission electron microscopy. | [80] |
| In vitro | 0.50–1.00 µL/mL | The permeability of the membrane increased in correlation with the concentration of citral; in addition, the application induced a decrease in the content of lipids and ergosterol in Penicillium italicum fungal cells. | [69] |
| In vitro | 2.0–4.0 µL/mL | Citral application reduces enzymes’ activity of citrate synthase, isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and succionadrogenase; these decreases in mitochondrial enzymes mark a deficiency in the electron transport chain components, a decrease in ATP synthesis and the ability to generate NADPH, and an increase in the oxidative stress in the growth of Penicillium digitatum. | [81] |
| In vitro | 0–100 µg/mL | Antifungal activity is related to the genes entailed in the chitin and uridine diphosphate synthesis pathways in the amino sugar and nucleotide metabolic pathways of Magnaporthe oryzae, causing a reduction in glucan in the cell wall. | [82] |
| In vivo | 0.6 µL/mL | The effect of citral treatment in kiwi fruits causes the antioxidant enzyme system, which includes catalase, peroxidase, and superoxide dismutase, to become active, besides physicochemical parameters which decrease weight loss, softening, and fruit respiration. On the other hand, post-harvest quality is maintained by preventing the breakdown of ascorbic acid content, total flavonoid content, and total phenolic content. | [83] |
| In vitro | 0–80 mg/mL | The properties of citral (α-β-unsaturated aldehyde) in the carbonyl group allow β-carbon to become positively polarized and easily reactant to nucleophiles, basing its ability to act as an alkylating agent, capable of influencing biological functions and possibly being harmful by covalently binding to nucleophilic groups within cells. | [73] |
| In vitro | 128–256 µg/mL | Citral treatment showed an affinity for ergosterol, inhibited ergosterol biosynthesis, and was related to cell wall alterations, interfering in the cellular metabolism and the loss of membrane integrity, indicating a strong antifungal activity in Cladosporium sphaerospermum. | [76] |
| In vivo | 0–200 µL/mL | Citral applications in citrus fruits increase the activities of phenylalanine ammonia-lyase, peroxidase, and polyphenol oxidase; moreover, metabolomic analyses induce the accumulation of plant hormones as methyl jasmonate, abscisic acid, and phenylpropanoid metabolites. On the other hand, RNA-seq revealed the expression of multiple genes related to jasmonic acid profiles and phenylpropanoid biosynthesis. | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayón-Díaz, E.; Hernández-Montiel, L.G.; Sánchez-Burgos, J.A.; Zamora-Gasga, V.M.; González-Estrada, R.R.; Gutiérrez-Martínez, P. Natural Compounds and Derivates: Alternative Treatments to Reduce Post-Harvest Losses in Fruits. AgriEngineering 2024, 6, 1022-1042. https://doi.org/10.3390/agriengineering6020059
Rayón-Díaz E, Hernández-Montiel LG, Sánchez-Burgos JA, Zamora-Gasga VM, González-Estrada RR, Gutiérrez-Martínez P. Natural Compounds and Derivates: Alternative Treatments to Reduce Post-Harvest Losses in Fruits. AgriEngineering. 2024; 6(2):1022-1042. https://doi.org/10.3390/agriengineering6020059
Chicago/Turabian StyleRayón-Díaz, Edson, Luis G. Hernández-Montiel, Jorge A. Sánchez-Burgos, Victor M. Zamora-Gasga, Ramsés Ramón González-Estrada, and Porfirio Gutiérrez-Martínez. 2024. "Natural Compounds and Derivates: Alternative Treatments to Reduce Post-Harvest Losses in Fruits" AgriEngineering 6, no. 2: 1022-1042. https://doi.org/10.3390/agriengineering6020059
APA StyleRayón-Díaz, E., Hernández-Montiel, L. G., Sánchez-Burgos, J. A., Zamora-Gasga, V. M., González-Estrada, R. R., & Gutiérrez-Martínez, P. (2024). Natural Compounds and Derivates: Alternative Treatments to Reduce Post-Harvest Losses in Fruits. AgriEngineering, 6(2), 1022-1042. https://doi.org/10.3390/agriengineering6020059










