Is It Possible to Measure the Quality of Sugarcane in Real-Time during Harvesting Using Onboard NIR Spectroscopy?
Abstract
1. Introduction
2. Materials and Methods
2.1. NIR System Framework in Sugarcane Harvester
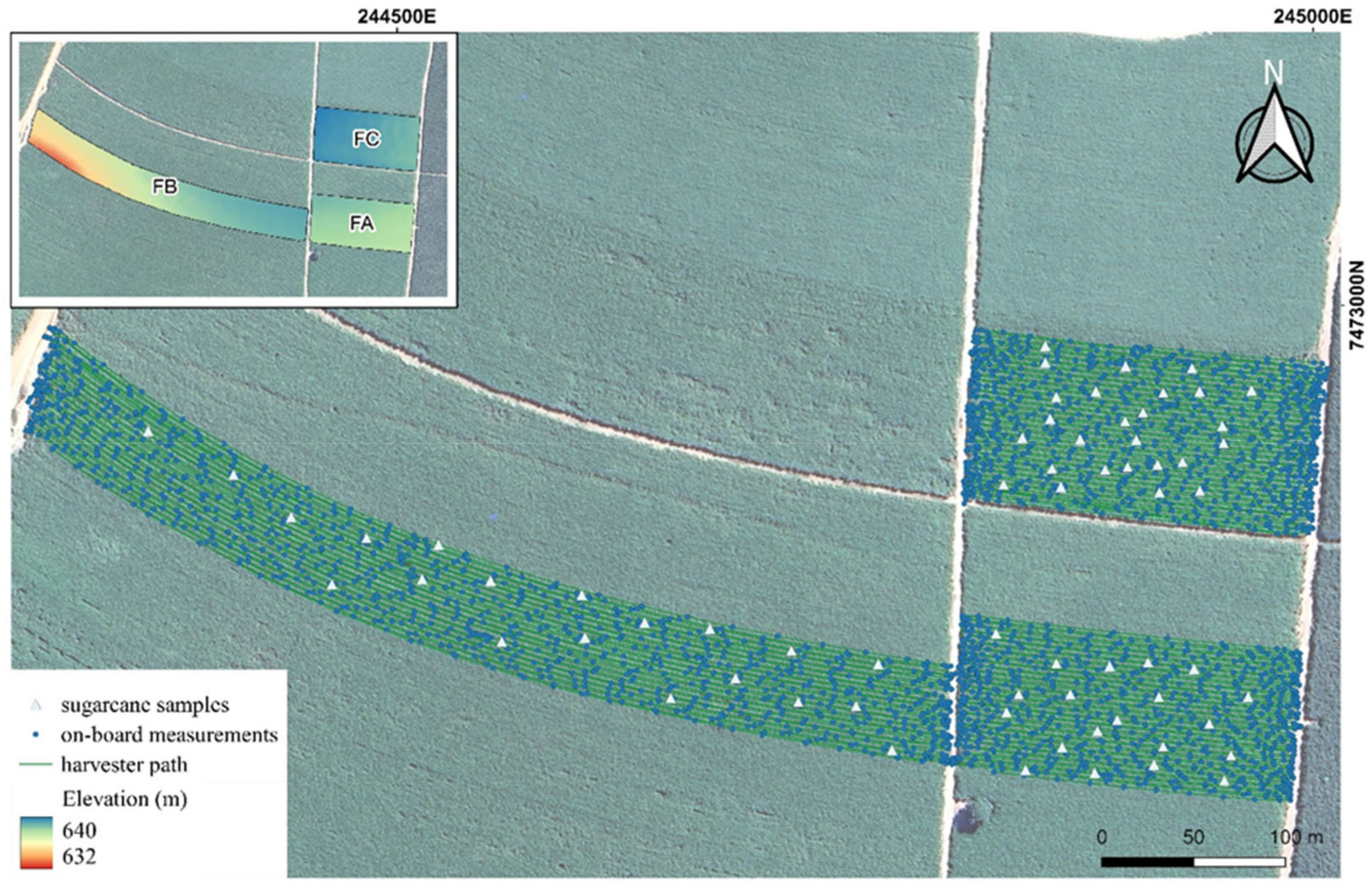
2.2. Field Experiment and Data Acquisition: On-the-Go Measurements
2.3. Reference Sugarcane Quality Analyses and Spectra Bench Acquisition
2.4. Multivariate Data Modeling for Sugarcane Quality Estimation
2.5. Spatial Variability and Site-Specific Assessment of the Relationships among Sugarcane Quality and Soil Attributes
3. Results and Discussion
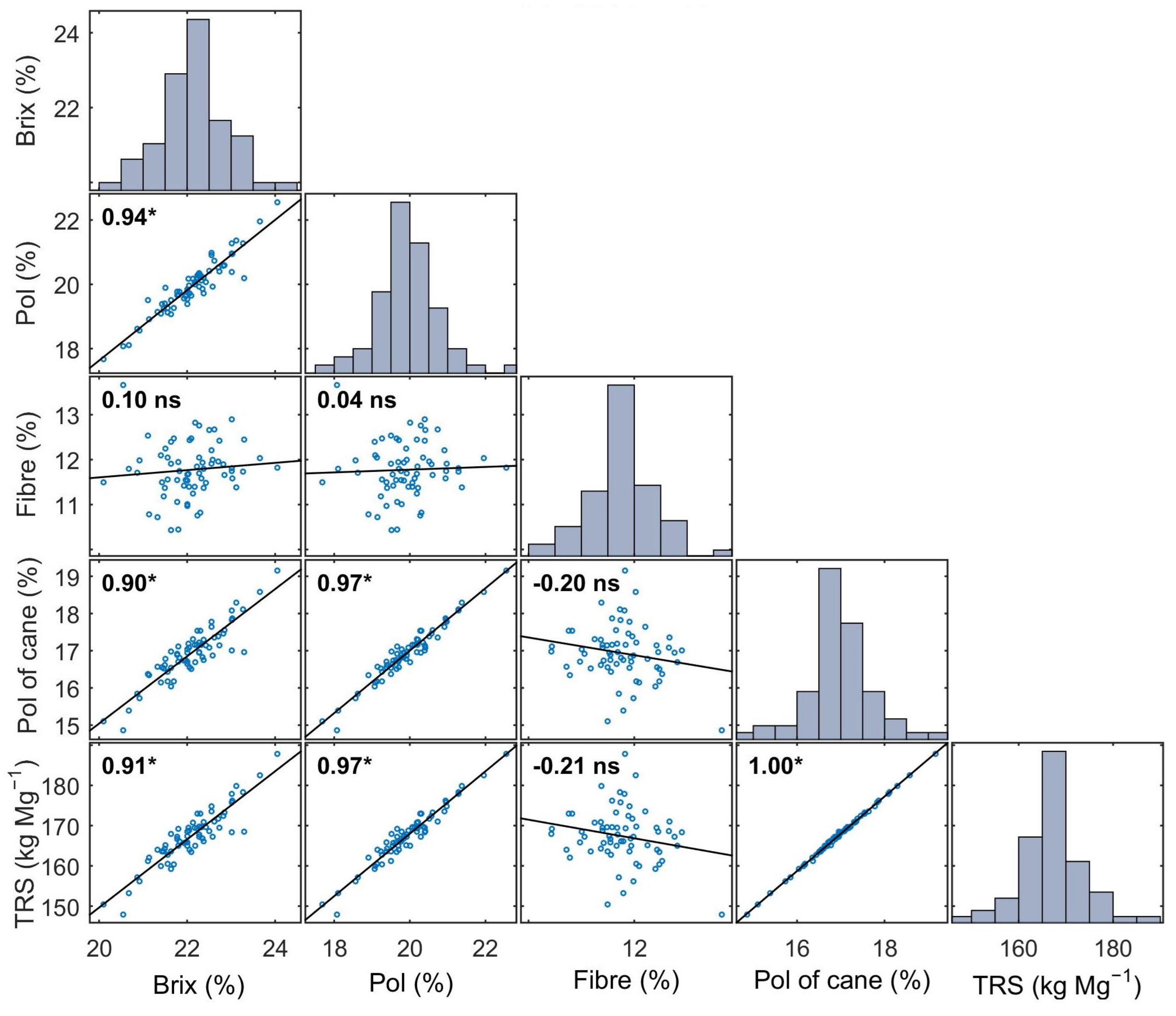
3.1. Sugarcane Quality Properties Characterization
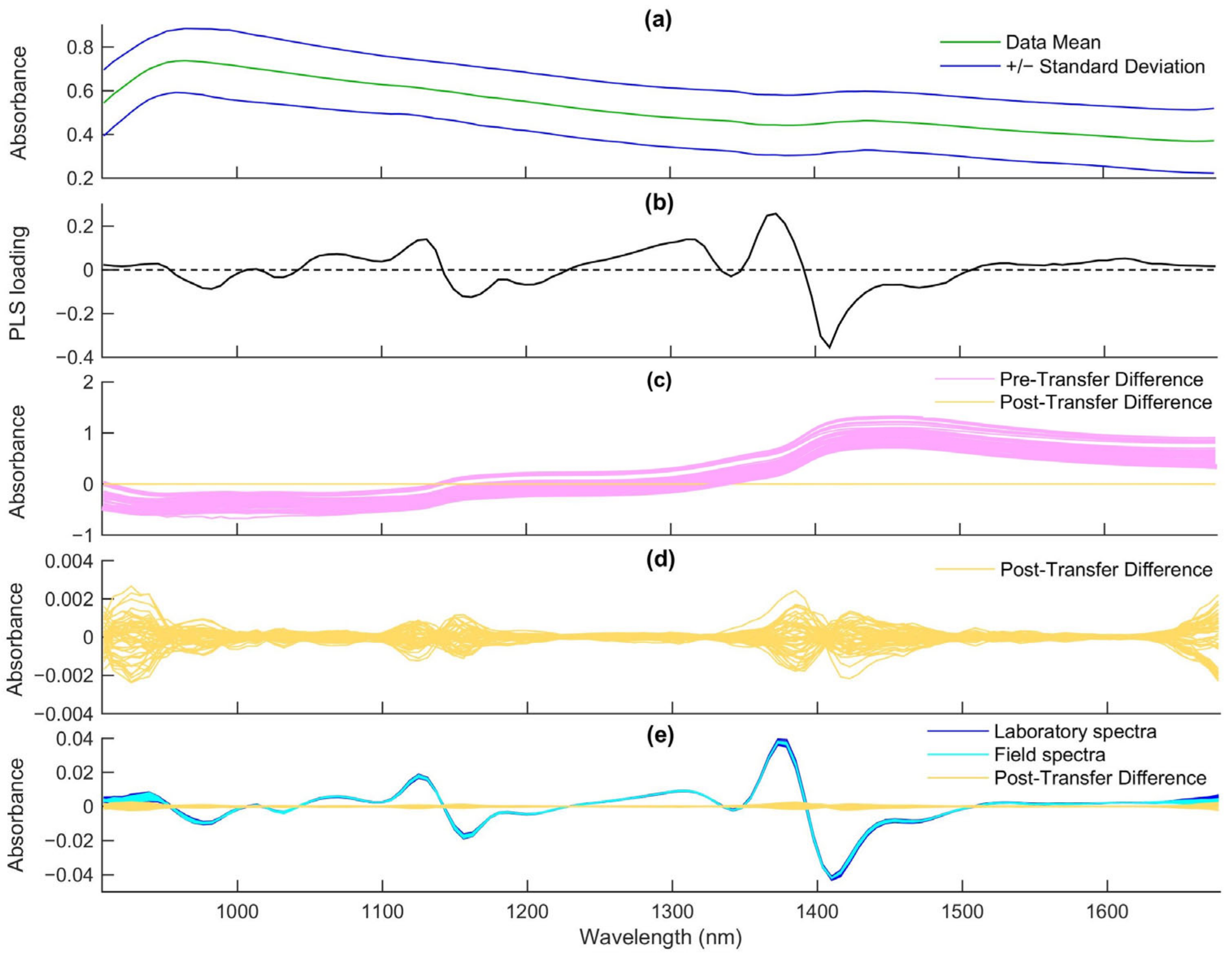
3.2. Processing of Spectral Data
3.3. Sugarcane Quality Properties Prediction Models
3.4. On-Board Data Analysis and Spatial Variability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Molin, J.P.; Bazame, H.C.; Maldaner, L.; Corredo, L.D.P.; Martello, M.; Canata, T.F.; de Paula Corredo, L.; Martello, M.; Canata, T.F. Precision Agriculture and the Digital Contributions for Site-Specific Management of the Fields. Rev. Ciência Agronômica 2020, 51, 1–10. [Google Scholar] [CrossRef]
- Cortés, V.; Blasco, J.; Aleixos, N.; Cubero, S.; Talens, P. Monitoring Strategies for Quality Control of Agricultural Products Using Visible and Near-Infrared Spectroscopy: A Review. Trends Food Sci. Technol. 2019, 85, 138–148. [Google Scholar] [CrossRef]
- Corrêdo, L.d.P.; Canata, T.F.; Maldaner, L.F.; de Lima, J.d.J.A.; Molin, J.P. Sugarcane Harvester for In-Field Data Collection: State of the Art, Its Applicability and Future Perspectives. Sugar Tech. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Bramley, R.G.V. Lessons from Nearly 20 Years of Precision Agriculture Research, Development, and Adoption as a Guide to Its Appropriate Application. Crop Pasture Sci. 2009, 60, 197–217. [Google Scholar] [CrossRef]
- Maldaner, L.F.; Corrêdo, L.d.P.; Canata, T.F.; Molin, J.P. Predicting the Sugarcane Yield in Real-Time by Harvester Engine Parameters and Machine Learning Approaches. Comput. Electron. Agric. 2021, 181, 105945. [Google Scholar] [CrossRef]
- CONSECANA. Sugarcane, Sugar, and Alcohol Producers Council of the State of São Paulo, Instruction Manual, 5th ed.; CONSECANA: Piracicaba, SP, Brazil; Consecana, SP, Brazil, 2006. [Google Scholar]
- Pasquini, C. Near Infrared Spectroscopy: A Mature Analytical Technique with New Perspectives—A Review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; Blasco, J.; Zude-Sasse, M.; Sun, X. Visible-NIR ‘Point’ Spectroscopy in Postharvest Fruit and Vegetable Assessment: The Science behind Three Decades of Commercial Use. Postharvest Biol. Technol. 2020, 168, 111246. [Google Scholar] [CrossRef]
- Sexton, J.; Everingham, Y.; Donald, D.; Staunton, S.; White, R. Investigating the Identification of Atypical Sugarcane Using NIR Analysis of Online Mill Data. Comput. Electron. Agric. 2020, 168, 105111. [Google Scholar] [CrossRef]
- Ruangratanakorn, J.; Suwonsichon, T.; Kasemsumran, S.; Thanapase, W. Installation Design of On-Line near Infrared Spectroscopy for the Production of Compound Fertilizer. Vib. Spectrosc. 2020, 106, 103008. [Google Scholar] [CrossRef]
- Xu, X.; Mo, J.; Xie, L.; Ying, Y. Influences of Detection Position and Double Detection Regions on Determining Soluble Solids Content (SSC) for Apples Using On-Line Visible/Near-Infrared (Vis/NIR) Spectroscopy. Food Anal. Methods 2019, 12, 2078–2085. [Google Scholar] [CrossRef]
- Salguero-Chaparro, L.; Peña-Rodríguez, F. On-Line versus off-Line NIRS Analysis of Intact Olives. LWT—Food Sci. Technol. 2014, 56, 363–369. [Google Scholar] [CrossRef]
- Porep, J.U.; Kammerer, D.R.; Carle, R. On-Line Application of near Infrared (NIR) Spectroscopy in Food Production. Trends Food Sci. Technol. 2015, 46, 211–230. [Google Scholar] [CrossRef]
- Munnaf, M.A.; Mouazen, A.M. Development of a Soil Fertility Index Using On-Line Vis-NIR Spectroscopy. Comput. Electron. Agric. 2021, 188, 106341. [Google Scholar] [CrossRef]
- Nawar, S.; Munnaf, M.A.; Mouazen, A.M. Machine Learning Based On-Line Prediction of Soil Organic Carbon after Removal of Soil Moisture Effect. Remote Sens. 2020, 12, 1308. [Google Scholar] [CrossRef]
- Tümsavaş, Z.; Tekin, Y.; Ulusoy, Y.; Mouazen, A.M. Prediction and Mapping of Soil Clay and Sand Contents Using Visible and Near-Infrared Spectroscopy. Biosyst. Eng. 2019, 177, 90–100. [Google Scholar] [CrossRef]
- Long, D.S.; McCallum, J.D. Adapting a Relatively Low-Cost Reflectance Spectrometer for on-Combine Sensing of Grain Protein Concentration. Comput. Electron. Agric. 2020, 174, 105467. [Google Scholar] [CrossRef]
- Yang, S.; Wu, Q.; Chang, H.; Li, Q.; Xu, H. Effect of Grain Density to near Infrared Spectra and Design of a Laboratory Evaluation System for Combine Harvester. In Proceedings of the 2017 ASABE Annual International Meeting, Spokane, WA, USA, 16–19 July 2017; pp. 1–8. [Google Scholar] [CrossRef]
- Risius, H.; Hahn, J.; Huth, M.; Tölle, R.; Korte, H. In-Line Estimation of Falling Number Using near-Infrared Diffuse Reflectance Spectroscopy on a Combine Harvester. Precis. Agric. 2015, 16, 261–274. [Google Scholar] [CrossRef]
- Franceschini, M.H.D.; Demattê, J.A.M.; Kooistra, L.; Bartholomeus, H.; Rizzo, R.; Fongaro, C.T.; Molin, J.P. Effects of External Factors on Soil Reflectance Measured On-the-Go and Assessment of Potential Spectral Correction through Orthogonalisation and Standardisation Procedures. Soil. Tillage Res. 2018, 177, 19–36. [Google Scholar] [CrossRef]
- Kuang, B.; Tekin, Y.; Mouazen, A.M. Comparison between Artificial Neural Network and Partial Least Squares for On-Line Visible and near Infrared Spectroscopy Measurement of Soil Organic Carbon, PH and Clay Content. Soil. Tillage Res. 2015, 146, 243–252. [Google Scholar] [CrossRef]
- Morellos, A.; Pantazi, X.E.; Moshou, D.; Alexandridis, T.; Whetton, R.; Tziotzios, G.; Wiebensohn, J.; Bill, R.; Mouazen, A.M. Machine Learning Based Prediction of Soil Total Nitrogen, Organic Carbon and Moisture Content by Using VIS-NIR Spectroscopy. Biosyst. Eng. 2016, 152, 104–116. [Google Scholar] [CrossRef]
- Ji, W.; Viscarra Rossel, R.A.; Shi, Z. Improved Estimates of Organic Carbon Using Proximally Sensed Vis-NIR Spectra Corrected by Piecewise Direct Standardization. Eur. J. Soil. Sci. 2015, 66, 670–678. [Google Scholar] [CrossRef]
- Ji, W.; Viscarra Rossel, R.A.; Shi, Z. Accounting for the Effects of Water and the Environment on Proximally Sensed Vis-NIR Soil Spectra and Their Calibrations. Eur. J. Soil. Sci. 2015, 66, 555–565. [Google Scholar] [CrossRef]
- Nawi, N.M.; Chen, G.; Jensen, T.; Mehdizadeh, S.A. Prediction and Classification of Sugar Content of Sugarcane Based on Skin Scanning Using Visible and Shortwave near Infrared. Biosyst. Eng. 2013, 115, 154–161. [Google Scholar] [CrossRef]
- Nawi, N.M.; Chen, G.; Jensen, T. Visible and Shortwave near Infrared Spectroscopy for Predicting Sugar Content of Sugarcane Based on a Cross-Sectional Scanning Method. J. Near Infrared Spectrosc. 2013, 21, 289–297. [Google Scholar] [CrossRef]
- Nawi, N.M.; Chen, G.; Jensen, T. In-Field Measurement and Sampling Technologies for Monitoring Quality in the Sugarcane Industry: A Review. Precis. Agric. 2014, 15, 684–703. [Google Scholar] [CrossRef]
- Phetpan, K.; Udompetaikul, V.; Sirisomboon, P. An Online Visible and Near-Infrared Spectroscopic Technique for the Real-Time Evaluation of the Soluble Solids Content of Sugarcane Billets on an Elevator Conveyor. Comput. Electron. Agric. 2018, 154, 460–466. [Google Scholar] [CrossRef]
- Udompetaikul, V.; Phetpan, K.; Sirisomboon, P. Development of the Partial Least-Squares Model to Determine the Soluble Solids Content of Sugarcane Billets on an Elevator Conveyor. Measurement 2021, 167, 107898. [Google Scholar] [CrossRef]
- Mayrink, G.O.; Valente, D.S.M.; Queiroz, D.M.; Pinto, F.A.C.; Teofilo, R.F. Determination of Chemical Soil Properties Using Diffuse Reflectance and Ion-Exchange Resins. Precis. Agric. 2019, 20, 541–561. [Google Scholar] [CrossRef]
- Delefortrie, S.; Saey, T.; De Pue, J.; Van De Vijver, E.; De Smedt, P.; Van Meirvenne, M. Evaluating Corrections for a Horizontal Offset between Sensor and Position Data for Surveys on Land. Precis. Agric. 2016, 17, 349–364. [Google Scholar] [CrossRef]
- Magalhães, P.S.G.; Cerri, D.G.P. Yield Monitoring of Sugar Cane. Biosyst. Eng. 2007, 96, 1–6. [Google Scholar] [CrossRef]
- Kennard, R.W.; Stone, L.A. Computer Aided Design of Experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-Trending of near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Savitzky, A.; Golay, M.J.E. Smoothing and Differentiation of Data by Simplified Least Squares Procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Oliveri, P.; Malegori, C.; Simonetti, R.; Casale, M. The Impact of Signal Pre-Processing on the Final Interpretation of Analytical Outcomes—A Tutorial. Anal. Chim. Acta 2019, 1058, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Veltkamp, D.J.; Kowalski, B.R. Multivariate Instrument Standardization. Anal. Chem. 1991, 63, 2750–2756. [Google Scholar] [CrossRef]
- Feudale, R.N.; Woody, N.A.; Tan, H.; Myles, A.J.; Brown, S.D.; Ferré, J. Transfer of Multivariate Calibration Models: A Review. Chemom. Intell. Lab. Syst. 2002, 64, 181–192. [Google Scholar] [CrossRef]
- Workman, J.J. A Review of Calibration Transfer Practices and Instrument Differences in Spectroscopy. Appl. Spectrosc. 2018, 72, 340–365. [Google Scholar] [CrossRef]
- Wold, S.; Martens, H.; Wold, H. The Multivariate Calibration Problem in Chemistry Solved by the PLS Method. In Matrix Pencils; Springer: Berlin/Heidelberg, Germany, 1983; pp. 286–293. [Google Scholar]
- Corrêdo, L.d.P.; Maldaner, L.F.; Bazame, H.C.; Molin, J.P. Evaluation of Minimum Preparation Sampling Strategies for Sugarcane Quality Prediction by Vis-Nir Spectroscopy. Sensors 2021, 21, 2195. [Google Scholar] [CrossRef]
- Pomares-Viciana, T.; Martínez-Valdivieso, D.; Font, R.; Gómez, P.; del Río-Celestino, M. Characterisation and Prediction of Carbohydrate Content in Zucchini Fruit Using near Infrared Spectroscopy. J. Sci. Food Agric. 2018, 98, 1703–1711. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- 44. ASTM E1655-17; Standard Practices for Infrared Multivariate Quantitative Analysis. ASTM International: West Conshohocken, PA, USA, 2017; Volume 05.
- Bellon-Maurel, V.; Fernandez-Ahumada, E.; Palagos, B.; Roger, J.M.; McBratney, A. Critical Review of Chemometric Indicators Commonly Used for Assessing the Quality of the Prediction of Soil Attributes by NIR Spectroscopy. TrAC—Trends Anal. Chem. 2010, 29, 1073–1081. [Google Scholar] [CrossRef]
- Maldaner, L.F.; Molin, J.P. Data Processing within Rows for Sugarcane Yield Mapping. Sci. Agric. 2020, 77, e20180391. [Google Scholar] [CrossRef]
- Da Costa, M.V.A.; Fontes, C.H.; Carvalho, G.; Júnior, E.C.d.M. UltraBrix: A Device for Measuring the Soluble Solids Content in Sugarcane. Sustainability 2021, 13, 1227. [Google Scholar] [CrossRef]
- Workman, J., Jr.; Weyer, L. Practical Guide and Spectral Atlas for Interpretive Near-Infrared Spectroscopy; Routledge: London, UK, 2012; ISBN 9781439875261. [Google Scholar]
- Golic, M.; Walsh, K.; Lawson, P. Short-Wavelength near-Infrared Spectra of Sucrose, Glucose, and Fructose with Respect to Sugar Concentration and Temperature. Appl. Spectrosc. 2003, 57, 139–145. [Google Scholar] [CrossRef]
- Osborne, B.G. Near-Infrared Spectroscopy in Food Analysis. Encycl. Anal. Chem. 2006, 14. [Google Scholar] [CrossRef]
- Phuphaphud, A.; Saengprachatanarug, K.; Posom, J.; Maraphum, K.; Taira, E. Prediction of the Fibre Content of Sugarcane Stalk by Direct Scanning Using Visible-Shortwave near Infrared Spectroscopy. Vib. Spectrosc. 2019, 101, 71–80. [Google Scholar] [CrossRef]
- Maraphum, K.; Chuan-Udom, S.; Saengprachatanarug, K.; Wongpichet, S.; Posom, J.; Phuphaphud, A.; Taira, E. Effect of Waxy Material and Measurement Position of a Sugarcane Stalk on the Rapid Determination of Pol Value Using a Portable near Infrared Instrument. J. Near Infrared Spectrosc. 2018, 26, 287–296. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Moorman, T.B.; Novak, J.M.; Parkin, T.B.; Karlen, D.L.; Turco, R.F.; Konopka, A.E. Field-Scale Variability of Soil Properties in Central Iowa Soils. Soil. Sci. Soc. Am. J. 1994, 58, 1501–1511. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Magalhães, P.S.G.; Franco, H.C.J. Soil Attributes and Leaf Nitrogen Estimating Sugar Cane Quality Parameters: Brix, Pol and Fibre. Precis. Agric. 2013, 14, 270–289. [Google Scholar] [CrossRef]
- Johnson, R.M.; Richard, E.P. Sugarcane Yield, Sugarcane Quality, and Soil Variability in Louisiana. Agron. J. 2005, 97, 760–771. [Google Scholar] [CrossRef]
- Catelan, M.G.; Marques Júnior, J.; Siqueira, D.S.; Gomes, R.P.; Bahia, A.S.R.d.S. Sugarcane Yield and Quality Using Soil Magnetic Susceptibility. Sci. Agric. 2022, 79. [Google Scholar] [CrossRef]






| Property | Data Set | Min. | Max. | Mean | S.D. | C.V. (%) | Q1 | Q3 | Kurt. | Skew. |
|---|---|---|---|---|---|---|---|---|---|---|
| Brix (%) | All data | 20.10 | 24.05 | 22.09 | 0.73 | 3.30 | 21.63 | 22.55 | 3.43 | −0.06 |
| Cross-val. | 20.10 | 24.05 | 22.14 | 0.79 | 3.57 | 21.63 | 22.58 | 3.34 | −0.13 | |
| Pred. | 20.92 | 23.03 | 21.98 | 0.57 | 2.59 | 21.51 | 22.37 | 2.24 | −0.09 | |
| Pol (%) | All data | 17.68 | 22.55 | 19.92 | 0.85 | 4.27 | 19.50 | 20.35 | 4.15 | 0.20 |
| Cross-val. | 17.68 | 22.55 | 19.93 | 0.93 | 4.67 | 19.50 | 20.39 | 3.99 | 0.21 | |
| Pred. | 18.57 | 20.94 | 19.88 | 0.65 | 3.27 | 19.40 | 20.30 | 2.30 | −0.07 | |
| Fiber (%) | All data | 10.43 | 13.66 | 11.77 | 0.61 | 5.18 | 11.42 | 12.04 | 3.54 | 0.22 |
| Cross-val. | 10.43 | 13.66 | 11.77 | 0.62 | 5.27 | 11.50 | 11.99 | 3.92 | 0.46 | |
| Pred. | 10.44 | 12.67 | 11.78 | 0.60 | 5.09 | 11.37 | 12.25 | 2.50 | −0.45 | |
| Pol of cane (%) | All data | 14.86 | 19.16 | 16.93 | 0.74 | 4.37 | 16.56 | 17.30 | 4.41 | 0.00 |
| Cross-val. | 14.86 | 19.16 | 16.95 | 0.79 | 4.66 | 16.57 | 17.19 | 4.42 | 0.03 | |
| Pred. | 15.73 | 17.87 | 16.90 | 0.59 | 3.49 | 16.37 | 17.36 | 2.05 | −0.29 | |
| All data | 147.84 | 187.82 | 167.37 | 6.82 | 4.07 | 163.98 | 170.77 | 4.41 | −0.05 | |
| TRS (kg Mg−1) | Cross-val. | 147.84 | 187.82 | 167.53 | 7.32 | 4.37 | 164.05 | 169.67 | 4.45 | −0.03 |
| Pred. | 156.13 | 176.15 | 166.98 | 5.55 | 3.32 | 161.25 | 171.01 | 2.02 | −0.28 |
| Field | Property | Model Fit | C0 | C0 + C1 | A (m) | C0/(C0 + C1) |
|---|---|---|---|---|---|---|
| FA | Brix (%) | Exponential | 0.060 | 0.098 | 33.62 | 0.61 |
| Pol (%) | Spherical | 0.091 | 0.223 | 71.53 | 0.41 | |
| Fiber (%) | Gaussian | 0.036 | 0.043 | 92.93 | 0.84 | |
| Pol of cane (%) | Spherical | 0.071 | 0.161 | 75.74 | 0.44 | |
| TRS (kg Mg−1) | Spherical | 6.250 | 13.019 | 73.78 | 0.48 | |
| FB | Brix (%) | Exponential | 0.252 | 0.363 | 64.48 | 0.69 |
| Pol (%) | Spherical | 0.349 | 0.444 | 62.98 | 0.79 | |
| Fiber (%) | Spherical | 0.108 | 0.117 | 264.23 | 0.92 | |
| Pol of cane (%) | Spherical | 0.293 | 0.360 | 66.12 | 0.81 | |
| TRS (kg Mg−1) | Spherical | 25.176 | 31.016 | 66.01 | 0.81 | |
| FC | Brix (%) | Exponential | 0.381 | 0.517 | 38.76 | 0.74 |
| Pol (%) | Exponential | 0.500 | 0.629 | 58.34 | 0.79 | |
| Fiber (%) | Spherical | 0.136 | 0.212 | 118.73 | 0.64 | |
| Pol of cane (%) | Spherical | 0.396 | 0.508 | 93.10 | 0.78 | |
| TRS (kg Mg−1) | Spherical | 34.424 | 44.395 | 97.67 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêdo, L.d.P.; Molin, J.P.; Canal Filho, R. Is It Possible to Measure the Quality of Sugarcane in Real-Time during Harvesting Using Onboard NIR Spectroscopy? AgriEngineering 2024, 6, 64-80. https://doi.org/10.3390/agriengineering6010005
Corrêdo LdP, Molin JP, Canal Filho R. Is It Possible to Measure the Quality of Sugarcane in Real-Time during Harvesting Using Onboard NIR Spectroscopy? AgriEngineering. 2024; 6(1):64-80. https://doi.org/10.3390/agriengineering6010005
Chicago/Turabian StyleCorrêdo, Lucas de Paula, José Paulo Molin, and Ricardo Canal Filho. 2024. "Is It Possible to Measure the Quality of Sugarcane in Real-Time during Harvesting Using Onboard NIR Spectroscopy?" AgriEngineering 6, no. 1: 64-80. https://doi.org/10.3390/agriengineering6010005
APA StyleCorrêdo, L. d. P., Molin, J. P., & Canal Filho, R. (2024). Is It Possible to Measure the Quality of Sugarcane in Real-Time during Harvesting Using Onboard NIR Spectroscopy? AgriEngineering, 6(1), 64-80. https://doi.org/10.3390/agriengineering6010005









