Abstract
Straw from no-till cropping systems, in addition to increasing the soil organic matter content, may also impede the movement of applied herbicides into the soil and, thus, alter the behavior and fate of these compounds in the environment. Rain or irrigation before or after an herbicide treatment can either help or hinder its movement through the straw, influencing weed control. Our objective was to develop a system for herbicide application and rain simulation, enabling the evaluation of the movement of various herbicides either in dry or wet straw under different rainfall volumes (25, 50, 75, and 100 mm). The amount of the applied herbicides that moved through the straw were collected and measured using a liquid chromatograph with a tandem mass spectrometry system (LC-MS/MS). Measurements obtained with the developed system showed a high herbicide treatment uniformity across all replications. The movement of the active ingredients through the straw showed variability that was a function of the applied herbicide, ranging from 17% to 99%. In wet straw, the collected herbicide remained constant from 50 to 100 mm of simulated rainfall. For the wet straw, the decreasing percentages of the herbicide movement through straw to the soil were sulfentrazone (99%), atrazine and diuron (91% each), hexazinone (84%), fomesafen (80.4%), indaziflam (79%), glyphosate (63%), haloxyfop-p-methyl (45%), and S-metolachlor (27%). On the dry straw, the decreasing percentages of the herbicide movement were fomesafen (88%), sulfentrazone (74%), atrazine (69.4%), hexazinone (69%), diuron (68.4%), glyphosate (48%), indaziflam (34.4%), S-metolachlor (22%), and haloxyfop-p-methyl (18%). Overall, herbicide movement was higher in wet straw (with a previous 25 mm simulated rainfall layer) than in dry straw. Some herbicides, like haloxyfop-p-methyl and indaziflam, exhibited over 50% higher movement in wet straw than dry straw after 100 mm of simulated rain. The developed system can be adapted for various uses, serving as a valuable tool to evaluate the behavior of hazardous substances in different agricultural and environmental scenarios.
1. Introduction
Understanding the behavior and fate of herbicides in the environment is a pivotal concern in both agriculture and ecology [1]. Herbicides are substances designed to control weeds, but their interaction with the environment can have significant impacts on non-target organisms [2]. These chemicals have the potential to be transported through water, air, or soil, extending their impact beyond the initially treated areas [3]. The mobility of herbicides is influenced by various physicochemical characteristics, including water solubility, soil adsorption, and persistence, among others [1,4]. Some herbicides may even leach into groundwater, posing a threat to the quality of drinking water and potentially affecting human health and surrounding vegetation [5,6]. In addition, the interaction of herbicides with non-target vegetation can lead to ecological impacts [7].
The soil, characterized as a complex, open, and dynamic system, plays a pivotal role as the primary fate for herbicides [1]. Within this intricate environment, the redistribution and degradation of these products take place at a variable rate for each herbicide. This process is rapid, usually taking only a few days for non-persistent herbicides, while it can extend over months or even years for moderately and highly persistent herbicides [8]. The widespread adoption of no-till systems in Brazil introduces an additional factor affecting the fate of herbicides due to the presence of straw (plant residues) [9,10].
Brazil is the world’s main producer of sugarcane and contributes to more than 40% of the production, which is mainly intended to meet the global demand for bioenergy with the aim of reducing dependence on crude oil [11]. Historically, burning straw was a common practice to facilitate the harvest of sugarcane; however, these fires emitted significant atmospheric pollutants, including aerosols, gases, and various hydrocarbons, posing a risk to public health [12]. Consequently, in 2007, the government of the state of São Paulo, the main national producer of sugarcane, established a ‘green ethanol’ production protocol, with the aim of ending pre-harvest burning by 2014 [13]. This protocol gave rise to the Green Harvest or Raw Cane production method [14], which involves the accumulation and deposition of substantial quantities of plant residues (straw) on the soil surface, forcing the direct sowing of sugarcane under no-till systems [11].
Straw diminishes weed competition by affecting the dormancy, germination, and mortality rates of seeds, leading to alterations in the weed community [15]. However, the nature of these changes is highly specific, contingent upon the type and quantity of straw, and particularly the weed species involved. Moreover, certain species have adapted to the presence of straw. For instance, herbicide-resistant weeds, including those from the genera Amaranthus, Conyza, and Lolium, as well as species like Digitaria insularis, Eleusine indica, and Euphorbia heterophylla are well adapted to non-tillage systems in Brazil [16,17], necessitating additional measures such as chemical control.
Despite the numerous agricultural and environmental benefits and functionalities of straw [18], its presence has a significant impact on the transport of herbicides into the soil. This is due to the herbicides adhering to the surface of the straw, which compromises the management of difficult-to-control weeds and significantly influences the final fate of herbicides in the environment [19,20]. This influence arises from the increase in the soil organic matter content, leading to a strong sorption of herbicides [21,22,23,24,25]. The retention of herbicides in the straw impedes the movement of these products through water, preventing them from reaching the soil; in addition to reducing their effectiveness, this can also prolong their persistence in the environment [26,27]. Therefore, precipitation or irrigation, whether before or after application, regulates the transfer of herbicides to the soil with straw acting as an intermediary [19,26].
The methodologies employed to investigate the movement of herbicides from straw to soil typically involve bioassay, stationary sprayers, and field rain simulators [26,27,28,29]. Bioassays usually simulate field conditions in a controlled environment using columns that allow the subdivision of the substrate or soil into layers after herbicide application and the simulation of rainfall. Following this, bioindicator plants are germinated in these soil layers, making it possible to determine the leaching depth of the herbicide through the soil profile based on the phytotoxic symptoms presented by these plants, which are often highly susceptible to the evaluated herbicide(s) [30,31]. In the case of field rainfall simulators, the area where rainfall is simulated is usually small (1 m2), requiring multiple simulators or additional time to apply all the desired rainfall layers in the experimental plots [29]. In both bioassays and field experiments, herbicides are applied using conventional methods, either with backpack or tractor sprayers. In contrast, stationary sprayer allows for both the application of the herbicide and the simulation of rain on columns or trays; however, it permits the evaluation of only one herbicide or one rain layer at a time. Some of these approaches are followed by the chromatographic analysis of the rearranged solution [26,27,28], but additional methods of extraction of the leached herbicide solution are required.
As can be seen, each of these approaches has several limitations, such as low precision, repeatability issues, logistical challenges, or a limited number of herbicides or rain layers to evaluate. Therefore, the objective of this study was to develop a system for herbicide application and rainfall simulation that allows the simultaneous assessment of the movement and transfer of various herbicides in dry or wet sugarcane straw under different rainfall volumes. Additionally, this system aims to expedite the collection of the moved herbicide solution samples for quantification using an LC-MS/MS system, ultimately enhancing the precision of the technique. In this initial study for the development of the system, sugarcane straw was used in consideration of the economic importance of this crop. Additionally, these results could serve as a basis to optimize the system and evaluate different types of straw, substrates, soils, and herbicides in future studies.
2. Materials and Methods
2.1. Experimental Conditions
The movement of atrazine, diuron, fomesafen, glyphosate, haloxyfop-p-methyl, hexazinone, indaziflam, S-metolachlor, and sulfentrazone was determined under two application conditions: (1) on wet straw, and (2) on dry straw. The main physicochemical and agronomic characteristics of these herbicides are summarized in Table S1. The herbicides were applied separately to sugarcane straw, cut into 0.5 × 0.5 cm sections from plants without herbicide application, and contained in polypropylene capsules (application area = 15.6 cm2). The amount of straw used corresponded to 10 t ha−1.
The herbicide solutions were prepared at their respective field doses, resulting in different concentrations (Table 1); nevertheless, a consistent volume of 40 µL, equivalent to 250 L·ha−1, was applied to each capsule. The solution was administered to the capsules using a repeating pipette. In treatments involving wet straw, a 25 mm rainfall layer preceded herbicide application to simulate the washing of target plants after rain or irrigation. In treatments on dry straw, 40 µL of herbicide solution was diluted in water (with a volume equivalent to 25 mm of rain) and applied simultaneously with the first layer of simulated rain. One-hour post-herbicide application, three distinct layers (50, 75, or 100 mm) of simulated rain were evaluated, both on dry and wet straw. The experiment adhered to a completely randomized design with four replications per treatment.

Table 1.
Herbicide, trade name, dose (g·ai·ha−1) and type of formulation of the products used to evaluate the movement of herbicides in sugarcane straw.
2.2. System of Herbicide Application and Rain Simulation
The herbicide application and rainfall simulation were conducted using a stationary simulator (Figure 1), consisting of a fixed table and a movable table.
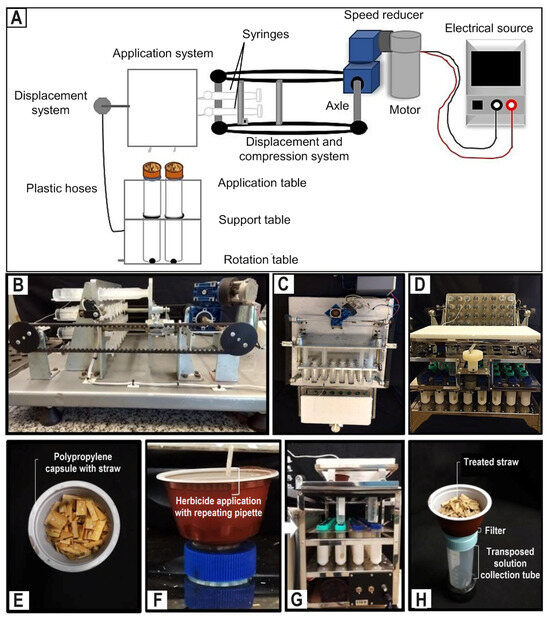
Figure 1.
(A) Scheme of the system developed for herbicide application and rain simulation. Sequence of the equipment structures: (B) side view of the syringe compression system; (C) top view of the rain application and simulation table; and (D) front view of the rain application and simulation table. Sequence of herbicide application or rain simulation: (E) polypropylene capsule with straw treated with repeat pipette; (F) Falcon collection tube with capsule when applying herbicides or simulating rain; (G) herbicide solution in movement to be collected (white arrow); and (H) collection of the herbicide solution for chromatographic analysis.
The equipment was powered using a 12-volt motor, driven by a power source and connected to two reducers. On the fixed table, 32 syringes of 60 mL, containing the desired volumes of water or herbicide solution, were securely affixed. The mobile table was horizontally displaced by 18 cm through the action of two straps that exert pressure on the pistons. To initiate the process, the volumes of water or herbicide solution from the syringes were injected into plastic hoses with a diameter of 3.3 mm. These hoses were positioned 1.5 cm above the surface of the capsules, where a repeating pipette was attached. These hoses were linked to a perforated structure that facilitated horizontal movement, while the collector, a 50 mL Falcon tube, along with the polypropylene capsule, rotated 360°. This design ensured a uniform distribution of the liquid across the entire capsule area. To prevent the passage of impurities, a perforated lid with a fixed filter was attached to the Falcon tube. Once the predetermined rainfall volumes were attained, the solutions that moved through the sugarcane straw were carefully collected within 10–15 min after the rain simulation. This collection took place once water ceased draining from the treated straw and was stored for subsequent chromatographic analysis.
2.3. Herbicide Analytical Analysis
Aliquots of the collected solution that passed through the sugarcane straw after each rainfall simulation were filtered (0.45 µm) and analyzed using LC-MS/MS. The analysis employed a system comprising a high-performance liquid chromatograph (Proeminence UFLC, Shimadzu Corporation, Kyoto, Japan) coupled to a hybrid triple quadrupole mass spectrometer (4500, Triple Quad, AB Sciex, Framingham, MA, USA). This system had high sensitivity and repeatability, with low noise and simultaneous measurement of several compounds, maintaining a constant relationship between signal intensity (chromatographic peak area) and the concentration of different compounds expressed in molar units.
The analysis involved a total running time of 20, 30, and 100 s for the studied herbicides, with a retention time (RT) in the chromatographic column of 0.71 for glyphosate and an average of 5.0 min for the other herbicides. Table 2 provides detailed information on the chromatographic conditions for quantifying the herbicides evaluated, and Figure S1 displays chromatograms illustrating them.

Table 2.
Chromatographic conditions used to quantify the herbicides moved through the sugarcane straw in different rainfall levels.
Calibration curves were established for each herbicide using high-purity analytical standards exceeding 98% purity. These curves facilitated the quantification of the transported amount of each herbicide. Herbicide concentrations were converted into mass (g), accounting for the volume of the rinsate solution.
2.4. Statistical Analysis
Data obtained from chromatographic analyses were corrected according to the volume of rainfall and converted into g·ia·ha−1. Subsequently, normality and homogeneity tests were conducted following statistical precepts.
The behavior of the herbicide movement concerning rainfall volumes was determined by fitting the data to Mitscherlich’s non-linear regression model: y = a(1 − 10−c(0+x)) [32], where a is the asymptote of the model, corresponding to the maximum amount of herbicide moved through the straw, c is the concavity of the curve, determining the exit speed of the herbicide from the straw, x is the volume of rain necessary to move the herbicide (mm), and y is the total amount of herbicide transferred from the 10 t·ha−1 layer of straw. This model is employed to express the relationship between a stimulus or time variable and a response, with applications particularly in the field of measurement reliability [33].
The data obtained from the conditions of application directly to the straw and through the rain volumes were compared using the Tukey test at a 5% probability. Statistical analyses were performed using the SAS program (SAS Institute, version 9.1.3, Cary, NC, USA), and the graphics were generated using the SigmaPlot program (Version 12.5, Systat Software, San Jose, CA, USA).
3. Results
This entire system and methodology developed for herbicide application and rain simulation in this work were devised to mimic realistic rain conditions and herbicide application on straw. The system demonstrated remarkable treatment uniformity (Figure 1), as confirmed via LC-MS/MS analysis, ensuring a robust level of results.
The movement of active ingredients into sugarcane straw exhibited variability depending on the herbicide, ranging from 17% (haloxyfop-p-methyl in dry straw) to 99% (sulfentrazone in wet straw). When herbicides were applied to wet straw, herbicide movement remained constant from 50 to 100 mm of simulated rain. This suggests that the initial 25 mm rain layer before treatment created a favorable environment for the movement of herbicides through straw, regardless of subsequent rainfall volume. The decreasing order of movement in relation to the theorical total applied herbicide (g·ai·ha−1) was: sulfentrazone (99%), atrazine (91%), diuron (91%), hexazinone (84%), fomesafem (80.4%), indaziflam (79%), glyphosate (63%), haloxyfop-P-methyl (45%), and S-metolachlor (27%) (Figure 2).
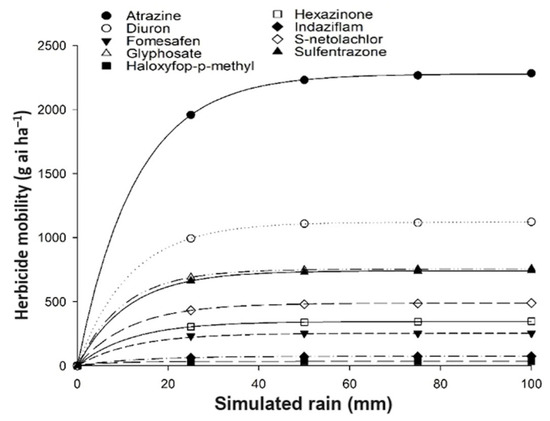
Figure 2.
Data adjusted using the Mitscherlich model for maximum amounts of herbicides extracted in 10 t·ha−1 of wet sugarcane straw (25 mm of simulated rainfall before treatment) of sugarcane after different simulations of rainfall (mm).
In dry straw, the herbicide movement rates followed a descending order: fomesafen (88%), sulfentrazone (74%), atrazine (69.4%), hexazinone (69%), diuron (68.4%), glyphosate (48%), indaziflam (34.4%), S-metolachlor (22%), and haloxyfop-p-methyl (18%) (Figure 3). Notably, the movement of atrazine, diuron, and sulfentrazone increased with the rise in simulated rainfall volume. In contrast, the movement rates of the remaining herbicides remained constant, irrespective of the simulated rainfall volume ranging from 50 mm to 100 mm. This shows how different herbicides interact with dry straw under varying rainfall conditions compared to wet straw.
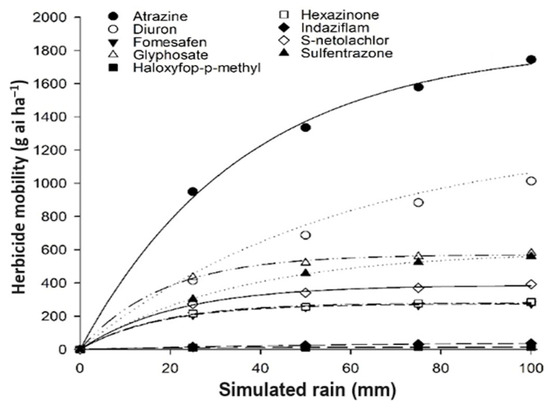
Figure 3.
Data adjusted using the Mitscherlich model for maximum extracted amounts of herbicides in 10 t ha−1 of dry sugarcane straw after different rainfall simulations (mm).
All herbicides present good fits to the nonlinear regression model in the two conditions evaluated (dry and wet straw) with R2 values greater than 99% (Table 3).

Table 3.
Parameters of Mitscherlich’s non-linear regression model fitted as a function of the herbicide content moved through sugarcane straw (10 t·ha−1) under two distinct application conditions, following the simulation of accumulated rainfall (mm).
Overall, herbicide movement was higher in wet straw than in dry straw. Some herbicides, such as haloxyfop-P-methyl and indaziflam, exhibited an increase of over 50% in wet straw compared to dry straw after accumulating 100 mm of simulated rain. Most other herbicides showed increases ranging from 17% to 25%. Diuron and fomesafen demonstrated increases of less than 10% in wet straw compared to dry straw (Table 4). This observed behavior may be linked to the formulation used and the solubility of the molecules in water, which can vary between high and low levels. The Koc values, ranging from moderately mobile to mobile (Table S1), influence herbicide movement under the evaluated conditions. Various factors, such as application period, application rate, type of coverage, intensity of irrigation or precipitation, infiltration rate, and physical-chemical properties of the product and soil, contribute to the concentration and distance of herbicide transport [1,3,4,20].

Table 4.
Percentage increase in the movement of herbicides applied to wet straw (previous simulated rain of 25 mm) in relation to dry straw, after accumulated rain of 100 mm.
4. Discussion
The herbicides studied were selected for their different physicochemical characteristics, which affect how they behave in the environment [1]. They come from different chemical groups, work in different ways, and can target specific weeds or a wide range of them, before or after they sprout. This diversity helps us understand how herbicides move in different levels of moisture and rainfall, giving us a broad perspective.
The literature provides various approaches to explore the movement of herbicides through straw. In a bioassay assessing the movement of atrazine in soils with two densities of straw (4.5 and 9.0 t·ha−1), herbicide application was conducted using doses ranging from 0 to 5 kg·ai·ha−1. The application was performed with a backpack sprayer, followed by a simulated 20 mm rain 24 h after treatment. The findings revealed that rain enhanced the movement of atrazine through the straw, leading to an improvement in control efficiency (high toxicity levels), especially at a dose of 1.25 kg ai ha−1 [28].
Several researchers have employed a stationary boom sprayer (conditioned within a room) equipped with four XR-11002 nozzles spaced 0.5 m apart and positioned 0.5 m above the target surface, including pots or polypropylene capsules containing 10 t·ha−1 of straw). The herbicide spray volume was set at 200 L·ha−1, and rain simulation was conducted using an automatic hydraulic pump with three high-flow TK-SS-20 nozzles spaced 0.5 m apart to ensure rain uniformity. Each pass of the simulator applied a 2.5 mm layer of rain. Following the rain simulations, the findings indicated that the herbicides diuron, sulfentrazone, and metribuzin moved through the straw after receiving 35 mm, 20 mm, and 21.5 mm of rain, respectively. These rain events resulted in extractions of 66%, 76.5%, and 99%, indicating the extent to which the herbicides moved through the straw under different rainfall conditions [27,34,35,36].
Souza [29] developed a rain simulator designed for field conditions. The apparatus consists of a rectangular structure with four adjustable legs, featuring a centrally positioned Veejet-type sprinkler nozzle (model 80100) at a height of 3 m above the ground. During operation, the nozzle’s oscillation, covering a designated 1 m area, is controlled using a mechanical system that determines the number of oscillations. A pump injects water into the nozzle, maintaining a constant pressure of 4 kgf·cm−2 (6 PSI), facilitating the movement of herbicide molecules. Evaluating the retention potential of herbicides hexazinone and diuron in straw, the presence of 7 t·ha−1 of sugarcane straw proved effective in reducing the loss of diuron after a three-day rain period. However, due to its high solubility, hexazinone exhibited a high rate of mobility [37]. Droplet impact energy and intensity, which can vary under simulated rainfall conditions, may influence the dynamics of herbicides in the environment [38]; therefore, comprehending the kinetic energy of precipitation from rain simulators is crucial. Natural conditions exert a higher impact on the soil compared to simulated rainfalls, lacking characteristics comparable to natural rainfall, such as droplet size, terminal velocity, and kinetic energy [39].
The movement of the herbicides assessed in this study was intricately linked to the moisture content in the straw. Application on dry straw resulted in herbicide movement aligned with precipitation intensity, meaning the transfer of these products through the straw increased or decreased depending on the volume of rain. Dry straw has the capacity to retain the herbicide on the surface and in macropores, impeding their movement and increasing its residuality [40]. Conversely, applying herbicides on wet straw led to notable movement, even with low rainfall intensity, signifying elevated environmental humidity. This condition facilitated the washing and leaching of herbicides in straw [1,41], suggesting that, under field conditions, larger amounts of herbicides could reach the soil. This wet condition is crucial for enhancing the effectiveness of pre-emergent herbicides [42]; therefore, applying herbicides like atrazine, diuron, hexazinone, S-metolachlor, and sulfentrazone on wet straw could result in improved performance in weed control. Conversely, the effectiveness of post-emergence herbicides with high leaching potential is impacted by humid conditions during application [43].
The system devised in this study differs significantly from conventional methods due to its exceptional precision in dosage. It enables volumetric application of simulated rainwater and regulation of water application rates, setting it apart from its counterparts. This device has the potential to analyze the movement of various herbicides across different types of straw, substrates, soils, and under various precipitation and irrigation conditions. In other words, it could be adapted for various applications, making it a valuable tool for understanding the behavior of other pesticides and harmful substances in different agricultural and environmental contexts. Additionally, the findings of this study can aid in establishing the optimal timing for applying the evaluated herbicides, whether it should be before or after rainfall or irrigation, depending on the specific compound to be used. Furthermore, the results can contribute to optimizing dosage by considering the amount of herbicide moved in relation to precipitation, with the objective of minimizing environmental impact. However, a limitation arises due to the minimal impact of the droplets on the straw, leading to limited input of kinetic energy into the system. This limitation seems to have compromised the transport of herbicides that do not form true solutions in water, such as indaziflam.
5. Conclusions
In this study, we developed a system to stimulate rainfall and investigate how herbicides move through sugarcane straw (10 t·ha−1) under dry and wet conditions, proving effective in both scenarios. By adjusting rainfall levels and ensuring consistent volumes in each experiment, we replicated the initial rainfall pattern following a field herbicide application across the treated area. The first method simulated rain without significantly affecting dry straw, ensuring uniformity. The second method represented conditions where the herbicide could have been washed from the plants or the top layers of straw. Herbicide movement was more pronounced when applied to wet straw (with a simulated rain layer of 25 mm) compared to dry straw, especially with subsequent layers of rain. It is important to note that our research focuses on understanding how easily herbicides, diluted or suspended in water, pass through or are retained by straw, rather than studying their removal from initial deposition targets. Therefore, interpreting herbicide movement into sugarcane straw through rainwater is crucial, always comparing results with conventional methodologies that consider the impact of raindrops on straw.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriengineering6010049/s1, Table S1. Main physicochemical and agronomic characteristics of herbicides evaluated in sugarcane straw transposition experiments; Figure S1. Chromatograms obtained for the different herbicides (50 L−1).
Author Contributions
Conceptualization, E.D.V. and C.A.C.; methodology, I.T.d.S., I.P.F.S.d.B., A.K.A.d.M., V.P.d.M. and G.C.M.; software, I.T.d.S. and R.A.-d.l.C.; validation, I.T.d.S., I.P.F.S.d.B., A.K.A.d.M., E.D.V. and C.A.C.; formal analysis, I.T.d.S., I.P.F.S.d.B., A.K.A.d.M. and R.A.-d.l.C.; investigation, I.T.d.S., I.P.F.S.d.B., A.K.A.d.M., V.P.d.M. and G.C.M.; resources, E.D.V. and C.A.C.; data curation, I.T.d.S., I.P.F.S.d.B. and A.K.A.d.M.; writing—original draft preparation, I.T.d.S.; writing—review and editing, R.A.-d.l.C.; visualization, I.T.d.S., I.P.F.S.d.B., A.K.A.d.M., V.P.d.M., G.C.M., P.O.d.A., E.D.V. and C.A.C.; supervision, I.P.F.S.d.B. and A.K.A.d.M.; project administration, E.D.V. and C.A.C.; funding acquisition, E.D.V. and C.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) through the granting of a postgraduate scholarship to the first author (I.T.d.S.).
Data Availability Statement
Data will be made available on request.
Acknowledgments
R.A.-d.l.C. thanks to the ‘FEPAF—Fundação de Estudos e Pesquisas Agrícolas e Florestais’ (Project 2224).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- De Prado, R.; Palma-Bautista, C.; Vázquez-García, J.G.; Alcántara-de la Cruz, R. Influence of herbicide environmental behavior on weed management. In Interactions of Biochar and Herbicides in the Environment, 1st ed.; Mendes, K., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 53–77. [Google Scholar]
- Havens, P.L.; Sims, G.K.; Erhardt-Zabik, S. Fate of herbicides in the environment. In Handbook of Weed Management Systems, 1st ed.; Smith, A.E., Ed.; Routledge: New York, NY, USA; pp. 245–278.
- Bertuzzo, E.; Thomet, M.; Botter, G.; Rinaldo, A. Catchment-scale herbicides transport: Theory and application. Adv. Water Resour. 2013, 52, 232–242. [Google Scholar] [CrossRef]
- Krähmer, H.; Walter, H.; Jeschke, P.; Haaf, K.; Baur, P.; Evans, R. What makes a molecule a pre-or a post-herbicide–how valuable are physicochemical parameters for their design? Pest Manag. Sci. 2021, 77, 4863–4873. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.; Jefferson, B.; Jarvis, P. Pesticide removal from drinking water sources by adsorption: A review. Environ. Technol. Rev. 2019, 8, 1–24. [Google Scholar] [CrossRef]
- Jayasumana, C.; Paranagama, P.; Agampodi, S.; Wijewardane, C.; Gunatilake, S.; Siribaddana, S. Drinking well water and occupational exposure to herbicides is associated with chronic kidney disease, in Padavi-Sripura, Sri Lanka. Environ. Health 2015, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Egan, J.F.; Bohnenblust, E.; Goslee, S.; Mortensen, D.; Tooker, J. Herbicide drift can affect plant and arthropod communities. Agric. Ecosyst. Environ. 2014, 185, 77–87. [Google Scholar] [CrossRef]
- Curran, W.S. Persistence of herbicides in soil. Crops Soils 2016, 49, 16–21. [Google Scholar] [CrossRef]
- Cessna, A.J.; McConkey, B.G.; Elliott, J.A. Herbicide transport in surface runoff from conventional and zero-tillage fields. J. Environ. Qual. 2013, 42, 782–793. [Google Scholar] [CrossRef]
- Fuentes-Llanillo, R.; Telles, T.S.; Junior, D.S.; de Melo, T.R.; Friedrich, T.; Kassam, A. Expansion of no-tillage practice in conservation agriculture in Brazil. Soil Tillage Res. 2021, 208, 104877. [Google Scholar] [CrossRef]
- Bordonal, R.O.; Carvalho, J.L.N.; Lal, R.; Figuereido, E.B.; Oliveira, B.G.; Scala, N.L., Jr. Sustainability of sugarcane production in Brazil. A review. Agron. Sustain. Dev. 2018, 38, 13. [Google Scholar] [CrossRef]
- Paraiso, M.L.D.S.; Gouveia, N. Health risks due to pre-harvesting sugarcane burning in São Paulo State, Brazil. Rev. Bras. Epidemiol. 2015, 18, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, D.A.; Rudorff, B.F.T.; Silva, W.F.; Adami, M.; Mello, M.P. Remote sensing images in support of environmental protocol: Monitoring the sugarcane harvest in São Paulo State, Brazil. Remote Sens. 2011, 3, 2682–2703. [Google Scholar] [CrossRef]
- Rachid, C.T.C.C.; Santos, A.L.; Piccolo, M.C.; Balieiro, F.C.; Coutinho, H.L.; Peixoto, R.S.; Tiedje, J.M.; Rosado, A.S. Effect of sugarcane burning or green harvest methods on the Brazilian Cerrado soil bacterial community structure. PLoS ONE 2013, 8, e59342. [Google Scholar] [CrossRef] [PubMed]
- Franczuk, J.; Kosterna, E.; Zaniewicz-Bajkowska, A. Weed-control effects on different types of cover-crop mulches. Acta Agric. Scand. B Soil Plant Sci. 2010, 60, 472–479. [Google Scholar] [CrossRef]
- Alcántara-de la Cruz, R.; Oliveira, G.M.; Carvalho, L.B.; Silva, M.F.G.F. Herbicide resistance in Brazil: Status, impacts, and future challenges. In Herbicides—Current Research and Case Studies in Use; Price, A., Kelton, J., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Gaines, T.A.; Slavov, G.T.; Hughes, D.; Küpper, A.; Sparks, C.D.; Oliva, J.; Vila-Aiub, M.M.; Garcia, M.A.; Merotto, A., Jr.; Neve, P. Investigating the origins and evolution of a glyphosate-resistant weed invasion in South America. Mol. Ecol. 2021, 30, 5360–5372. [Google Scholar] [CrossRef]
- Iqbal, R.; Raza, M.A.S.; Valipour, M.; Saleem, M.F.; Zaheer, M.S.; Ahmad, S.; Toleikiene, M.; Haider, I.; Nazar, M.A. Potential agricultural and environmental benefits of mulches—A review. Bull. Natl. Res. Cent. 2020, 44, 75. [Google Scholar] [CrossRef]
- Prado, A.B.C.A.; Obara, F.E.B.; Brunharo, C.A.G.; Melo, M.S.C.; Christoffoleti, P.J.; Alves, M.C. Dynamic of herbicides applied in preemergence on sugarcane straw under different water regimes. Rev. Bras. Herbic. 2013, 12, 179–187. [Google Scholar]
- Tonieto, T.A.P.; Regitano, J.B. Effects of straw decomposition degree on leaching and weed control efficacy of tebuthiuron and hexazinone in green sugarcane harvesting. Planta Daninha 2014, 32, 809–815. [Google Scholar] [CrossRef]
- Sun, K.; Gao, B.; Ro, K.S.; Novak, J.M.; Wang, Z.; Herbert, S.; Xing, B. Assessment of herbicide sorption by biochars and organic matter associated with soil and sediment. Environ. Pollut. 2012, 163, 167–173. [Google Scholar] [CrossRef]
- Takeshita, V.; Mendes, K.F.; Alonso, F.G.; Tornisielo, V.L. Effect of organic matter on the behavior and control effectiveness of herbicides in soil. Planta Daninha 2019, 37, e019214401. [Google Scholar] [CrossRef]
- Bonfleur, E.J.; Kookana, R.S.; Tornisielo, V.L.; Regitano, J.B. Organomineral interactions and herbicide sorption in Brazilian tropical and subtropical Oxisols under no-tillage. J. Agric. Food Chem. 2016, 64, 3925–3934. [Google Scholar] [CrossRef]
- Reddy, K.N.; Locke, M.A.; Wagner, S.C.; Zablotowicz, R.M.; Gaston, L.A.; Smeda, R.K. Chlorimuron ethyl sorption and desorption kinetics in soil and herbicide-desiccated cover crop residues. J. Agric. Food Chem. 1995, 10, 2752–2757. [Google Scholar] [CrossRef]
- Munhoz-Garcia, G.V.; Takeshita, V.; Pimpinato, R.F.; de Moraes, N.G.; Nalin, D.; Tornisielo, V.L. Cover crop straw interferes in the retention and availability of diclosulam and diuron in the environment. Agronomy 2023, 13, 1725. [Google Scholar] [CrossRef]
- Fontes, J.R.A.; Silva, A.A.; Vieira, R.F.; Ramos, M.M. Leaching of herbicides applied with irrigation water under no-till systems. Planta Daninha 2004, 22, 623–631. [Google Scholar] [CrossRef]
- Carbonari, C.A.; Gomes, G.L.G.C.; Trindade, M.L.B.; Edivaldo, J.R.M.; Velini, E.D. Dynamics of sulfentrazone in sugarcane straw. Weed Sci. 2016, 64, 201–206. [Google Scholar] [CrossRef]
- Fornarolli, D.A.; Rodrigues, B.N.; Lima, J.; Valério, M.A. Influence of the mulch on the behavior of atrazine. Planta Daninha 1998, 16, 97–107. [Google Scholar] [CrossRef]
- Souza, M.D. Desenvolvimento e Utilização de um Simulador de Chuvas Para Estudos de Atributos Físicos e Químicos do Solo Relacionados a Impactos Ambientais; Documentos, 37; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2004. [Google Scholar]
- Futch, S.; Singh, M. Herbicide Mobility Using Soil Leaching Columns. Bull. Environ. Contam. Toxicol. 1999, 62, 520–529. [Google Scholar] [CrossRef]
- Silva Junior, A.C.D.; Gonçalves, C.G.; Queiroz, J.R.G.; Martins, D. Evaluation of leaching potential of tebuthiuron using bioindicator plants. Arq. Inst. Biol. 2018, 85, e0692015. [Google Scholar] [CrossRef]
- Mitscherlich, E.A. Das gesetz des minimums und das gesetz des abnehmenden bodenertrages. Landwirtsch. Jahrbücher 1909, 38, 537–552. [Google Scholar]
- Heidari, M.; Manju, M.A.; IJzerman-Boon, P.C.; van den Heuvel, E.R. D-Optimal Designs for the Mitscherlich Non-Linear Regression Function. Math. Methods Stat. 2022, 31, 1–17. [Google Scholar] [CrossRef]
- Jiang, L.; Dami, I.; Mathers, H.M.; Dick, W.A.; Doohan, D. The effect of straw mulch on simulated simazine leaching and runoff. Weed Sci. 2011, 59, 580–586. [Google Scholar] [CrossRef]
- Rossi, C.V.S.; Velini, E.D.; Luchini, L.C.; Negrisoli, E.; Correa, M.R.; Pivetta, J.P.; Costa, A.G.F.; Silva, F.M.L. Performance of metribuzin apllied on sugarcane straw. Planta Daninha 2013, 31, 223–230. [Google Scholar] [CrossRef]
- Tropaldi, L.; Carbonari, C.A.; de Brito, I.P.F.S.; de Matos, A.K.A.; de Moraes, C.P.; Velini, E.D. Dynamics of clomazone formulations combined with sulfentrazone in sugarcane (Saccharum spp.) straw. Agriculture 2021, 11, 854. [Google Scholar] [CrossRef]
- Vaz, L.R.L.; Barizon, R.R.M.; de Souza, A.J.; Regitano, J.B. Runoff of hexazinone and diuron in green cane systems. Water Air Soil Pollut. 2021, 232, 116. [Google Scholar] [CrossRef]
- Confessor, J.G.; Silva, L.L.; Araújo, P.M.S.D. An assessment of water and soil losses in pastures of the Brazilian Savanna using simulated rainfall. Soc. Nat. 2022, 34, e65618. [Google Scholar] [CrossRef]
- Jiang, F.; Zhan, Z.; Chen, J.; Lin, J.; Wang, M.K.; Ge, H.; Huang, Y. Rill erosion processes on a steep colluvial deposit slope under heavy rainfall in flume experiments with artificial rain. Catena 2018, 169, 46–58. [Google Scholar] [CrossRef]
- Patel, F.; Trezzi, M.M.; Nunes, A.L.; Bittencourt, H.V.H.; Diesel, F.; Pagnoncelli, F.D.B., Jr. The straw presence preceding soybean crop increases the persistence of residual herbicides. Adv. Weed Sci. 2023, 41, e020200051. [Google Scholar] [CrossRef]
- Albers, C.N.; Jacobsen, O.S.; Bester, K.; Jacobsen, C.S.; Carvalho, P.N. Leaching of herbicidal residues from gravel surfaces–A lysimeter-based study comparing gravels with agricultural topsoil. Environ. Pollut. 2020, 266, 115225. [Google Scholar] [CrossRef]
- Khalil, Y.; Flower, K.; Siddique, K.H.; Ward, P. Rainfall affects leaching of pre-emergent herbicide from wheat residue into the soil. PLoS ONE 2019, 14, e0210219. [Google Scholar] [CrossRef]
- Stewart, C.L.; Soltani, N.; Nurse, R.E.; Hamill, A.S.; Sikkema, P.H. Precipitation influences pre-and post-emergence herbicide efficacy in corn. Am. J. Plant Sci. 2012, 3, 1193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).