Abstract
In this work, hydrogen production from the co-digestion of sugarcane straw and sugarcane vinasse in the dark fermentation (DF) process was monitored using a cost-effective hydrogen detection system. This system included a sensor of the MQ-8 series, an Arduino Leonardo board, and a computer. For the DF, different concentrations of sugarcane vinasse and volumetric ratios of vinasse/hemicellulose hydrolysate were used together with a thermally pretreated inoculum, while the hydrogen detection system stored the hydrogen concentration data during the fermentation time. The results showed that a higher concentration of vinasse led to higher inhibitors for the DF, resulting in a longer lag phase. Additionally, the hydrogen detection system proved to be a useful tool in monitoring the DF, showcasing a rapid response time, and providing reliable information about the period of adaptation of the inoculum to the substrate. The measurement system was assessed using the error metrics SE, RMSE, and MBE, whose values ranged 0.6 and 5.0% as minimum and maximum values. The CV (1.0–8.0%) and SD (0.79–5.62 ppm) confirmed the sensor’s robustness, while the ANOVA at the 5% significance level affirmed the repeatability of measurements with this instrument. The RMSE values supported the accuracy of the sensor for online measurements (6.08–14.78 ppm). The adoption of this straightforward and affordable method sped up the analysis of hydrogen in secluded regions without incurring the expenses associated with traditional measuring instruments while offering a promising solution for biomass valorization, contributing to the advancement of rural green energy initiatives in remote areas.
1. Introduction
In recent years, renewable energy sources have become increasingly important due to the environmental compromises of countries with a limitation on carbon emissions. Among the energy sources for the energy transition towards a non-carbon economy, hydrogen has been classified as one of the most promising and the cleanest fuel owing to its high energy content per unit mass (142 kJ/g) and the production of water as the product of its oxidation [1]. Hydrogen can be produced using several methods including natural gas refining, water electrolysis, biomass pyrolysis, and biological processes such as biophotolysis by green algae, photofermentation by cyanobacteria, and dark fermentation (DF) by strict or facultative anaerobic bacteria [2].
Anaerobic digestion is a biological process widely used in biogas production from biogenic residues and involves a minimum of two stages of biological reactions, acidogenesis (or DF) and methanogenesis. In the first phase, acid-forming bacteria hydrolyze organic matter into simpler compounds (carbohydrates, lipids, amino acids) and produce hydrogen gas (H2), carbon dioxide (CO2), and organic acids like acetic acid, propionic acid, and butyric acid as the metabolic by-product, while in methanogenesis, methanogens consume hydrogen and acetic acid to produce methane [3,4,5,6]. Studies have been made to investigate the application of anaerobic digestion in rural settings and assess its potential as a sustainable energy solution [7,8]. Although there is not much progress in the case of DF, it is also known that anaerobic digestion can be directed towards DF by manipulating operational parameters like pH and retention time and by inhibiting methanogenesis [9]; this adaptation suggests that DF, with controlled parameters, holds promise for implementation in rural areas.
Over the past few years, there has been a growing interest in enhancing biohydrogen production using both simple (optimization of carbon/nitrogen (C/N) ratio, pH, and temperature) and complex techniques (Bacterial immobilization, nanotechnology, genetic engineering) [10]. A prevalent methodology employed in DF to enhance biohydrogen production is the application of inoculum pretreatment techniques. The most used method is heat-shock pretreatment (HSP), which consists of heating the sludge to temperatures between 80 and 121 °C for an exposure time of 10–120 min; this practice delays the time of emergence of methanogens [11,12,13]. Moreover, in instances where the substrate employed in DF lacks a substantial carbohydrate concentration, a complementary strategy known as co-digestion can be applied, which consists of the union of two or more different biogenic residues to increase the composition of useful substrates in the process and improve the C/N ratio [14,15].
Another recurring problem in DF is the measurement of hydrogen produced during the bioprocess. On the analytical scale, numerous precise methods exist for quantifying hydrogen, including but not limited to gas chromatography, commercial gas meters, respirometers, and an extensive array of hydrogen sensors such as electrochemical or resistive sensors. The choice of method depends on the specific application and the maximum concentration of hydrogen in the sample [16,17,18]. On the one hand, analytical technologies like gas chromatography are widely used to measure the composition of hydrogen produced on the laboratory scale, but it is very expensive compared to other alternatives. On the other hand, hydrogen sensors are cheap, require low or no maintenance, are easily replaceable, have a fast response time, and are easy to install and use [16]. In particular, the MQ-8 series sensor is a low-cost sensor used to detect hydrogen in concentrations between 200 and 10,000 ppm and it is classified as a resistive sensor [19]. Its detection system relies on the alteration in the electrical resistance of a sensitive layer in response to the presence of hydrogen in a gas flow; the sensitive part of the MQ-8 series comprises a ceramic tube made of Al2O3, enveloped by a SnO2 layer. In the presence of air, an oxidation reaction occurs between the gas and oxygen, resulting in a reduction in the resistance of the sensor [19]. Due to its fast response time, low cost, and ease of installation and use, in this contribution, the potential of the MQ-8 sensor as a feasible option for monitoring hydrogen production in a DF process is explored.
Currently, the needs for circular and zero-waste processes motivate the application of valorization alternatives like the utilization of anaerobic processes for energy production. Some residues are of great interest due to their valorization potential such as food waste, wastewater, and crop residues from wheat, corn, rice, and sugarcane [2,3]. During 2021, Colombia produced 24 million tons of sugarcane [20], placing this country as one of the largest producers of sugarcane worldwide. Despite the importance of this activity in agro-industrial and economic terms, such large production leads to an abundant generation of waste, especially lignocellulosic residues like sugarcane straw and liquid waste like vinasse.
Sugarcane straw is a fibrous and heterogeneous residue composed mostly of plant tops and dry leaves and represents approximately 15.6% of the sugarcane crop [21,22]. Although part of this residue is used to prepare the soil, about 50% is left in the field to feed livestock or is usually burned, increasing the emissions of carbon and particulate material in rural areas and wasting the potential of this lignocellulosic residue [22,23]. One of the biotechnological uses of sugarcane straw is as a carbon source for fermentation processes; however, due to the recalcitrant nature of this residue, a hydrolysis pretreatment is necessary to break the complex interaction between the lignocellulosic fractions [24]. The diluted acid pretreatment is one of the most used for high hemicellulose solubilization and high yield in the deconstruction of cellulose [25,26]. The diluted acid pretreatment consists of using a dilute acid solution, preferably sulfuric acid, with a concentration less than 4% wt in a temperature range of 120–210 °C and a residence time between 5 and 90 min [25].
Sugarcane vinasse is a by-product of the sugar–ethanol industry, characterized as an acidic suspension, with a high organic content, unpleasant odor, and dark brown color [27]. In this sector, fertilization and irrigation with diluted vinasse are widely practiced; however, the infiltration of this complex residue in soils and water bodies leads to several environmental hazards like soil salinization, soil instability, the permanent acidification of water sources, and insect spreading [28]. Consequently, efforts have been made to explore new applications for this residue [24,29]. Among the treatments used to reduce the environmental impact of vinasse are physicochemical (coagulation/flocculation, electrocoagulation, adsorption, advanced oxidation, and membrane processes), thermal (evaporation/combustion), and biological treatments (aerobic and anaerobic) [30]. In particular, the biologic treatments have attracted the attention for valorization through biogas production and, hence, as a source of bioenergy [30].
Although sugarcane vinasse and sugarcane straw have been widely explored as individual substrates for DF [22,31,32], research on the co-digestion of these residues is notably scarce. The absence of studies on the co-digestion of these wastes underscores the necessity to identify a suitable co-digestion ratio for enhancing biohydrogen production. Additionally, sugarcane vinasse harbors inhibitors (polyphenols) that, at high concentration levels, may adversely impact the metabolic activity of the bacterial consortium [33]. This emphasizes the importance of determining an appropriate dilution level to strike a balance between reducing inhibitors and supplying essential nutrients for efficient biohydrogen production. Therefore, the aim of this work is to monitor hydrogen production from vinasse and sugarcane straw co-digestion as low-cost substrates in a DF process, by utilizing an MQ-8 sensor as a prospective tool for low-sophistication installations with potential application for rural and remote areas.
2. Materials and Methods
2.1. Material Acquisition
The sugarcane vinasse was obtained from a distillery with an initial concentration of ~33 Brix. The sugarcane straw used in this study came from a sugar mill, while the inoculum and the slaughterhouse wastewater used as a substrate in the adaption phase were collected from a Waste-Water Treatment Plant (WWTP).
2.2. Sugarcane Straw Pretreatment
Sugarcane straw pretreatment was adapted according to [22] and pre-experimental tests. The sugarcane straw underwent size reduction to approximately 3–5 cm using a blade mill, followed by diluted acid pretreatment at 120 °C for 60 min in a closed vessel, without agitation. During the pretreatment process, a 1.5% (v/v) H2SO4 solution was employed at a solid-to-liquid ratio of 1:10 (g/mL). After the diluted acid pretreatment, the liquid fraction obtained, known as hemicellulose hydrolysate (HH), was subjected to vacuum filtration, its pH was adjusted to 5.0 using a 1N NaOH solution, and it was stored in the refrigerator at 4 °C.
2.3. Vinasse Characterization
The analyses and methods used in the characterization of the initial vinasse are detailed in Table 1. Conceição et al. demonstrated that raw sugarcane vinasse hinders bacterial consortium growth compared to diluted vinasse [34]. Therefore, diluted solutions were prepared at 5, 10, and 15% v/v of the original concentration using distilled water as the solvent.

Table 1.
Methods used in the characterization of the initial sugarcane vinasse.
2.4. Inoculum Adaptation
The inoculum used in this study was derived from a microbial sludge obtained from a WWTP. Since the sludge was acclimated to slaughterhouse wastewater within the WWTP, it was progressively subjected to different substrate concentrations for adaptation purposes. The related concentrations are shown in Table 2. Using HI93754B-25 Kits (Hanna Instruments, Venice, Italy), COD measurements were conducted on the first day and four days after the initial feed of substrate; a 60% reduction in the initial COD was considered a good measure for replacing the initial substrates with more vinasse–HH-rich substrates.

Table 2.
Substrate concentrations used in inoculum adaptation.
Once the adaptation phase ended, the inoculum was constantly fed with substrate rich in vinasse–HH, and prior to being used in the DF process, it was subjected to heat-shock pretreatment at 121 °C for 20 min in an autoclave to delay the time of emergence of methanogens.
2.5. Dark Fermentation, Biohydrogen Production, and Measurement
The substrate concentrations used in the DF process are indicated in Table 3. Each substrate was pH-adjusted to 6.0 with a 1N Na2HPO4 solution.

Table 3.
Substrate concentrations used in DF.
The experimental setup was placed in a closed room with a constant temperature of 27 °C. A 500 mL amber flask was used as a batch reactor, the lid of the flask was adjusted to direct the outflow of the gas, and the hose that transported the gas was connected to the hydrogen detection and data storage system. Online measurement was performed by using a low-cost hydrogen sensor of the MQ-8 series (pre-heated for 6 h), an Arduino Leonardo board, and a computer. A water column was employed to apply hydrostatic pressure on the gas for better detection. The experimental setup for DF and hydrogen detection is shown in Figure 1.
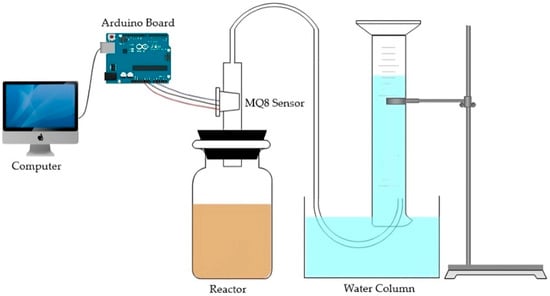
Figure 1.
Experimental setup for DF and hydrogen detection.
During the DF process, 200 mL of substrate and 100 mL of pretreated inoculum were added to the reactor, stirred at 90 rpm, and monitored for 6 days. The hydrogen concentration data provided by the sensor were recorded on the computer. Lastly, the water level in the cylinder was verified during the 6 days of the experiment. Each experiment was performed in triplicate.
2.6. Data Analysis for Sensor Validation
Statistics were calculated to assess the reliability, accuracy, and consistency of sensor measurements. For statistical analysis, samples taken from the line were compared with gas chromatography for biohydrogen quantification as described elsewhere [39,40]. The linear relationship between resistance and hydrogen concentration was independently confirmed, considering the resistance range indicated by the supplier (10–60 kΩ) and the calibration procedure.
For accuracy and repeatability assessment, five discrete samples of different and known concentrations were repeatedly measured spaced by 0.5 h (n = 11 times). The fast response and stability of the measurement provided by the MQ-8 was checked by recording the first three values returned by the sensor; then, the average value was considered as the single data point. For each statistical sample of measurements formed by the n = 11 data points, the following statistics metrics were calculated, mean, standard deviation, coefficient of variation (CV), standard error (SE), root-mean-square error (RMSE), and mean bias error (MBE), as previously applied by Abhiram et al. in the validation of a lysimeter [41]. The error metrics, i.e., SE, RMSE, and MBE, allowed the overall accuracy of the sensor’s predictions to be evaluated against the reference method. The one-way analysis of variance (ANOVA) at the 5% significance level was used in the evaluation of concentration differences for two samples formed by n = 11 measurements each. Hypothesis testing was carried out under the null hypothesis of means equality, thus assessing the statistical significance of the measurements. Additionally, the coefficient of variation (CV) serves as an indicator of the precision and stability of the sensor’s measurements.
3. Results and Discussion
3.1. Vinasse Characterization
The analysis of sugarcane vinasse revealed a COD value of 228,220 mg O2/L. This high organic load often leads to increased microbial competition for resources. In DF, where specific microorganisms are desired to have the higher metabolic activity for production of biohydrogen, a high COD concentration could disrupt the microbial community dynamics. This possible disruption may lead to growth inhibition, decreased metabolic activity, and low production yields [42]. Additionally, the sugarcane vinasse showed a high concentration of polyphenols, which are toxic for the anaerobic microorganisms [33]. Thus, the dilution of sugarcane vinasse contributed to reduce the inhibitors’ concentration as well as the organic load of the substrate, leading to a more favorable environment for DF. Conversely, using excessively low sugarcane vinasse concentrations may hinder the bacterial growth due to an insufficient amount of carbon sources. This highlights the need to find an optimal dilution level to find a balance between reducing inhibitors and providing essential nutrients for efficient biohydrogen production. The characterization of the initial sugarcane vinasse is shown in Table 4.

Table 4.
Characterization of the initial sugarcane vinasse.
3.2. Sensor Validation
The log–linear relationship between H2 concentration and resistance ratio was confirmed with slope = −1.9151 and R2 = 0.99, as previously shown by other authors for this kind of sensor [19,43,44]. The statistics calculated for the validation set are displayed in Table 5. The CV is associated with the repeatability of measurements. In this case, the CV range was 1.0–8.0%, showing the highest variability at the lowest concentrations (15 and 19 ppm, respectively). At the highest concentrations tested, the CV decreased to 1–2%, indicating low variability in the sensor’s measurements across multiple trials at concentrations higher than 60 ppm. The standard deviation as a measure of dispersion was found to be between 0.8 and 5.62 ppm, with the highest value being for the highest concentration measured (370 ppm), further confirming the sensor’s consistent performance. In all cases, the ANOVA results (95% of significance, Fα = 2.85, p < 0.05) for every independent set validated the null hypothesis of means equality. These results support the consistency of sensor measurements under different experimental conditions, thus assuring reproducibility. Additionally, the confirmed error metrics RMSE, SE, and MBE suggest a good accuracy of the method in the range of concentrations produced during the DF. The error values were in the range of −0.1 and 4.6 ppm as minimum and maximum values, respectively. As observed for the standard deviation, the higher dispersion on data registered for the highest concentration led to a wider interval of confidence (369 ± 4.6 ppm). On average, the error of measurement for the validation set was 2 ppm. Additionally, the RSME calculated for the time courses of hydrogen concentration are displayed in Figure 2. On average, the error for the hydrogen concentration for the online monitoring of biohydrogen production was 14.44 ppm. Previous studies with similar approaches found errors varying by 0.6 and 1% of the H2 concentration measured through the reference method [19,43,44]. Those results are in the range of our measurements for the different error metrics calculated (SE, RMSE, and MBE). Our results collectively suggest that the sensor exhibits acceptable accuracy and internal variability, providing relatively reliable and precise measurements, considering the simplicity of the measurement instrument.

Table 5.
Metrics of sensor validation.
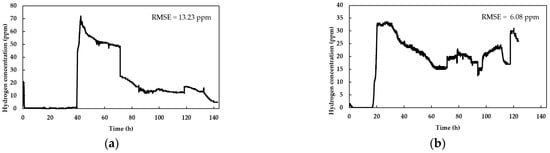
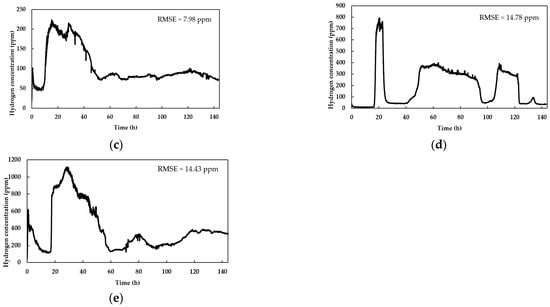
Figure 2.
Hydrogen detection rate from a thermally pretreated inoculum fed with a substrate composed of sugarcane vinasse and HH: (a) volumetric ratio of 3:1 (sugarcane vinasse/HH) and sugarcane vinasse diluted to 15% of the original; (b) volumetric ratio of 3:1 (sugarcane vinasse/HH) and sugarcane vinasse diluted to 5% of the original; (c) volumetric ratio of 1:1 (sugarcane vinasse/HH) and sugarcane vinasse diluted to 10% of the original; (d) volumetric ratio of 1:3 (sugarcane vinasse/HH) and sugarcane vinasse diluted to 5% of the original; (e) volumetric ratio of 1:3 (sugarcane vinasse/HH) and sugarcane vinasse diluted to 15% of the original.
3.3. Hydrogen Measurement and Detection
The mean time courses of triplicates of hydrogen production during the co-digestion process at different sugarcane vinasse concentrations and volumetric sugarcane vinasse/HH ratios are displayed in Figure 2.
All graphs displayed small perturbations in the data, suggesting the low RSME calculated for every experiment. Online variations in hydrogen concentration data are directly linked to oscillations in the signals transmitted by the sensor and they are not related to the DF dynamics. The water displacement apparatus is essential for hydrogen measurement by contributing to maintain the approximately constant pressure in the line (1.2 kPa) and, hence, the detection and registration of hydrogen concentration. In contrast, when the fluid column was omitted, no readings were registered by the sensor.
The experiments in Figure 2b,d,e showed a similar lag phase of ~20 h associated with biohydrogen production, aligning with findings from previous studies employing batch reactors [45,46,47]. Figure 2c presents a shorter lag phase compared to Figure 2b despite having similar substrate concentrations. When using high vinasse concentrations, higher concentrations of polyphenols inhibit the bacterial growth, thus delaying the onset of DF [48]. This inhibition effect is clearly observed in Figure 2a, where the experiments with the highest concentration of sugarcane vinasse and sugarcane vinasse/HH ratio led to pronounced lag phases (More than 40 h). Therefore, further studies focusing on polyphenol removal may contribute to take advantage of the high sugar content of vinasse with shorter lag times.
After the lag period, all the fermentations showed an exponential phase at different times, in connection with the substrate’s composition. The implemented low-cost sensor successfully registered the production phase and concentration in the line with a fast response time (in the mS scale). However, the measurements present some instabilities in short timespans; therefore, it is convenient to consider the average readings during a time period, e.g., 30 min. This is noticed in Figure 2b–e, where intermittent peaks in hydrogen concentration were evident. Such behavior of the sensor is expected due to the limited selectivity of metal–oxide–semiconductor-based resistive sensors when exposed to different gas compositions, such as those produced in the process of DF [49]. Particularly, biohydrogen is produced alongside gases such as CO2 and, in latter stages, with methane; these by-products influence the sensor readings, generating reading anomalies that can be compensated for by averaging the readings during a given timespan.
3.4. Advantages and Disadvantages of the Low-Cost Hydrogen Sensor
The implementation of a hydrogen detection system in a DF permits the continuous monitoring of hydrogen production across the entire process. Our approach provides reliable information on the period of adaptation of the inoculum to the substrate. Additionally, real-time data enable timely corrective measures if deviations occur, making the system usable for process control. Compared to the reference method used in the laboratory scale, i.e., gas chromatography, this technique also offers the advantages of easy handling and low-cost. In addition, the use of this method speeds up the analysis of DF gases in remote areas. If implemented in the mentioned areas, operators of biomass valorization systems could manage and enhance their setups without incurring high costs associated with the acquisition of traditional measuring instruments.
It is worth noting that the main drawbacks of the measurement system, compared with the industrial instruments, are the data perturbation, the variability among replicates, and the susceptibility to decalibration. Furthermore, the effectiveness of this detection system would diminish when dealing with hydrogen concentrations below 200 ppm and above 10,000 ppm. In contrast to gas chromatography, this method exhibits lower reliability in the accuracy of the obtained data. Nevertheless, if the acquisition prices of the MQ-8-based H2 detection system is compared to commercial hydrogen online gas analyzers, it is at least 24-fold lower, being easily and cheaply replaceable. Moreover, the import costs for developing countries substantially increase the final cost of instruments, thus affecting the accessibility and implementation of this kind of technology in the field.
3.5. Future Perspectives
In recent years, research focused on biohydrogen production has increased, especially using DF with different kinds of substrates. This tendency has prompted the exploration of novel technologies for measuring critical parameters, such as hydrogen concentration, and the use of techniques to enhance hydrogen production, like co-digestion and inoculum pretreatments. In this case, implementing co-digestion as a treatment alternative for waste treatment could favor the sustainability of the sugarcane industry. By efficiently utilizing these residues to produce value-added products, such as hydrogen, industries can establish a sustainable waste management system while generating additional revenue. This method not only reduces the environmental impact of waste disposal but also aligns the sugarcane industry with sustainable practices, ensuring a greener and more eco-friendly future for the sector.
While the utilization of the MQ-8 sensor offers a practical and cost-effective experimental setup, it is imperative to conduct studies aimed at improving the reliability of the data it generates. Fakra et al. proved that the MQ-8 sensor inside a partially enclosed capsule improved the repeatability of measurements due to better control of the environment in which the sensor was placed, providing more relevant and stable measured values. Furthermore, the authors declared that to obtain more accurate data, calibration of the MQ-8 sensor with a gas of known hydrogen concentration is necessary to avoid inconsistencies in the data [19]. In this study, calibration points other than those suggested by the manufacturer were not considered for DF monitoring; this decision was made considering that implementing field calibration with hydrogen in remote areas might not be feasible. Nevertheless, our approach remains flexible, allowing for the possibility of sensor calibration using portable devices.
Even though improvements to the experimental setup are still necessary, the hydrogen detection system can provide good monitoring of hydrogen production in a DF process in rural areas where biomass resources are accessible (food waste, manure, etc.) but the equipment or materials available in a laboratory are scarce. Additionally, this technology has the potential to transform sustainable energy production in remote regions, empowering local communities to efficiently harness clean energy sources. The study and implementation of biohydrogen decentralized projects at small scales would be facilitated with the use simple low-cost control systems like that proposed in this contribution. The deployment of such systems could contribute to advancing clean energy solutions and reducing energy disparities in rural communities.
4. Conclusions
A study of hydrogen production through the co-digestion between sugarcane vinasse and sugarcane straw in a DF process was performed. It was shown that a higher concentration of sugarcane vinasse leads to higher inhibitors present in the DF, resulting in a longer lag phase. The hydrogen detection system used in this study proved to be useful for monitoring the process, giving information about the period of adaptation of the inoculum to the substrate. The low CV (1.0–8.0%) and standard deviation values (0.79–5.62 ppm) registered affirm the sensor’s robustness, while the ANOVA confirmed the repeatability of measurements with this instrument by validating the null hypothesis of means equality. The low error values (0.6–5.0%) supported the accuracy of the sensor for both offline and online measurement. Overall, these results point out the effectiveness of the sensor in consistently and accurately measuring the target variable under diverse conditions, establishing its reliability for practical applications. While further improvements to the measurement system are still necessary, the system’s ease of access and user-friendly design generate optimistic expectations for future experimentation and research. This technology also offers a promising solution for the control of waste valorization processes oriented towards biohydrogen production, especially relevant for rural areas, where agroindustry resources are available but equipment accessibility is very limited.
Author Contributions
Conceptualization, H.R.-M.; methodology, A.B. and H.R.-M.; software, A.B.; validation, D.G.-R., A.B. and H.R.-M.; formal analysis, A.B., D.G.-R. and H.R.-M.; investigation, A.B., D.G.-R. and H.R.-M.; data curation, A.B.; writing—original draft preparation, A.B.; writing—review and editing, D.G.-R. and H.R.-M.; visualization, A.B.; supervision, D.G.-R. and H.R.-M.; project administration, H.R.-M.; funding acquisition, H.R.-M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge partial funding by Vicerrectoría de Investigaciones, Universidad del Valle, Project CI-21191.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hay, J.X.W.; Wu, T.Y.; Juan, J.C.; Jahim, J.M. Biohydrogen Production through Photo Fermentation or Dark Fermentation Using Waste as a Substrate: Overview, Economics, and Future Prospects of Hydrogen Usage. Biofuels Bioprod. Biorefining 2013, 7, 334–352. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrre, H.; Steyer, J.P. Hydrogen Production from Agricultural Waste by Dark Fermentation: A Review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Nayal, F.S.; Mammadov, A.; Ciliz, N. Environmental Assessment of Energy Generation from Agricultural and Farm Waste through Anaerobic Digestion. J. Environ. Manag. 2016, 184, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Baral, S.S.; Dey, R.; Mutnuri, S. Biogas Generation Potential by Anaerobic Digestion for Sustainable Energy Development in India. Renew. Sustain. Energy Rev. 2010, 14, 2086–2094. [Google Scholar] [CrossRef]
- Massé, D.I.; Talbot, G.; Gilbert, Y. On Farm Biogas Production: A Method to Reduce GHG Emissions and Develop More Sustainable Livestock Operations. Anim. Feed. Sci. Technol. 2011, 166, 436–445. [Google Scholar] [CrossRef]
- Ferrer, I.; Garfí, M.; Uggetti, E.; Ferrer-Martí, L.; Calderon, A.; Velo, E. Biogas Production in Low-Cost Household Digesters at the Peruvian Andes. Biomass Bioenergy 2011, 35, 1668–1674. [Google Scholar] [CrossRef]
- Garfí, M.; Gelman, P.; Comas, J.; Carrasco, W.; Ferrer, I. Agricultural Reuse of the Digestate from Low-Cost Tubular Digesters in Rural Andean Communities. Waste Manag. 2011, 31, 2584–2589. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Trably, E.; Dumas, C.; Steyer, J.P.; Carrère, H. Lignocellulosic Materials into Biohydrogen and Biomethane: Impact of Structural Features and Pretreatment. Crit. Rev. Environ. Sci. Technol. 2013, 43, 260–322. [Google Scholar] [CrossRef]
- Goveas, L.C.; Nayak, S.; Kumar, P.S.; Vinayagam, R.; Selvaraj, R.; Rangasamy, G. Recent Advances in Fermentative Biohydrogen Production. Int. J. Hydrogen Energy 2024, 54, 200–217. [Google Scholar] [CrossRef]
- Mohammadi, P.; Ibrahim, S.; Mohamad Annuar, M.S.; Law, S. Effects of Different Pretreatment Methods on Anaerobic Mixed Microflora for Hydrogen Production and COD Reduction from Palm Oil Mill Effluent. J. Clean. Prod. 2011, 19, 1654–1658. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pontoni, L.; d’Antonio, G.; Lens, P.N.L.; Esposito, G.; Pirozzi, F. Dark Fermentation of Complex Waste Biomass for Biohydrogen Production by Pretreated Thermophilic Anaerobic Digestate. J. Environ. Manag. 2015, 152, 43–48. [Google Scholar] [CrossRef]
- Mahata, C.; Ray, S.; Das, D. Optimization of Dark Fermentative Hydrogen Production from Organic Wastes Using Acidogenic Mixed Consortia. Energy Convers. Manag. 2020, 219, 113047. [Google Scholar] [CrossRef]
- Sen, B.; Aravind, J.; Kanmani, P.; Lay, C.H. State of the Art and Future Concept of Food Waste Fermentation to Bioenergy. Renew. Sustain. Energy Rev. 2016, 53, 547–557. [Google Scholar] [CrossRef]
- Zhou, P.; Elbeshbishy, E.; Nakhla, G. Optimization of Biological Hydrogen Production for Anaerobic Co-Digestion of Food Waste and Wastewater Biosolids. Bioresour. Technol. 2013, 130, 710–718. [Google Scholar] [CrossRef]
- Boshagh, F.; Rostami, K. A Review of Measurement Methods of Biological Hydrogen. Int. J. Hydrogen Energy 2020, 45, 24424–24452. [Google Scholar] [CrossRef]
- Kyrpel, T.; Saska, V.; de Poulpiquet, A.; Luglia, M.; Soric, A.; Roger, M.; Tananaiko, O.; Giudici-Orticoni, M.T.; Lojou, E.; Mazurenko, I. Hydrogenase-Based Electrode for Hydrogen Sensing in a Fermentation Bioreactor. Biosens. Bioelectron. 2023, 225, 115106. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; He, J.; Gao, X.; Yang, T.; Zeng, X. Continuous Amperometric Hydrogen Gas Sensing in Ionic Liquids. Analyst 2018, 143, 4136–4146. [Google Scholar] [CrossRef] [PubMed]
- Fakra, D.A.H.; Andriatoavina, D.A.S.; Razafindralambo, N.A.M.N.; Amarillis, K.A.; Andriamampianina, J.M.M. A Simple and Low-Cost Integrative Sensor System for Methane and Hydrogen Measurement. Sens. Int. 2020, 1, 100032. [Google Scholar] [CrossRef]
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 19 December 2023).
- Carvalho, J.L.N.; Nogueirol, R.C.; Menandro, L.M.S.; Bordonal, R.d.O.; Borges, C.D.; Cantarella, H.; Franco, H.C.J. Agronomic and Environmental Implications of Sugarcane Straw Removal: A Major Review. GCB Bioenergy 2017, 9, 1181–1195. [Google Scholar] [CrossRef]
- Tomasini, M.; Faber, M.d.O.; Ferreira-Leitão, V.S. Sequential Production of Hydrogen and Methane Using Hemicellulose Hydrolysate from Diluted Acid Pretreatment of Sugarcane Straw. Int. J. Hydrogen Energy 2023, 48, 9971–9987. [Google Scholar] [CrossRef]
- Sindhu, R.; Kuttiraja, M.; Binod, P.; Janu, K.U.; Sukumaran, R.K.; Pandey, A. Dilute Acid Pretreatment and Enzymatic Saccharification of Sugarcane Tops for Bioethanol Production. Bioresour. Technol. 2011, 102, 10915–10921. [Google Scholar] [CrossRef]
- Sindhu, R.; Gnansounou, E.; Binod, P.; Pandey, A. Bioconversion of Sugarcane Crop Residue for Value Added Products—An Overview. Renew. Energy 2016, 98, 203–215. [Google Scholar] [CrossRef]
- Hu, F.; Ragauskas, A. Pretreatment and Lignocellulosic Chemistry. Bioenergy Res. 2012, 5, 1043–1066. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment Technologies for an Efficient Bioethanol Production Process Based on Enzymatic Hydrolysis: A Review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.E.R.; Hu, B. Vinasse from Sugarcane Ethanol Production: Better Treatment or Better Utilization? Front. Energy Res. 2017, 5, 7. [Google Scholar] [CrossRef]
- Carrilho, E.N.V.M.; Labuto, G.; Kamogawa, M.Y. Destination of Vinasse, a Residue from Alcohol Industry: Resource Recovery and Prevention of Pollution; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128039069. [Google Scholar]
- Fuess, L.T.; Rodrigues, I.J.; Garcia, M.L. Fertirrigation with Sugarcane Vinasse: Foreseeing Potential Impacts on Soil and Water Resources through Vinasse Characterization. J. Environ. Sci. Health Part A 2017, 52, 1063–1072. [Google Scholar] [CrossRef]
- Mikucka, W.; Zielińska, M. Distillery Stillage: Characteristics, Treatment, and Valorization. Appl. Biochem. Biotechnol. 2020, 192, 770–793. [Google Scholar] [CrossRef] [PubMed]
- Fuess, L.T.; Ferraz, A.D.N.; Machado, C.B.; Zaiat, M. Temporal Dynamics and Metabolic Correlation between Lactate-Producing and Hydrogen-Producing Bacteria in Sugarcane Vinasse Dark Fermentation: The Key Role of Lactate. Bioresour. Technol. 2018, 247, 426–433. [Google Scholar] [CrossRef]
- Magrini, F.E.; de Almeida, G.M.; da Maia Soares, D.; Fuentes, L.; Ecthebehere, C.; Beal, L.L.; da Silveira, M.M.; Paesi, S. Effect of Different Heat Treatments of Inoculum on the Production of Hydrogen and Volatile Fatty Acids by Dark Fermentation of Sugarcane Vinasse. Biomass Convers. Biorefinery 2021, 11, 2443–2456. [Google Scholar] [CrossRef]
- Freitas, P.V.; Da Silva, D.R.; Beluomini, M.A.; Da Silva, J.L.; Stradiotto, N.R. Determination of Phenolic Acids in Sugarcane Vinasse by HPLC with Pulse Amperometry. J. Anal. Methods Chem. 2018, 2018, 4869487. [Google Scholar] [CrossRef]
- Conceição, G.R.; da Silva, C.S.; do Vale, T.O.; dos Santos, J.N.; Matos, J.B.T.L.; Almeida, P.F.d.; Chinalia, F.A. Culture Operational Strategies for the Production of Methane and Algal Oil Using Ethanol Vinasse Effluent. J. Appl. Phycol. 2023, 35, 2135–2149. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. AOAC: Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Lane, H.; Eynon, L. Determination of Reducing Sugar by Means of Fehling’s Solution with Methylene Blue as Internal Indicator. J. Soc. Chem. Ind. 1923, 42, 32–37. [Google Scholar]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Garcia Martínez, E.; Fernandez Segovia, I.; Fuentes López, A. Determinación de Polifenoles Totales Por El Método de FolinCiocalteu; Food Technology Department, ETSIAMN, Universitat Politècnica de València: València, Spain, 2015. [Google Scholar]
- Davila-Vazquez, G.; Alatriste-Mondragón, F.; de León-Rodríguez, A.; Razo-Flores, E. Fermentative Hydrogen Production in Batch Experiments Using Lactose, Cheese Whey and Glucose: Influence of Initial Substrate Concentration and PH. Int. J. Hydrogen Energy 2008, 33, 4989–4997. [Google Scholar] [CrossRef]
- Purwanto, H.; Akiyama, T. Hydrogen Production from Biogas Using Hot Slag. Int. J. Hydrogen Energy 2006, 31, 491–495. [Google Scholar] [CrossRef]
- Abhiram, G.; McCurdy, M.; Davies, C.E.; Grafton, M.; Jeyakumar, P.; Bishop, P. An Innovative Lysimeter System for Controlled Climate Studies. Biosyst. Eng. 2023, 228, 105–119. [Google Scholar] [CrossRef]
- Pouresmaeil, S.; Nosrati, M.; Ebrahimi, S. Operating Control for Enrichment of Hydrogen-Producing Bacteria from Anaerobic Sludge and Kinetic Analysis for Vinasse Inhibition. J. Environ. Chem. Eng. 2019, 7, 103090. [Google Scholar] [CrossRef]
- Tavera-Romero, F.; Ríos-Maravilla, A.; Granados-Samaniego, J.; Turpin, S. Gas Measurement System for Bio-Hydrogen Production Based on Nejayote’s Dark Phase Fermentation. J. Phys. Conf. Ser. 2021, 1723, 012058. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Wu, N.; Zhang, Y.; Svoronos, S.; Pullammanappallil, P. Low-Cost, Arduino-Based, Portable Device for Measurement of Methane Composition in Biogas. Renew. Energy 2019, 138, 224–229. [Google Scholar] [CrossRef]
- Sharma, Y.; Li, B. Optimizing Hydrogen Production from Organic Wastewater Treatment in Batch Reactors through Experimental and Kinetic Analysis. Int. J. Hydrogen Energy 2009, 34, 6171–6180. [Google Scholar] [CrossRef]
- Logan, B.E.; Oh, S.E.; Kim, I.S.; Van Ginkel, S. Biological Hydrogen Production Measured in Batch Anaerobic Respirometers. Environ. Sci. Technol. 2002, 36, 2530–2535. [Google Scholar] [CrossRef]
- Wicher, E.; Seifert, K.; Zagrodnik, R.; Pietrzyk, B.; Laniecki, M. Hydrogen Gas Production from Distillery Wastewater by Dark Fermentation. Int. J. Hydrogen Energy 2013, 38, 7767–7773. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as Antimicrobial Agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).