Effects of Bicarbonate Addition and N:P Ratio on Microalgae Growth and Resource Recovery from Domestic Wastewater
Abstract
1. Introduction
2. Materials and Methods
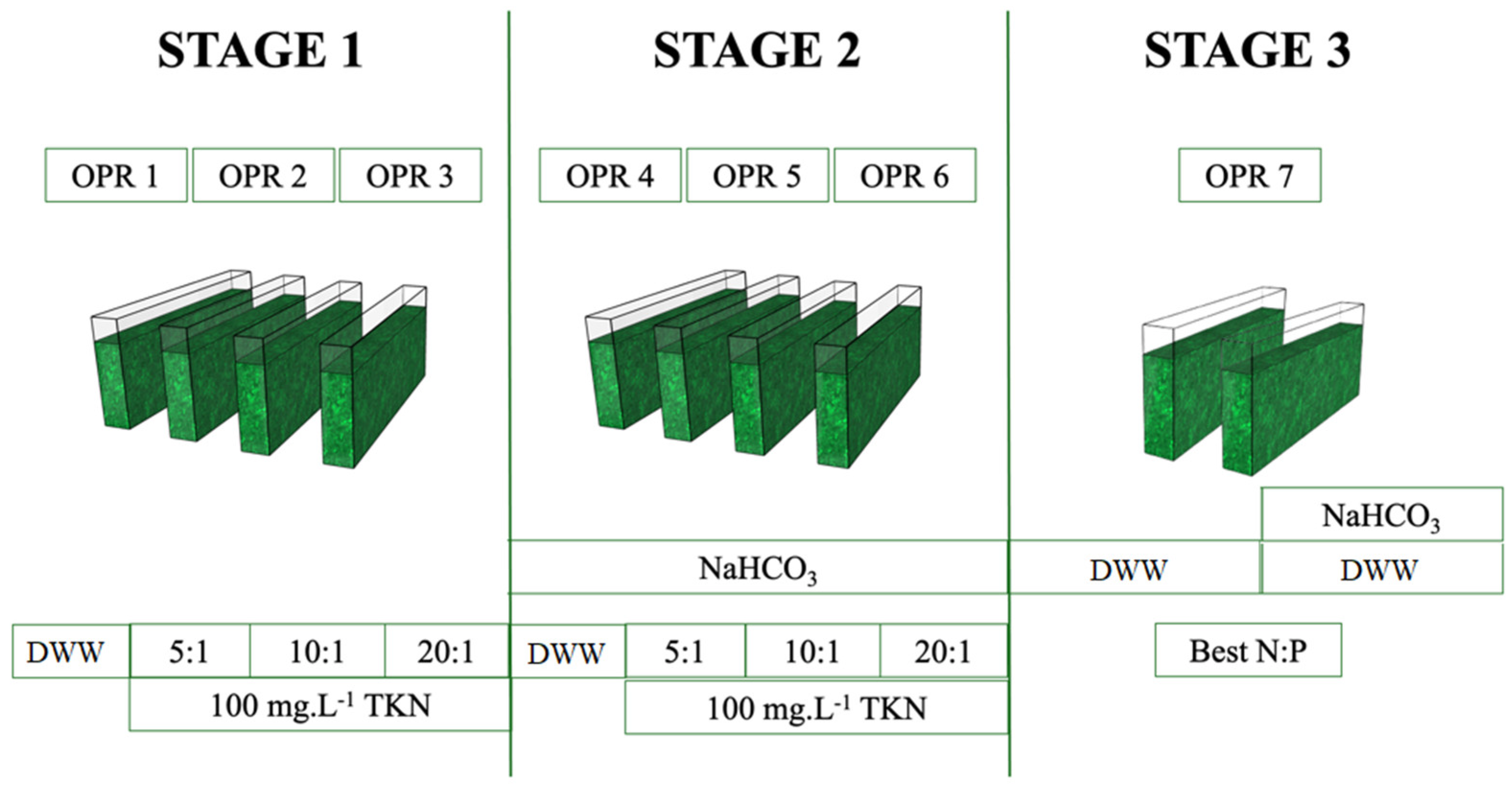
2.1. Biomass Production
2.2. Monitoring of Microalgae Growth and Wastewater Treatment
2.3. Statistical Analyses
3. Results and Discussion
3.1. Inoculum Characterization
3.2. Microalgae Cultivation
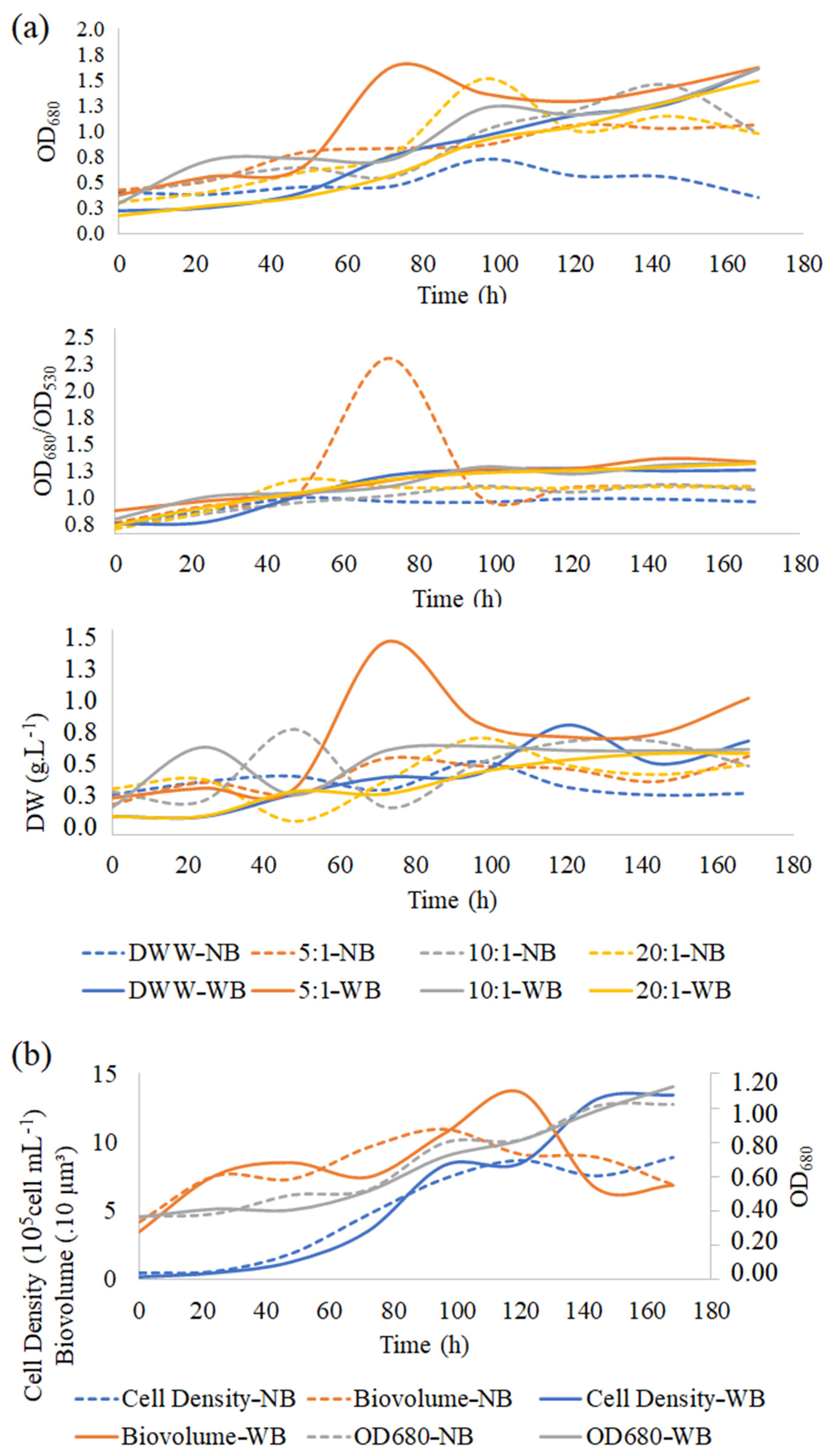
3.3. Microalgae Growth
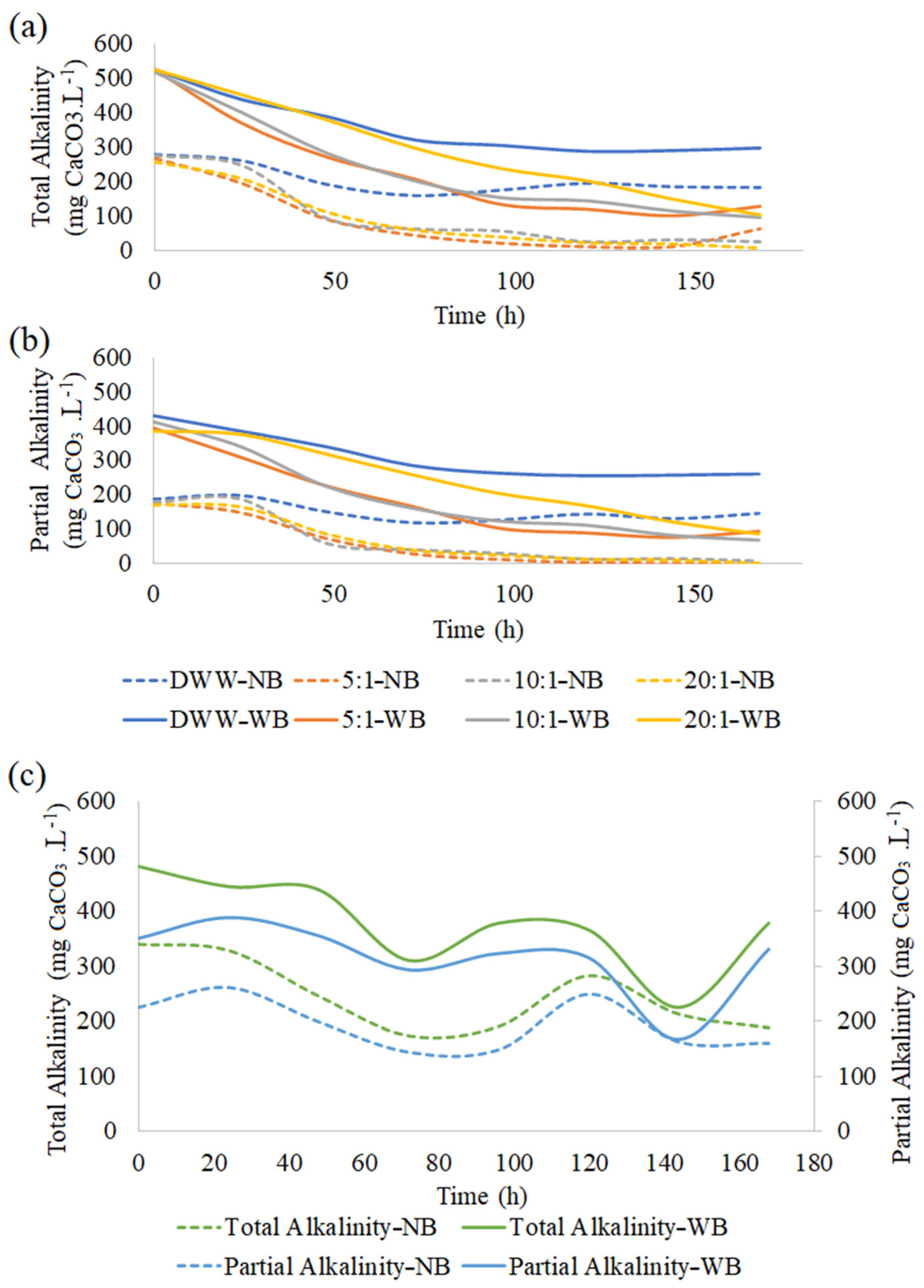
3.4. Wastewater Treatment Monitoring
3.4.1. Nitrogen
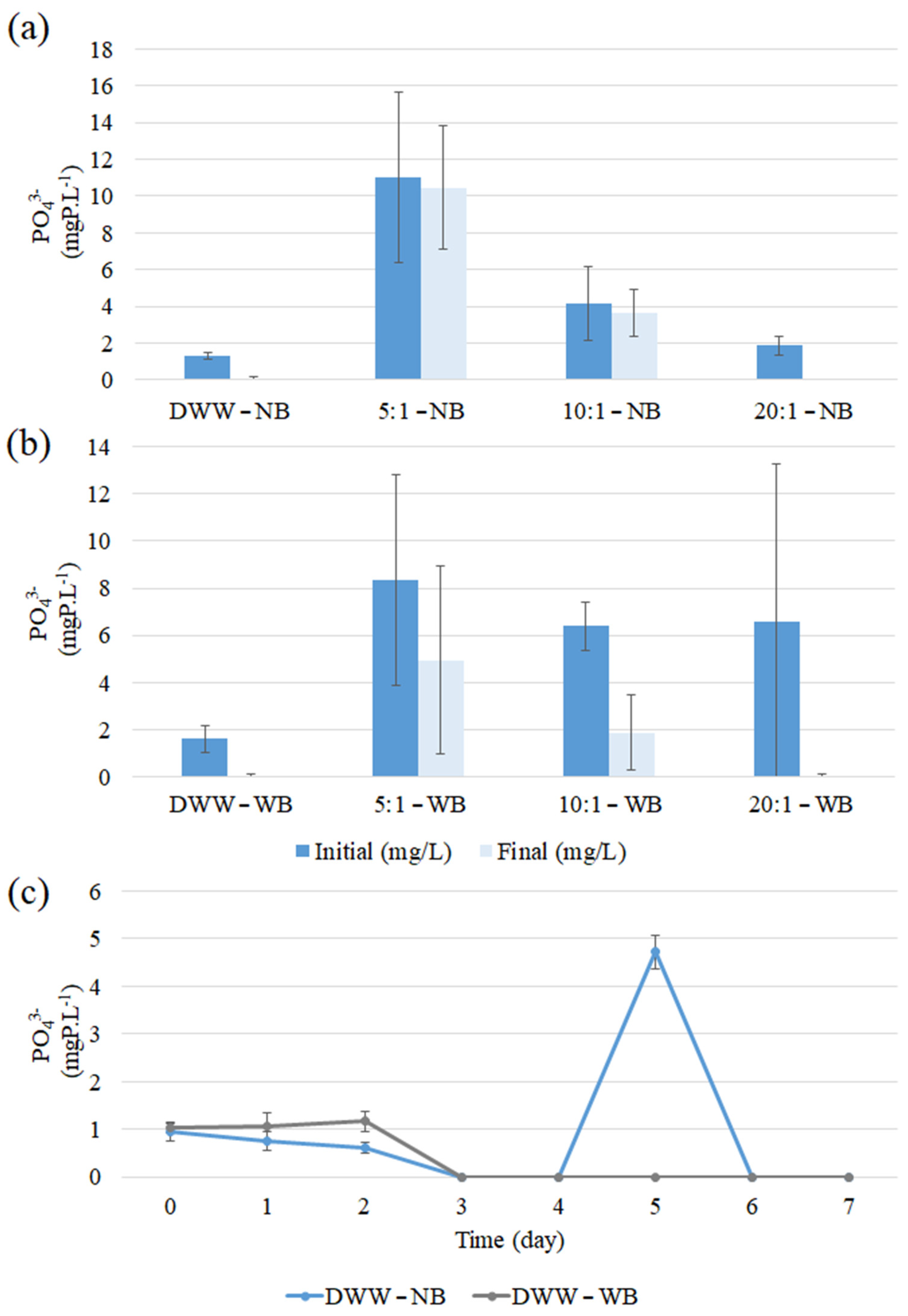
3.4.2. Orthophosphate
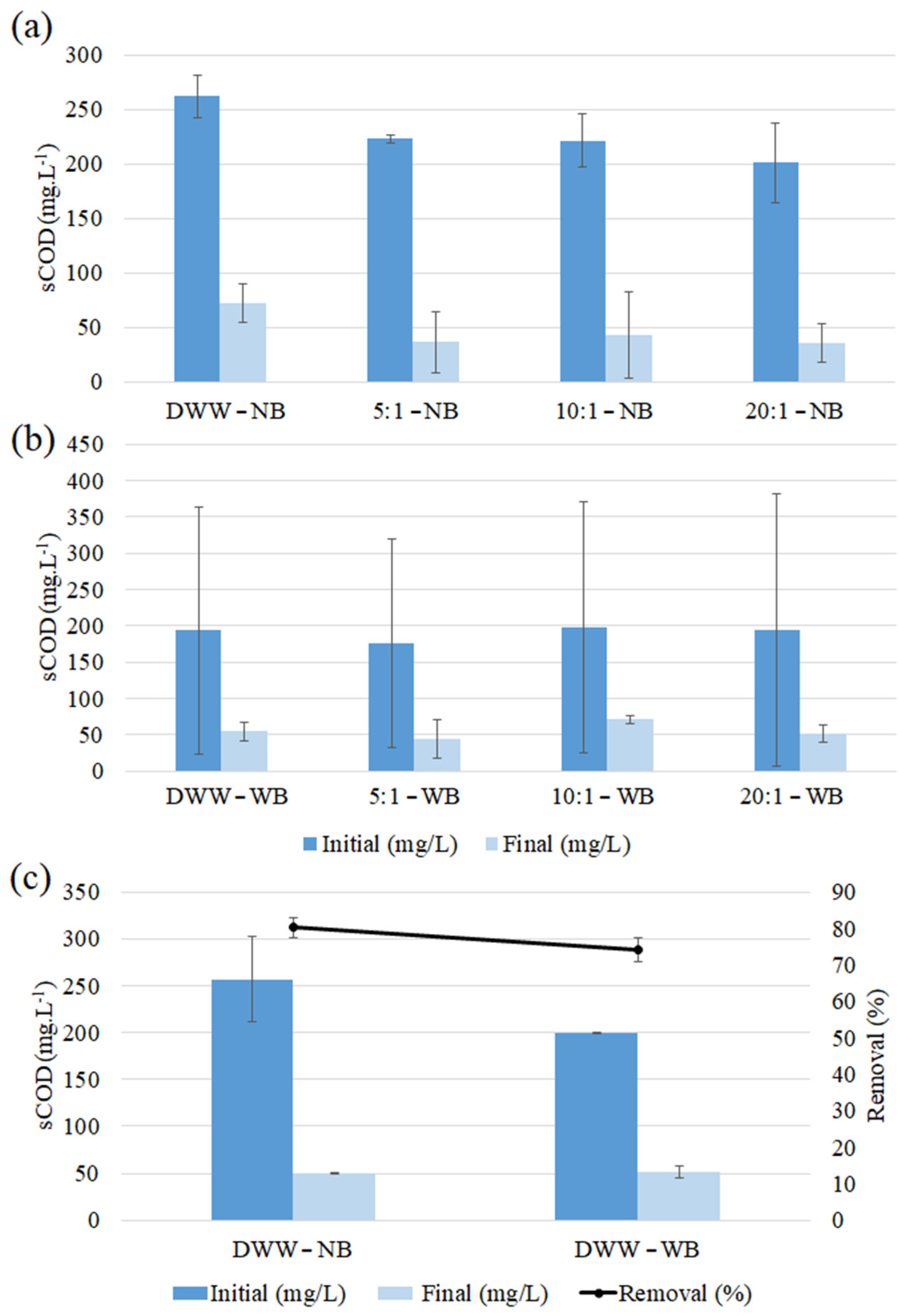
3.4.3. sCOD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drosou, F.; Kekes, T.; Boukouvalas, C.L. Life cycle assessment of the canned fruits industry: Sustainability through waste valorization and implementation of innovative techniques. AgriEngineering 2023, 5, 395–412. [Google Scholar] [CrossRef]
- Álvarez-González, A.; Uggetti, E.; Serrano, L.; Gorchs, G.; Escolà Casas, M.; Matamoros, V.; Gonzalez-Flo, E.; Díez-Montero, R. The potential of wastewater grown microalgae for agricultural purposes: Contaminants of emerging concern, heavy metals and pathogens assessment. Environ. Pollut. 2023, 324, 121399. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Gutiérrez, R.; Uggetti, E.; Garfí, M.; García, J.; Ferrer, I. Towards energy neutral microalgae-based wastewater treatment plants. Algal Res. 2017, 28, 235–243. [Google Scholar] [CrossRef]
- Hussain, F.; Shah, S.Z.; Ahmad, H.; Abubshait, S.A.; Abubshait, H.A.; Laref, A.; Manikandan, A.; Kusuma, H.S.; Iqbal, M. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. A review. Renew. Sustain. Energy Rev. 2021, 137, 110603. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, L.; Qi, Y. Enhancing the productivity of microalgae cultivated in wastewater toward biofuel production: A critical review. Appl. Energy 2015, 137, 282–291. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Show, P.L.; Yap, Y.J.; Ling, T.C.; Chang, J.-S. Effects of water culture medium, cultivation systems and growth modes for microalgae cultivation: A review. J. Taiwan Inst. Chem. Eng. 2018, 91, 332–344. [Google Scholar] [CrossRef]
- Leite, L.D.S.; Hoffmann, M.T.; Daniel, L.A. Microalgae cultivation for municipal and piggery wastewater treatment in Brazil. J. Water Process Eng. 2019, 31, 100821. [Google Scholar] [CrossRef]
- Gao, P.; Guo, L.; Zhao, Y.; Jin, C.; She, Z.; Gao, M. Enhancing microalgae growth and product accumulation with carbon source regulation: New perspective for the coordination between photosynthesis and aerobic respiration. Chemosphere 2021, 278, 130435. [Google Scholar] [CrossRef]
- Choi, H.J.; Lee, S.M. Effect of the N/P ratio on biomass productivity and nutrient removal from municipal wastewater. Bioprocess Biosyst. Eng. 2015, 38, 761–766. [Google Scholar] [CrossRef]
- Hong, J.W.; Kim, O.H.; Jo, S.W.; Cho, H.W.; Yoon, H.S. Mass Cultivation from a Korean Raceway Pond System of Indigenous Microalgae as Potential Biofuel Feedstock. Oil Gas Res. 2016, 2, 108. [Google Scholar]
- Rani, S.; Ojha, C.S.P. Chlorella sorokiniana for integrated wastewater treatment, biomass accumulation and value-added product estimation under varying photoperiod regimes: A comparative study. J. Water Process Eng. 2021, 39, 101889. [Google Scholar] [CrossRef]
- Leite, L.S.; Daniel, L.A. Optimization of microalgae harvesting by sedimentation induced by high pH. Water Sci. Technol. 2020, 82, 1227–1236. [Google Scholar] [CrossRef]
- Jánoska, A. A Liquid Foam-Bed Photobioreactor for Microalgae Production; Wageningen University and Research: Wageningen, The Netherlands, 2018. [Google Scholar]
- Leite, L.D.S.; Hoffmann, M.T.; De Vicente, F.S.; Dos Santos, D.V.; Daniel, L.A. Adsorption of algal organic matter on activated carbons from alternative sources: Influence of physico-chemical parameters. J. Water Process Eng. 2021, 44, 102435. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; Water Environment Federation: Washington, DC, USA, 2017. [Google Scholar]
- Riccio, G.; De Luca, D.; Lauritano, C. Monogalactosyldiacylglycerol and Sulfolipid Synthesis in Microalgae. Mar. Drugs 2020, 18, 237. [Google Scholar] [CrossRef]
- Phinyo, K.; Pekkoh, J.; Peerapornpisal, Y. Distribution and ecological habitat of Scenedesmus and related genera in some freshwater resources of Northern and North-Eastern Thailand. Biodiversitas J. Biol. Divers. 2017, 18, 1092–1099. [Google Scholar] [CrossRef]
- NEN 6520:1981; Water: Spectrofotometrische Bepaling van Het Gehalte aan Chlorofyl-a. Nederlands Normalisatie Instituut: Delft, The Netherlands, 1981.
- Nush, E. Comparison of differents methods for chlorophyll and phaeropigments determination. Arch. Hydrobiol. 1980, 4, 14–36. [Google Scholar]
- Hillebrand, H.; Dürselen, C.D.; Kirschtel, D.; Pollingher, D.; Zohary, T. Biovolume calculation for pelagic and benthic microalgae. J. Phycol. 1999, 35, 403–424. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Ferragut, C.; Tucci, A.; Crossetti, L.O.; Ferrari, F.; Bicudo, D.d.C.; Sant’Anna, C.L.; Bicudo, C.E.d.M. Biovolume de cianobactérias e algas de reservatórios tropicais do Brasil com diferentes estados tróficos. Hoehnea 2014, 41, 9–30. [Google Scholar] [CrossRef]
- Bicudo, C.E.D.M.; Menezes, M. Gêneros de Algas de Águas Continentais do Brasil (Chave Para Identificação e Descrições), 3rd ed.; RiMa: São Carlos, Brazil, 2017. [Google Scholar]
- Oliveira, R.S. Família Scenedesmaceae (Chlorophyceae, Sphaeropleales) no Estado de Goiás: Biodiversidade e distribuição geográfica; Universidade Federal de Goiás: Goiânia, Brazil, 2015. [Google Scholar]
- Lage, S.; Toffolo, A.; Gentili, F.G. Microalgal growth, nitrogen uptake and storage, and dissolved oxygen production in a polyculture based-open pond fed with municipal wastewater in northern Sweden. Chemosphere 2021, 276, 130122. [Google Scholar] [CrossRef]
- Leite, L.S.; Daniel, L.A. Microalgae Production Integrated with the Wastewater Treatment: A Management Approach. Cost-efficient Wastewater Treatment Technologies. In The Handbook of Environmental Chemistry; Nasr, M., Negm, A.M., Eds.; Springer: Cham, Switzerland, 2022; Volume 117. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Assemany, P.P.; Calijuri, M.L.; Tango, M.D.; Couto, E.A. Energy potential of algal biomass cultivated in a photobioreactor using effluent from a meat processing plant. Algal Res. 2016, 17, 53–60. [Google Scholar] [CrossRef]
- Foladori, P.; Petrini, S.; Nessenzia, M.; Andreottola, G. Evolution of real municipal wastewater treatment in photobioreactors and microalgae-bacteria consortia using real-time parameters. Chem. Eng. J. 2018, 345, 507–516. [Google Scholar] [CrossRef]
- Couto, E.A. Produção de Biomassa em Lagoas de Alta Taxa com Diferentes Profundidades e Seu Aproveitamento para Geração de Energia via Liquefação Hidrotérmica. Ph.D. Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2016. [Google Scholar]
- Umetani, I.; Janka, E.; Sposób, M.; Hulatt, C.J.; Kleiven, S.; Bakke, R. Bicarbonate for microalgae cultivation: A case study in a chlorophyte, Tetradesmus wisconsinensis isolated from a Norwegian lake. J. Appl. Phycol. 2021, 33, 1341–1352. [Google Scholar] [CrossRef]
- Whangchai, K.; Mathimani, T.; Sekar, M.; Shanmugam, S.; Brindhadevi, K.; Van Hung, T.; Chinnathambi, A.; Alharbi, S.A.; Pugazhendhi, A. Synergistic supplementation of organic carbon substrates for upgrading neutral lipids and fatty acids contents in microalga. J. Environ. Chem. Eng. 2021, 9, 105482. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Barragan, J.; Perales, J.A. Photobiotreatment: Influence of Nitrogen and Phosphorus Ratio in Wastewater on Growth Kinetics of Scenedesmus Obliquus. Int. J. Phytoremediat. 2013, 15, 774–788. [Google Scholar] [CrossRef]
- Ratomski, P.; Hawrot-Paw, M.; Koniuszy, A. Utilization of CO2 from Sodium Bicarbonate to produce Chlorella vulgaris biomass in tubular photobioreactors for biofuel purposes. Sustainability 2021, 13, 9118. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ahn, C.-Y.; Jo, B.-H.; Lee, S.-A.; Park, J.-Y.; An, K.-G.; Oh, H.-M. Increased microalgae growth and nutrient removal using balanced N:P ratio in wastewater. J. Microbiol. Biotechnol. 2013, 23, 92–98. [Google Scholar] [CrossRef]
- Alketife, A.M.; Judd, S.; Znad, H. Synergistic effects and optimization of nitrogen and phosphorus concentrations on the growth and nutrient uptake of a freshwater Chlorella vulgaris. Environ. Technol. 2017, 38, 94–102. [Google Scholar] [CrossRef]
- Sacristán de Alva, M.; Luna-Pabello, V.M.; Cadena, E.; Ortíz, E. Green microalga Scenedesmus acutus grown on municipal wastewater to couple nutrient removal with lipid accumulation for biodiesel production. Bioresour. Technol. 2013, 146, 744–748. [Google Scholar] [CrossRef]
- Koreivienė, J.; Valčiukas, R.; Karosienė, J.; Baltrėnas, P. Testing of Chlorella/Scenedesmus microalgae consortia for remediation of wastewater, co₂ mitigation and algae biomass feasibility for lipid production. J. Environ. Eng. Landsc. Manag. 2014, 22, 105–114. [Google Scholar] [CrossRef]
- Gupta, S.; Pawar, S.; Pandey, R.A. Current practices and challenges in using microalgae or treatment of nutrient rich wastewater from agro-based industries. Sci. Total Environ. 2019, 687, 1107–1126. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Hong, Y.; Liu, X.; Zhao, G.; Zhang, H.; Zhai, Q. Microalgae cultivation in domestic wastewater for wastewater treatment and high value-added production: Species selection and comparison. Biochem. Eng. J. 2022, 185, 108493. [Google Scholar] [CrossRef]
- Lu, W.; Liu, S.; Lin, Z.; Lin, M. Enhanced Microalgae Growth for Biodiesel Production and Nutrients Removal in Raw Swine Wastewater by Carbon Sources Supplementation. Waste Biomass Valorization 2021, 12, 1991–1999. [Google Scholar] [CrossRef]
- Chen, C.; Song, Y.; Yuan, Y. The operating characteristics of partial nitrification by controlling pH and alkalinity. Water 2021, 13, 286. [Google Scholar] [CrossRef]
- Mao, Y.; Xiong, R.; Gao, X.; Jiang, L.; Peng, Y.; Xue, Y. Analysis of the status and improvement of microalgal phosphorus removal from municipal wastewater. Processes 2021, 9, 1486. [Google Scholar] [CrossRef]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef]
- Fito, J.; Alemu, K. Microalgae—Bacteria consortium treatment technology for municipal wastewater management. Nanotechnol. Environ. Eng. 2018, 4, 4. [Google Scholar] [CrossRef]



| Daily Analyses | Abbreviation | Unit | Reference | Stage | PBR Input and Output Analysis | Abbreviation | Unit | Reference | Stage | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1, 2 | 3 | 1, 2 | 3 | ||||||||
| Maximum and minimum temperature | TMAX and TMIN | °C | [15] | X | X | Cell Biovolume | - | µm3 | [20,21] | X | X |
| Dissolved Oxygen | DO | mg O2·L−1 | 4500-O [15] | X | X | Genus and Species Identification | - | - | [22,23] | X | X |
| pH | - | - | 4500-H+ [15] | X | X | Volatile Suspended Solid | VSS | mg·L−1 | 2540 D [15] | X | X |
| Partial and Total Alkalinity | - | mg CaCO3·L−1 | 2320-B [15] | X | X | Total Suspended Solid | TSS | mg·L−1 | 2540 D [15] | X | X |
| Optical Density at 530 and 680 nm | OD530 and OD680 | - | [16,17] | X | X | Fixed Suspended Solid | FSS | mg·L−1 | 2540 D [15] | X | X |
| Chlorophyll-a | - | mg·L−1 | [18,19] | X | Ammonia Nitrogen | NH3 | mg N·L−1 | 4500-NH3C [15] | X | ||
| Dry Weight | DW | mg·L−1 | [7] | X | Total Kjeldahl Nitrogen | TKN | mg N·L−1 | 4500-Norg.B [15] | X | ||
| Ammonia Nitrogen | NH3 | mg N·L−1 | 4500-NH3C [15] | X | Nitrate | NO3− | mg N·L−1 | Hach® 10020 [15] | X | ||
| Total Kjeldahl Nitrogen | TKN | mg N·L−1 | 4500-Norg.B [15] | X | Nitrite | NO2− | mg N·L−1 | Hach® 10019 [15] | X | ||
| Nitrate | NO3− | mg N·L−1 | Hach® 10020 | X | Total Phosphorus | TP | mg P·L−1 | 4500-P E [15] | X | ||
| Nitrite | NO2− | mg N·L−1 | Hach® 10019 | X | Orthophosphate | PO43− | mg P·L−1 | 4500-P E [15] | X | ||
| Total Phosphorus | TP | mg P·L−1 | 4500-P E [15] | X | Soluble Chemical Oxygen Demand | sCOD | mg O2·L−1 | 5220 B [15] | X | ||
| Orthophosphate | PO43− | mg P·L−1 | 4500-P E [15] | X | |||||||
| Soluble Chemical Oxygen Demand | sCOD | mg O2·L−1 | 5220 B [15] | X | |||||||
| Condition | DW 96 h | OD 96 h (680 nm) | sCOD Removal (%) | TKN Removal (%) | NH3 Removal (%) | NO2− | NO3− | PO43− Removal | VSS Increment (%) |
|---|---|---|---|---|---|---|---|---|---|
| (g·L−1) | (mg·L−1) | (mg·L−1) | (%) | ||||||
| Stage 1 | |||||||||
| DWW-NB | 0.54 ± 0.29 a | 0.73 ± 0.16 a | 72.09 ± 7.99 a | 73.98 ± 32.08 a | 100 a | 1.20 ± 1.79 a | 1.28 ± 0.10 b | 94.09 ±9.16 a | 8.82 ± 96.51 a |
| 5:1-NB | 0.51 ± 0.22 a | 0.86 ± 0.03 a | 83.65 ± 12.53 a | 52.80 ± 18.33 a | 79.55 ± 16.97 a | 1.09 ± 1.63 a | 19.72 ± 7.45 a | 1.99 ± 9.65 b | 288.90 ± 530.97 a |
| 10:1-NB | 0.51 ± 0.57 a | 1.01 ± 0.46 a | 81.40 ± 15.96 a | 46.76 ± 17.90 a | 81.16 ± 27.29 a | 6.83 ± 5.79 a | 3.21 ± 1.94 b | 5.13 ± 24.53 b | 108.42 ± 161.13 a |
| 20:1-NB | 0.71 ± 0.01 a | 1.5 ± 0.88 a | 81.68 ± 10.64 a | 42.51 ± 21.87 a | 80.41 ± 25.88 a | 2.28 ± 2.33 a | 9.13 ± 5.18 ab | 100 a | 322.63 ± 498.63 a |
| Stage 2 | |||||||||
| DWW-WB | 0.44 ± 0.11 c | 0.95 ± 0.18 c | 45.21 ± 35.07 c | 62.87 ± 21.04 c | 100 c | 0.20 ± 0.04 e | 0.76 ± 0.10 c | 97.46 ± 4.00 c | 440.95 ± 496.29 c |
| 5:1-WB | 0.82 ± 0.42 c | 1.36 ± 0.58 c | 41.39 ± 50.26 c | 68.58 ± 8.97 c | 100 c | 7.05 ± 4.88 cd | 5.22 ± 6.64 c | −30.60 ± 162.25 c | 63.29 ± 52.65 c |
| 10:1-WB | 0.64 ± 0.38 c | 1.22 ± 0.84 c | 19.53 ± 59.21 c | 72.06 ± 7.52 c | 95.97 ± 6.9 c | 10.14 ± 0.15 c | 6.33 ± 3.94 c | 73.32 ± 21.78 c | 301.35 ± 74.99 c |
| 20:1-WB | 0.45 ± 0.21 c | 0.91 ± 0.33 c | 35.21 ± 47.33 c | 70.08 ± 6.54 c | 100 c | 2.54 ± 0.63 de | 4.38 ± 2.97 c | 98.33 ± 2.58 c | 331.86 ± 18.28 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tango, M.D.; Zanetoni Filho, J.A.; Daniel, L.A.; Leite, L.d.S.; Hoffmann, M.T.; Moutinho, F.H.M. Effects of Bicarbonate Addition and N:P Ratio on Microalgae Growth and Resource Recovery from Domestic Wastewater. AgriEngineering 2023, 5, 1178-1195. https://doi.org/10.3390/agriengineering5030075
Tango MD, Zanetoni Filho JA, Daniel LA, Leite LdS, Hoffmann MT, Moutinho FHM. Effects of Bicarbonate Addition and N:P Ratio on Microalgae Growth and Resource Recovery from Domestic Wastewater. AgriEngineering. 2023; 5(3):1178-1195. https://doi.org/10.3390/agriengineering5030075
Chicago/Turabian StyleTango, Mariana Daniel, José Antônio Zanetoni Filho, Luiz Antonio Daniel, Luan de Souza Leite, Maria Teresa Hoffmann, and Fellipe Henrique Martins Moutinho. 2023. "Effects of Bicarbonate Addition and N:P Ratio on Microalgae Growth and Resource Recovery from Domestic Wastewater" AgriEngineering 5, no. 3: 1178-1195. https://doi.org/10.3390/agriengineering5030075
APA StyleTango, M. D., Zanetoni Filho, J. A., Daniel, L. A., Leite, L. d. S., Hoffmann, M. T., & Moutinho, F. H. M. (2023). Effects of Bicarbonate Addition and N:P Ratio on Microalgae Growth and Resource Recovery from Domestic Wastewater. AgriEngineering, 5(3), 1178-1195. https://doi.org/10.3390/agriengineering5030075







