Abstract
Glyphosate is the most widely used herbicide for weed control in citrus orchards in Brazil; therefore, it is likely that several species have gained resistance to this herbicide and that more than one resistant species can be found in the same orchard. The objective was to identify weeds resistant to glyphosate in citrus orchards from different regions of the São Paulo State (SP) and determine how many resistant species are present within the same orchard. Seeds of Amaranthus deflexus, A. hybridus, Bidens pilosa, Chloris elata, Conyza bonariensis, Digitaria insularis, Solanum Americanum, and Tridax procumbens, which, as reported by growers, are suspected to be resistant to glyphosate, were collected from plants that survived the last application of this herbicide (>720 g of acid equivalent [ae] ha–1) in sweet orange and Tahiti acid lime orchards. Based on dose–response and shikimic acid accumulation assays, all populations of A. deflexus, A. hybridus, B. pilosa, and T. procumbens were sensitive to glyphosate. However, populations of B. pilosa from the Olimpia region (R-NS, R-PT and R-OdA) showed signs of resistance based on plant mortality rates by 50% within a population (LD50 = 355–460 g ae ha−1). All populations of C. bonariensis, C. elata, and D. insularis were resistant to glyphosate, presenting resistance ratios from 1.9 to 27.6 and low shikimate accumulation rates. Solanum americanum also showed resistance, with resistance ratios ranging from 4.3 to 25.4. Most of the citrus orchards sampled presented the occurrence of more than one species resistant to glyphosate: Nossa Senhora—one species; Olhos D’agua and Passatempo—two species; Araras—four species; and Cordeiropolis and Mogi-Mirim—up to five species. The results reported in this paper provide evidence of multiple species in citrus orchards from São Paulo that have exhibited resistance to glyphosate. This underscores the difficulties in managing glyphosate-resistant weeds which are prevalent throughout the country, such as C. bonariensis and D. insularis. The presence of these resistant species further complicates the control of susceptible species that may also develop resistance. In addition, the glyphosate resistance of S. americanum was identified for the first time.
1. Introduction
By producing 1.1 million tons of orange juice, Brazil will produce three quarters of the world’s orange juice in the 2022/2023 season [1]. Citrus production is mainly concentrated in the Brazilian southeastern region, especially in the state of São Paulo, which is responsible for more than 75% of national production in 366,446 ha (60% of the citrus planted area) [2]. In addition to competing with citrus trees, weeds causing yield reductions of up to 50% can host vector insects and pathogens and hinder cultural practices; thus, their management is essential in order to maintain healthy and highly productive orchards [3,4].
Although some non-chemical weed management practices are being adopted on a small scale by citrus growers [5], weed management in citrus is mainly based on herbicides, directing applications in the intra- (under the canopy of the trees) and inter-row areas of the orchards [6]. However, due to the fact that, in Brazil, there are few authorized herbicides (only 10 active ingredients) for weed management in citrus orchards [7], the most used herbicide is glyphosate, both for the wide spectrum of weeds that it is capable of controlling and for its low cost [5,6,8,9].
Glyphosate is a homolog of phosphoenolpyruvate in the shikimic acid pathway, interrupting 5-enolpyruvylshikimate-3-phosphate (EPSP) biosynthesis by inhibiting the EPSP synthase activity [10]. The interruption of this metabolic pathway causes the accumulation of shikimic acid, affecting the synthesis of three essential aromatic amino acids: phenylalanine, tyrosine, and tryptophan [10]. Therefore, it is possible to determine the susceptibility/resistance to glyphosate through the concentration of shikimic acid present in the plants [11]. This herbicide, in addition to being cheap and effective, has other interesting properties, such as low residual activity in the soil, low toxicity to mammals, minimal environmental impact, among others [12,13], making it the most used agricultural chemical pesticide in the world [10].
Low herbicide rotation and the absence of economically viable integrated weed management strategies have led to an extensive and excessive reliance on glyphosate. As a result, reports of low efficacy with respect to glyphosate in the application of controlling weeds have increased year after year. In addition, most citrus growers commonly increase the rate and frequency of glyphosate application instead of using herbicides with other mechanisms of action [3,6]. Surveys have revealed that 98% of Brazilian citrus growers use glyphosate, with 73% applying doses between 1000 and 2000 g acid equivalent (ae) ha−1 and 11% doses > 2000 g ae ha−1. The frequency of application varies, and only 11% of the producers apply glyphosate once a year; the majority (78%) apply glyphosate two–four times annually, while 11% apply it more than five times annually [9].
In Brazilian citrus orchards, populations of Conyza bonariensis, Conyza canadensis, and Digitaria insularis with resistance to glyphosate have been reported since before 2010 [14,15]. The tropical conditions in which citrus are produced also favor the growth of annual and perennial weeds [16,17]. Species of the genera Amaranthus, Bidens, Chloris, Conyza, Eleusine, Lolium, among others, are weeds that present a high risk of evolving herbicide resistance [18], and some of them have already reportedly become resistant to herbicides in other crops in Brazil [19]. Because species of these genera occur in high densities in citrus orchards [14] and also present high rates of survival in the field after herbicide applications, the possibility that new weed species have developed resistance to glyphosate is likely. In addition, citrus growers have exerted strong selection pressure on weed populations with glyphosate [6,9], so it is likely that more than one resistant species will occur in the same orchard. The occurrence of several herbicide-resistant weed species in the same area considerably increases the costs of resistance management [20].
Since glyphosate is the most used herbicide in Brazil, the aim of this work was to identify glyphosate-resistant weeds in citrus groves from different regions of the São Paulo State and to verify how many resistant weed species can be found within the same orchard. The resistance level was determined by dose–response and shikimic acid accumulation assays.
2. Materials and Methods
2.1. Plant Material
Field surveys were carried out in sweet orange [Citrus sinensis (L.) Osbeck] and Tahiti acid lime (C. latifolia Tan.) orchards in the municipalities of Araras, Cordeirópolis, Mogi-Mirim, and Olimpia, São Paulo State. In these orchards, seeds of at least 20 specimens of each species that survived the last application of this herbicide (>720 g ae ha–1) were harvested, which, according to growers, showed signs of resistance to glyphosate due to major control failures. Seeds were identified and stored in paper bags. Susceptible seeds of the same species were collected in an ecologically managed Tahiti acid lime orchard (without the use of herbicides) in the municipality of Mogi-Mirim and on the campus of the Federal University of São Carlos in uncultivated areas. The list of weed species collected at each site are detailed in Table 1.

Table 1.
Identification of weed populations with suspected resistance to glyphosate collected in different citrus orchards from the State of São Paulo, Brazil.
Seeds from each population were germinated in plastic trays (31 × 15 × 10 cm) containing a mixture of moistened-at-field-capacity commercial substrate and sand (1:1 v/v) and covered with expanded vermiculite. The trays were sealed and kept in a greenhouse until germination (3–6 d). Seedlings of 1–2 true leaves were transplanted individually into 250 mL pots containing substrate, sand, and vermiculite (2:2:1 v/v/v) and fertilized with ~100 mg of 14-14-14 (NPK) (Forth Cote, Osmocote, Froth Jardim Ltd., Cerquilho, Brazil). The plants were kept in a greenhouse until they were required for use in the experiments.
2.2. Dose–Response Assays
Seedlings of different species with 3–4 true leaves (rosette of 6–9 leaves in the case of C. bonariensis) were treated with glyphosate (Roundup® Original DI, 370 g ae L–1, Monsanto, Sao Paulo, Brazil). The different doses of glyphosate tested were as follows: 0, 23.12, 46.25, 92.5, 185, 370, 740, 1110, 1480, 2220, and 2960 g ae ha–1. Applications were carried out with a pneumatic knapsack sprayer equipped with a flat fan nozzle (110,015, KGF Spray Nozzles, Vinhedo, Brazil) at 50 cm canopy height. The application pressure was 30 psi, as measured with a glycerin manometer (Model GCN, Cotergavi Instrumentos de Medição Ltd.a., São Paulo, Brazil) and adapted to the application bar to deliver 200 L of ha−1 herbicide solution. Sets of 10 plants from each weed population were treated per herbicide dose. Doses of 23.12 and 46.25 g ha–1 were applied only to dicot weeds, and the highest one (2960 g ha–1) only in monocots.
At 21 d after treatment, fresh weight and plant mortality were recorded, and the values were converted to percentages relative to the untreated control to estimate the GR50 (dose that reduces fresh weight by 50%) and/or LD50 (dose that kills 50% of the individuals in a population) via nonlinear regression analysis and calculate the resistance factor (RF = R/S). The experiments were repeated for populations that showed glyphosate resistance.
2.3. Shikimic Acid Accumulation
Sets of at least 10 plants from each weed population were treated with 370 and 740 g ae ha–1 of glyphosate. A set of untreated plants from each population was reserved as a control group to construct the calibration curve with known concentrations of shikimic acid (0, 0.01, 0.05, 0.1 and 0.2 µg). At 96 h after treatment, 50 mg samples of fresh tissue (from the second and third leaves) of the treated and untreated plants were collected and placed in 1.5 mL tubes containing 1 mL of 0.25 N HCl, frozen in liquid N2, and stored at −40 °C until required for use. Shikimic acid accumulation was determined following the methodology of Cromartie and Polge [21]. The samples were thawed at room temperature and subsequently incubated for 45 min at 37 °C. Aliquots of 50 µL were transferred to new tubes containing 200 µL of 0.25% (w/v) periodic acid and 0.25% (w/v) sodium m-periodate. After a new incubation at 37 °C for 30 min, 200 µL 0.6 N sodium hydroxide and 0.22 N sodium sulfite was added and homogenized. Volumes of 300 µL were transferred to spectrophotometric cuvettes, which were then supplemented with 600 µL of distilled water.
The absorbance of samples was measured at a wavelength of 380 nm in a diode array spectrophotometer (HP 8425A, Palo Alto, CA, USA). Shikimic acid accumulation was determined from the difference between treated and untreated plants, and the results were expressed in µg of shikimic acid g−1 fresh tissue. Five samples with three technical replicates were analyzed by population in a completely randomized design.
2.4. Statistical Analysis
The GR50 and LD50 values were calculated using the following equation: y = ([(d)/1 + (x/g)b]) [22]. In this equation, y is the response at 50% of the parameter of interest, d is the upper limit of the curve, c is the lower limit of the curve, b is the slope of the curve, x is the dose(s) of herbicide, and g is the inflection point of the curve (GR50 or LD50). Regression analyses were performed using SigmaPlot 10.0 (Systat Software Inc. San Jose, CA, USA).
After checking the normality of the shikimic acid accumulation data by using the Shapiro–Wilk test, the data were submitted to ANOVA. When necessary, Tukey’s test at probability α = 0.05 was performed to compare means. Statistical analyses were performed using Statistics 9.0 software (Analytical Software, Tallahassee, FL, USA).
3. Results
3.1. Dose–Response Assays
Populations with suspected resistance (A. deflexus, A. hybridus, B. Pilosa, and T. procumbens) turned out to be sensitive to glyphosate, presenting GR50 and LD50 values similar to those determined for their susceptible counterparts. In all cases, these values were close to or below 100 g ae ha–1, except the B. pilosa populations from the Olimpia region (R-NS, R-PT and R-OdA), which presented LD50 values that ranged from 355 to 460 g ae ha–1. All populations of C. bonariensis, C. elata, and D. insularis were resistant to glyphosate. For C. elata and D. insularis, the RF’s ranged from 1.9 to 8.3 in relation to their susceptible counterparts. Conyza bonariensis was the most resistant species, with RF’s of up to 27.6, and in some cases, it was not possible to determine the LD50 since 50% of the plants in a population did not die with the maximum dose evaluated for dicots (2220 g ae ha–1). Solanum americanum presented RF’s ranging from 4.3 to 32.5 for GR50 and from 6.8 to 25.4 for LD50 when compared with their respective susceptible populations (Figure 1, Table 2).
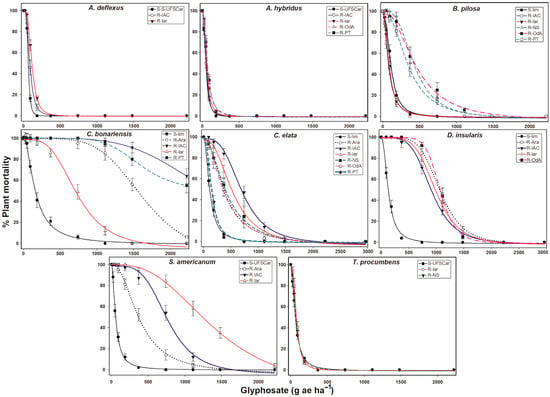
Figure 1.
Dose–response curves of the percentage of plant mortality within a population of weed species with suspected glyphosate resistance collected in citrus orchards from different regions of the São Paulo State, Brazil. Vertical bars ± standard (n = 10).

Table 2.
Effective mean doses of glyphosate to reduce dry weight (GR50) and/or plant survival (LD50) by 50% in weeds collected in citrus orchards from São Paulo State, Brazil.
Most of the citrus orchards sampled with weed management problems with glyphosate presented the occurrence of more than one species resistant to this herbicide. In the orange orchards of the Nossa Senhora ranch, resistance was identified only for C. elata. The citrus orchards from the Olhos D’agua (C. elata and D. insualris) and Passatempo (C. bonariensis and C. elata) ranches presented two glyphosate-resistant species. However, all the orchards from the Olimpia region showed signs of resistance to B. pilosa. Four species (C. bonariensis, C. elata, D. insularis, and S. americanum) with different glyphosate resistance levels were identified in the Taiti acid lime orchard in the Araras region and, in the Pêra, the sweet orange orchards of the Cordeiropolis and Mogi-Mirim municipalities exhibited four resistant species (C. bonariensis, C. elata, D. insualris, and S. americanum) (Table 2).
3.2. Shikimic Acid Accumulation
The accumulation of shikimic acid differs between species and populations within species. Similar to the dose–response results, A. deflexus, A. hybridus, and T. procumbens were susceptible to glyphosate, as they accumulated large amounts of shikimic acid both at 370 and 740 g ae ha–1. Plants of B. pilosa from Olimpia and C. elata treated with 370 g ae ha–1 accumulated between 2 and 4 less shikimate than their susceptible counterparts (S-lim); however, when treated with a dose of 740 g ae ha–1, these species accumulated amounts of shikimate similar to S-lim populations. Resistant populations of C. bonariensis, D. insularis, and S. americanum accumulated up to 10 times less shikimate than their susceptible counterparts when treated at 370 g ae ha–1, and even when there was an increase at a dose of 740 g ae ha–1, the accumulation was lower than in the susceptible populations (Figure 2).
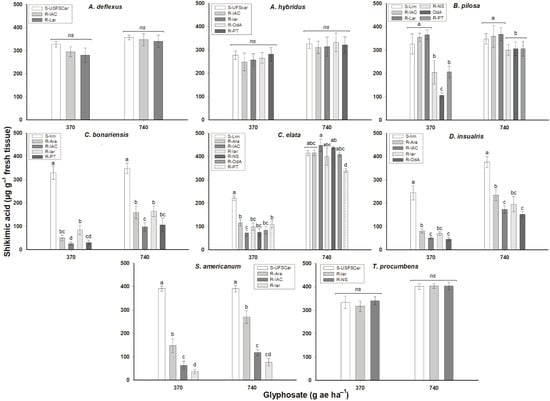
Figure 2.
Shikimic acid accumulation in weeds collected in citrus orchards from different regions of the São Paulo State, Brazil. Same letter denotes no differences among treatments as determined by Tukey’s test (p < 0.05). ns = non-significant. Vertical bars ± standard (n = 3).
4. Discussion
The recommended dose of glyphosate by manufacturers varies from 370 to 2220 g ae ha–1, depending on the crop and the weed species to be controlled [23]. Due to the need for multiple applications per agricultural year, many growers have adopted a dose of 740 g ae ha–1, which was seen as the standard dose in previous years [8]. However, due to increasing weed management failures, most growers apply doses of at least 1080–1110 g ae ha–1 [9]. Although growers reported glyphosate resistance in A. deflexus, A. hybridus, B. Pilosa, and T. procumbens, the tested populations of these species were susceptible in both the dose–response and shikimate acid accumulation assays. The control failures observed may be due to the variability involved in the phenological stage of weeds. Although manufacturers recommend application at the early phenological stage of 4–6 true leaves [23] (as in this work), the tropical climate of Brazil [24] and the staggered germination of weeds [25] make it impossible to apply herbicides on uniform populations of weeds. The phenological stage directly influences the efficacy of herbicides. For example, susceptible populations of C. bonariensis in the rosette stage presented a GR50 of 15.7 g ae ha–1; however, in bolting and flowering, the values were 87 and 118 g ae ha–1, respectively, i.e., the susceptibility to glyphosate in these phenological stages was 5.4 and 7.3 times less than in the rosette stage [26]. The umbrella (shadding) effect imposed by taller plants on smaller plants also affects herbicide efficacy [27], and many growers end up associating resistance with the high plant survival rates of their orchards; however, in many cases, the plants may not have been reached by the herbicide or were reached in non-lethal concentrations.
The populations of B. pilosa observed in this study, based on their high weight reduction with low doses of glyphosate, can be classified as susceptible. They require special attention because the populations from the Olimpia region showed signs of glyphosate resistance, with LD50 values ranging between 350 and 450 g ae ha–1. Bidens pilosa is a species that is already reportedly highly resistant to glyphosate, according to studies regarding citrus orchards in Mexico [28]. In a parallel study of this research group, resistant populations of this species were identified with LD50 values of up to 880 g ae ha–1 [9]. This suggests that there are already populations of B. pilosa with different levels of resistance in citrus orchards in São Paulo. In contrast, all populations of C. bonariensis, C. elata, and D. insularis tested in this study were found to be resistant to glyphosate. Conzya bonariensis and D. insualris had already been identified as having glyphosate resistance in Brazilian citrus orchards [14,15], and C. elata in soybean crops [29]. There are no studies that report the distribution of resistance to glyphosate in species in Brazilian citrus orchards, unlike for other crops [30]. However, based on our results, it can be concluded that resistant populations of these species are widely distributed in the citrus production region of São Paulo. In addition to seed dispersal, resistance may have been produced by independent events [31] since the levels of resistance varied between the populations of each species.
Solanum americanum is an unprecedented case of glyphosate resistance in Brazil and in the world. In 2020, Amaranthus viridis was also reported to be glyphosate-resistant in some of the citrus orchards evaluated in this study (R-lar and R-IAC) [8], i.e., these two new glyphosate-resistant species join widely distributed resistant species such as C. bonariensis, C. elata, and D. insularis, increasing the challenge of weed management in citrus orchards in Brazil. The identification of up to five resistant species reiterates the complex situation of glyphosate resistance in some citrus orchards in São Paulo. Several studies have already characterized the resistance mechanisms involved in glyphosate-resistant populations of C. bonariensis, C. elata, and D. insularis from Brazil [14,29,32], and although it is possible that the populations in this study present different mechanisms than those reported in the literature, our future studies will focus on characterizing the resistance mechanisms in S. americanum, as well as those of A. viridis.
The occurrence of multiple species resistant to glyphosate within the same citrus orchard is worrisome since weed management is more difficult and costly. The average cost of chemical weed management in soybean under Brazilian conditions was estimated to be BRL 120 ha–1 year–1 [20]. That cost increases based on the presence of herbicide resistant weeds. When only one resistant species occurs, the cost increases by BRL 83.5 for Conyza sp. species, BRL 98 for Lolium sp., and BRL 227 for D. insularis; however, when multiple species occur (Conyza sp. + D. insularis), the average increase is BRL 312 [20], i.e., the cost of resistance management nearly quadruples (BRL 120 + 312) since the application of herbicides with different mechanisms of action is required.
Growers are generally reluctant to adopt integrated weed management strategies [33] because these costs often increase, and it is understandable that they would want to strive to reduce production costs. In addition, most of the time, growers do not have a projection of the increase in productivity and profit margins [34]. Research conducted by this research group shows that, although rising weed management costs regarding citrus orchards can complicate weed management strategies, the productive potential of orchards and the potential economic returns also rise [3]. Mechanical weed control is inefficient and expensive (BRL 696 ha–1), but by implementing ecological mowing, economic returns can be doubled compared to unmanaged orchards (BRL 7212 vs. 3344 ha–1). Weed management with post-emergence herbicides was three times cheaper than with pre-emergence herbicides (BRL 435 vs. 148 ha–1); however, the economic return was similar, and most farmers adopt this method because, in addition to being cheap, it is also easier to execute from a practical point of view. However, the association of pre- and post-emergence herbicides and ecological mowing (BRL 500 ha–1) increased yield, improved the quality of fruits, and tripled economic returns (BRL 11,124 ha–1). Therefore, researchers have the responsibility of producing and transferring information on herbicide resistance management strategies that are logistically, technically, and economically viable since, most of the time, studies that evaluate management alternatives do not evaluate the costs of each strategy, much less the productive potential.
5. Conclusions
The occurrence of multiple species of glyphosate-resistant weeds was identified in citrus orchards from different regions of São Paulo, Brazil. The incorrect management of widely distributed glyphosate-resistant species such as C. bonariensis and D. insularis make it difficult to control susceptible species, as observed with A. deflexus, A. hybridus, B. pilosa, and T. procumbens. Furthermore, this work identified glyphosate resistance in S. americanum for the first time. Our future research will be focus on characterizing the resistance mechanisms of resistant populations, mainly of S. americanum, aiming to develop resistance management strategies that consider the cost and productive potential of citrus orchards.
Author Contributions
Conceptualization, R.A.-d.l.C. and M.F.d.G.F.d.S.; methodology, G.d.S.A., R.A.-d.l.C., R.M., L.R.R.J., and M.F.d.G.F.d.S.; software, G.d.S.A. and R.A.-d.l.C.; validation, L.B.d.C., F.A.d.A., and M.F.d.G.F.d.S.; formal analysis, G.d.S.A. and R.A.-d.l.C.; investigation, G.d.S.A., R.A.-d.l.C., R.M., and L.R.R.J.; resources, F.A.d.A. and M.F.d.G.F.d.S.; data curation, G.d.S.A. and R.A.-d.l.C.; writing—original draft preparation, G.d.S.A., R.A.-d.l.C., R.M., and L.R.R.J.; writing—review and editing, G.d.S.A., R.A.-d.l.C., L.B.d.C., F.A.d.A., and M.F.d.G.F.d.S.; visualization, L.B.d.C., F.A.d.A., and M.F.d.G.F.d.S.; supervision, G.d.S.A., R.A.-d.l.C., R.M., L.R.R.J., L.B.d.C., F.A.d.A., M.F.d.G.F.d.S.; project administration, L.B.d.C., F.A.d.A., and M.F.d.G.F.d.S.; funding acquisition, M.F.d.G.F.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
RAC and MFGFS thank the “Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP” for the financial support (main-grant: 2014/50918-7, subgrant: 2018/15910-6).
Data Availability Statement
Data sharing is not applicable.
Acknowledgments
The authors thank Guilherme Moraes de Oliveira for her technical assistance in the greenhouse experiments. Ricardo Alcántara-de la Cruz thanks to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (grant: 105187/2023-2).
Conflicts of Interest
The authors declare no conflict of interest.
References
- USDA—United States Department of Agriculture. Citrus. 2023. Available online: https://apps.fas.usda.gov/psdonline/circulars/citrus.pdf (accessed on 31 March 2023).
- SIDRA—Sistema IBGE de Recuperação Automática. Levantamento Sistemático da Produção Agrícola—Fevereiro. 2023. Available online: https://sidra.ibge.gov.br/home/lspa/sao-paulo (accessed on 31 March 2023).
- Martinelli, R.; Rufino, L.R., Jr.; Alcántara-de la Cruz, R.; Monquero, P.A.; Azevedo, F.A. Ecological Mowing with Residual Herbicides: A Viable Weed Management Tool for Citrus Orchards. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3997512 (accessed on 31 March 2023).
- Atakan, E.; Pehlivan, S. Influence of weed management on the abundance of thrips species (Thysanoptera) and the predatory bug, Orius niger (Hemiptera: Anthocoridae) in citrus mandarin. Appl. Entomol. Zool. 2020, 55, 71–81. [Google Scholar] [CrossRef]
- Azevedo, F.A.; Almeida, R.F.; Martinelli, R.; Próspero, A.G.; Licerre, R.; Conceição, P.M.; Arantes, A.C.C.; Dovis, V.L.; Boaretto, R.M.; Mattos, D., Jr. No-Tillage and high-density planting for Tahiti acid lime grafted onto flying dragon trifoliate orange. Front. Sustain. Food Syst. 2020, 4, 108. [Google Scholar] [CrossRef]
- Martinelli, R.; Rufino, L.R., Jr.; Melo, A.C.; Alcántara-de la Cruz, R.; Silva, M.F.G.F.; Silva, J.R.; Boaretto, R.M.; Monquero, P.A.; Mattos, D., Jr.; Azevedo, F.A. Glyphosate excessive use chronically disrupts the shikimate pathway and can affect photosynthesis and yield in citrus trees. Chemosphere 2022, 308, 136468. [Google Scholar] [CrossRef] [PubMed]
- Fundecitrus—Produtos para Proteção da Citricultura. Available online: https://www.fundecitrus.com.br/protecitrus (accessed on 2 May 2023).
- Alcántara-de la Cruz, R.; Amaral, G.S.; Oliveira, G.M.; Rufino, L.R.; Azevedo, F.A.; Carvalho, L.B.; Silva, M.F.G.F. Glyphosate resistance in Amaranthus viridis in Brazilian citrus orchards. Agriculture 2020, 10, 304. [Google Scholar] [CrossRef]
- Martinelli, R.; Rufino, L.R., Jr.; Alcántara-de la Cruz, R.; da Conceição, P.M.; Monquero, P.A.; de Azevedo, F.A. Glyphosate excessive use affects citrus growth and yield: The vicious (and unsustainable) circle in Brazilian orchards. Agronomy 2022, 12, 453. [Google Scholar] [CrossRef]
- Duke, S.O. The history and current status of glyphosate. Pest Manag. Sci. 2018, 74, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Shaner, D.L.; Nadler-Hassar, T.; Henry, W.B.; Koger, C.H. A rapid in vivo shikimate accumulation assay with excised leaf discs. Weed Sci. 2005, 53, 769–774. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate: Environmental fate and impact. Weed Sci. 2020, 68, 201–207. [Google Scholar] [CrossRef]
- Lacroix, R.; Kurrasch, D.M. Glyphosate toxicity: In vivo, in vitro, and epidemiological evidence. Toxicol. Sci. 2023, 192, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.B.; Alves, P.L.C.A.A.; Gonzalez-Torralva, F.; Cruz-Hipolito, H.E.; Rojano-Delgado, A.M.; De Prado, R.; Gil-Hammanes, J.; Barro, F.; Luque de Castro, M.D. Pool of resistance mechanisms to glyphosate in Digitaria insularis. J. Agric. Food Chem. 2012, 60, 615–622. [Google Scholar] [CrossRef]
- Moreira, M.S.; Nicolai, M.; Carvalho, S.J.P.; Christoffoleti, P.J. Glyphosate-resistance in Conyza canadensis and C. Bonariensis. Planta Daninha 2008, 25, 157–164. [Google Scholar] [CrossRef]
- Caetano, R.S.X.; Christoffoleti, P.J.; Victoria-Filho, R. Weed seed bank of a ’Pera’ citrus orchard. Sci. Agric. 2001, 58, 509–517. [Google Scholar] [CrossRef]
- Passos, O.S.; Souza, J.D.S.; Bastos, D.C.; Girardi, E.A.; Gurgel, F.L.; Garcia, M.V.B.; Oliveira, R.P.; Soares Filho, W.S. Citrus industry in Brazil with emphasis on tropical areas. In Citrus-Health Benefits and Production Technology, 1st ed.; Sajid, M., Ed.; IntechOpen: London, UK, 2019; pp. 59–78. [Google Scholar]
- Andres, A.; Concenço, G.; Schreiber, F.; Agostinetto, D.; Vargas, L.; Behenck, G.; Antoniaci, C.; Alves, Y.S. Predictions for weed resistance to herbicides in Brazil: A botanical approach. In Herbicide Resistance in Weeds and Crops; Pacanoski, Z., Ed.; IntechOpen: London, UK, 2017; pp. 134–157. [Google Scholar]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 3 April 2023).
- Adegas, F.S.; Vargas, L.; Gazziero, D.L.P.; Karam, D.; Silva, A.F.; Agostinetto, D. Impacto econômico da resistência de planta daninhas a herbicidas no Brasil. EMBRAPA-Circular Técnica. 2017, 32, 1–11. [Google Scholar]
- Cromartie, T.H.; Polge, N. Method of Detecting Shikimic Acid. U.S. Patent 006482654B1, 8 February 2002. Available online: https://patents.google.com/patent/US6482654B1/en (accessed on 30 March 2023).
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed]
- ADAPAR—Agência de Defesa Agropecuária do Paraná. Trade Label of Roundup Original DI. 2023. Available online: https://www.adapar.pr.gov.br/sites/adapar/arquivos_restritos/files/documento/2023-02/rounduporiginaldi.pdf (accessed on 3 April 2023).
- Assunção, J.; Chein, F. Climate change and agricultural productivity in Brazil: Future perspectives. Environ. Dev. Econ. 2016, 21, 581–602. [Google Scholar] [CrossRef]
- Batlla, D.; Benech-Arnold, R.L. Weed seed germination and the light environment: Implications for weed management. Weed Biol. Manag. 2014, 14, 77–87. [Google Scholar] [CrossRef]
- González-Torralva, F.; Cruz-Hipolito, H.; Bastida, F.; Mülleder, N.; Smeda, R.J.; De Prado, R. Differential susceptibility to glyphosate among the Conyza weed species in Spain. J. Agric. Food Chem. 2010, 58, 4361–4366. [Google Scholar] [CrossRef]
- Xie, H.S.; Hsiao, A.I.; Quick, W.A. Effect of shading on activity of imazamethabenz and fenoxaprop in wild oat (Avena fatua). Weed Sci. 1994, 42, 66–69. [Google Scholar] [CrossRef]
- Alcántara-de la Cruz, R.; Fernández-Moreno, P.T.; Ozuna, C.V.; Rojano-Delgado, A.M.; Cruz-Hipolito, H.E.; Domínguez-Valenzuela, J.A.; Barro, F.; De Prado, R. Target and non-target site mechanisms developed by glyphosate-resistant hairy beggarticks (Bidens pilosa L.) populations from Mexico. Front. Plant Sci. 2016, 7, 1492. [Google Scholar] [CrossRef]
- Brunharo, C.A.; Patterson, E.L.; Carrijo, D.R.; Melo, M.S.C.; Nicolai, M.; Gaines, T.A.; Nissen, S.J.; Christoffoleti, P.J. Confirmation and mechanism of glyphosate resistance in tall windmill grass (Chloris elata) from Brazil. Pest Manag. Sci. 2016, 72, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Lucio, F.R.; Kalsing, A.; Adegas, F.S.; Rossi, C.V.S.; Correia, N.M.; Gazziero, D.L.P.; Silva, A.F. Dispersal and frequency of glyphosate-resistant and glyphosate-tolerant weeds in soybean-producing edaphoclimatic microregions in Brazil. Weed Technol. 2019, 33, 217–231. [Google Scholar] [CrossRef]
- Takano, H.K.; Oliveira, R.S., Jr.; Constantin, J.; Mangolim, C.A.; Machado, M.D.; Bevilaqua, M.R. Spread of glyphosate-resistant sourgrass (Digitaria insularis): Independent selections or merely propagule dissemination? Weed Biol. Manag. 2018, 18, 50–59. [Google Scholar] [CrossRef]
- Ferreira, E.A.; Galon, L.; Aspiazú, I.; Silva, A.A.; Concenço, G.; Silva, A.F.; Oliveira, J.A.; Vargas, L. Glyphosate translocation in hairy fleabane (Conyza bonariensis) biotypes. Planta Daninha 2008, 26, 637–643. [Google Scholar] [CrossRef]
- Moss, S. Integrated weed management (IWM): Why are farmers reluctant to adopt non-chemical alternatives to herbicides? Pest Manag. Sci. 2019, 75, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Livingston, M.; Fernandez-Cornejo, J.; Frisvold, G.B. Economic returns to herbicide resistance management in the short and long run: The role of neighbor efects. Weed Sci. 2016, 64, 595–608. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).