Abstract
This review appraised current research on enzyme-embedded biodegradable agricultural plastics and microbial degradation, given that the increased use of fossil-fuel-based plastics in agriculture involved significant environmental tradeoffs. Over 370 million tons of plastics were produced in 2019, releasing over 400 million tons of greenhouse gases during production, transportation, consumption, burning, and exposure to sunlight biodegradation. Less than 10% of bags are recycled at the end of their life, leading to environmental pollution. Thus, it is imperative to summarize studies that have suggested solutions of this problem. The scoping review approach was preferred, given that it established current practices and uncovered international evidence on bio-based solutions and conflicting outcomes. Bioplastics with low greenhouse warming potential had a small market share (approximately 1%). The accumulation of fossil-fuel-based plastics and poor post-use management releases mercury, dioxins, furans, and polychlorinated biphenyls (PCBs). Enzyme-embedded polymers degrade fast in the environment but lack the desired mechanical properties. Even though polylactic acid (PLA) and other bioplastics are better alternatives to synthetic polymers, they persist in the environment for years. Fast degradation is only practical under special conditions (elevated temperatures and humidity), limiting bioplastics’ practical benefits. The research and development of plastics that could degrade under ambient conditions through enzyme-catalyzed reactions and soil-inoculated microbes are ongoing. However, there are no guarantees that the technology would be profitable in commercial agriculture. Other limiting factors include the geographical disparities in agricultural plastic waste management. Future perspectives on the waste management of agricultural plastics require smart technologies, such as artificial intelligence (AI), machine learning (ML), and enzyme-embedded plastics that degrade under ambient conditions. The replacement of synthetic plastics with polylactic acid and polycaprolactone/Amano lipase (PCL/AL) composite films would offset the negative ecological effects. A major drawback was the slow research and development and commercial adoption of bio-based plastics. The transition to bioplastics was resource- and time-intensive.
1. Introduction
Plastics are widely used in agricultural production, including using them in mulch films, packaging, and plastic-coated seeds. Most agricultural plastics are fossil-based polymers, with a few biopolymers available in the market [1]. The production of fossil-based agricultural plastics is based on depolymerization, the process through which a polymer is disassembled into its constituent monomers or oligomers, which are then either reused or degraded as building blocks for newly generated polymers [2]. However, depolymerization in the production of traditional plastics is based on either chemical or thermal processes, which are energy-intensive and costly. According to Greene et al. [1], the costs associated with the production of traditional plastics can be avoided by adopting bioplastics, though many biodegradable polymers have been criticized for their slow biodegradability, thereby offering no environmental benefits. The solution to this problem is the adoption of enzyme-embedded bioplastics, which refer to a group of biopolymers embedded with known and verified hydrolases that can act on fossil-based polymers to fasten the degradation process [1]. Examples of enzymes commonly known and verified for this purpose include polyethylene terephthalate (PET)-active enzymes (see Table 1 in [1], for specific examples). Considering the negative environmental impact of fossil-based polymers, embedding enzymes in them can improve their biodegradation, thereby reducing this negative effect on the environment.
Thus, before presenting the purpose and objectives of this review, it is imperative to perform the life-cycle assessment (LCA) of non-biodegradable plastics versus biodegradable plastics. The world produced approximately 370 million tons of plastics in 2019 alone, with only 9% being recycled, 12% being incinerated, and the remaining being dumped in landfills or the environment [3]. The production of plastics, especially fossil-fuel based, their transportation, incineration, burning, or biodegradation under the sun produces large amounts of greenhouse gases (GHGs), currently estimated to contribute up to 400 million tons of GHGs per year, and this is expected to increase exponentially soon if the issue is not addressed urgently [4]. When non-bioplastics are left in the environment, they take several years to biodegrade. Since fossil-based polymers account for the biggest share of plastics produced every day and their high production rates coupled with high consumption, non-bioplastics accumulate in the environment, leading to pollution [5]. As elaborated on later, the production of bioplastics is currently limited in the commercial space, meaning most of the plastics currently produced are non-biodegradable. Due to the low production of bioplastics and their relatively faster biodegradation compared to traditional plastics, it can be anticipated that they are rarely found in the environment and are not a major concern for pollution.
1.1. Purpose and Objectives
The review article synthesized recent research on enzyme-embedded agricultural bioplastics and the utilization of microbial degradation processes to provide a nuanced understanding of the cost–benefits of the technique relative to the traditional chemical and mechanical recycling of synthetic polymers. Recent studies have provided contrasting narratives on the subject. On one hand, critics argued that the transition toward the production of enzyme-embedded agricultural bioplastics would only offer limited benefits in the long term [1]. On the other hand, proponents of the technique argue that it can enhance the biodegradation of plastic materials used in agricultural processes [6,7,8]. Even though there was extensive evidence to the contrary, the criticism of enzyme-embedded plastics by [1] was grounded on the unresolved issues concerning the performance of enzyme-embedded bioplastics. The disagreement between studies regarding the costs and benefits of enzyme-embedded agricultural bioplastics raises concerns and hence should be treated as a significant knowledge gap, owing to the importance of the subject matter. In addition, there is an urgent need to shift to highly degradable bioplastics amid climate change advocacy voices. In this review, it was hypothesized that, although enzyme-embedded agricultural bioplastics have not attained commercial maturity, they have shown great potential in achieving faster biodegradation in agricultural use. The complementary hypothesis is that enzyme-embedded agricultural bioplastics will attain slow commercial maturity because they have not shown commercial benefits.
The novelty of this review is based on the finding that current bioplastics in the market have not achieved what they were initially intended for. They remain non-biodegraded for years, making little to no change from traditional fossil-based polymers [1]. With emerging technologies, such as enzyme-embedded agricultural bioplastics, artificial intelligence, and machine learning, it is imperative to map the current state-of-the-art literature to investigate the maturity level of these technologies and their potential to solve the presenting challenges. A quick literature survey reveals contrasting views regarding the effectiveness of enzyme-embedded agricultural bioplastics [1,6]. That itself is a significant literature gap. This review is the first to map literature focusing on AI, ML, and enzyme-embedded agricultural bioplastics. The review will focus on the following objectives to address the knowledge gaps:
Objectives
- To review current research on microbial degradation of agricultural plastics using microorganisms such as bacteria, fungi, and enzymes;
- To explore the role of enzyme-embedded agricultural plastics in improving commercial recyclability;
- To explore the suitability of intelligent technologies, including artificial intelligence (AI) and machine learning (ML), in agricultural plastic waste management.
1.2. State-of-the-Art Research on Enzyme-Embedded Biodegradable Agricultural Plastics and Microbial Degradation
There is no consensus on the best solutions for agricultural plastic waste management. One group of researchers advocated for the gradual phase-out of synthetic plastic materials with biodegradable films and lesser uses of plastics in agriculture [9,10]. Even though Adhikari et al. [9] and Marí et al. [10] supported the use of bioplastics in agriculture, critics such as DelRe et al. [7] argued that the strategy had minimal environmental benefits because polylactic acid (PLA) and other plastics did not decompose under natural conditions. PLA persisted in the environment for the same duration as polyethylene (PE), polypropylene (PP), high-density polyethylene (HDPE), low-density polyethylene (LDPE), and polyvinyl chloride (PVC). If the PLA was discarded with non-bioplastics, there was a higher risk of contamination—it would affect the effectiveness of mechanical recycling. For PLA to degrade fast, the degradation process must be catalyzed by heat and humid conditions via expensive processes.
The complexity of PLA recycling could explain why stakeholders in the polymer industry hesitated to produce biodegradable agricultural plastics in large quantities. Sintim and Flury [11] adopted a cautious approach towards agricultural plastic waste management, suggesting that the environmental cost–benefits of the polymers should be thoroughly investigated before widespread commercial adoption to prevent a recurrence of the plastic microbead scenario in healthcare and cosmetic products, which led to the late adoption of the Microbead-Free Waters Act of 2015. The cautious approach did not align with (Adhikari et al. [9]; Marí et al. [10])’s worldviews, which advocated for the aggressive use of bioplastics. Despite the recommendations made by (Adhikari et al. [9]; Marí et al. [10]) and other researchers, Vanderreydt et al. [12], noted that the share of bioplastics had remained low (about 1%). The low adoption of bioplastics could be attributed to the inadequate mechanical properties relative to synthetic polymers.
The shortcomings of PLA and other bioplastics have catalyzed research into enzyme-embedded bioplastics [13,14,15], 3D printing [2], and the 4D printing of bioplastics. The support for bioplastics by [13,14,15] negated the tradeoff between ease of biodegradation and mechanical performance (TS, EB, and YM). The need for robust mechanical performance was highlighted by La Mantia et al. [16]. Despite the inverse relationship between the biodegradation of agricultural plastic and mechanical performance, recent research and development (RandD) led to widespread usage of bioplastics in mulching, protective nets, and greenhouse covering [17,18]. However, the production-related constraints must be addressed moving forward.
The limitations of PLA and other bioplastics reinforce the need to invest in enzyme-embedded plastics. On the downside, Chow et al. [1], and Greene et al. [2], suggested that the technology for enzyme-embedding plastics was in its infancy, and there were multiple unknown variables. Additionally Chow et al. [1], noted that recent R&D projects failed to produce functional biocatalysts with broad-spectrum enzymes that can act on different subtypes of synthetic plastics such as polystyrene, polyamide, polyurethane, PVC, polypropylene, and polyethylene. Furthermore, the commercially viable pilot projects reported in the literature were specific to low-density polymers with limited crystallinity. The concerns raised by Chow et al. [1] about the limits of enzyme-embedded biodegradable agricultural plastics and microbial degradation contrast with DelRe et al. [7], who suggested that the existing constraints could be resolved through the introduction of biocatalysts coupled with interfacing enzymes and biomachinery. The proposed approach would provide the on-demand modification and/or programmable degradation of plastics.
Even though introducing biocatalysts by DelRe et al. [7] may help offset selected challenges highlighted by Chow et al. [1], there were other barriers to the widespread adoption of the enzyme-embedded biodegradable agricultural plastics and microbial degradation technology. For example, current technologies are only effective with low-density synthetic plastics. However, the high density and highly crystalline synthetic plastics represent about 95% of all polymers in the market [19,20]. The preliminary observations drawn from Bilal et al. [6]; DelRe et al. [7]; González-Henríquez et al. [8] and Chow et al. [1] show that there was no consensus on the case for enzyme-embedded biodegradable agricultural plastics and microbial degradation as ideal alternatives to the traditional process.
Empirical studies do not address the gaps in knowledge about the superiority and suitability of microbial degradation and enzyme-embedded techniques in different classes of agricultural plastics. For example, Greene et al. [2] research on 3D-printed enzyme-embedded agricultural plastics only focused on polycaprolactone/Amano lipase (PCL/AL) composite films. Similarly, Huang et al.’s [13] study was confined to one class of agricultural plastics, namely poly(L-lactic acid) (PLLA) (see Figure 1). The focus on two classes of plastics by Greene et al. [2] and Huang et al. [13] does not yield conclusive evidence that may help shape agricultural policies and practices. The criticism of recent experimental research was validated by the availability of other agricultural plastics with near equal or superior efficiency.
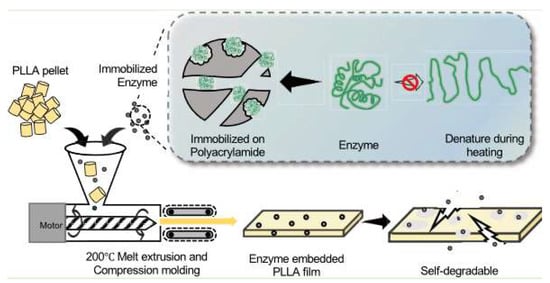
Figure 1.
Production of enzyme-embedded bioplastics [13].
The intensification of agricultural production has exacerbated the accumulation of agricultural plastic waste on farms to satisfy the growing demand for food. Conservative estimates indicate that nearly 7.4 million tons of agricultural plastic waste comprising polyvinyl chloride (PVC), polymethylmethacrylate (PMMA), polyethylene, and polypropylene were used and disposed of on farms [21]. The true estimates could be higher, considering that there was poor data documentation on remote farms. The utilization of agricultural plastics is expected to increase fourfold by 2050 (from a low of 9.2 billion to 34 billion). The projected increase in the demand for bioplastics will create an enormous ecological challenge, considering the delayed transition to ecologically suitable bioplastics. As of 2021, only 1% of commercially available plastics were bio-based [22]. Considering that the demand for agricultural plastics was projected to remain low due to the less favorable mechanical, optical, and chemical properties, it was necessary to explore alternative interventions, including developing enzyme-embedded agricultural plastics and microbial-mediated biodegradation. Agricultural plastics have an extended lifespan. For example, PMMA plastics have a lifespan of 25 years [23]. The agricultural plastic PMMA degradation rate could be even higher, considering that the degradation process is slowed by the presence of agricultural residues and pesticides [24]. The high rates of plastic accumulation underscore the need for customized and localized enzyme-embedded biodegradable agricultural plastics and microbial degradation.
Huang et al. [13] successfully synthesized self-degradable plastic using poly(L -lactic acid) (PLLA) pellets and enzymes immobilized on polyacrylamide. The observations made by Huang et al. [13] were in tandem with Greene et al. [2], who reported a 70% polymer degradation (weight loss) following the enzyme-mediated breakdown of the polymer structure. The demonstrated benefits of enzyme-embedded biodegradable agricultural plastics and microbial degradation will compel stakeholders to abandon traditional methods of agricultural plastic waste management, such as incineration, which releases mercury, dioxins, furans, polychlorinated biphenyls (PCBs) into the atmosphere [24]. However, at the moment, there has been a minimal transition towards benign agricultural plastics; this is despite the immense potential of agricultural plastics in recent studies. The researcher posits the challenge may be resolved through better delineation of the benefits and the limits of alternative plastic waste management options, particularly enzyme-embedded biodegradable agricultural plastics and microbial degradation vis-à-vis incineration, chemical, and mechanical recycling processes. The following sections present the research methodology (data collection procedures and extraction), the arguments in favor of replacing synthetic plastics, bioplastic products in the market, microbial degradation and enzyme-embedded plastics, and the incorporation of intelligent technologies in plastic waste management.
2. Research Method
2.1. Justification for the Scoping Review
The choice of a scoping review was grounded on the following considerations. First, scoping reviews are valid approaches for examining emerging evidence [25] when the use of microorganisms is still developing, and the emerging evidence is inadequate to catalyze a complete industry transition. Second, mapping current literature on a subject based on an a priori protocol helps to reduce bias by enhancing transparency in the review process [26]. In the current case, the appraisal of the current research on agricultural plastic waste management methods helped to identify gaps and inadequacies of existing agricultural plastic waste recycling technologies and to inform future policies.
2.2. Research Questions
Three questions guided the review process.
Research question 1. Are biodegradable agricultural plastics appropriate alternatives to synthetic polymers?
Research question 1.1. Can enzyme-embedded microbial plastics replace traditional synthetic plastic materials?
Research Question 2. Which techniques can improve an enzyme’s ability to break down high-density plastics such as polystyrene, polyamide, polyurethane, PVC, polypropylene, and polyethylene?
Research question 2.1: What are the tradeoffs associated with enzyme-embedded biodegradable agricultural plastics and microbial degradation?
2.3. Search Strategy
The review approach was critical to addressing gaps in the knowledge on synthetic plastic waste using enzyme-embedded biodegradable agricultural plastics and microbial degradation to achieve better sustainability. Brings et al. [27] noted that in-depth reviews were reliable and less biased than ad-hoc reviews. Additionally, conducting systematic reviews in agricultural engineering is an established practice [18,28,29]. A key step in the review was determining the search strategy approaches for extracting literature from scholarly databases, such as Springer, Nature, IEEE, Taylor and Francis, and Web of Science. The three search strategies (manual, automated, and snowballing) recommended by Bogner et al. [30], were employed to reduce the number of missed articles on enzyme-embedded and microbial degradation. Automated searches involve the application of pre-defined search strings. The manual searches were limited to hard-copy books and articles.
The search process was aligned with E. Navarro et al.’s [31] review of technology solutions in smart farming. The publication window of interest was between 2012 and 2022. However, exceptions were made for seminal articles on microbial degradation and enzyme embedding. The seminal articles made an original contribution to the subject.
2.4. Study Selection
The search processes were guided by the following keywords: enzyme-embedded, agricultural plastics, microbial, degradation, sustainability, farming, and agriculture. The listed search terms were expanded using Boolean operators AND/OR. For example, the search phrases’ ‘enzyme-embedded’ AND ‘agricultural plastics’ were combined to extract additional literature from the scholarly databases. The term ‘microbial degradation’ was not employed in isolation, given that it yielded information that was not specific to this research. The inclusion and exclusion criteria are highlighted in Table 1.

Table 1.
Inclusion and exclusion criteria.
Since the inclusion and exclusion criteria listed above did not demonstrate the number of studies extracted from different journals, the PRISMA approach was incorporated. The illustration in Figure 2 shows that 20 core publications (seminal research) were identified. In addition, five articles were sourced from non-peer-reviewed sources. Additional articles were cited to support the key observations made in the seminal research. The abstract, title, and full-text screening process led to the exclusion of duplicate and non-value-adding research. The case for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) framework in research was affirmed by Page et al. [32] and other scholars. In particular, Page et al. [32] recommended the tool for reporting systematic review evidence.
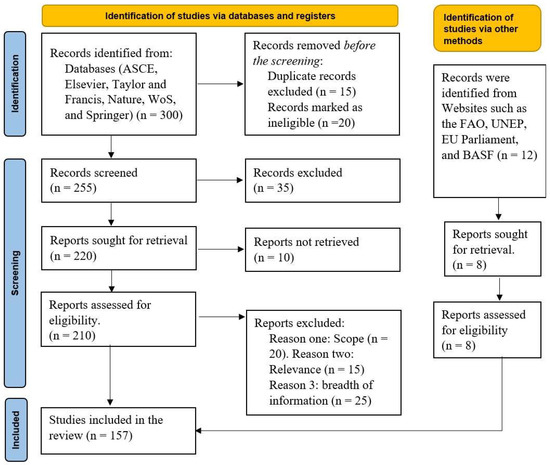
Figure 2.
PRISMA diagram illustrating the article selection process.
The 20 seminal articles were identified via the PRISMA screening process, and the additional five publications were subjected to forward and backward snowballing using the guidelines highlighted by Wohlin et al. [33]. Finally, an iteration of the snowballing process was performed to confirm the relevance of the extracted findings.
2.5. Data Extraction and Synthesis
Only the researcher was involved in the data extraction process. The full-text version of each article was reviewed, after which it was ranked based on relevance. The ranking represented the extent to which the findings addressed the study questions and themes on synthetic plastic waste using enzyme-embedded biodegradable agricultural plastics and microbial degradation to achieve better sustainability. The articles were synthesized using the thematic analysis of qualitative information and descriptive analysis of quantitative data. Thematic analysis is an established method for data analysis [34,35]. Thematic analysis and inductive reasoning established patterns in emerging research. The core themes were microbial degradation, methanotrophs, Acinetobacter, Pseudomonas, Bacillus, and biodegradable agricultural plastics. Each theme was reviewed in detail under Section 3.2.
3. Results and Discussion
3.1. Replacing Fossil-Fuel-Based Agricultural Plastics with Bio-Based Polymers
The replacement of fossil-fuel-based agricultural plastics with biodegradable, enzyme-embedded, 3D/4D-printed materials has drawn significant research attention in the past decade. The R&D of biodegradable, sprayable, and compostable materials was investigated by Castillo-Díaz et al. [36] and Marí et al. [10] in Spain and Adhikari et al. [9] in Australia. On the one hand, Castillo-Díaz et al. [36] noted that it was feasible to replace non-recyclable agricultural plastics with alternative plastics, including biodegradable polymers in flower pots, trellising rings, and chronotropic traps. On the other hand, Adhikari et al. [9] supported using biodegradable plastic films with reservations because of the limited mechanical properties, cost, and ineffective biodegradation processes. The concerns raised by Adhikari et al. [9] contrast with Maier [37], who suggested that the mechanical properties of biodegradable materials could be enhanced by increasing the ratios of PBAT and TPS. On the downside, enhancing the elastic modulus, tensile strength, and elongation before break involves a tradeoff with the biodegradation rate.
Even though there were valid arguments raised by Maier [37], the management of non-biodegradable plastic films after each planting season was expensive and small quantities of the plastics were retained in the soils, impacting subsequent crop yields [9,10,38]. The arguments made by Adhikari et al. [9], Marí et al. [10] and LeMoine et al. [38] validated Castillo-Díaz et al.’s [36] proposal on the substitution of fossil-fuel-based plastics with biodegradable polymers.
The commercial viability of substitution was demonstrated by BASF (the world’s leading chemical company), which developed Ecoflex—a biodegradable material comprising aromatic and aliphatic polyesters blended with poly (lactic acid) and starch [39]. In line with Siegenthaler et al. [39], Maier [37] supported using other biodegradable polymer brands such as Ecovia and Mater-Bi. In addition, the films were ideal for agricultural applications because of the higher biodegradation degree (>90%) and short composting timelines (~30 days) Maier [37]. On the downside, Siegenthaler et al. [39] and Maier [37] negated the potential tradeoff between mechanical performance and degradation.
Ecovia and Mater-Bi were used in stiff-formed packaging, coated paper boards, shrink films, knitted nets, mulch films, organic waste bags, and retail shopping bags. The broad scope of application illustrated that Ecoflex could partially substitute agricultural plastics manufactured using polyethylene (PE) and polypropylene (PP). Apart from Ecoflex, there were other successful interventions, such as the Label Agri-Waste project in the EU [40,41]. In line with Briassoulis et al. [40,41], Bos et al. [42] underscored the importance of the Label Agri-Waste project in the future management of agricultural plastic waste through proper labeling. The project enabled upcycling in the eight participating EU countries (Spain, Italy, Greece, Germany, France, Finland, Belgium, and Cyprus). However, the program’s long-term effectiveness would depend on the inclusion of all EU member states.
Since agricultural plastics were employed in many applications beyond agricultural packaging, exploring alternative IPM strategies that do not rely on synthetic plastic was necessary [43]. For example, adhesive/sticky traps could be replaced by ultrasound emitters in the management of pests, especially leaf-miner adults, whiteflies, and aphids [36]. Furthermore, the utility of ultrasound emitters as complementary IPM tools to sticky traps was demonstrated by Mankin [44]. Therefore, a key question was whether replacing sticky plastic traps with ultrasound emitters reduced agricultural plastic waste on farms.
Two worldviews were advanced concerning the subject. The first school of thought suggested that replacing sticky plastic traps was feasible [36]. The second school of thought advanced by Mankin [44] did not support the former because costs limited the practical benefits, and the acoustics were only effective in the control of insects that exhibit orientation behavior or phonotaxis, including Tephritid fruit flies, cockroaches, moths, field and mole crickets, midges, and mosquitoes. Based on the latter findings, ultrasonic emitters cannot entirely replace sticky plastic traps on farms.
The poor agricultural plastic waste management practices in Europe raise fundamental concerns about the future of precision farming, given the extension of areas under greenhouses did not translate to better concern for the environment. Spain had 71,783 ha under greenhouses; this was among the highest in Europe [36]. Lim and Thian [45] linked the perceived unwillingness to implement agricultural plastic waste management best practices to the lack of economic incentives. The economic value of plastic recyclate was about 5% of the virgin polymers. On the contrary, recycled steel and iron were 70–90% the value of virgin metals, while recycled paper was 58% the value of new paper. The claims advanced by Lim and Thian [45] contradict Shamsuyeva and Endres [46], who projected strong market demand for recyclates in Europe. The mixed observations suggest that the value of polymer recyclates may improve with the advances in technology.
3.2. Bioplastics for Smart Agriculture and Replacement of Fossil-Fuel-Based Plastics
3.2.1. Theme 1: Biodegradable Agricultural Plastics (Thermoplastic Starch (TPS), Polyhydroxyalkanoates (PHA), Polybutylene Adipate Terephthalate (PBAT), and PLA)
Thermoplastic starch (TPS) and PLA were among the leading biodegradable materials available in the market [9,10,47]. The demand and widespread use of PLA and TPS could be partly linked to the high biodegradation rate. A 90% degradation rate was achieved within 30 days at slightly higher temperatures (55 °C) [37]. The hydrolysis of the polymer was amplified at elevated temperatures, but the degree of amplification depended on the material’s crystallinity [48]. Both Maier [37] and Shi and Palfery [48] concurred there was an inverse relationship between the biodegradable polymer’s crystallinity and the biodegradation rate. There was no contrary evidence presented in the literature. The temperature and water-dependent anaerobic and aerobic biodegradation limit the economic benefits of bioplastics. The biodegradation rate was slightly lower for PHA/PBAT (45%) and PBAT (35%). The degradation rate of other bioplastics is presented in Table 2. Furthermore, Iwamoto and Tokiwa [49] argued that the parameters could be modified by adjusting the glass transition temperature, wettability, and composition of the polymers. The latter study confirmed that higher wettability and lower GTP yield resulted in higher degradation. In contrast, Maier [37], Shi and Palfery [48] and Huang et. al. [13] proposed better alternatives for enhancing degradation based on state-of-the-art research. For example, Proteinase K-derived Tritirachium album enzyme-embedded PLA films are promising, compared to the traditional processes.

Table 2.
Biodegradation rates of different types of plastics [37].
The commercial brands include Ecovio, Arrosi 69/240, Mimgreen, Bioflex, Mater-Bi, and Sphere (Marí et al., 2019). Each offers unique benefits and challenges on farms. For example, Ecovio was cheaper relative to fossil-fuel-based alternatives. Mater-Bi and Sphere were profitable in the long term, given the ease of degradation (Marí et al., 2019). On the downside, Ecovio had lower tensile strength than Mater-Bi bioplastics (9 versus 22 MPa) [16]. In addition, the elastic modulus of Mater-Bi was higher (110 MPa versus 98 MPa). In contrast, Ecovio had slightly better performance in terms of elongation at break (EB) (Mater-Bi’s EB was 617, while Ecovio’s was 617 MPa), but it was lower than synthetic plastics [16]. The unique mechanical properties highlighted by La Mantia et al. [16] depended on the polymer ratios. The influence of chemical composition on bioplastic utility was confirmed by La Mantia et al. [16] and Maier [37]. Ecovia was made of PBAT/PLA, while Mater-Bi was made of thermoplastic starch (TPS) and PCL.
Engineers were faced with a dilemma as they attempted to engineer durable bioplastics, given that any modifications in the polymer composition would influence the chemical properties (oxygen permeability and wettability and the rate of biodegradation) [9,10,37,47]. For example, Ecovio was 94% composted within 181 days (at ambient temperatures—28 °C). In contrast, a 30 µm-thick film of PLA was degraded after 11 months [37]. Achieving optimal biodegradation performance involves balancing the heat distortion temperature, water absorption rate, softening point, glass transition temperature, and melting point. The observations made by Maier [37] were partly inconsistent with Iwamoto and Tokiwa [49], who noted the water absorption rate for nylon was superior compared to PLC; this was a limiting factor because water helps catalyze biodegradation in the environment. From an economic perspective, plastics with the shortest degradation timeframe were best for commercial farms because the seasonal clearing of non-biodegradable films from soils was expensive in the long term.
On a positive note, biodegradation could be enhanced by inoculating soils with microorganisms (bacteria and fungi) [36]. In cases where inoculation was not feasible, enzyme-embedded plastics could be deployed [13,14,15]. However, current technologies do not support the commercial upscaling of enzyme-embedded polymers in farms [14,15]. Most commercial farms could either rely on fossil-fuel-based plastics or bioplastics, which were not easily degraded under normal conditions. Shi and Palfery’s [48] research helped to address the problem by modifying the glass transition temperature of the polymers. Moving forward, a fundamental question was whether it was best to invest in enzyme-embedded bioplastics or inoculate microorganisms in soils [7,14,15,48]. Such questions remain unresolved because scholars had no consensus on the technique best suited for commercial farms.
Since the bioplastics’ elastic modulus was lower than the biodegradable alternatives, and the biodegradation rates varied significantly, the mechanical performance must be enhanced prior to matching existing plastics in the market. The estimates provided by La Mantia et al. [16] were comparable to Aldas et al. [47]. However, in the latter study, the tensile strength and Young’s modulus were enhanced with the NF 866 polymeric matrix [47]. For example, the recycling of agricultural films was impacted by the accumulation of inert contaminants (water, organic matter, soil, minerals, sand, pesticides, and fertilizer), also referred to as exogenous materials [40,50]. The inert contaminants increase agricultural plastics’ weight, making recycling expensive and less suited for certain applications. On average, recycling conventional plastics costs EUR 100–300/ton (see Table 3). If the current subsidies increased by 50%, the production of biodegradable agricultural plastics might become profitable (10). In contrast to LeMoine et al. [38] and Marí et al. [10] other studies postulated that the economic costs in isolation do not predict the choice of the recycling method [51,52,53]; this was due to the limited commercial applications of the recycled materials, as noted by Lange [54] and Schyns and Shaver [55]. Plus, the recycling cycles were constrained by the chemical composition and additives in the recycling stream.

Table 3.
Specific application of agricultural plastics and the estimated cost of waste management [10,36].
The accumulation of exogenous materials increased the cost of plastic waste recycling and logistics (transportation of the plastic waste from the source to the disposal site or recycling plant) [36]. The conservative estimates provided by LeMoine et al. [38] suggested slight variations in waste management costs. For example, the recycling of anti-hail protective nets was expensive compared to the recycling of mulching films. The estimates provided by LeMoine et al. [38] contrast with Marí et al.’s research [10], which adopted a multi-dimensional view of plastic waste recycling. According to the latter worldview, the cost of biodegradable and non-biodegradable plastics should be the lifetime cost benefits (with and without subsidies) and the commercial brand available. Based on the data presented in Table 4, biodegradable films’ net margins were higher than non-biodegradable films. However, adoption in commercial agriculture was unfeasible due to limited production [12]. The concerns raised by Vanderreydt et al. [12] were not in agreement with the EU network of environment protection agencies, which advocated for the rapid adoption of bioplastics to offset the negative ecological impact [37]. The lack of consensus and growing criticism of bioplastics might be detrimental to the gradual phase-out of fossil-fuel-based alternatives.

Table 4.
Cost benefits and net margins associated with biodegradable and non-biodegradable plastic materials [10].
The review of the cost benefits of biodegradable (thermoplastic starch, PLA, and PCL) as a substitute for fossil-fuel-based alternatives (HDPE, LDPE, PP, PS, PE) demonstrated the following. First, current technologies for bioplastics are not well-established. The low cost of fossil-fuel-based alternatives explains why PE was extensively used in agriculture. Nonetheless, the gradual substitution of PE and other fossil-fuel-based plastics was feasible, given that Sphere and Ecovio were profitable alternatives. However, there were various constraints on widespread adoption, including higher market prices, which impeded commercial adoption, and lower subsidies. In the meantime, the production of bioplastics, especially from agricultural biomass, would remain a challenge.
The EU was leading in managing agricultural plastics [40,41] this was attributed to the intensive production of plastic waste through intensive greenhouse farming. The area under greenhouses within the EU accounts for about 40% of the global area under greenhouses (175,000 out of 405,000 ha) [36]. As noted by Castillo-Díaz et al. [36], greenhouse-cultivated areas were concentrated in the Netherlands, Italy, Greece, France, and Spain (71,783 ha) [36]. Similar patterns were documented by [56]. The large production capacity for agricultural plastics within the EU could partly explain why Germany, Denmark, and Switzerland led in energy recovery from plastic waste [56]. On a positive note, the intensification of greenhouse agriculture in Spain, Italy, and the Netherlands may result in a lesser ecological impact, given the tangible investments in plastic waste recycling—less than 10% were discarded in landfills [57]. The progress in agricultural and non-agricultural plastic waste collection in the Netherlands was further highlighted by Dijkgraaf and Gradus [58]. On the downside, this was not the case in Greece. The cost–benefits associated with different waste management practices for agricultural practices, including mechanical recycling, pyrolysis [59], landfilling and incineration, and product redesign, are reviewed in the next section. The utility and environmental sustainability of incineration versus landfilling are discussed.
3.2.2. Theme 2: Microbial Degradation and Enzyme/Microorganism-Embedded Biodegradable Agricultural Plastics
Microbial degradation of plastic waste involves bio-deterioration, bio-fragmentation, and assimilation [60]. Researchers isolated different bacteria and enzymes in the biodegradation of plastic materials [61,62,63,64]. Pilot studies yielded promising data on the future of microbial degradation [65,66]. Greene et al. [2] demonstrated the commercial viability of degrading polycaprolactone/Amano lipase (PCL/AL) composite films using microorganisms. The role of microorganisms (particularly lipase enzymes) in the biodegradation of PLC was also confirmed [49]. The progress made in Western countries contrasts with Africa, where the biodegradation of plastic waste was poorly investigated [67]. The inconsistent investment in next-generation research would negatively impact environmental conservation, given that explosive population growth increased the demand for plastics.
Even in cases where there was a robust investment in plastic biodegradation, stakeholders contended with the poor discarding and mixing of plastic waste. One school of thought claimed that the functionality of the microorganisms was impaired by polymer blending. For example, blending PLC with PET and PS compromised the biodegradability rate [45,46,57,68]. Another school of thought suggested that the role of bacteria, enzymes, or fungi in the biodegradation of plastics would be beneficial. On the one hand, scholars supported using enzymes, fungi, or bacteria [64,69,70,71]. On the other hand, considering that the accidental mixing of polymers was common during the collection and recycling of agricultural waste plastics, the problem could be offset by pairing the advanced 3D/4D printing of agricultural plastics and Al with microorganisms for the optimal decomposition of plastics.
The case for the 3D/4D printing of agricultural plastics was corroborated by Greene et al. [2] who used 3D printing techniques to embed enzymes into polycaprolactone/Amano lipase (PCL/AL) composite films to enhance the biodegradation rate. The enzyme-embedded bioplastics were degraded within seven days at near-normal temperatures. Furthermore, 100% weight loss was achieved in the presence of phosphate buffer solutions [2]. In contrast to Greene et al. [2], Huang et al. [13] developed proteinase K-derived Tritirachium album enzyme-embedded PLA films that were degraded within 96 h. The viability of the enzyme integration options by Greene et al. [2] and Huang et al. [13] in large-scale production remains questionable, considering the lack of data in the public domain concerning commercial viability. Additionally, recent studies, including Tang et al. [15], recommended the use of alternative materials and strategies such as carbon-particle–enzyme complexes (which were effective at adsorbing and entrapping the enzymes) and metal nanoparticle–enzyme complexes (see Figure 3) [15]. The need for further R&D was further augmented by the fact that he enzyme-embedded degradation of plastics had critical drawbacks, including the need to constantly cultivate new bacteria cultures to provide larvae that consume synthetic plastics, the high cost of maintaining the cultures, and the possibility of incomplete microbial degradation and poor mineralization generating microplastics that are toxic to the environment [72]. The drawbacks of microbial degradation highlighted by Mohanan et al. [72] underscore the need for further research to build upon Greene et al.’s [2] and Huang et al.’s [13] preliminary observations. New options for enzyme encapsulation should be achieved through further R&D.
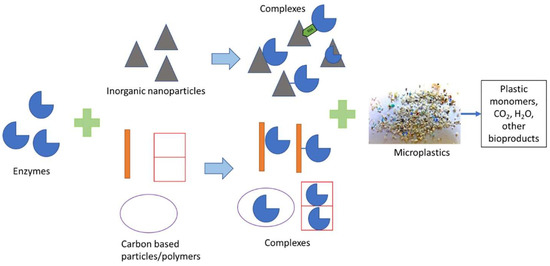
Figure 3.
Illustration of carbon-particle–enzyme complexes for enhancing the degradation of plastics [15].
The positive observations by Greene et al. [2] and Huang et al. [13] show that replacing fossil-fuel agricultural plastics with biodegradable enzyme-embedded alternatives was feasible. However, the one-dimensional perspectives limited the case for bioplastics advanced by Greene et al. [2] and Huang et al. [13]. A life-cycle analysis of the entire biodegradable plastic supply chain established and identified critical sustainability issues that should be addressed moving forward, including recyclability potential and end-of-life (EoL) management practices [73]. Despite bioplastics perceived ecologically benign nature, the EoL practices could threaten the environment. The potentially harmful effects were under-emphasized in research. The reservations made by Gerassimidou et al. [73] concerning the disposal and management of biodegradable agricultural plastic waste should be addressed before the complete phase-out of fossil-fuel-based alternatives. From another point of view, the case for enzyme-embedded agricultural plastics advanced by Greene et al. [2] and Huang et al. [13] only provides a partial solution to the problem. The critical shortcomings of bioplastics, including limited mechanical performance, validated the latter worldview. A comparative analysis of the mechanical performance of the biodegradable and non-bioplastics by La Mantia et al. [16] confirmed that bioplastics, such as Mater-Bi and Ecovio, had lower tensile strength and elongation at the break and EM compared to the non-biodegradable alternatives.
Considering that mechanical strength predicted the durability of agricultural plastics, it may be uneconomical for farmers to invest in biodegradable polymers; this view was corroborated by Marí et al. [10], who recommended the use of Sphere and Mater-Bi plastics in agriculture. The tradeoff between biodegradability and the mechanical performance of agricultural plastics remains a concern for researchers and stakeholders in smart farming. In theory, technological advances would make it possible to develop robust biodegradable polymers. On the downside, R&D and the commercialization of such innovations would be time-intensive, a fact that is evident from the time taken to manufacture enzyme-embedded bioplastics [13,14,15]. Fraunhofer Institute for Applied Polymer Research developed the first commercially viable prototypes in 2021 [14]. Such innovations were yet to be commercialized in the agricultural sector. Drawing from the theory of technology adoption/technology acceptance model, consumer willingness to purchase the materials was not guaranteed [14]. The reservations highlighted by Granić and Marangunić [74] were justified by the inconsistent adoption of precision agriculture technology despite the perceived benefits [75]. The externalities could compromise the adoption of new plastic materials despite the progress made in R&D.
The nascent biological degradation technology could further complicate the ease of adoption for enzyme-embedded polymers [76,77,78]. Scholarly findings recommended different strategies for enzyme infusion into the polymer structure. On the one hand, Greene et al. [2] opted to integrate enzymes into polycaprolactone/Amano lipase (PCL/AL), while Huang et al. [13] emphasized the suitability of proteinase K-derived Tritirachium album enzyme-embedded PLA films, which recorded a 78% weight loss after 96 h. On the other hand, Tang et al. [15] used a different strategy (metal-nanoparticles–enzyme complexes and carbon-particle–enzyme complexes).
From the researcher’s point of view, the biodegradation rate could be enhanced by including light-reactive smart materials in the polymer microstructure [79]. Such materials were easily degraded using UV light. Alternatively, microbial degradation could be enhanced using enzymes-hydrophobins and the incorporation of nanoparticles [15]. The selection of suitable techniques to enhance the degradation process should factor in the tradeoffs. For example, the widespread use of nanoparticles in agriculture could deactivate beneficial soil microbes [80]. The potential dilapidating effects on the environment must be addressed before metal-nanoparticle–enzyme complexes and carbon-particle–enzyme complexes are used on a broader scale in agricultural plastics.
3.2.3. Theme 3: Methanotrophs, Acinetobacter, Pseudomonas aeruginosa, Bacillus, for Biological Degradation of Agricultural Plastic Waste
Methanotrophs were a unique class of bacteria that decomposed atmospheric methane and methane compounds in synthetic polymers. The viability of methanotrophs was justified, considering that the enzyme-embedded biodegradable polymers were new and expensive. Moreover, the introduction of bacterial species in landfills was a viable alternative. A pilot study by Muenmee et al. [81] used methane oxidation to introduce methanotrophs, autotrophs, and heterotrophs capable of naturally biodegrading plastic waste. In contrast to Muenmee et al. [81], Jeon et al. [82] recommended Lysinibacillus species JJY0216.
Following the assessment of the suitability of different bacterial consortiums, the type I/II methanotrophs (Methylobacter sp./Methylocella sp.) were effective primary decomposers for HDPE and other polymers. A 15% weight reduction was observed within days [81]. Furthermore, the biodegradation efficiency of the methanotrophs was higher than Lysinibacillus species JJY0216, which decomposed 4–9% of PE and PP after 26 days [82]. Apart from the decomposition of plastics in landfills, the methanotrophs may help reverse the accumulation of methane in the atmosphere [83] or catalyze biochemical processes that secret biodegradable PHA polymer [84]. Furthermore, the accumulation of CH4 greenhouse gas was partly linked to the production and disposal of agricultural and non-agricultural plastic waste [83]. Considering that Guerrero-Cruz et al. [83], Muenmee et al. [81], and Nielsen and Miller [84] proposed unique applications of methanotrophs ranging from the secretion of PHA, the biodegradation of plastic waste in landfills, and the removal of CH4 from the atmosphere, the following issues should be addressed.
The performance of the methanotrophs was lower than Pseudomonas aeruginosa, which achieved a 25% weight reduction of PHA [85]. However, the comparative analysis of different microorganisms justified the deployment of Pseudomonas aeruginosa and methanotrophs rather than Lysinibacillus species JJY0216 [82]. The concerns raised about microbial degradation efficiency were resolved using H2O2 to stimulate microbial function [86]. In contrast to Mohammadi et al. [86], C. F. Yin et al. [87], recommended the use of both Acinetobacter and Bacillus species to achieve better degradation rates (14–24% reduction in weight). A major drawback was that the effectiveness of hydrogen peroxide in other bacterial strains beyond Bacillus and Pseudomonas species was unknown. Moreover, relying on a consortium of Acinetobacter and Bacillus strains may not be feasible, considering the performance was comparable to one strain (Pseudomonas aeruginosa).
First, the technological readiness of methanotrophs, Acinetobacter, Pseudomonas aeruginosa, and Bacillus species may compromise responsible plastic waste management, considering that the bacteria effectively removed greenhouse gases and degraded fossil-fuel-based polymers. Second, methanotrophs had a limited methane removal efficiency (64–80%) [83]. The challenge was not unique to methanotrophs—all plastic-degrading bacteria must be incubated for a specified period under specific culture conditions. Similar to Guerrero-Cruz et al. [83], Jeon et al. [82] demonstrated a positive relationship between the culture time and the culture media and gravimetric weight losses attributed to bacterial decomposition. Higher weight losses were correlated with longer incubation periods and the optimization of the incubation periods [82]. The experimental evidence drawn from the two studies affirmed the role of microorganisms in agricultural plastic waste management. However, Guerrero-Cruz et al.’s [83] and Jeon et al. ’s [82] researches were inconclusive, given that they did not demonstrate the relationship between bacteria sources and biodegradation efficiency.
Richert and Dąbrowska [88] observed that environmental microorganisms were less effective than proteinase K enzymes in the biodegradation of PLA and PLC; this was evident from the weight loss%. The superior performance of the enzymes was linked to their ability to function best under different conditions and variable oxygen requirements. Like Richert and Dabrowska [88], Evode et al. [89] encouraged the use of enzyme’s catabolic reaction for the bioremediation and biological recycling of PET. On the negative side, Richert and Dabrowska [88] and Evode et al. [89] disregard the specificity of enzyme-mediated biodegradation. Lange [54] highlighted critical barriers to enzyme degradation. For example, the process was only feasible if the plastics had low crystallinity and high hydrophilicity and were susceptible to enzyme hydrolysis. On the downside, synthetic polymers were hydrophobic—a property that limited biofilm formation [90]. The lack of hydrophilic functional groups improved the durability of PET but impacted the rate of biodegradation.
The issues raised by Lange [54] concerning the commercial viability of enzyme-mediated degradation align with Chen et al. [62], who noted that the enzyme-substrate binding must match the geometry of the synthetic polymer; this explains why there were limited enzymes suitable to degrade PS. On a positive note, the crystallinity, hydrophilicity, and susceptibility to enzyme hydrolysis were achieved via chemical modifications. A fundamental barrier to the enzyme and bacteria-mediated biodegradation was limited efficiency. Microcellular bacterial systems did not operate at 100% efficiency; alternative interventions were required to complement bacterial decomposition. The viable alternatives included mechanical and chemical recycling. Each alternative had unique environmental tradeoffs and greenhouse warming potential.
Following the appraisal of the different methods of recycling agricultural plastic wastes, the following observations were made. First, bacteria-derived enzymes were highly effective in managing solid agricultural waste plastics. The case for microorganisms was validated by the versatility and scalability of the process compared to mechanical and chemical recycling. Moreover, microorganisms were multipurpose—they could degrade synthetic agricultural plastics in landfills, facilitate the development of bioplastics, or be embedded into the polymer microstructure. Finally, the viability of polycaprolactone/Amano lipase (PCL/AL) composite films developed using microorganisms to replace synthetic agricultural polymers was demonstrated in recent studies.
Similarly, type I/II methanotrophs could degrade HDPE (15% of the weight) and other polymers in less than 30 days. The methanotrophs were considered more effective relative to Lysinibacillus species JJY0216, which degraded less than 10% of the plastic waste. Even though there was a growing consensus on the immense potential of methanotrophs and enzymes, their function depended on the agricultural polymers’ chemical properties. For example, the action of different bacterial species was influenced by the crystallinity, high hydrophilicity, and susceptibility to enzyme hydrolysis. Moreover, the enzyme-mediated degradation of microorganisms in landfills was depth-dependent. Enzymes performed sub-optimally below depths of 15 cm. Since municipal landfills were large and deep, the enzyme-mediated composting of polymers was ineffective.
The differences in the biodegradation efficiency of the microorganisms reinforced the need for alternative agricultural plastic waste management strategies. Both mechanical and chemical recycling emerged as viable alternatives to the landfilling and incineration of agricultural plastic waste. Pilot projects demonstrated the unique benefits and shortcomings of these techniques. On the one hand, mechanical recycling had a lower carbon footprint than landfilling and incineration. However, the quality of the recyclate was a major concern. The recycled polymer cannot match the performance of virgin polymers. On the other hand, the efficiency of mechanical recycling was dependent on the proper sorting of polymers before processing; this was challenging given the inadequacy of the existing sorting techniques. On the other hand, chemical recycling had a higher carbon footprint than biodegradation because nearly half of the carbon in the polymers was lost during chemical treatment. Moreover, the process relied on physical and chemical activating agents.
3.3. Intelligent Farming Technologies, Geospatial, and Enzyme-Embedded Plastics and Bioplastics
Future perspectives on agricultural plastic waste recycling should be aligned with environment protection agencies (EPA Network) network recommendations. The EPA advocated for better communication between industry stakeholders and other actors, the proper labeling of agricultural plastic waste, further research on the technical possibilities of agricultural plastics, improvements in the aerobic and anaerobic decomposition of plastic waste, the development of infrastructure for biodegradable and non-biodegradable agricultural plastic waste recycling, and restrictions on the production of agricultural plastic waste [37]. Beyond the EPA recommendations, recent R&D supports the exploration of the 4D printing of agricultural plastics noted by [18]. The case for the 3D/4D printing of agricultural plastics was corroborated by Greene et al. [2] who used 3D printing techniques to embed enzymes into polycaprolactone/Amano lipase (PCL/AL) composite films to enhance the biodegradation rate. However, not all scholars support using the 3D/4D printing of agricultural plastics because the printable filaments are difficult to recycle [91,92]. Even though there were justifiable grounds for investing in next-generation technologies for polymer recycling (depolymerization, gasification, mechanical recycling, pyrolysis, and hydrocracking) and the development of enzyme-embedded and 4D printed biodegradable agricultural plastics, the cost–benefits of the technologies were poorly delineated.
3.3.1. Artificial Intelligence, Machine Learning, and Smart Technologies in Agricultural Plastic Waste Management
Smart technologies for agriculture plastic waste management include artificial intelligence, big data, and IoT technologies [93,94,95]. Historically, smart technologies have been extensively employed in precision agriculture [28,75,96]. Specific applications encompass smart technologies to remotely monitor humidity, temperature, and other parameters [97,98,99,100]. The positive influence of precision technologies in agriculture outlined by Ouammi et al. [99] and Villa-Henriksen et al. [100] was corroborated by (Maraveas et al. [28]; Maraveas and Bartzanas [29]). Even though the use of precision agriculture technologies in agriculture was established, there was no consensus on whether these technologies would effectively manage agricultural plastic waste.
According to A. Kumar and Verma [4]; Sarc et al. [101], technology transfer in managing agricultural and non-agricultural plastic waste was either delayed or limited. In contrast, Abdallah et al. [102] supported using AI in managing plastic waste. A similar argument was made by Henriksen et al. [103] concerning the viability of machine learning in agricultural and non-agricultural plastic waste sorting and recycling. The positive outlook advanced by Henriksen et al. [103] was in line with Yu et al. [104], who postulated that AI systems could enhance the efficiency of machines in the sorting and recycling of plastic waste. Alternatively, the AI systems could be coupled with high-resolution satellite imagery for remote monitoring and mapping plastic waste accumulation [105,106]. In other cases, AI and ML could guide energy modeling and generation. Therefore, the concerns raised about the inadequacies of precision technologies do not outweigh the benefits [28]. The body of evidence in support of AL and ML validated the emphasis on the benefits of precision technologies in agricultural plastic waste management.
The development of the aquatic plastic litter detection, classification, and quantification system (APLASTIC-Q) and ZenRobotics Recycler were cases in point [107,108]. AI and machine learning in plastic waste management attracted significant scholarly attention, but commercial deployment would be delayed by variable performance. For example, the APLASTIC-Q system (based on CNN and high-resolution satellite imagery) had an accuracy of 83% accuracy [108]. In line with Wolf et al. [108], Sunny et al. [109] affirmed that CNN was suitable for smart waste management systems [109]. In other cases, CNN-guided DenseNet, SchuffleNet, Resnet-50, ResNeXt, MobileNet_v2, and AlexNet were used to classify plastic waste based on the polymer composition [110]. The correct image classification of polymers was important in managing agricultural plastic waste and removing microplastics from the environment.
Drawing from the applications enumerated by Chazhooretal [110], CNN-based smart systems could be deployed in the agricultural sector to reduce the contamination of agricultural soils. On the downside, there was a 17% probability of inaccurate data using neural networks [108]. The use of inaccurate data may impact decision-making. The variable accuracy was not unique to the APLASTIC-Q system because the high-resolution imagery accuracy levels reported by Wolf et al. [108] were comparable to Lanorte et al. [111] and Sun et al. [112]. The reliance on high-resolution satellite imagery had mixed benefits. On the one hand, it was one of the most reliable techniques for obtaining high-resolution images. On the other hand, the reliability of satellite imagery data was variable and influenced by multiple variables. The later concerns about the reliability of satellite imagery were confirmed by Sun et al. [112], who deployed 1D-CNN. Similarly, Lanorte et al. [111] mixed levels of accuracy with the Landsat 8 satellite imagery. The observations made by Lanorte et al. [111] and Sun et al. [112] show that AI is a reliable technique for mapping plastic waste, but accuracy levels should be improved with better algorithms.
The problem was partly resolved by developing a hybrid algorithm (ensemble model with decision trees, SVM, and CNN). The hybrid algorithm model classified plastic waste with an accuracy of 99% [113]. The findings documented by Gruber et al. [113] about the suitability of SVM–CNN algorithms in plastic waste management were aligned with Jude et al. [114], whose research reported 100% accuracy in the training and validation phases. In the SVM–CNN case, higher accuracy was not an indication of the better reliability of the technology, considering the system only detected 18 out of the 41 different polymers available [113]. The inverse relationship between higher iterations (>9) and accuracy levels was a major concern in real-life applications [114]. On a positive note, the shortcomings of AI systems in plastic waste management identified by Gruber et al. [113] and Wolf et al. [108] should not be a barrier to commercial adoption in the agricultural sector. The reliable modeling of hydrogen energy generation from plastics using machine learning tools indicated that the benefits outweighed the risks [115]. The case for AI and machine learning was further reinforced by the fact that all waste management techniques had limitations, and smart systems were highly adaptable. The utility of smart technologies in plastic waste can further be enhanced with the advances made with bio-inspired algorithms for agriculture [116,117,118]. For example, swarm robots may reduce the need for plastic insect control nets and agrochemicals packed in plastics [119,120,121,122]. The proposal was in line with recent evidence, which confirmed that swarm robots reduce the traditional use of pesticides [123,124,125,126]. In other cases, the technologies have enabled farmers to detect hazards such as fire [127,128,129]; this reduces the need for plastic water pipes and water detectors. Even though APLASTIC-Q, SVM-CNN-ENSEMBLE, and ZenRobotics Recycler were not designed for agricultural plastic waste, they could be adapted for such functions. However, the widespread use of robotic equipment may be detrimental to agriculture. The concerns were informed by Nguyen and Seibel’s [130] research, which noted that the commercialization of soft robots would have mixed effects. On the one hand, robotic systems would reduce the need for plastics by automating key processes. On the other hand, robotic systems were made of synthetic elastomers, which were often difficult to recycle.
The transfer of smart technologies in managing agricultural and non-agricultural plastic waste via sorting and collection was delayed [131,132]. Even though the viability of AI, robots, and smart systems was demonstrated in the 1990s, commercial adoption and advances in R&D were delayed; this was confirmed by Rabold [133], Ward [134] and Barolli et al. [135]. Extensive research on the role of AI in plastic waste management was published by Anitha et al. [136]. Within the same period, another study was published by Andeobu et al. [137] concerning the role of AI in plastic waste management. Both studies acknowledged the immense potential of precision technologies in properly managing biodegradable and non-biodegradable waste.
On the one hand, Anitha et al. [136] suggested that simulations could help identify the best polymer waste microbial degradation strategy. Alternatively, AI could reduce errors in the segregation of different polymers. The correct segregation of polymers was a vital step in mechanical recycling; including different polymer blends reduced the quality of the final product. The efficiency of AI in sorting plastic waste was threefold higher than human labor (170 versus 50 cycles per minute). On the other hand Andeobu et al. [137] supported the use of AI technologies (including genetic algorithms, artificial neural networks, and support vector machines) in waste management but cautioned that the benefits would be minimal due to the lack of data. The case for AI technologies in plastic waste sorting made by Andeobu et al. [137] did not align with Lubongo and Alexandridis [138], who noted that intelligent sorting was best designed to handle single-component polymer materials, not blends.
Like all smart agriculture technologies, the deployment of AI and ML in waste management would be limited by cost. For example, the interoperability between different smart technologies depends on the availability of expensive cloud and IoT infrastructure [139,140]. In addition, the cost of operation would be amplified by higher energy requirements for IoT devices and resource scarcity [140,141]. In contrast, Jain and Bagherwal [140] and Ziouzios et al. [141] noted other constraints, such as limited transmission ranges and low accuracy. However, on a positive note, the cost- and energy-related barriers to adoption may be offset by the commercial maturity of the technology and the transition to renewable energy.
3.3.2. Geospatial Estimation of the Distribution of Agricultural Plastics and Development of Well-Fit Collection Procedures
Geospatial techniques facilitated the visualization of global agricultural plastic waste distribution [11,112,142]. Such information can help develop an optimized agricultural plastic waste supply chain design to reduce energy needs [142]. However, real-time use was contingent on the refinement of the technology to achieve satisfactory accuracy and reproducible outcomes. A survey conducted in Bari, southern Italy, achieved a mapping accuracy of 94.5%, user accuracy of 87%, and Kappa coefficient of 0.94 using the Landsat 8 satellite coupled with support vector machines [111]. The accuracy reported by Lanorte et al. [111] was slightly higher than the data published by Sun et al. [112] using alternative tools. On the downside, the reliability of the Landsat 8 satellite imagery in mapping agricultural plastic waste was challenged because the agricultural plastic waste estimates drawn from the satellite were 19.9 tons lower compared to data collected using regional maps [111]. The underestimation of agricultural plastic waste could compromise waste management interventions. The risk of plastic waste underestimation was further amplified by the impact of exogenous materials on plastic waste volume. According to LeMoine et al. [38], soil, organic matter, and pesticides adhered on the surface of the plastic films increase the weight threefold to fourfold, increasing the cost of waste management.
Lanorte et al.’s [111] review of the potential limitations of the Landsat 8 satellite in the mapping of plastic waste could explain why Sun et al. [112] opted for 2-temporal sentinel-2 and 1D-CNN deep learning. The latter technique had user and producer accuracy levels of 79.96 and 86.32%, respectively. The reported accuracy was lower than Lanorte et al. [111] due to the utilization of different geospatial mapping techniques. Despite the lower Kappa coefficient and mapping accuracy of the 2-temporal sentinel-2 and 1D-CNN deep learning technique, it offered unique advantages, such as high-precision images derived using near-IR narrow bands [112]. Such images were useful in precision agriculture (see Figure 4). Based on the false-color VHI images, the area under plastic greenhouses could be accurately estimated (represented in yellow). The utility of the mapping approach employed by Sun et al. [112] was corroborated by Cillis et al. [143] in a survey conducted in eight municipalities in Basilicata, southern Italy. In contrast to Sun et al. [112], Cillis et al. [143] relied on a QGIS geospatial mapping technique. The QGIS approach was equally useful in commercial agriculture.
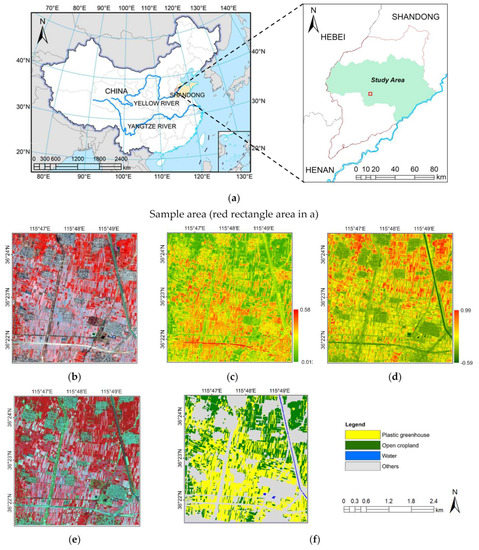
Figure 4.
Satellite imagery of the study area. (a) The geographical location of the study area; (b) a two-temporal satellite image with false colors for better visualization and contrast; (c) the retrogressive PGI drawn from the two-temporal satellite image; (d) NDVI drawn from the two-temporal satellite image (e) false-color VHR images (f) PG mapping [112].
The mapping of agricultural plastic waste in eight municipalities in Basilicata, southern Italy, by Cillis et al. [143], corroborated earlier outcomes observed on the need for satellite mapping to complement ground-based measurements [111,112,142]. However, satellite imaging should be customized using machine learning algorithms. Furthermore, despite the comparable outcomes, there were slight variations in data [111,112,142], a phenomenon linked to weather changes and technical issues. On a positive note, there was room for improvement, given that the volume of agricultural plastics could be estimated from the crop under cultivation (see Table 5).

Table 5.
Geospatial mapping of agricultural plastics in eight municipalities [143].
Despite the promising outcomes by Sun et al. [112], the researcher noted it was impractical to determine whether QGIS was superior to Landsat 8 satellite and 1D-CNN deep learning because the former had notable shortcomings. For example, the incorrectly classified area under agricultural plastics was large—the differences could be observed from a visual inspection of the RGB representation and the RPGI index [143]. In addition, the concerns raised by Cillis et al. [143] and Sun et al. [112] about the reliability of satellite mapping indicated that the techniques required further improvements before widespread commercial adoption; this was possible with advances in high-resolution satellite imagery.
3.3.3. Cost Benefits of Geospatial Mapping of Agricultural Plastic Waste
The Landsat 8 satellite was superior to the 1D-CNN deep learning techniques if user accuracy and mapping accuracies were the primary criteria for selection. The perceived superiority was confirmed by L. W. Liu et al. [144]; Villa-Henriksen et al. [100] in estimating plant growth rates and data-driven production. The case for Landsat 8 was valid despite the shortcomings identified by Lanorte et al. [111]; Sun et al. [112]. After comparing the different geospatial techniques for mapping agricultural waste in farms, each technique had unique benefits and drawbacks. Mapping accuracy was not the sole determinant—secondary uses were also important.
The broader view of geospatial mapping techniques was justified considering that high-precision images might be more useful than higher mapping and user accuracy. On the downside, the widespread use of the Landsat 8 satellite remains questionable, given that it relied on one metric, namely land-use mapping and the indices of waste production to crop type (including vineyards, olive, orchard, and vegetables) and plastic application (films, anti-hail nets, fertilizer bags, agrochemical containers, and irrigation pipe [111]. Other metrics predicted the size and volume of agricultural plastics, including farming practices (precision versus traditional), local weather, and location. In contrast to Lanorte et al. [111], Jawad et al. [145]; R. K. Singh et al. [146] demonstrated that precision agriculture relies on communication between wireless sensors, automated machines using artificial neural networks, and other infrastructure made of plastic and non-plastic components. Based on the latter findings, the accumulation of agricultural plastics should be higher in precision farming. The limitation of geospatial planning in this regard is the fact that their accuracy is mostly below 95%, which means that there is still a need to improve their accuracy even further; for example, Landsat Enhanced Thematic Mapper Plus (ETM+) achieved an accuracy of 91% in mapping plastic greenhouses, but it only detected them when the greenhouse fraction was greater than 12% in a mixed pixel [142]. Therefore, besides improving on monitoring metrics of geospatial mapping technologies, their detection accuracy also needs to be improved for better outcomes. Otherwise, the current applications may suffer under detection in plastic mapping. Additionally, it is unclear if the geospatial mapping technologies can detect the type of plastics, since the life cycles of non-biodegradable and bioplastics differ significantly. Thus, the geospatial data may be of limited use in making policy decisions in agricultural plastics, because the policy landscape must be influenced by the type of plastics widely deployed in agricultural production.
4. Conclusions
This review advanced the current understanding of the practical benefits and drawbacks of leading agricultural plastic waste management strategies, such as substituting fossil-fuel-based polymers with enzyme-embedded polymers and bioplastics (TPS, PLA, and PLC). The accumulation of agricultural plastic waste poses new threats to the environment and may limit agricultural yields. Using 2-temporal sentinel-2 and 1D-CNN deep learning and Landsat 8 imaging techniques provides accurate information on the geographic distribution of agricultural plastic waste resources in selected EU countries. The geospatial mapping techniques affirmed the relationship between farming intensification and agricultural plastic waste accumulation.
Microorganisms, such as enzymes and bacteria, effectively manage solid agricultural waste plastics. The case for microorganisms was validated by the versatility and scalability of the process compared to mechanical and chemical recycling. Moreover, microorganisms were multipurpose—they could degrade synthetic agricultural plastics in landfills, facilitate the development of bioplastics, or be embedded into the polymer microstructure. The viability of polycaprolactone/Amano lipase (PCL/AL) composite films developed using microorganisms to replace synthetic agricultural polymers was demonstrated in recent studies. Similarly, type I/II methanotrophs could degrade HDPE (15% of the weight) and other polymers in less than 30 days. The degradation timelines depended on the physical conditions—elevated temperature enhanced degradation [37]. The methanotrophs were considered more effective relative to Lysinibacillus species JJY0216, which degraded less than 10% of the plastic waste. Even though there was a growing consensus on the immense potential of methanotrophs and enzymes, their function depended on the agricultural polymers’ chemical properties. For example, the action of different bacterial species was influenced by the crystallinity, high hydrophilicity, and susceptibility to enzyme hydrolysis.
Moreover, the enzyme-mediated degradation of microorganisms in landfills was depth-dependent. Enzymes performed sub-optimally below the depths of 15 cm. Since municipal landfills were large and deep, the enzyme-mediated composting of polymers was ineffective. The differences in the biodegradation efficiency of the microorganisms reinforced the need for alternative strategies for agricultural plastic waste management, including responsible consumption and further research and development.
Author Contributions
C.M. Manuscript preparation; M.I.K. read and comment; T.B. supervisor. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No data available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chow, J.; Perez-Garcia, P.; Dierkes, R.; Streit, W.R. Microbial enzymes will offer limited solutions to the global plastic pollution crisis. Microb. Biotechnol. 2022, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Greene, A.F.; Vaidya, A.; Collet, C.; Wade, K.R.; Patel, M.; Gaugler, M.; West, M.; Petcu, M.; Parker, K. 3D-Printed Enzyme-Embedded Plastics. Biomacromolecules 2021, 22, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S.; et al. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, S.K. Design and development of e-smart robotics-based underground solid waste storage and transportation system. J. Clean. Prod. 2022, 343, 130987. [Google Scholar] [CrossRef]
- Nielsen, T.D.; Hasselbalch, J.; Holmberg, K.; Stripple, J. Politics and the plastic crisis: A review throughout the plastic life cycle. WIREs Energy Environ. 2020, 9, e360. [Google Scholar] [CrossRef]
- Bilal, M.; Qamar, S.A.; Ashraf, S.S.; Rodríguez-Couto, S.; Iqbal, H.M.N. Robust nanocarriers to engineer nanobiocatalysts for bioprocessing applications. Adv. Colloid Interface Sci. 2021, 293, 102438. [Google Scholar] [CrossRef] [PubMed]
- DelRe, C.; Jiang, Y.; Kang, P.; Kwon, J.; Hall, A.; Jayapurna, I.; Ruan, Z.; Ma, L.; Zolkin, K.; Li, T.; et al. Near-complete depolymerization of polyesters with nano-dispersed enzymes. Nature 2021, 592, 558–563. [Google Scholar] [CrossRef] [PubMed]
- González-henríquez, C.M.; Sarabia-vallejos, M.A.; Rodriguez-hernandez, J. Progress in Polymer Science Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116. [Google Scholar] [CrossRef]
- Adhikari, R.; Bristow, K.L.; Casey, P.S.; Freischmidt, G.; Hornbuckle, J.W.; Adhikari, B. Preformed and sprayable polymeric mulch film to improve agricultural water use efficiency. Agric. Water Manag. 2016, 169, 1–13. [Google Scholar] [CrossRef]
- Marí, A.I.; Pardo, G.; Cirujeda, A.; Martínez, Y. Economic evaluation of biodegradable plastic films and paper mulches used in open-air grown pepper (Capsicum annum L.) crop. Agronomy 2019, 9, 36. [Google Scholar] [CrossRef]
- Sintim, H.Y.; Flury, M. Is Biodegradable Plastic Mulch the Solution to Agriculture’s Plastic Problem? Environ. Sci. Technol. 2017, 51, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Vanderreydt, I.; Rommens, T.; Tenhunen, A.; Mortensen, L.F.; Tange, I. Greenhouse gas emissions and natural capital implications of plastics (including biobased plastics). Eur. Environ. Agency 2021. Eionet Report—ETC/WMGE 2021/3. [Google Scholar]
- Huang, Q.; Hiyama, M.; Kabe, T.; Kimura, S.; Iwata, T. Enzymatic Self-Biodegradation of Poly(l-lactic acid) Films by Embedded Heat-Treated and Immobilized Proteinase K. Biomacromolecules 2020, 21, 3301–3307. [Google Scholar] [CrossRef] [PubMed]
- Mehlhase, S. Enzymes successfully embedded in plastics. Fraunhofer Institute for Applied Polymer Research IAP. 2021. Available online: https://www.fraunhofer.de/content/dam/zv/en/press-media/2021/june-2021/iap-enzymes-embedded-in-plastics.pdf (accessed on 25 November 2022).
- Tang, K.H.D.; Lock, S.S.M.; Yap, P.-S.; Cheah, K.W.; Chan, Y.H.; Yiin, C.L.; Ku, A.Z.E.; Loy, A.C.M.; Chin, B.L.F.; Chai, Y.H. Immobilized enzyme/microorganism complexes for degradation of microplastics: A review of recent advances, feasibility and future prospects. Sci. Total Environ. 2022, 832, 154868. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Ceraulo, M.; Testa, P.; Morreale, M. Biodegradable polymers for the production of nets for agricultural product packaging. Materials 2021, 14, 323. [Google Scholar] [CrossRef]
- Maraveas, C. Environmental Sustainability of Greenhouse Covering Materials. Sustainability 2019, 11, 6129. [Google Scholar] [CrossRef]
- Maraveas, C.; Bayer, I.S.; Bartzanas, T. 4D printing: Perspectives for the production of sustainable plastics for agriculture. Biotechnol. Adv. 2021, 54, 107785. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.G. A comparative study on the distribution behavior of microplastics through FT-IR analysis on different land uses in agricultural soils. Environ. Res. 2022, 215, 114404. [Google Scholar] [CrossRef]
- You, X.; Wang, S.; Li, G.; Du, L.; Dong, X. Microplastics in the soil: A review of distribution, anthropogenic impact, and interaction with soil microorganisms based on meta-analysis. Sci. Total Environ. 2022, 832, 154975. [Google Scholar] [CrossRef]
- Batista, T.; Cansado, I.P.d.P.; Tita, B.; Ilhéu, A.; Metrogos, L.; Mourão, P.A.M.; Nabais, J.M.V.; Castanheiro, J.; Borges, C.; Matos, G. Dealing with Plastic Waste from Agriculture Activity. Agronomy 2022, 12, 134. [Google Scholar] [CrossRef]
- Narancic, T.; Cerrone, F.; Beagan, N.; O’Connor, K.E. Recent advances in bioplastics: Application and biodegradation. Polymers 2020, 12, 920. [Google Scholar] [CrossRef]
- Yin, W.; Xie, Z.; Yin, Y.; Yi, J.; Liu, X.; Wu, H.; Wang, S.; Xie, Y.; Yang, Y. Aging behavior and lifetime prediction of PMMA under tensile stress and liquid scintillator conditions. Adv. Ind. Eng. Polym. Res. 2019, 2, 82–87. [Google Scholar] [CrossRef]
- World Bank Agricultural Pollution Plastics. 2022. Available online: https://documents1.worldbank.org/curated/en/122161521208357388/pdf/124346-repl-WB-Knowledge-Plastic.pdf (accessed on 12 July 2022).
- Gray, A. Body as voice: Restorative dance/movement psychotherapy with survivors of relational trauma. In The Routledge International Handbook of Embodied Perspectives in Psychotherapy; Routledge: London, UK, 2019; pp. 147–160. [Google Scholar] [CrossRef]
- Sargeant, J.M.; O’Connor, A.M. Scoping Reviews, Systematic Reviews, and Meta-Analysis: Applications in Veterinary Medicine. Front. Vet. Sci. 2020, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brings, J.; Salmon, A.; Saritas, S. Context uncertainty in requirements engineering: Definition of a search strategy for a systematic review and preliminary results. CEUR Workshop Proc. 2015, 1342, 171–178. [Google Scholar]
- Maraveas, C.; Piromalis, D.; Arvanitis, K.G.; Bartzanas, T.; Loukatos, D. Applications of IoT for optimized greenhouse environment and resources management. Comput. Electron. Agric. 2022, 198, 106993. [Google Scholar] [CrossRef]
- Maraveas, C.; Bartzanas, T. Sensors for structural health monitoring of agricultural structures. Sensors 2021, 21, 314. [Google Scholar] [CrossRef]
- Bogner, J.; Verdecchia, R.; Gerostathopoulos, I. Characterizing Technical Debt and Antipatterns in AI-Based Systems: A Systematic Mapping Study. In Proceedings of the 2021 IEEE/ACM International Conference on Technical Debt (TechDebt), Madrid, Spain, 19–21 May 2021; pp. 64–73. [Google Scholar] [CrossRef]
- Navarro, E.; Costa, N.; Pereira, A. A Systematic Review of IoT Solutions for Smart Farming. Sensors 2020, 20, 4231. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Wohlin, C.; Kalinowski, M.; Romero Felizardo, K.; Mendes, E. Successful combination of database search and snowballing for identification of primary studies in systematic literature studies. Inf. Softw. Technol. 2022, 147, 106908. [Google Scholar] [CrossRef]
- Kiger, M.E.; Varpio, L. Thematic analysis of qualitative data: AMEE Guide. Med. Teach. 2020, 42, 846–854. [Google Scholar] [CrossRef]
- Vaismoradi, M.; Turunen, H.; Bondas, T. Content analysis and thematic analysis: Implications for conducting a qualitative descriptive study. Nurs. Health Sci. 2013, 15, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Díaz, F.J.; Belmonte-Ureña, L.J.; Camacho-Ferre, F.; Tello-Marquina, J.C. The management of agriculture plastic waste in the framework of circular economy. Case of the almeria greenhouse (Spain). Int. J. Environ. Res. Public Health 2021, 18, 12042. [Google Scholar] [CrossRef] [PubMed]
- Maier, N.; Biodegradable Plastics Approaches and experiences from 16 Members of the EPA Network. European Network of the Heads of Environment Protection Agencies (EPA Network)—Interest Group on Plastics. 2018. Available online: file:///C:/Users/MDPI/Downloads/IG%20Plastics_Working%20paper_Biodegradable%20plastics.pdf (accessed on 22 November 2022).
- LeMoine, B.; Erälinna, L.; Trovati, G.; Casallo, I.M.; Amate, J.J.; Zlatar, K.; Butlewski, K.; Ojanpera, M.; Picuno, P.; EIP-AGRI Focus Group. Reducing the plastic footprint of agriculture. EIP-Agric. Netw. 2021. Available online: https://ec.europa.eu/eip/agriculture/en/publications/eip-agri-focus-group-plastic-footprint-final (accessed on 22 November 2022).
- Siegenthaler, K.O.; Künkel, A.; Skupin, G.; Yamamoto, M. Ecoflex® and Ecovio®: Biodegradable, Performance-Enabling Plastics. In Synthetic Biodegradable Polymers. Advances in Polymer Science; Rieger, B., Künkel, A., Coates, G.W., Reichardt, R., Dinjus, E., Zevaco, T.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 91–136. [Google Scholar]
- Briassoulis, D.; Hiskakis, M.; Babou, E.; Antiohos, S.K.; Papadi, C. Experimental investigation of the quality characteristics of agricultural plastic wastes regarding their recycling and energy recovery potential. Waste Manag. 2012, 32, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, D.; Hiskakis, M.; Babou, E. Technical specifications for mechanical recycling of agricultural plastic waste. Waste Manag. 2013, 33, 1516–1530. [Google Scholar] [CrossRef]
- Bos, U.; Makinshi, C.; Fischer, M. Life Cycle Assessment of Common Used Agricultural Plastic Products in the E.U. Acta Hortic. 2008, 801, 227–236. [Google Scholar]
- Singh, P.; Sharma, V.P. Integrated Plastic Waste Management: Environmental and Improved Health Approaches. Procedia Environ. Sci. 2016, 35, 692–700. [Google Scholar] [CrossRef]
- Mankin, R.W. Applications of acoustics in insect pest management. CABI Rev. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Lim, B.K.H.; Thian, E.S. Biodegradation of polymers in managing plastic waste—A review. Sci. Total Environ. 2022, 813, 151880. [Google Scholar] [CrossRef]
- Shamsuyeva, M.; Endres, H.J. Plastics in the context of the circular economy and sustainable plastics recycling: Comprehensive review on research development, standardization and market. Compos. Part C Open Access 2021, 6, 100168. [Google Scholar] [CrossRef]
- Aldas, M.; Rayón, E.; López-Martínez, J.; Arrieta, M.P. A deeper microscopic study of the interaction between gum rosin derivatives and a mater-Bi type bioplastic. Polymers 2020, 12, 226. [Google Scholar] [CrossRef]
- Shi, B.; Palfery, D. Temperature-dependent polylactic acid (PLA) anaerobic biodegradability. Int. J. Environ. Waste Manag. 2012, 10, 297–306. [Google Scholar] [CrossRef]
- Iwamoto, A.; Tokiwa, Y. Enzymatic degradation of plastics containing polycaprolactone. Polym. Degrad. Stab. 1994, 45, 205–213. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Ye, Y.; Zhang, B. On the degradation of (micro)plastics: Degradation methods, influencing factors, environmental impacts. Sci. Total Environ. 2022, 806, 151312. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E. The Biggest Source of Plastic Trash You’ve Never Heard of. 2015. Available online: https://ensia.com/features/the-biggest-source-of-plastic-trash-youve-never-heard-of/ (accessed on 12 November 2022).
- Jee, C. A New Chemical Process Could Turn a Quarter of Our Plastic Waste into Clean Fuel. MIT Technol. Rev. 2019. Available online: https://www.technologyreview.com/2019/02/11/137483/a-new-chemical-process-could-turn-a-quarter-of-our-plastic-waste-into-clean-fuel/ (accessed on 14 October 2022).
- UNEP. How Plastic Is Infiltrating the World’s Soils. 2021. Available online: https://www.unep.org/news-and-stories/story/how-plastic-infiltrating-worlds-soils (accessed on 2 October 2022).
- Lange, J.P. Managing Plastic Waste-Sorting, Recycling, Disposal, and Product Redesign. ACS Sustain. Chem. Eng. 2021, 9, 15722–15738. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Dijkgraaf, E.; Gradus, R. Post-collection Separation of Plastic Waste: Better for the Environment and Lower Collection Costs? Environ. Resour. Econ. 2020, 77, 127–142. [Google Scholar] [CrossRef]
- Ali, M.F.; Siddiqui, M.N. Thermal and catalytic decomposition behavior of PVC mixed plastic waste with petroleum residue. J. Anal. Appl. Pyrolysis 2005, 74, 282–289. [Google Scholar] [CrossRef]
- Kumar Sen, S.; Raut, S. Microbial degradation of low density polyethylene (LDPE): A review. J. Environ. Chem. Eng. 2015, 3, 462–473. [Google Scholar] [CrossRef]
- Bahl, S.; Dolma, J.; Singh, J.J.; Sehgal, S. Biodegradation of plastics: A state of the art review. Mater. Today Proc. 2020, 39, 31–34. [Google Scholar] [CrossRef]
- Chen, C.C.; Dai, L.; Ma, L.; Guo, R.T. Enzymatic degradation of plant biomass and synthetic polymers. Nat. Rev. Chem. 2020, 4, 114–126. [Google Scholar] [CrossRef]
- Jaiswal, S.; Sharma, B.; Shukla, P. Integrated approaches in microbial degradation of plastics. Environ. Technol. Innov. 2020, 17, 100567. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. Recent insight into enzymatic degradation of plastics prevalent in the environment: A mini-review. Clean. Eng. Technol. 2021, 2, 100083. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Al-Tohamy, R.; Zhu, D.; Mahmoud, Y.A.G.; Koutra, E.; Metwally, M.A.; Kornaros, M.; Sun, J. Plastic wastes biodegradation: Mechanisms, challenges and future prospects. Sci. Total Environ. 2021, 780, 146590. [Google Scholar] [CrossRef] [PubMed]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic biodegradation: Frontline microbes and their enzymes. Sci. Total Environ. 2021, 759, 143536. [Google Scholar] [CrossRef]
- Akan, O.D.; Udofia, G.E.; Okeke, E.S.; Mgbechidinma, C.L.; Okoye, C.O.; Zoclanclounon, Y.A.B.; Atakpa, E.O.; Adebanjo, O.O. Plastic waste: Status, degradation and microbial management options for Africa. J. Environ. Manag. 2021, 292, 112758. [Google Scholar] [CrossRef]
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Elisabetta, M.; Temporiti, E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal Enzymes Involved in Plastics Biodegradation. Microorganisms 2022, 10, 1180. [Google Scholar]
- Hou, L.; Majumder, E.L.W. Potential for and distribution of enzymatic biodegradation of polystyrene by environmental microorganisms. Materials 2021, 14, 503. [Google Scholar] [CrossRef]
- Rana, K.I. Usage of Potential Micro-organisms for Degradation of Plastics. Open J. Environ. Biol. 2019, 4, 7–15. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef] [PubMed]
- Gerassimidou, S.; Martin, O.V.; Chapman, S.P.; Hahladakis, J.N.; Iacovidou, E. Development of an integrated sustainability matrix to depict challenges and trade-offs of introducing bio-based plastics in the food packaging value chain. J. Clean. Prod. 2021, 286, 125378. [Google Scholar] [CrossRef]
- Granić, A.; Marangunić, N. Technology acceptance model in educational context: A systematic literature review. Br. J. Educ. Technol. 2019, 50, 2572–2593. [Google Scholar] [CrossRef]
- Kendall, H.; Clark, B.; Li, W.; Jin, S.; Jones, G.D.; Chen, J.; Taylor, J.; Li, Z.; Frewer, L.J. Precision agriculture technology adoption: A qualitative study of small-scale commercial “family farms” located in the North China Plain. Precis. Agric. 2022, 23, 319–351. [Google Scholar] [CrossRef]
- Moharir, R.V.; Kumar, S. Challenges associated with plastic waste disposal and allied microbial routes for its effective degradation: A comprehensive review. J. Clean. Prod. 2019, 208, 65–76. [Google Scholar] [CrossRef]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Shent, H.; Pugh, R.J.; Forssberg, E. A review of plastics waste recycling and the flotation of plastics. Resour. Conserv. Recycl. 1999, 25, 85–109. [Google Scholar] [CrossRef]
- Leist, S.K.; Zhou, J. Current status of 4D printing technology and the potential of light-reactive smart materials as 4D printable materials. Virtual Phys. Prototyp. 2016, 11, 249–262. [Google Scholar] [CrossRef]
- Khanna, K.; Kohli, S.K.; Handa, N.; Kaur, H.; Ohri, P.; Bhardwaj, R.; Yousaf, B.; Rinklebe, J.; Ahmad, P. Enthralling the impact of engineered nanoparticles on soil microbiome: A concentric approach towards environmental risks and cogitation. Ecotoxicol. Environ. Saf. 2021, 222, 112459. [Google Scholar] [CrossRef]
- Muenmee, S.; Chiemchaisri, W.; Chiemchaisri, C. Microbial consortium involving biological methane oxidation in relation to the biodegradation of waste plastics in a solid waste disposal open dump site. Int. Biodeterior. Biodegrad. 2015, 102, 172–181. [Google Scholar] [CrossRef]
- Jeon, J.M.; Park, S.J.; Choi, T.R.; Park, J.H.; Yang, Y.H.; Yoon, J.J. Biodegradation of polyethylene and polypropylene by Lysinibacillus species JJY0216 isolated from soil grove. Polym. Degrad. Stab. 2021, 191, 109662. [Google Scholar] [CrossRef]
- Guerrero-Cruz, S.; Vaksmaa, A.; Horn, M.A.; Niemann, H.; Pijuan, M.; Ho, A. Methanotrophs: Discoveries, Environmental Relevance, and a Perspective on Current and Future Applications. Front. Microbiol. 2021, 12, 1–28. [Google Scholar] [CrossRef]
- Nielsen, C.L.; Miller, C.D. Secretion of Bioplastic Polymers from Methanotrophic Bacteria Grown Using Natural Gas; Utah State University Student Research Symposium: Logan, UT, USA, 2016; Available online: https://digitalcommons.usu.edu/cgi/viewcontent.cgi?article=1300&context=researchweek (accessed on 1 October 2022).
- Qin, Z.H.; Mou, J.H.; Chao, C.Y.H.; Chopra, S.S.; Daoud, W.; Yuan Leu, S.; Ning, Z.; Tso, C.Y.; Chan, C.K.; Tang, S.; et al. Biotechnology of Plastic Waste Degradation, Recycling, and Valorization: Current Advances and Future Perspectives. ChemSusChem 2021, 14, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Moussavi, G.; Rezaei, M. Enhanced peroxidase-mediated biodegradation of polyethylene using the bacterial consortia under H2O2-biostimulation. Polymer 2022, 240, 124508. [Google Scholar] [CrossRef]
- Yin, C.F.; Xu, Y.; Zhou, N.Y. Biodegradation of polyethylene mulching films by a co-culture of Acinetobacter sp. strain NyZ450 and Bacillus sp. strain NyZ451 isolated from Tenebrio molitor larvae. Int. Biodeterior. Biodegrad. 2020, 155, 105089. [Google Scholar] [CrossRef]
- Richert, A.; Dąbrowska, G.B. Enzymatic degradation and biofilm formation during biodegradation of polylactide and polycaprolactone polymers in various environments. Int. J. Biol. Macromol. 2021, 176, 226–232. [Google Scholar] [CrossRef]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M.N. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Sangale, M.K.; Ade, A.B. Bioremediation Technology for Plastic Waste; Springer: Singapore, 2019. [Google Scholar]
- Koffi, A.; Toubal, L.; Jin, M.; Koffi, D.; Döpper, F.; Schmidt, H.-W.; Neuber, C. Extrusion-based 3D printing with high-density polyethylene Birch-fiber composites. J. Appl. Polym. Sci. 2022, 139, 51937. [Google Scholar] [CrossRef]
- Mikula, K.; Skrzypczak, D.; Izydorczyk, G.; Warchoł, J.; Moustakas, K.; Chojnacka, K.; Witek-Krowiak, A. 3D printing filament as a second life of waste plastics-a review. Environ. Sci. Pollut. Res. Int. 2021, 28, 12321–12333. [Google Scholar] [CrossRef]
- Chowdhury, M.H. Development of Automatic Smart Waste Sorter Machine. In Proceedings of the International Conference on Mechanical, Industrial and Materials Engineering 2013 (ICMIME2013), Rajshahi, Bangladesh, 1–3 November 2013. Paper ID: AM-012. [Google Scholar]
- Glouche, Y.; Couderc, P. A Smart Waste Management with Self-Describing objects. In Proceedings of the SMART 2013: The Second International Conference on Smart Systems, Devices and Technologies, Rome, Italy, 23–28 June 2013; p. 9. [Google Scholar]
- Thakker, S.; Narayanamoorthi, R. Smart and wireless waste management. In Proceedings of the ICIIECS 2015—2015 IEEE International Conference on Innovations in Information, Embedded and Communication Systems, Coimbatore, India, 19–20 March 2015; pp. 2–5. [Google Scholar] [CrossRef]
- Rial-Lovera, R. Agricultural Robots: Drivers, barriers and opportunities for adoption. In Proceedings of the 14th International Conference on Precision Agriculture, Montreal, QC, Canada, 24–27 June 2018; pp. 1–5. [Google Scholar]
- Bie, W.; Gradziuk, P.; Golonko, M.; Gołasa, P.; Wysoki, M.; Gradziuk, B.; Siedlecka, A.; Gromada, A. Sources of Greenhouse Gas Emissions in Agriculture, with Particular Emphasis on Emissions from Energy Used. Energies 2021, 14, 3784. [Google Scholar]
- Junaid, M.; Shaikh, A.; Hassan, M.U.; Alghamdi, A.; Rajab, K.; Al Reshan, M.S.; Alkinani, M. Smart agriculture cloud using AI based techniques. Energies 2021, 14, 5129. [Google Scholar] [CrossRef]
- Ouammi, A.; Achour, Y.; Dagdougui, H.; Zejli, D. Energy for Sustainable Development Optimal operation scheduling for a smart greenhouse integrated microgrid. Energy Sustain. Dev. 2020, 58, 129–137. [Google Scholar] [CrossRef]
- Villa-Henriksen, A.; Edwards, G.T.C.; Pesonen, L.A.; Green, O.; Sørensen, C.A.G. Internet of Things in arable farming: Implementation, applications, challenges and potential. Biosyst. Eng. 2020, 191, 60–84. [Google Scholar] [CrossRef]
- Sarc, R.; Curtis, A.; Kandlbauer, L.; Khodier, K.; Lorber, K.E.; Pomberger, R. Digitalisation and intelligent robotics in value chain of circular economy oriented waste management—A review. Waste Manag. 2019, 95, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.; Abu Talib, M.; Feroz, S.; Nasir, Q.; Abdalla, H.; Mahfood, B. Artificial intelligence applications in solid waste management: A systematic research review. Waste Manag. 2020, 109, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, M.L.; Karlsen, C.B.; Klarskov, P.; Hinge, M. Plastic classification via in-line hyperspectral camera analysis and unsupervised machine learning. Vib. Spectrosc. 2022, 118, 103329. [Google Scholar] [CrossRef]
- Yu, K.H.; Zhang, Y.; Li, D.; Montenegro-Marin, C.E.; Kumar, P.M. Environmental planning based on reduce, reuse, recycle and recover using artificial intelligence. Environ. Impact Assess. Rev. 2021, 86, 106492. [Google Scholar] [CrossRef]
- Gnann, N.; Garaba, S.; Zielinski, O. Plastic Waste Detection Assisted by Artifical Intelligence. In Proceedings of the 22nd EGU General Assembly, Online, 4–8 May 2020; Available online: https://meetingorganizer.copernicus.org/EGU2020/EGU2020-975.html?pdf (accessed on 1 October 2022).
- Hidalgo-Crespo, J.; Álvarez-Mendoza, C.I.; Soto, M.; Amaya-Rivas, J.L. Quantification and mapping of domestic plastic waste using GIS/GPS approach at the city of Guayaquil. Procedia CIRP 2022, 105, 86–91. [Google Scholar] [CrossRef]
- Lukka, T.J.; Tossavainen, T.; Kujala, J.V.; Raiko, T. ZenRobotics Recycler—Robotic Sorting using Machine Learning. In Proceedings of the International Conference on Sensor-Based Sorting (SBS), Aachen, Germany, 11–13 March 2014; Available online: https://users.ics.aalto.fi/praiko/papers/SBS14.pdf (accessed on 1 October 2022).
- Wolf, M.; Van Den Berg, K.; Garaba, S.P.; Gnann, N.; Sattler, K.; Stahl, F.; Zielinski, O. Machine learning for aquatic plastic litter detection, classification and quantification (APLASTIC-Q). Environ. Res. Lett. 2020, 15, 114042. [Google Scholar] [CrossRef]
- Sunny, M.S.H.; Dipta, D.R.; Hossain, S.; Faruque, H.M.R.; Hossain, E. Design of a convolutional Neural Network Based Smart Waste Disposal System. In Proceedings of the 1st International Conference on Advances in Science, Engineering and Robotics Technology, Dhaka, Bangladesh, 3–5 May 2019; pp. 2–6. [Google Scholar] [CrossRef]
- Chazhoor, A.A.P.; Ho, E.S.L.; Gao, B.; Woo, W.L. Deep transfer learning benchmark for plastic waste classification. Intell. Robot. 2022, 2, 1–19. [Google Scholar] [CrossRef]
- Lanorte, A.; De Santis, F.; Nolè, G.; Blanco, I.; Loisi, R.V.; Schettini, E.; Vox, G. Agricultural plastic waste spatial estimation by Landsat 8 satellite images. Comput. Electron. Agric. 2017, 141, 35–45. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Lin, R.; Zhang, Z.; Zhang, B. Mapping Plastic Greenhouses with Two-Temporal Sentinel-2 Images and 1D-CNN Deep Learning. Remote Sens. 2021, 13, 2820. [Google Scholar] [CrossRef]
- Gruber, F.; Grählert, W.; Wollmann, P.; Kaskel, S. Classification of black plastics waste using fluorescence imaging and machine learning. Recycling 2019, 4, 40. [Google Scholar] [CrossRef]
- Jude, A.B.; Singh, D.; Islam, S.; Jameel, M.; Srivastava, S.; Prabha, B.; Kshirsagar, P.R. An Artificial Intelligence Based Predictive Approach for Smart Waste Management. Wirel. Pers. Commun. 2021, 1–21. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Mustapa, S.I.; Kanthasamy, R.; Zwawi, M.; Cheng, C.K. Modeling the prediction of hydrogen production by co-gasification of plastic and rubber wastes using machine learning algorithms. Int. J. Energy Res. 2021, 45, 9580–9594. [Google Scholar] [CrossRef]
- Karar, M.E.; Alsunaydi, F.; Albusaymi, S.; Alotaibi, S. A new mobile application of agricultural pests recognition using deep learning in cloud computing system. Alex. Eng. J. 2021, 60, 4423–4432. [Google Scholar] [CrossRef]
- Navarro, I.; Matía, F. An Introduction to Swarm Robotics. ISRN Robot. 2012, 2013, 1–10. [Google Scholar] [CrossRef]
- Tropea, M.; Santamaria, A.F.; Potrino, G.; Rango, F. De Bio-Inspired Recruiting Protocol for FANET in Precision Agriculture Domains: Pheromone Parameters Tuning. In Proceedings of the IFIP Wireless Days, Manchester, UK, 24–26 April 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Brambilla, M.; Ferrante, E.; Birattari, M.; Dorigo, M.; Intell, S. Swarm Intell Swarm robotics: A review from the swarm engineering perspective. Swarm Intell. 2013, 7, 1–41. [Google Scholar] [CrossRef]
- Carbone, C.; Garibaldi, O.; Kurt, Z. Swarm Robotics as a Solution to Crops Inspection for Precision Agriculture. KnE Eng. 2018, 3, 552–562. [Google Scholar] [CrossRef]
- Tran Ngoc, N.H.; Jefferson, N.K.; Raspe, P.H. Agricultural Swarm Robotics with Distributed Sensing. Undergraduate Project Report; Worcester Polytechnic Institute: Worcester, MA, USA, 2017. [Google Scholar]
- Schranz, M.; Umlauft, M.; Sende, M.; Elmenreich, W. Swarm Robotic Behaviors and Current Applications. Front. Robot. AI 2020, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Borrás, J. Swarm of Tiny Robots Could Help Eliminate Pesticides. Clean Tech. 2020. Available online: https://cleantechnica.com/2020/08/07/swarm-of-tiny-robots-could-help-eliminate-pesticides/ (accessed on 1 October 2022).
- European Commission. Agricultural-Robotics Technology Company Enabling a Modular Swarm of Autonomous Drones for Spraying. 2022. Available online: https://cordis.europa.eu/project/id/836621 (accessed on 1 October 2022).
- Gul, F.; Rahiman, W.; Alhady, S.S.N.; Ali, A.; Mir, I.; Jalil, A. Meta-heuristic approach for solving multi-objective path planning for autonomous guided robot using PSO–GWO optimization algorithm with evolutionary programming. J. Ambient Intell. Humaniz. Comput. 2020, 12, 7873–7890. [Google Scholar] [CrossRef]
- Swarm Robotics for Agricultural Applications. SAGA Exp. 2022. Available online: https://echord.eu/saga.html (accessed on 1 October 2022).
- Arif, M.; Alghamdi, K.K.; Sahel, S.A.; Alosaimi, S.O.; Alsahaft, M.E. Role of Machine Learning Algorithms in Forest Fire Management: A Literature Review. J. Robot. Autom. 2021, 5, 212–226. [Google Scholar] [CrossRef]
- Aspragathos, N.; Dogkas, E.; Konstantinidis, P.; Koutmos, P.; Lamprinou, N.; Moulianitis, V.C.; Paterakis, G.; Psarakis, E.Ζ.; Sartinas, E.; Souflas, K.; et al. From Pillars to AI Technology-Based Forest Fire Protection Systems. In Intelligent System and Computing; Intechopen: London, UK, 2019; p. 13. [Google Scholar]
- Park, J.H.; Lee, S.; Yun, S.; Kim, H.; Kim, W.T. Dependable fire detection system with multifunctional artificial intelligence framework. Sensors 2019, 19, 2025. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.M.; Seibel, A. Recycling-oriented fabrication of soft robots. In Proceedings of the 2022 IEEE 5th International Conference on Soft Robotics (RoboSoft), Edinburgh, UK, 4–8 April 2022; pp. 571–576. [Google Scholar] [CrossRef]
- Pahl, C. How Machine Learning and Robotics Are Solving the Plastic Sorting Crisis. 2020. Available online: https://datafloq.com/read/using-ai-robotics-solve-plastic-sorting-crisis/ (accessed on 1 October 2022).
- Rojas, J. Plastic waste is exponentially filling our oceans, but where are the robots? In Proceedings of the 2018 IEEE Region 10 Humanitarian Technology Conference (R10-HTC), Malambe, Sri Lanka, 6–8 December 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Rabold, E. Robotics and Remote Systems. 1995. Available online: https://rrsd.ans.org/ (accessed on 1 October 2022).
- Ward, C. Overview of the DOE Waste Facilities Operations Robotic Technology Development Program. 1992. Available online: https://www.osti.gov/biblio/5046250 (accessed on 1 October 2022).
- Barolli, L.; Yim, K.; Chen, H.-C. Advances in Automation and Robotics Research: Proceedings of the 3rd Latin American Congress on Automation and Robotics; Moreno, H.A., Carrera, I.G., Ramírez-Mendoza, R.A., Baca, J., Banfield, I.A., Eds.; Springer: Cham, Switzerland, 2021; Volume 2, pp. 1–45. [Google Scholar]
- Anitha, R.; Maruthi, R.; Sudha, S. Automated Segregation and Microbial Degradation of Plastic Wastes: A Greener Solution to Waste Management Problems. Glob. Transit. Proc. 2022, 3, 100–103. [Google Scholar] [CrossRef]
- Andeobu, L.; Wibowo, S.; Grandhi, S. Artificial intelligence applications for sustainable solid waste management practices in Australia: A systematic review. Sci. Total Environ. 2022, 834, 155389. [Google Scholar] [CrossRef]
- Lubongo, C.; Alexandridis, P. Assessment of Performance and Challenges in Use of Commercial Automated Sorting Technology for Plastic Waste. Recycling 2022, 7, 11. [Google Scholar] [CrossRef]
- Aazam, M.; St-Hilaire, M.; Lung, C.H.; Lambadaris, I. Cloud-based smart waste management for smart cities. In Proceedings of the IEEE International Workshop on Computer Aided Modeling and Design of Communication Links and Networks (CAMAD), Toronto, ON, Canada, 23–25 October 2016; pp. 188–193. [Google Scholar] [CrossRef]
- Jain, A.; Bagherwal, R. Design and implementation of a smart solid waste monitoring and collection system based on Internet of Things. In Proceedings of the 2017 8th International Conference on Computing, Communication and Networking Technologies (ICCCNT), Delhi, India, 3–5 July 2017. [Google Scholar] [CrossRef]
- Ziouzios, D.; Tsiktsiris, D.; Baras, N.; Dasygenis, M. A Distributed Architecture for Smart Recycling Using Machine Learning. Future Internet 2020, 12, 141. [Google Scholar] [CrossRef]
- Georgali, P.Z.M.; Afxentiou, N.; Kylili, A.; Fokaides, P.A. Definition of optimal agricultural plastic waste collection centers with advanced spatial analysis tools. Clean. Eng. Technol. 2021, 5, 100326. [Google Scholar] [CrossRef]
- Cillis, G.; Statuto, D.; Schettini, E.; Vox, G.; Picuno, P. Implementing a GIS-Based Digital Atlas of Agricultural Plastics to Reduce Their Environmental Footprint. Part I: A Deductive Approach. Appl. Sci. 2022, 12, 1330. [Google Scholar] [CrossRef]
- Liu, L.W.; Ma, X.; Wang, Y.M.; Lu, C.T.; Lin, W.S. Using artificial intelligence algorithms to predict rice (Oryza sativa L.) growth rate for precision agriculture. Comput. Electron. Agric. 2021, 187, 106286. [Google Scholar] [CrossRef]
- Jawad, H.M.; Nordin, R.; Gharghan, S.K.; Jawad, A.M.; Ismail, M. Energy-efficient wireless sensor networks for precision agriculture: A review. Sensors 2017, 17, 1781. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Aernouts, M.; De Meyer, M.; Weyn, M.; Berkvens, R. Leveraging LoRaWAN technology for precision agriculture in greenhouses. Sensors 2020, 20, 1827. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).