Vestibular Evoked Myogenic Potentials (VEMPs) in Parkinson’s Disease Patients with Monopolar Deep Brain Stimulation
Abstract
1. Introduction
- To ascertain whether oVEMP and cVEMP responses could be recorded using the µVEMP device during simultaneous monopolar DBS;
- To post-filter the signals in order to identify VEMP responses;
- To validate the post-filtering analysis by recording the VEMP response in the same participant with their DBS device switched ON and OFF.
2. Materials and Methods
2.1. Participants
2.2. VEMP Testing
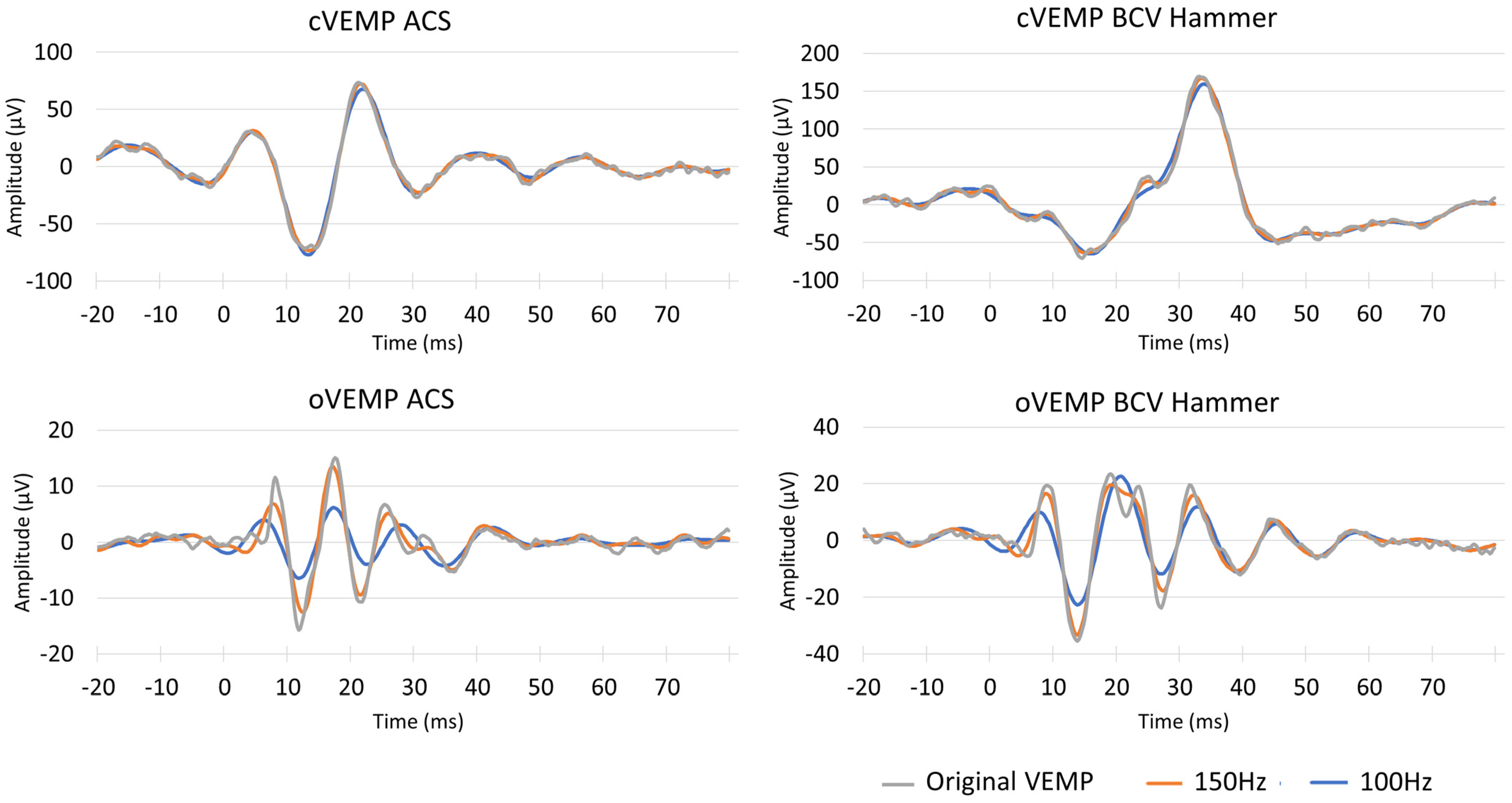
2.3. μVEMP Device and Filtering After VEMP Data Acquisition
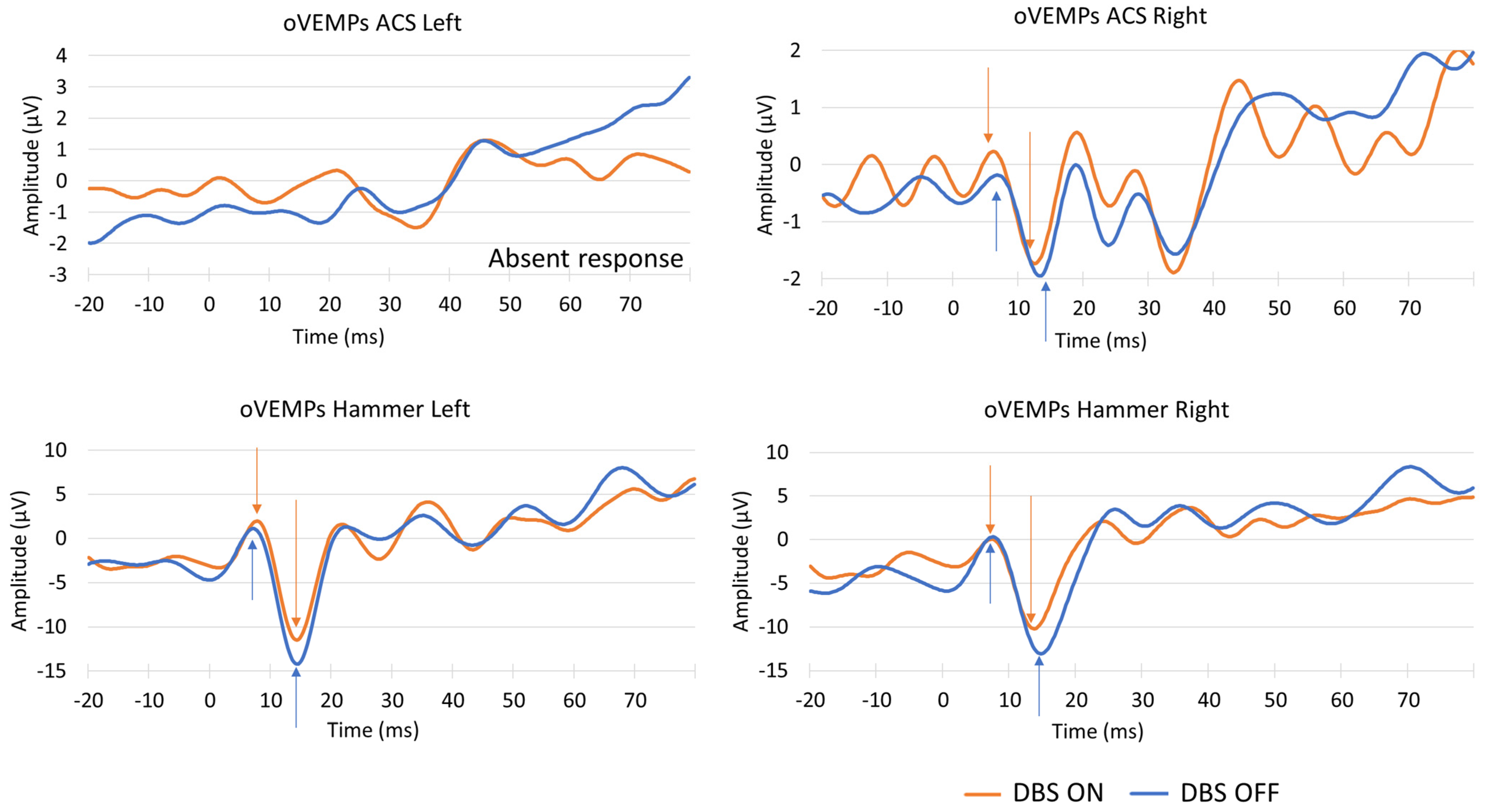
3. Results
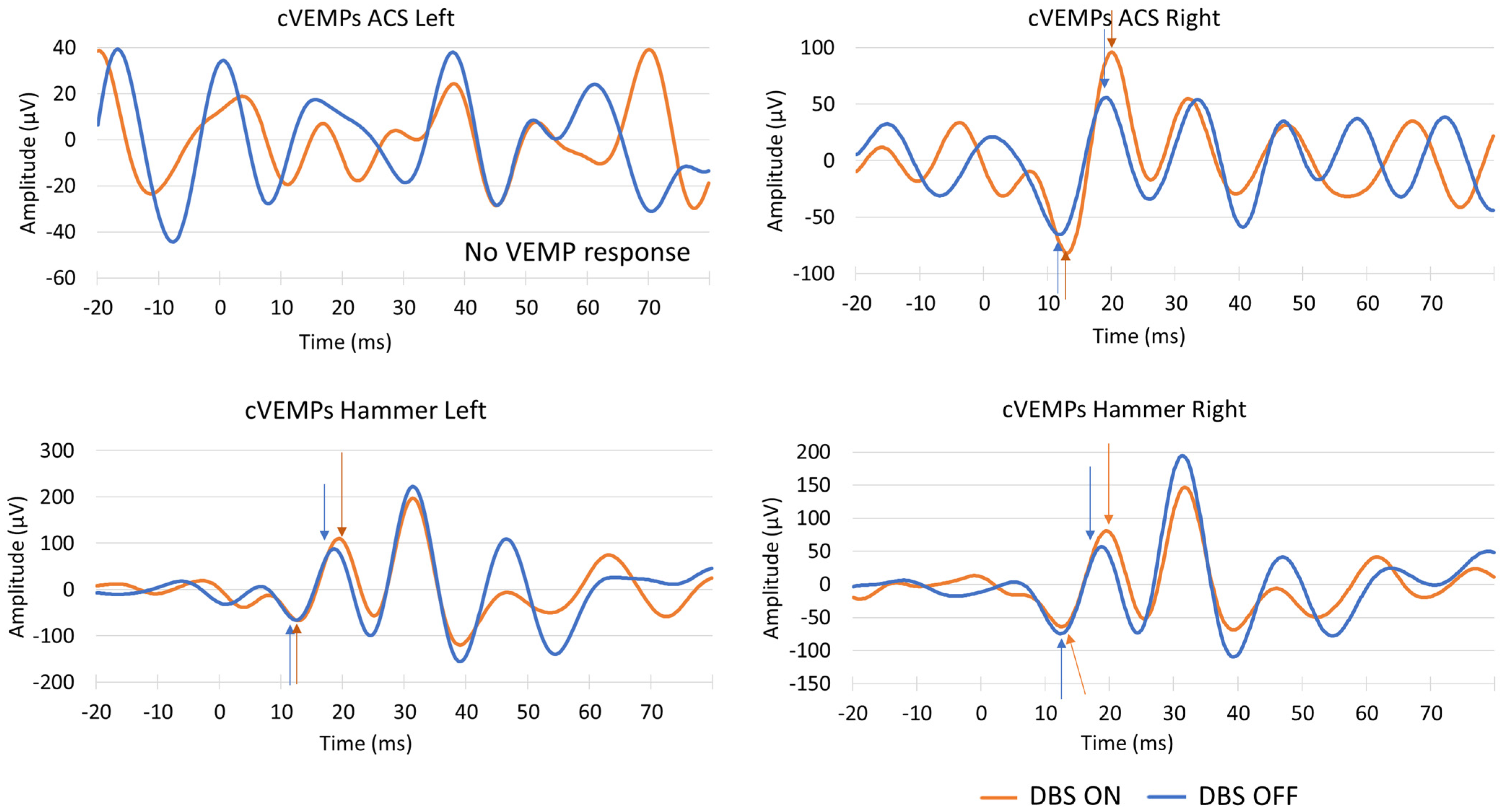
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reich, S.G.; Savitt, J.M. Parkinson’s Disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Hancu, I.; Boutet, A.; Fiveland, E.; Ranjan, M.; Prusik, J.; Dimarzio, M.; Rashid, T.; Ashe, J.; Xu, D.; Kalia, S.K.; et al. On the (Non-)equivalency of monopolar and bipolar settings for deep brain stimulation fMRI studies of Parkinson’s disease patients. J. Magn. Reson. Imaging 2019, 49, 1736–1749. [Google Scholar] [CrossRef] [PubMed]
- Potter-Nerger, M.; Reich, M.M.; Colebatch, J.G.; Deuschl, G.; Volkmann, J. Differential effect of dopa and subthalamic stimulation on vestibular activity in Parkinson’s disease. Mov. Disord. 2012, 27, 1268–1275. [Google Scholar] [CrossRef]
- Colebatch, J.G.; Rosengren, S.M.; Welgampola, M.S. Vestibular-evoked myogenic potentials. Handb. Clin. Neurol. 2016, 137, 133–155. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Vulovic, V.; Burgess, A.M.; Manzari, L.; Sokolic, L.; Pogson, J.; Robins, M.; Mezey, L.E.; Goonetilleke, S.; Cornell, E.D.; et al. Neural basis of new clinical vestibular tests: Otolithic neural responses to sound and vibration. Clin. Exp. Pharmacol. Physiol. 2014, 41, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Wellings, T.P.; Brichta, A.M.; Lim, R. Altered neurofilament protein expression in the lateral vestibular nucleus in Parkinson’s disease. Exp. Brain Res. 2017, 235, 3695–3708. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.E.; Schwarzel, A.K.; Canning, C.G. Recurrent Falls in Parkinson’s Disease: A Systematic Review. Park. Dis. 2013, 2013, 906274. [Google Scholar] [CrossRef]
- Smith, P.F. Vestibular Functions and Parkinson’s Disease. Front. Neurol. 2018, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Baik, K.; Lee, S.U.; Park, E.; Lee, C.N.; Woo, D.; Kim, Y.; Kwag, S.; Park, H.; Kim, J.S. Ocular Vestibular-Evoked Myogenic Potential Assists in the Differentiation of Multiple System Atrophy From Parkinson’s Disease. J. Mov. Disord. 2024, 17, 398–407. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Vulovic, V.; Burgess, A.M.; Sokolic, L.; Goonetilleke, S.C. The response of guinea pig primary utricular and saccular irregular neurons to bone-conducted vibration (BCV) and air-conducted sound (ACS). Hear. Res. 2016, 331, 131–143. [Google Scholar] [CrossRef]
- Temperli, P.; Ghika, J.; Villemure, J.G.; Burkhard, P.R.; Bogousslavsky, J.; Vingerhoets, F.J. How do parkinsonian signs return after discontinuation of subthalamic DBS? Neurology 2003, 60, 78–81. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, H.G.; Holden, J.; Rosengren, S.M.; Chiarovano, E. μVEMP: A Portable Interface to Record Vestibular Evoked Myogenic Potentials (VEMPs) With a Smart Phone or Tablet. Front. Neurol. 2018, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, K.E.; Chiarovano, E.; Paul, S.S.; MacDougall, H.G.; Curthoys, I.S. Static and dynamic otolith reflex function in people with Parkinson’s disease. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 2057–2065. [Google Scholar] [CrossRef]
- Hawkins, K.E.; Paul, S.S.; Chiarovano, E.; Curthoys, I.S. Using virtual reality to assess vestibulo-visual interaction in people with Parkinson’s disease compared to healthy controls. Exp. Brain Res. 2021, 239, 3553–3564. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.K. Data cleaning for dynamic modeling and control. In Proceedings of the European Control Conference, Karlsruhe, Germany, 31 August–3 September 1999; ETH Zurich: Zurich, Switzerland, 1999. [Google Scholar]
- Gustafsson, F. Determining the initial states in forward-backward filtering. IEEE Trans. Signal Process. 1996, 44, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Lozano, A.M.; Lipsman, N. Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 2013, 77, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Budman, E.; Deeb, W.; Martinez-Ramirez, D.; Pilitsis, J.G.; Peng-Chen, Z.; Okun, M.S.; Ramirez-Zamora, A. Potential indications for deep brain stimulation in neurological disorders: An evolving field. Eur. J. Neurol. 2018, 25, 434-e30. [Google Scholar] [CrossRef] [PubMed]
- Welgampola, M.S.; Colebatch, J.G. Vestibulocollic reflexes: Normal values and the effect of age. Clin. Neurophysiol. 2001, 112, 1971–1979. [Google Scholar] [CrossRef]
- de Natale, E.; Ginatempo, F.; Paulus, K.; Manca, A.; Mercante, B.; Pes, G.; Agnetti, V.; Tolu, E.; Deriu, F. Paired neurophysiological and clinical study of the brainstem at different stages of Parkinson’s Disease. Clin. Neurophysiol. 2015, 126, 1871–1878. [Google Scholar] [CrossRef]
- Shalash, A.S.; Hassan, D.M.; Elrassas, H.H.; Salama, M.M.; Méndez-Hernández, E.; Salas-Pacheco, J.M.; Arias-Carrión, O. Auditory-and Vestibular-Evoked Potentials Correlate with Motor and Non-Motor Features of Parkinson’s Disease. Front. Neurol. 2017, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Venhovens, J.; Meulstee, J.; Bloem, B.R.; Verhagen, W.I.M. Neurovestibular analysis and falls in Parkinson’s disease and atypical parkinsonism. Eur. J. Neurosci. 2016, 43, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, S.M.; Govender, S.; Colebatch, J.G. Ocular and cervical vestibular evoked myogenic potentials produced by air- and bone-conducted stimuli: Comparative properties and effects of age. Clin. Neurophysiol. 2011, 122, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Tramontani, O.; Gkoritsa, E.; Ferekidis, E.; Korres, S.G. Contribution of Vestibular-Evoked Myogenic Potential (VEMP) testing in the assessment and the differential diagnosis of otosclerosis. Med. Sci. Monit. 2014, 20, 205–213. [Google Scholar] [CrossRef]
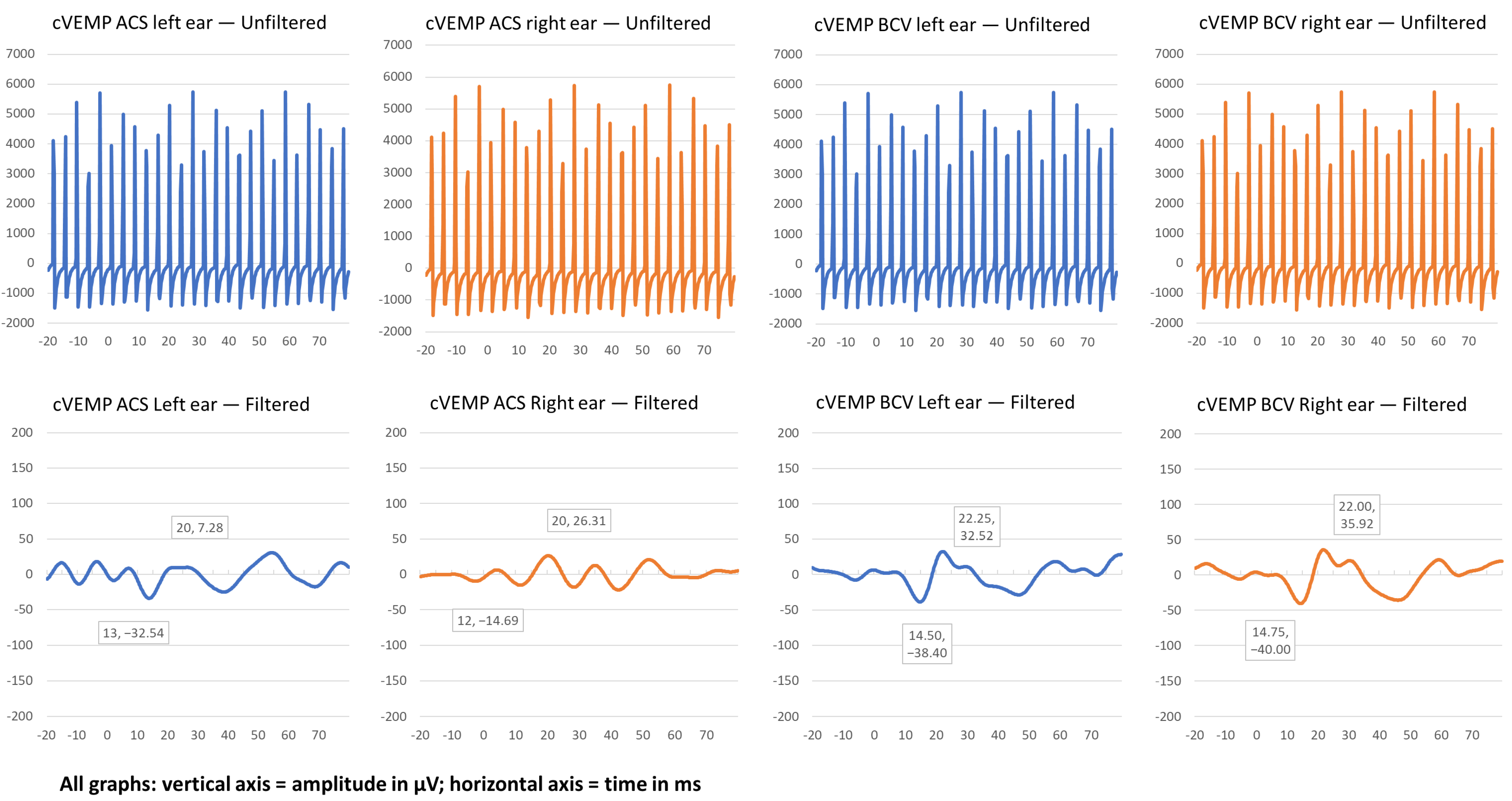
| cVEMP ACS Left Ear | cVEMP ACS Right Ear | cVEMP BCV Left Ear | cVEMP BCV Right Ear | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subject | Pp Amp μV | p1 Latency ms | n1 Latency ms | Pp Amp μV | p1 Latency ms | n1 Latency ms | Pp Amp μV | p1 Latency ms | n1 Latency ms | Pp Amp μV | p1 Latency ms | n1 Latency ms |
| P1 | 88.07 | 12.75 | 20.75 | 89.35 | 13.0 | 20.25 | 30.12 | 13.5 | 18.50 | 105.20 | 14.5 | 23.0 |
| P2 | 39.82 | 13.0 | 20.0 | 41.0 | 12.0 | 20.00 | 70.92 | 15.0 | 22.25 | 75.92 | 14.75 | 22.0 |
| P3 | 0 | - | - | 0 | - | - | 29.41 | 16.25 | 25.25 | 0 | - | - |
| P4 | 0 | - | - | 0 | - | - | 30.83 | 13.75 | 20.25 | 71.13 | 13.75 | 20.75 |
| P5 | 0 | - | - | 177.31 | 13 | 19.25 | 176.67 | 13 | 19.25 | 143.00 | 13 | 19.5 |
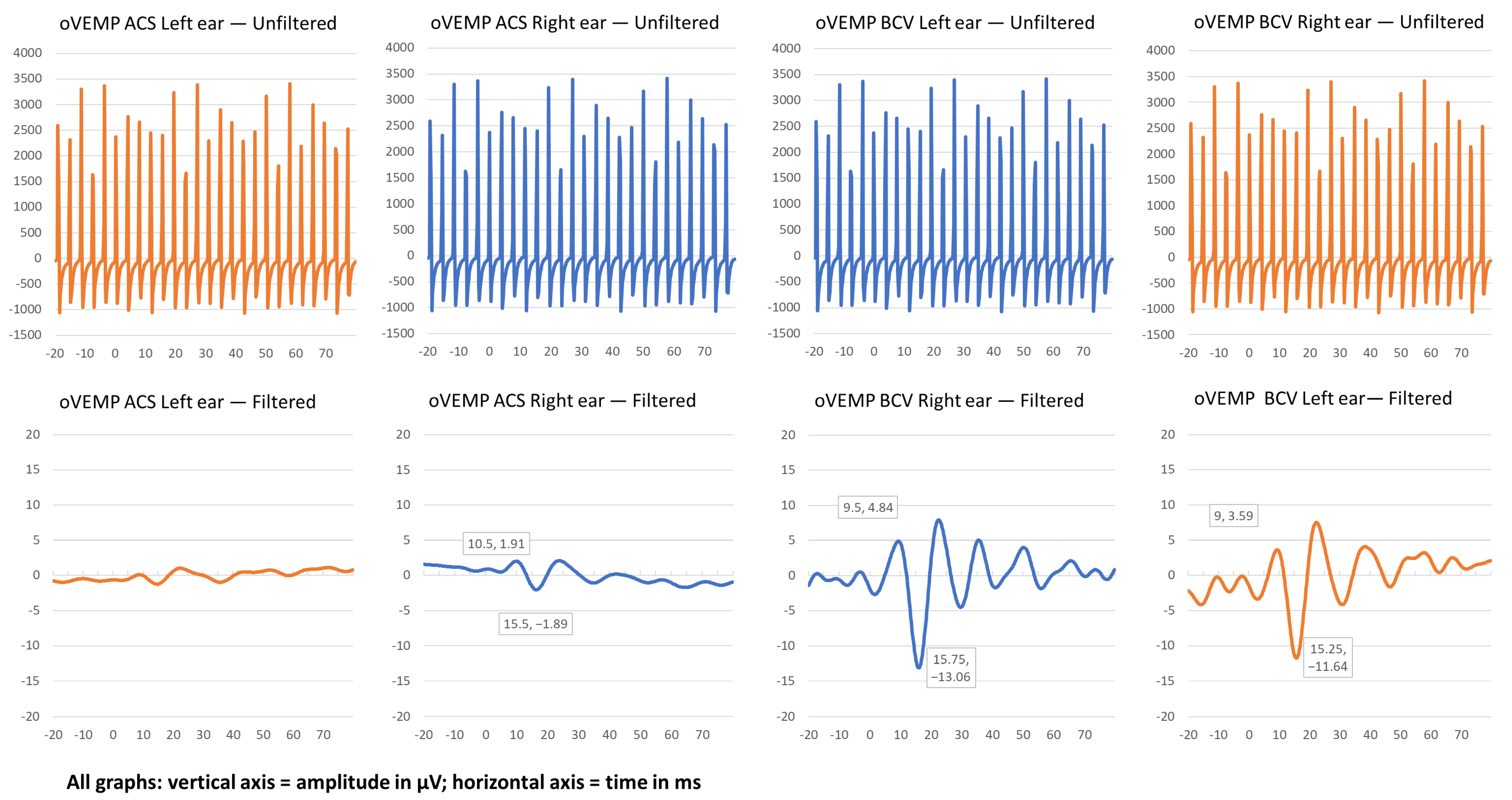
| oVEMP ACS Left Ear | oVEMP ACS Right Ear | oVEMP BCV Left Ear | oVEMP BCV Right Ear | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Pp Amp μV | n1 Latency | P1 Latency | Pp Amp μV | n1 Latency | p1 Latency | Pp Amp μV | n1 Latency | p1 Latency | Pp Amp μV | n1 Latency | p1 Latency |
| P1 | 0 | - | - | 0 | - | - | 2.01 | 9.25 | 14.50 | 2.0 | 8.5 | 14.0 |
| P2 | 0 | - | - | 3.80 | 10.5 | 15.5 | 15.39 | 9.5 | 15.75 | 15.23 | 9.0 | 15.25 |
| P3 | 0 | - | - | 0 | - | - | 0 | - | - | 4.61 | 10 | 16.5 |
| P4 | 0 | - | - | 0 | - | - | 0 | - | - | 0 | - | - |
| P5 | 0 | - | - | 2.12 | 7 | 13.5 | 13.21 | 8.25 | 14.5 | 10.62 | 8.25 | 13.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawkins, K.E.; Holden, J.; Chiarovano, E.; Lewis, S.J.G.; Curthoys, I.S.; MacDougall, H.G. Vestibular Evoked Myogenic Potentials (VEMPs) in Parkinson’s Disease Patients with Monopolar Deep Brain Stimulation. Signals 2025, 6, 10. https://doi.org/10.3390/signals6010010
Hawkins KE, Holden J, Chiarovano E, Lewis SJG, Curthoys IS, MacDougall HG. Vestibular Evoked Myogenic Potentials (VEMPs) in Parkinson’s Disease Patients with Monopolar Deep Brain Stimulation. Signals. 2025; 6(1):10. https://doi.org/10.3390/signals6010010
Chicago/Turabian StyleHawkins, Kim E., John Holden, Elodie Chiarovano, Simon J. G. Lewis, Ian S. Curthoys, and Hamish G. MacDougall. 2025. "Vestibular Evoked Myogenic Potentials (VEMPs) in Parkinson’s Disease Patients with Monopolar Deep Brain Stimulation" Signals 6, no. 1: 10. https://doi.org/10.3390/signals6010010
APA StyleHawkins, K. E., Holden, J., Chiarovano, E., Lewis, S. J. G., Curthoys, I. S., & MacDougall, H. G. (2025). Vestibular Evoked Myogenic Potentials (VEMPs) in Parkinson’s Disease Patients with Monopolar Deep Brain Stimulation. Signals, 6(1), 10. https://doi.org/10.3390/signals6010010






