A Comparison between Ultrasonic Guided Wave Leakage and Half-Cell Potential Methods in Detection of Corrosion in Reinforced Concrete Decks
Abstract
:1. Introduction
Significance
2. Background
2.1. Causes and Mechanism of Corrosion
2.2. Chloride Content Analysis
2.3. Non-Destructive Corrosion Monitoring
3. Methodology
3.1. Ultrasonic Guided Wave Leakage (UGWL) Method
- The raw data is stored in a “4K9” format using the Pulsonic Ultrasonic Pulse Analyzer.
- The raw data is then converted to CSV files using the Ultrasonic Pulse Analyzer Data Converter. This raw data in the form of velocities in the time domain.
- The CSV files are converted to Microsoft Excel documents and time domain data is transformed into frequency domain data using Fourier Analysis.
- The peak amplitude values in the frequency domain are plotted against distance (i.e., the location of the receiver). This allows to determine the attenuation of the UGWL waves along the wave guide and observe any deviations from the expected (theoretical) attenuation curve that can be due to flaws on the rebar or the rebar-concrete interface.
3.2. Half-Cell Potential Method
- To locate the corroding reinforcing bars, and thus, to assess the condition of reinforcement during the testing process.
- To define the position for further destructive analysis such as cores for chloride analysis and inspection windows to visually assess the corrosion condition of the rebars.
- To evaluate the corrosion condition of the rebar after the repair procedure, and thus, to assess the efficiency and durability of the repair.
- To design an anode layout of cathodic protection systems.
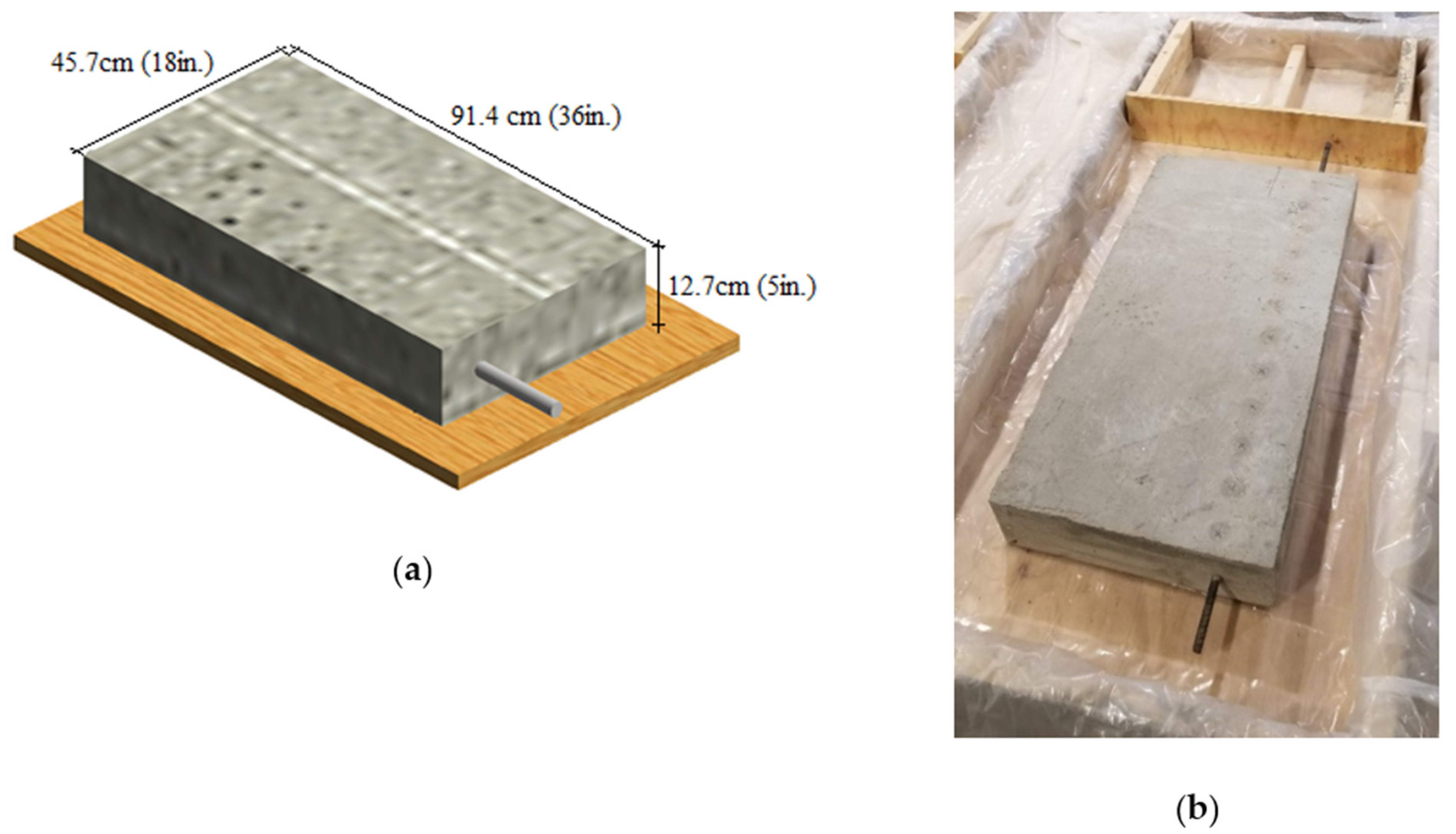
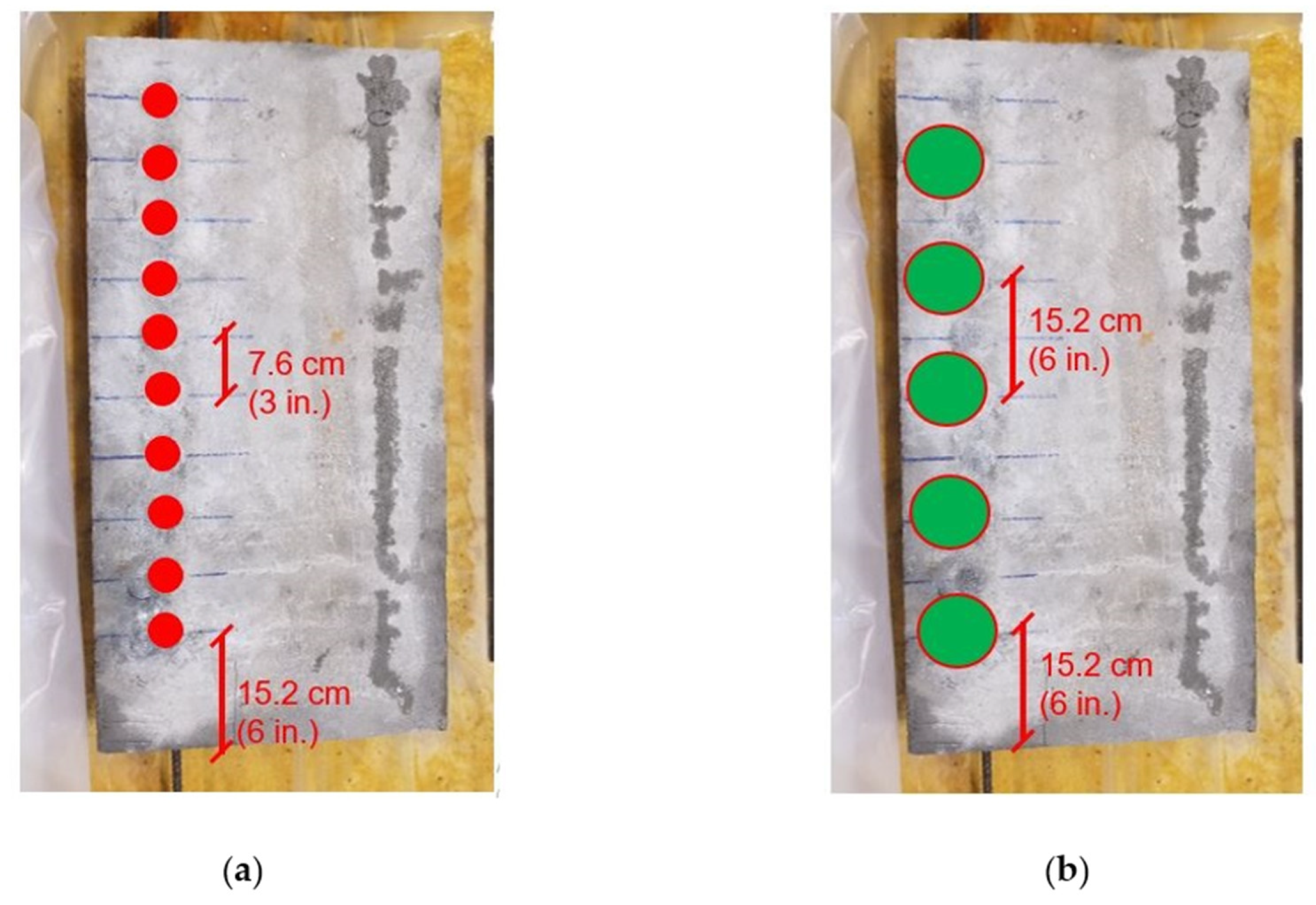
3.3. Specimen Details and Data Collection
3.4. Measurement of Chloride Content
4. Results
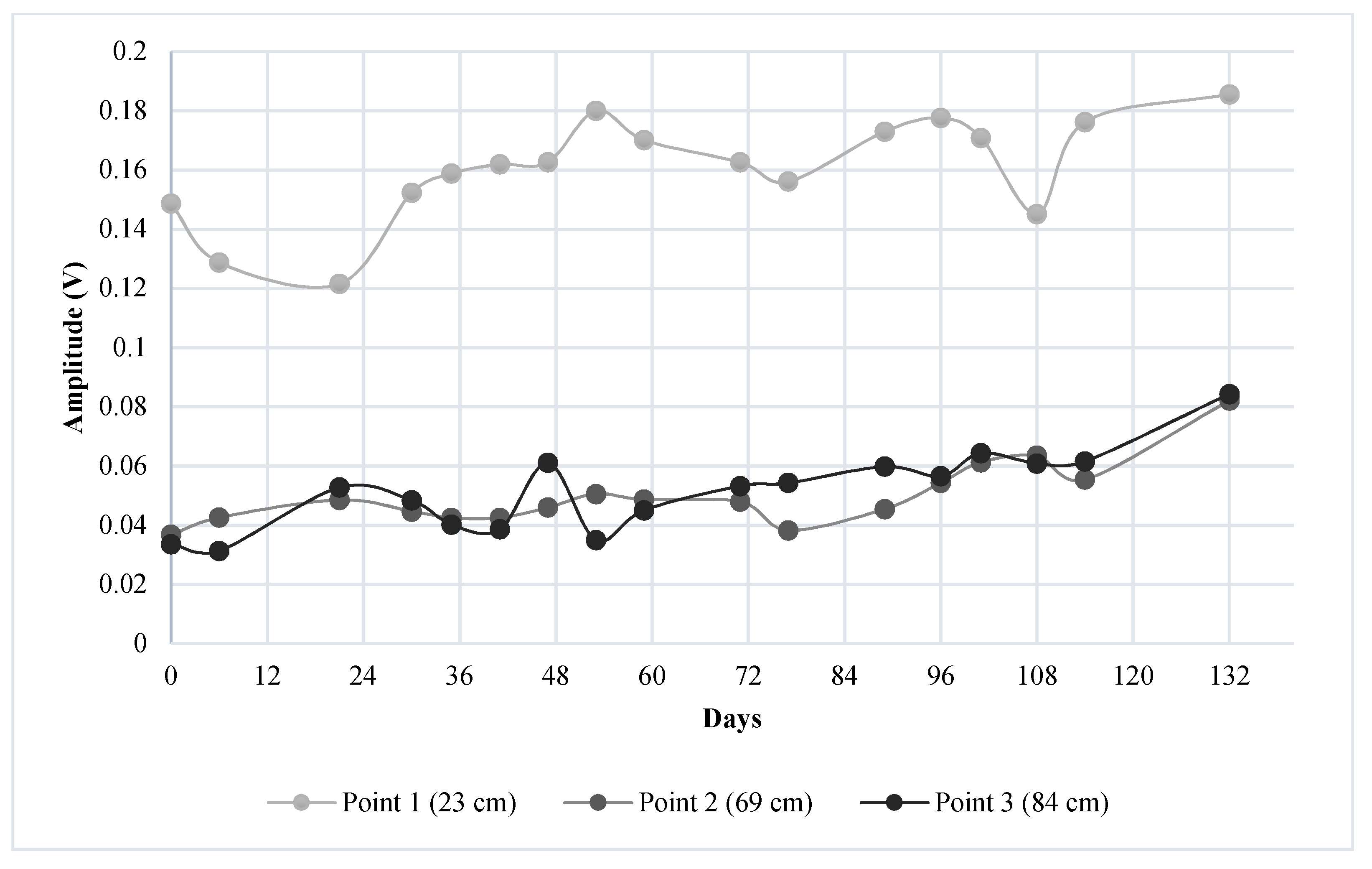
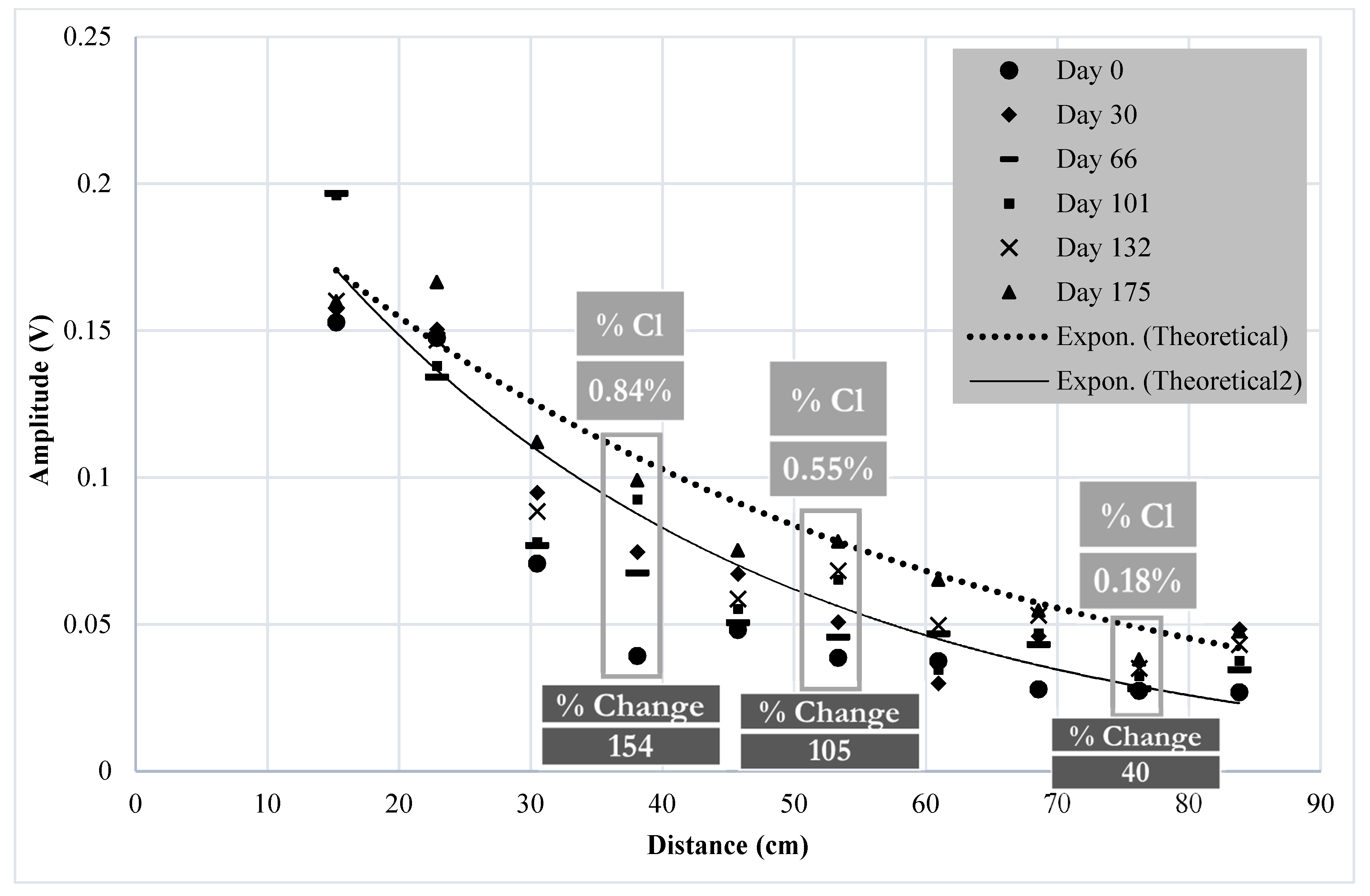
4.1. Results of Monitoring Corrosion Using UGWL
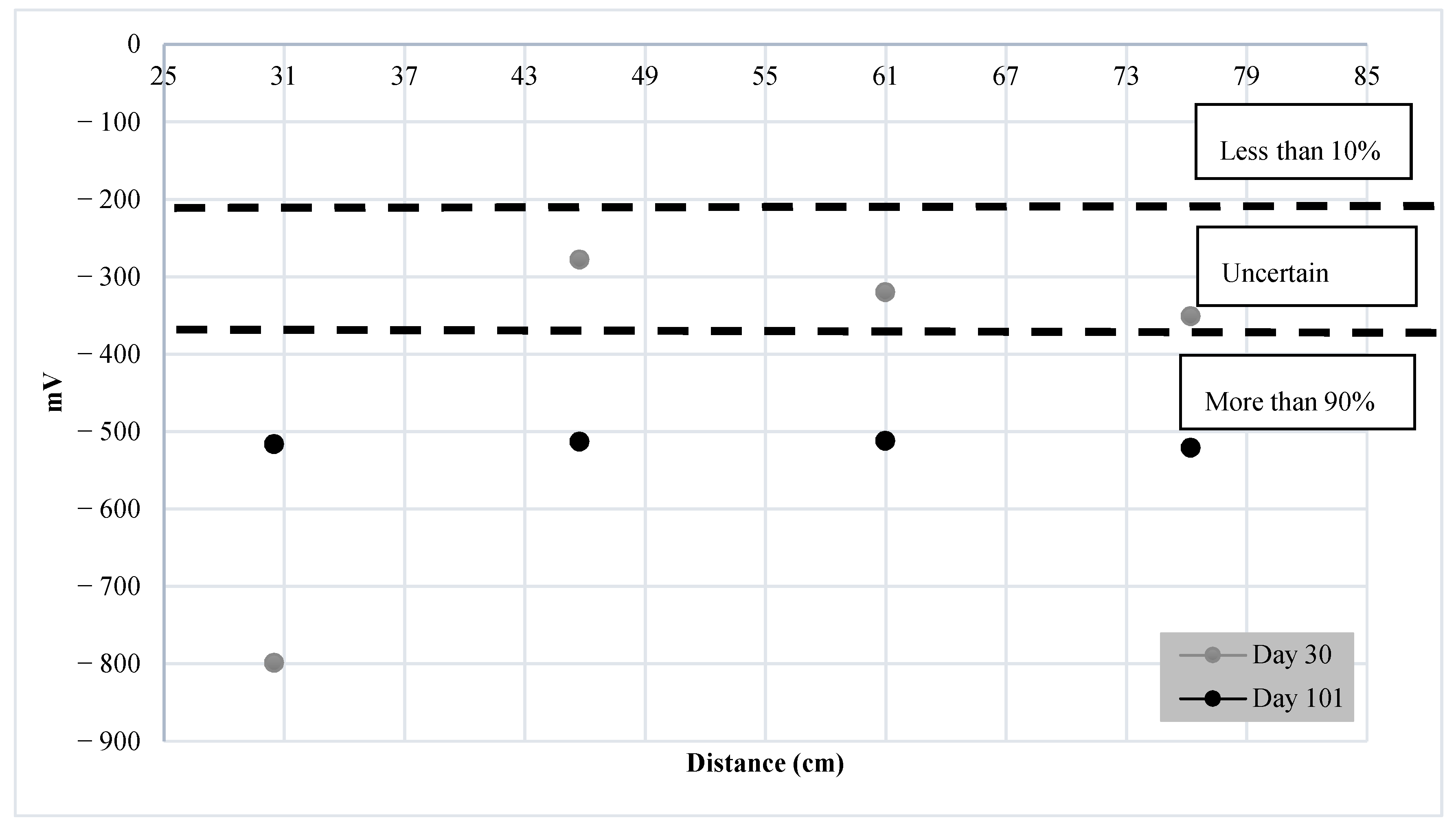
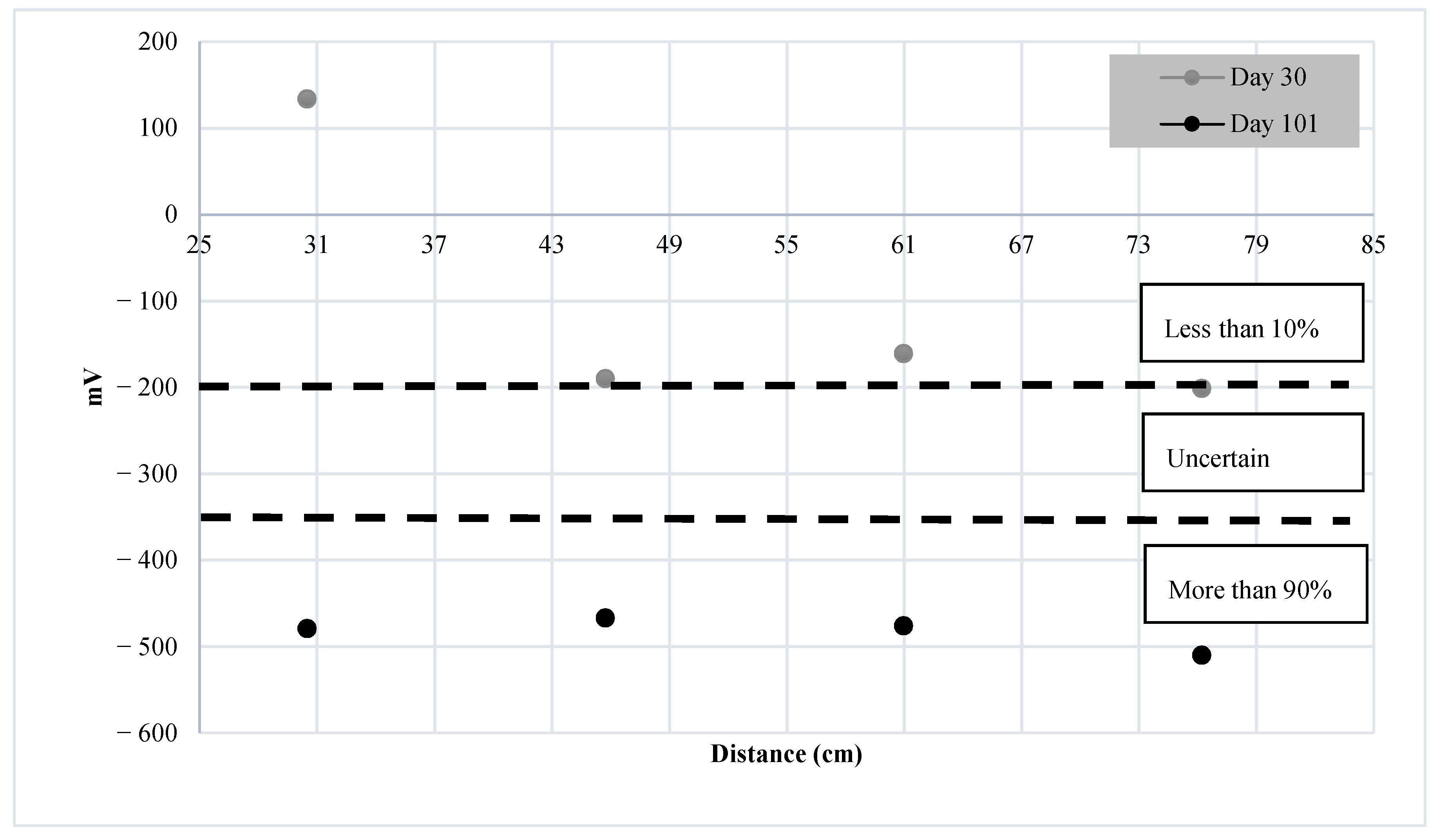
4.2. Results of Monitoring Corrosion Using HCP
4.3. Comparison of UGWL and HCP
4.4. Measurement of Chloride Content
5. Conclusions
- The experimental results suggest that a 50% change in UGWL data may be the threshold to be considered as a “significant change” in the condition of the rebar or the rebar–concrete interface with respect to corrosion progression in RC bridge decks. While a 50% change provided a permanent change in the data that presents a higher certainty of deterioration, it was verified by visual inspection that UGWL amplitude changes as small as 17% can indicate an onset of corrosion activity.
- While a larger data population is needed to confirm this finding, less than a 40% change in the UGWL amplitudes correlates to the CTL threshold of 0.1% by weight of concrete established by ACI 318.
- The findings suggest that when UGWL and HCP are benchmarked, most of the data were in good agreement. The UGWL measurements presented notablechanges in amplitude as early as 9 days, compared to 30 days with HCP. As such, UGWL may have the ability to detect corrosion activity sooner than HCP. In addition to earlier detection, UGWL allows for higher certainty in predictions compared to HCP’s large region of uncertainty, ranging between a 10 and 90% probability of corrosion activity.
- The experimental results also indicate that noticeable changes in the UGWL readings were recorded as early as 9 days for the lab specimens submerged in 10% NaCl. Over 175 days, the change in the UGWL amplitudes were as high as 154% with respect to the baseline. The 154% increase in the UGWL amplitude change correlated to a 0.84% chloride content, which is higher than the threshold of 0.1% by concrete weight specified by ACI 318. This finding suggests a strong potential for the proposed UGWL method for corrosion monitoring.
- UGWL has the potential to become a method that can detect multiple flaws with a single setup, because several other advantages of UGWL over HCP are identified in previous work, such as (1) UGWL can be used on epoxy-coated bars, while HCP is limited to use with black bars; and (2) UGWL can detect various flaws (corrosion, delamination, and independent cracks) with one setup, whereas HCP only detects corrosion. As such, UGWL has the potential to become a method that can detect multiple flaws with a single setup.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| UGWL: Specimen A | ||||
|---|---|---|---|---|
| Corrosion Data | ||||
| Day 30 | ||||
| File name | Distance (in) | Amplitude | Avg. | Standard Deviation |
| 1 | 9 | 0.149528903 | 0.150427285 | 0.001433856 |
| 2 | 9 | 0.149136852 | ||
| 3 | 9 | 0.148896135 | ||
| 4 | 9 | 0.152310975 | ||
| 5 | 9 | 0.151683608 | ||
| 6 | 9 | 0.151007237 | ||
| 7 | 12 | 0.096250092 | 0.094785525 | 0.002047222 |
| 8 | 12 | 0.095550813 | ||
| 9 | 12 | 0.096919853 | ||
| 10 | 12 | 0.094149955 | ||
| 11 | 12 | 0.09507235 | ||
| 12 | 12 | 0.094267489 | ||
| 13 | 12 | 0.096491907 | ||
| 14 | 12 | 0.095200269 | ||
| 15 | 12 | 0.09623313 | ||
| 16 | 12 | 0.093337921 | ||
| 17 | 15 | 0.074725763 | 0.074667792 | 0.000678115 |
| 18 | 15 | 0.074688041 | ||
| 19 | 15 | 0.075483172 | ||
| 20 | 15 | 0.075671486 | ||
| 21 | 15 | 0.07415704 | ||
| 22 | 15 | 0.074486913 | ||
| 23 | 15 | 0.074871704 | ||
| 24 | 15 | 0.074649849 | ||
| 25 | 15 | 0.074428593 | ||
| 26 | 15 | 0.073593579 | ||
| 27 | 18 | 0.064233128 | 0.067169366 | |
| 28 | 18 | 0.066504016 | ||
| 29 | 18 | 0.066432182 | ||
| 30 | 18 | 0.06655954 | ||
| 31 | 18 | 0.066835837 | ||
| 32 | 18 | 0.068642605 | ||
| 33 | 18 | 0.067258192 | ||
| 34 | 18 | 0.068421715 | ||
| 35 | 18 | 0.069637081 | ||
| 36 | 21 | 0.035443079 | 0.035098723 | 0.000395379 |
| 37 | 21 | 0.034969048 | ||
| 38 | 21 | 0.035155169 | ||
| 39 | 21 | 0.035073283 | ||
| 40 | 21 | 0.035794221 | ||
| 41 | 21 | 0.035327647 | ||
| 42 | 21 | 0.03501156 | ||
| 43 | 21 | 0.034839785 | ||
| 44 | 21 | 0.035085114 | ||
| 45 | 21 | 0.034288329 | ||
| 46 | 24 | 0.032787719 | 0.02989137 | 0.00239568 |
| 47 | 24 | 0.032139173 | ||
| 48 | 24 | 0.03236147 | ||
| 49 | 24 | 0.032947254 | ||
| 50 | 24 | 0.028859457 | ||
| 51 | 24 | 0.029445851 | ||
| 52 | 24 | 0.027741543 | ||
| 53 | 24 | 0.028028783 | ||
| 54 | 24 | 0.027311966 | ||
| 55 | 24 | 0.027290484 | ||
| 56 | 27 | 0.04866844 | 0.046021439 | 0.001493144 |
| 57 | 27 | 0.048405063 | ||
| 58 | 27 | 0.044544714 | ||
| 59 | 27 | 0.045752979 | ||
| 60 | 27 | 0.04466398 | ||
| 61 | 27 | 0.044244212 | ||
| 62 | 27 | 0.045965955 | ||
| 63 | 27 | 0.045768383 | ||
| 64 | 27 | 0.045952916 | ||
| 65 | 27 | 0.046247749 | ||
| 66 | 30 | 0.029307697 | 0.026814223 | 0.001186757 |
| 67 | 30 | 0.028548822 | ||
| 68 | 30 | 0.029121009 | ||
| 69 | 30 | 0.027857985 | ||
| 70 | 30 | 0.027242585 | ||
| 71 | 30 | 0.026570321 | ||
| 72 | 30 | 0.026159675 | ||
| 73 | 30 | 0.025861586 | ||
| 74 | 30 | 0.02592397 | ||
| 75 | 30 | 0.02577665 | ||
| 76 | 33 | 0.049846079 | 0.048366968 | 0.002623562 |
| 77 | 33 | 0.045668294 | ||
| 78 | 33 | 0.045455227 | ||
| 79 | 33 | 0.044984574 | ||
| 80 | 33 | 0.045906164 | ||
| 81 | 33 | 0.048896116 | ||
| 82 | 33 | 0.052287372 | ||
| 83 | 33 | 0.050199868 | ||
| 84 | 33 | 0.050795235 | ||
| 85 | 33 | 0.049630746 | ||
| HCP: Specimen A | ||
|---|---|---|
| Corrosion Data | ||
| Day 30 | ||
| Distance (in) | Potential (mV) | Average |
| 12 | −799 | −799 |
| 18 | −278 | −278 |
| 24 | −320 | −320 |
| 30 | −351 | −351 |
References
- Garcia, E.; Erdogmus, E.; Schuller, M.; Harvey, D. Novel method for the detection of onset of delamination in reinforced concrete bridge decks. J. Perform. Constr. Facil. 2017, 31, 04017102. [Google Scholar] [CrossRef]
- Garcia, E.; Erdogmus, E.; Schuller, M.; Harvey, D. Detecting onset of different types of flaws in reinforced concrete. ACI Mater. J. 2019, 116, 73–82. [Google Scholar] [CrossRef]
- Erdogmus, E.; Garcia, E.; Amiri, A.S.; Schuller, M. A novel structural health monitoring method for reinforced concrete bridge decks using ultrasonic guided waves. Infrastructures 2020, 5, 49. [Google Scholar] [CrossRef]
- Hartt, W.H.; Powers, R.G.; Leroux, V.; Lysogorski, D.K. Critical Literature Review of High.-Performance Corrosion Reinforcements in Concrete Bridge. Applications; United States Federal Highway Administration, Office of Infrastructure, Center for Marine Materials Florida Atlantic University—Sea Tech Campus: Dania Beach, FL, USA, 2004. [Google Scholar]
- Hema, J.; Guthrie, W.S.; Fonseca, F.S. Concrete Bridge. Deck Condition Assessment and Improvement Strategies; Utah Department of Transportation: Salt Lake City, UT, USA, 2004.
- Tesfamariam, S.; Bastidas-Arteaga, E.; Lounis, Z. Seismic retrofit screening of existing highway bridges with consideration of chloride-induced deterioration: A bayesian belief network model. Front. Built Environ. 2018, 4. [Google Scholar] [CrossRef]
- Basheer, P.A.M.; Chidiact, S.E.; Long, A.E. Predictive models for deterioration of concrete structures. Constr. Build. Mater. 1996, 10, 27–37. [Google Scholar] [CrossRef]
- Ji, Y.; Zhao, W.; Zhou, M.; Ma, H.; Zeng, P. Corrosion current distribution of macrocell and microcell of steel bar in concrete exposed to chloride environments. Constr. Build. Mater. 2013, 47, 104–110. [Google Scholar] [CrossRef]
- Cui, J. Multiple Sensor Periodic Nondestructive Evaluation on Concrete Bridge. Deck Maintenance. Ph.D. Thesis, University of Vermont, Burlington, VT, USA, 2012. [Google Scholar]
- Bentz, E.C. Probabilistic modeling of service life for structures subjected to chlorides. Mater. J. 2003, 100, 391–397. [Google Scholar]
- Sharma, A.; Sharma, S.; Sharma, S.; Mukherjee, A. Monitoring invisible corrosion in concrete using a combination of wave propagation techniques. Cem. Concr. Compos. 2018, 90, 89–99. [Google Scholar] [CrossRef]
- Valcarce, M.B.; Vázquez, M. Carbon steel passivity examined in alkaline solutions: The effect of chloride and nitrite ions. Electrochim. Acta 2008, 53, 5007–5015. [Google Scholar] [CrossRef]
- Melchers, R.E.; Li, C.Q. Phenomenological modeling of reinforcement corrosion in marine environments. ACI Mater. J. 2006, 103, 25. [Google Scholar]
- Modares, M.; Waksmanski, N. Overview of structural health monitoring for steel bridges. Pract. Period. Struct. Des. Constr. 2013, 18, 187–191. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Ann, K.Y.; Song, H.-W. Chloride threshold level for corrosion of steel in concrete. Corros. Sci. 2007, 49, 4113–4133. [Google Scholar] [CrossRef]
- Glass, G.K.; Buenfeld, N.R. Chloride-induced corrosion of steel in concrete. Prog. Struct. Eng. Mater. 2000, 2, 448–458. [Google Scholar] [CrossRef]
- ACI Committee, and International Organization for Standardization. Building Code Requirements for Structural Concrete (ACI 318-08) and Commentary; American Concrete Institute: Farmington Hills, MI, USA, 2008; Chapter 4; p. 60. [Google Scholar]
- Standard, B. Structural Use of Concrete: Code of Practice for Design and Construction, Part. 1, BS 8110; British Standard Institution: London, UK, 1997. [Google Scholar]
- ACI Committee 357. 357R-84: Guide for the Design and Construction of Fixed Offshore Concrete Structures (Reapproved 1997); Technical Documents. Published Online January 1; American Concrete Institute: Farmington Hills, MI, USA, 1984. [Google Scholar]
- ASTM C. Standard Test. Method for Acid-Soluble Chloride in Mortar and Concrete; Published Online; ASTM: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Zaki, A.; Chai, H.K.; Aggelis, D.G.; Alver, N. Non-destructive evaluation for corrosion monitoring in concrete: A review and capability of acoustic emission technique. Sensors 2015, 15, 19069–19101. [Google Scholar] [CrossRef]
- Elsener, B.; Andrade, C.; Gulikers, J.; Polder, R.; Raupach, M. Hall-cell potential measurements—Potential mapping on reinforced concrete structures. Mater. Struct. 2003, 36, 461–471. [Google Scholar] [CrossRef]
- Gholizadeh, S. A review of non-destructive testing methods of composite materials. Procedia Struct. Integr. 2016, 1, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Ohtsu, M.; Tomola, Y. Corrosion monitoring in reinforced concrete by Acoustic Emission. J. Acoust. Emiss. 2003, 21, 157–169. [Google Scholar]
- Li, W.; Xu, C.; Ho, S.C.; Wang, B.; Song, G. Monitoring concrete deterioration due to reinforcement corrosion by integrating acoustic emission and FBG strain measurements. Sensors 2017, 17, 657. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.V.C. Identifying the Onset, Type, and Location of Deterioration in Reinforced Concrete Using Ultrasonic Testing. Ph.D. Dissertation, University of Nebraska-Lincoln, Lincoln, NE, USA, 2016. [Google Scholar]
- Amiri, A.S. A Comparison between Ultrasonic Guided Wave Leakage and Half-Cell Potential Methods in Detection of Corrosion in Reinforced Concrete Structures. Master’s Thesis, University of Nebraska, Lincoln, NE, USA, 2020. [Google Scholar]
- Ervin, B.L. Monitoring Corrosion of Rebar Embedded in Mortar Using Guided Ultrasonic Waves (Doctor of Philosophy). Ph.D. Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2007. [Google Scholar]
- Mustapha, S.; Lu, Y.; Li, J.; Ye, L. Damage detection in rebar-reinforced concrete beams based on time reversal of guided waves. Struct. Health Monit. 2014, 13, 347–358. [Google Scholar] [CrossRef]
- Manning, D.G. Detecting Defects and Deterioration in Highway Structures; NCHRP Synthesis of Highway Practice; Transportation Research Board: Washington, DC, USA, 1985; Volume 118. [Google Scholar]
- Stratfull, R.F. The corrosion of steel in a reinforced concrete bridge. Corrosion 1957, 13, 43–48. [Google Scholar] [CrossRef]
- ASTM C. 876-99 Standard, Test. Method for Half-Cell Potentials of Uncoated Reinforcing Steel in Concrete; American Society for Testing and Materials: West Conshohocken, PA, USA, 1999. [Google Scholar]
- Frølund, T.; Klinghoffer, O.; Sørensen, H.E.; Denmark, D.D. Pro’s and con’s of half-cell potentials and corrosion rate measurements. In Proceedings of the International Conference on Structural Faults, London, UK, 1–3 July 2003. [Google Scholar]
- Pradhan, B.; Bhattacharjee, B. Half-cell potential as an indicator of chloride-induced rebar corrosion initiation in RC. J. Mater. Civ. Eng. 2009, 21, 543–552. [Google Scholar] [CrossRef]
- Zou, Z.H.; Wu, J.; Wang, Z.; Wang, Z. Relationship between half-cell potential and corrosion level of rebar in concrete. Corros. Eng. Sci. Technol. 2016, 51, 588–595. [Google Scholar] [CrossRef]
- Yodsudjai, W.; Pattarakittam, T. Factors influencing half-cell potential measurement and its relationship with corrosion level. Measurement 2017, 104, 159–168. [Google Scholar] [CrossRef]
- Abbas, S.; Soliman, A.M.; Nehdi, M.L. Chloride ion penetration in reinforced concrete and steel fiber-reinforced concrete precast tunnel lining segments. Mater. J. 2014, 111, 613–622. [Google Scholar]
- Akhtar, S. Review of nondestructive testing methods for condition monitoring of concrete structures. J. Constr. Eng. 2013. [Google Scholar] [CrossRef] [Green Version]







| Standard | Reference | Chloride Threshold Level (CTL) (%, Cement) | |
|---|---|---|---|
| Reinforced Concrete | Pre-Stressed Concrete | ||
| British Standard | [19] | 0.4 | 0.1 |
| ACI 357 (Water-soluble Cl-) | [20] | 0.1 | 0.06 |
| ASTM 1152 (Acid-soluble Cl-) | [21] | 0.2 | 0.08 |
| Half-Cell Potential Measurements (Mv) | Probability of Rebar Corrosion Activity |
|---|---|
| >−200 | Less than 10% |
| −200 to −350 | Uncertain |
| <−350 | More than 90% |
| UGWL Data Points | Day 0 | Day 9 | Day 30 | Day 101 | Day 175 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Amp. (v) | Amp. (v) | % change vs. Day 0 | Amp. (v) | % change vs. Day 0 | Amp. (v) | % change vs. Day 0 | Amp. (v) | % Change vs. Day 0 | |
| 38 cm (15 in) | 0.039 | 0.061 | 56 | 0.074 | 90 | 0.092 | 136 | 0.099 | 154 |
| 53 cm (21 in) | 0.038 | 0.055 | 45 | 0.051 | 34 | 0.065 | 71 | 0.078 | 105 |
| Parameters | UGWL | HCP |
|---|---|---|
| Application for other purposes | Can be used to detect cracks and delamination in addition to corrosion [2]; | n/a |
| Influence of temperature | No information | Influenced by temperature and humidity [37,39] |
| Need for coupling material | It needs coupling gel between the sensors and the test materials (i.e., concrete) | Not needed |
| Applicability on structures involving epoxy-coated bar | It was successfully used for structures involving an epoxy-coated rebar [1] | Not suitable for structures that involves an epoxy-coated rebar [33] |
| Attachment to the rebar | Required | Required |
| Sample | Mass (g) | Volume (L) | Solution (ppm) | %Cl per Concrete Weight |
|---|---|---|---|---|
| Method blank | 0 | 0.2500 | 0.13 | -- |
| 38 cm (15 in) | 2.1008 | 0.2500 | 70.6 | 0.84% |
| 53 cm (21 in) | 2.1037 | 0.2500 | 47.5 | 0.56% |
| 76 cm (30 in) | 1.9880 | 0.2500 | 15.2 | 0.19% |
| Sample | Mass (g) | Volume (L) | Solution (ppm) | %Cl per Concrete Weight |
|---|---|---|---|---|
| Method blank | 0 | 0.2500 | Not Detected | -- |
| 38 cm (15 in) | 2.0425 | 0.2500 | 68.7 | 0.84% |
| 53 cm (21 in) | 1.9692 | 0.2500 | 42.7 | 0.54% |
| 76 cm (30 in) | 2.0100 | 0.2500 | 14.2 | 0.18% |
| Sample | Average %Cl per Concrete Weight |
|---|---|
| 38 cm (15 in) | 0.84% |
| 53 cm (21 in) | 0.55% |
| 76 cm (30 in) | 0.185% |
| Measurement Location | UGWL Data | HCP Data | ||||
|---|---|---|---|---|---|---|
| Day 0 (Amplitude) | Day 175 (Amplitude) | Percentage Change in UGWL Reading (%) | Day 175 Reading (mV) | Day 175 Probability of Corrosion Activity | Average %Cl per Concrete Weight | |
| 38 cm (15 in) | 0.039 | 0.099 | 154% | −624 | >90% | 0.84% |
| 53 cm (21 in) | 0.038 | 0.078 | 105% | −595 | >90% | 0.55% |
| 76 cm (30 in) | 0.027 | 0.038 | 40% | −574 | >90% | 0.185% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiri, A.S.; Erdogmus, E.; Richter-Egger, D. A Comparison between Ultrasonic Guided Wave Leakage and Half-Cell Potential Methods in Detection of Corrosion in Reinforced Concrete Decks. Signals 2021, 2, 413-433. https://doi.org/10.3390/signals2030026
Amiri AS, Erdogmus E, Richter-Egger D. A Comparison between Ultrasonic Guided Wave Leakage and Half-Cell Potential Methods in Detection of Corrosion in Reinforced Concrete Decks. Signals. 2021; 2(3):413-433. https://doi.org/10.3390/signals2030026
Chicago/Turabian StyleAmiri, Ahmad Shoaib, Ece Erdogmus, and Dana Richter-Egger. 2021. "A Comparison between Ultrasonic Guided Wave Leakage and Half-Cell Potential Methods in Detection of Corrosion in Reinforced Concrete Decks" Signals 2, no. 3: 413-433. https://doi.org/10.3390/signals2030026
APA StyleAmiri, A. S., Erdogmus, E., & Richter-Egger, D. (2021). A Comparison between Ultrasonic Guided Wave Leakage and Half-Cell Potential Methods in Detection of Corrosion in Reinforced Concrete Decks. Signals, 2(3), 413-433. https://doi.org/10.3390/signals2030026






