Implication of Altered Acoustic Active Space for Cetacean Species That Result from Soundscape Changes and Noise Additions
Abstract
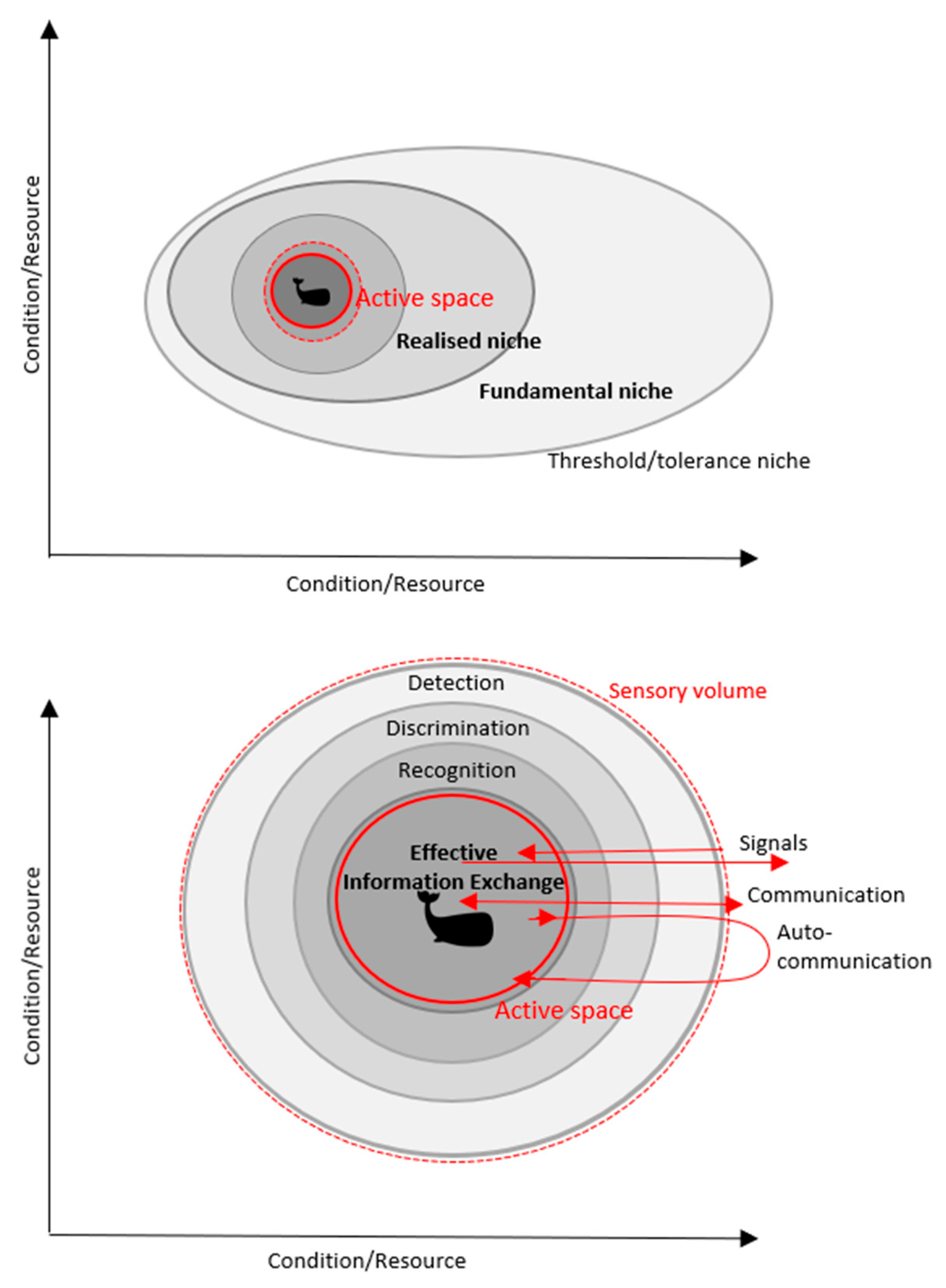
1. Introduction
2. Background
3. Active Space and Marine Mammals
4. Anthropogenic Noise Effects on a Cetacean
5. Application of Active Space in Ecological Study
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Creanza, N.; Fogarty, L.; Feldman, M.W. Cultural niche construction of repertoire size and learning strategies in songbirds. Evol. Ecol. 2016, 30, 285–305. [Google Scholar] [CrossRef]
- Hart, P.J.; Ibanez, T.; Paxton, K.; Tredinnick, G.; Sebastian-Gonzalez, E.; Tanimoto-Johnson, A. Timing is everything: Acoustic niche partitioning in two tropical wet forest bird communities. Front. Ecol. Evol. 2021, 9, 753363. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Concluding remarks. In Cold Spring Harbour Symposium on Quantitative Biology; Cold Spring Harbour Laboratory Press: Cold Spring Harbour, NY, USA, 1957; Volume 22, pp. 415–427. [Google Scholar]
- Burnham, R. Acoustic disturbance, risk estimates and mitigation strategies: An animal-centric approach. In Proceedings for the Effects of Noise on Aquatic Life Conference, Berlin, Germany, 10–15 July 2022; Popper, A., Hawkins, A., Thomsen, F., Eds.; Aquaticnoise: Copenhagen, Denmark, 2023. [Google Scholar]
- Parker, B.C.; Turner, B.L. Operational niches and community interaction values as determined from in vitro studies of some soil algae. Evolution 1961, 15, 228–238. [Google Scholar]
- Bosser, W.H.; Wilson, E.O. The analysis of olfactory communication among animals. Theor. Biol. 1963, 5, 443–469. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.A. Active space of pheromone plume and its relationship to effective attraction radius in applied models. J. Chem. Ecol. 2008, 34, 1134–1145. [Google Scholar] [CrossRef]
- Byers, J. Modeling distributions of flying insects: Effective attraction radius of pheromone in two and three dimensions. J. Theor. Biol. 2009, 256, 81–89. [Google Scholar] [CrossRef]
- Wilson, D.R.; Ratcliffe, L.M.; Mennill, D.J. Black-capped chickadees, Poecile atricapillus, avoid song overlapping: Evidence for the acoustic interference hypothesis. Anim. Behav. 2016, 114, 219–229. [Google Scholar] [CrossRef]
- Cannon, W.B. The Wisdom of the Body; Norton: New York, NY, USA, 1932. [Google Scholar]
- Hofman, V.; Sanguinetti-Scheck, J.I.; Kunzel, S.; Geurten, B.; Gomez-Sena, L.; Engelmann, J. Sensory flow shaped by active sensing: Sensorimotor strategies in electric fish. J. Exp. Biol. 2013, 216, 2487–2500. [Google Scholar] [CrossRef]
- Torres, L.G. A sense of scale: Foraging cetaceans’ use of scale-dependent multimodal sensory systems. Mar. Mamm. Sci. 2017, 33, 1170–1193. [Google Scholar] [CrossRef]
- Schoemann, R.P.; Erbe, C.; Pavan, G.; Righini, R.; Thomas, J.A. Analysis of soundscapes as an ecological tool. In Exploring Animal Behaviour through Sound; Erbe, C., Thomas, J.A., Eds.; Springer: Basel, Switzerland, 2022; Volume 1, pp. 217–267. [Google Scholar]
- Slabbekoorn, H.; Bouton, N. Soundscape orientation: A new field in need of sound investigation. Anim. Behav. 2008, 76, e5–e8. [Google Scholar] [CrossRef]
- Allen, N.A. An Investigation of the Roles of Geomagnetic and Acoustic Cues in Whale Navigation and Orientation. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2013. [Google Scholar]
- Clark, C.W.; Ellison, W.T.; Southall, B.L.; Hatch, L.; Van Parijs, S.M.; Frankel, A.; Ponirakis, D. Acoustic masking in marine ecosystems: Intuitions, analysis, and implication. Mar. Ecol. Prog. Ser. 2009, 395, 201–222. [Google Scholar] [CrossRef]
- Erbe, C.; Reichmuth, C.; Cunningham, K.; Lucke, K.; Dooling, R. Communication masking in marine mammals: A review and research strategy. Mar. Poll. Bull. 2015, 103, 15–38. [Google Scholar] [CrossRef]
- Payne, R.; Webb, D. Orientation by means of long range acoustic signaling in baleen whales. Ann. N. Y. Acad. Sci. 1971, 2317, 110–141. [Google Scholar] [CrossRef]
- Au, W.W.L.; Ford, J.K.B.; Horne, J.K.; Allman, K.A.N. Echolocation signals of free-ranging killer whales (Orcinus orca) and modeling of foraging for chinook salmon (Oncorhynchus tshawytscha). J. Acoust. Soc. Am. 2004, 115, 901–909. [Google Scholar] [CrossRef]
- Vagle, S.; Burnham, R.E.; O’Neill, C.; Yurk, H. Variability in anthropogenic underwater noise due to bathymetry and sound speed characteristics. J. Mar. Sci. Eng. 2021, 9, 1047. [Google Scholar] [CrossRef]
- Burnham, R.E.; Vagle, S.; Thupaki, P.; Thornton, S. Implications of wind and vessel noise on the sound fields experienced by southern resident killer whales Orcinus orca in the Salish Sea. Endanger. Spec. Res. 2023, 50, 31–40. [Google Scholar] [CrossRef]
- Farina, A. Soundscape Ecology, Principles, Patterns, Methods and Applications; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Burnham, R.E. Whale Geography: A species-centred approach applied to migration behaviours. Prog. Phys. Geog. 2020, 44, 419–434. [Google Scholar] [CrossRef]
- Ryan, M. Energy, calling, and selection. Anim. Zool. 1988, 28, 885–898. [Google Scholar] [CrossRef]
- Prestwich, K. The energetics of acoustic signaling in anurans and insects. Anim. Zool. 1994, 34, 625–643. [Google Scholar] [CrossRef]
- Hauser, M. The Evolution of Communication; MIT Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Oberweger, K.; Goller, F. The metabolic cost of birdsong production. J. Exp. Biol. 2001, 204, 3379–3388. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Ophir, A.G. The energetic basis of acoustic communication. Proc. R. Soc. Biol. Sci. 2010, 277, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.D.; Schwartz, J.J. The behavioural ecology of Anuran communication. In Hearing and Sound Communication in Amphibians; Narins, P.M., Feng, A.S., Fay, R.R., Popper, A.N., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 44–86. [Google Scholar]
- Wallschager, D. Correlation of song frequency and body weight in passerine birds. Experientia 1980, 36, 412. [Google Scholar] [CrossRef]
- Ryan, M. Factors influencing the evolution of acoustic communication: Biological constraints. Brain Behav. Evol. 1986, 28, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, K.N.; Brugger, K.E.; Topping, M. Energy and communication in three species of hylid frogs: Power input, power output and efficiency. J. Exp. Biol. 1989, 144, 53–80. [Google Scholar] [CrossRef]
- Sanborn, A. Body temperature and the acoustic behavior of the cicada Tibicen winnemanna (Homoptera: Cicadidae). J. Insect Behav. 1997, 10, 257–264. [Google Scholar] [CrossRef]
- Jacobs, E.R. The Active Space of Sperm Whale Codas. Master’s Thesis, Aarhus University, Aarhus, Denmark, 2019. [Google Scholar]
- Markl, H. Manipulation, modulation, information, cognition: Some of the riddles of communication. Fortschr. Zool. 1985, 31, 163–194. [Google Scholar]
- Maynard-Smith, J.; Harper, D. Animal Signals; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Wiley, R.H. Specificity and multiplicity in the recognition of individuals: Implications for the evolution of social behaviour. Biol. Rev. 2013, 88, 179–195. [Google Scholar] [CrossRef]
- Knörnschild, M.; Jung, K.; Nagy, M.; Metz, M.; Kalko, E. Bat echolocation calls facilitate social communication. Proc. R. Soc. B Biol. Sci. 2012, 279, 4827–4835. [Google Scholar] [CrossRef]
- Barclay, R.M.R. Interindividual use of echolocation calls: Eavesdropping by bats. Behav. Ecol. Sociobiol. 1982, 10, 271–275. [Google Scholar] [CrossRef]
- Fenton, M.B. Communication in the Chiroptera; Indiana University Press: Bloomington, IN, USA, 1985. [Google Scholar]
- Masters, W.M.; Raver, K.A.S.; Kazial, K.A. Sonar signals of big brown bats, Eptesicus fuscus, contain information about individual identity, age and family affiliation. Anim. Behav. 1995, 50, 1243–1260. [Google Scholar] [CrossRef]
- Dusenbery, D.B. Sensory Ecology: How Organisms Acquire and Respond to Information; W. H. Freeman and Company: New York, NY, USA, 1992. [Google Scholar]
- Griffin, D.R. Listening in the Dark: The Acoustic Orientation of Bats and Men; Cornell University Press: New York, NY, USA, 1958. [Google Scholar]
- Kazial, K.A.; Pacheco, S.; Zielinski, K.N. Information Content of Sonar Calls of Little Brown Bats (Myotis lucifugus): Potential for Communication. J. Mammal. 2008, 89, 25–33. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Dobkin, D.S.; Wheye, D. Bird Voices and Vocal Development. In Birds of Stanford Essays; Stanford University Press: Redwood City, CA, USA, 2008. [Google Scholar]
- Marler, P. Characteristics of some animal calls. Nature 1955, 176, 6–8. [Google Scholar] [CrossRef]
- Harcourt, R. Maternal aggression in the South American fur seal in Peru. Can. J. Zool. 1992, 70, 320–325. [Google Scholar] [CrossRef]
- Falk, D. Prelinguistic evolution in early hominins: Whence motherese? Behav. Brain Sci. 2004, 27, 491–541. [Google Scholar] [CrossRef]
- Charrier, I.; Burlet, A.; Aubin, T. Social vocal communication in captive Pacific walruses Odobenus rosmarus divergens. Mamm. Biol. 2011, 76, 622–627. [Google Scholar] [CrossRef]
- Poole, J.H. Behavioral contexts of elephant acoustic communication. In The Amboseli Elephants: A Long-Term Perspective on a Long-Lived Mammal; Moss, C.J., Croze, H., Lee, P.C., Eds.; The University of Chicago: Chicago, IL, USA, 2011; pp. 125–161. [Google Scholar]
- Sauvé, C.C.; Beauplet, G.; Hammill, M.O.; Charrier, I. Acoustic Analysis of Airborne, Underwater, and Amphibious Mother Attraction Calls by Wild Harbor Seal Pups (Phoca vitulina). J. Mamm. 2015, 96, 591–602. [Google Scholar] [CrossRef]
- Lakshminarayanan, K.; Ben Shalom, D.; van Wassenhove, V.; Orbelo, D.; Houde, J.; Poeppel, D. The effect of spectral manipulations on the identification of affective and linguistic prosody. Brain Lang. 2003, 84, 250–263. [Google Scholar] [CrossRef]
- Rickheit, G.; Herrmann, T.; Deutsch, W. Psycholinguistik: Eininternationales Handbuch (Psycholinguistics: An International Handbook); Walter de Gruyter: Berlin, Germany, 2003. [Google Scholar]
- Sidtis, D.; Kreiman, J. In the beginning was the familiar voice: Personally familiar voices in the evolutionary and contemporary biology of communication. Int. Psych. Behav. 2012, 46, 146–159. [Google Scholar] [CrossRef]
- Bignotte-Giro, I.; Fong, A.; Lopez-Iborra, G. Acoustic niche partitioning in five Cuban frogs of the genus Eleutherodactylus. Amphib. Reptil. 2018, 40, 1–11. [Google Scholar] [CrossRef]
- Rukstalis, M.; French, J.A. Vocal buffering of the stress response: Exposure to conspecific vocalizations moderates urinary cortisol excretion in isolated marmosets. Horm. Behav. 2005, 47, 1–7. [Google Scholar] [CrossRef]
- Hennessy, M.B.; Hornschuh, G.; Kaiser, S.; Sachser, N. Cortisol responses and social buffering: A study throughout the life span. Horm. Behav. 2006, 49, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Kilner, R.M.; Noble, D.G.; Davies, N.B. Signals of need in parent-offspring communication and their exploitation by the common cuckoo. Nature 1999, 397, 667–672. [Google Scholar] [CrossRef]
- Magrath, R.D.; Platzen, D.; Kondo, J. From nestling calls to fledgling silence: Adaptive timing of change in response to aerial alarm calls. Proc. R. Soc. Lond. B Biol. Sci. 2006, 273, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Nagasawa, M.; Mogi, K. The importance of mother-infant communication for social bond formation in mammals. Anim. Sci. J. 2012, 83, 446–452. [Google Scholar] [CrossRef]
- Langbauer, W.R., Jr.; Payne, K.; Charif, R.; Rapport, L.; Osborne, F. African elephants respond to distant playback of low-frequency conspecific calls. J. Exp. Biol. 1991, 157, 35–46. [Google Scholar] [CrossRef]
- Langbauer, W.R. Elephant communication. Zoo Biol. 2000, 19, 425–445. [Google Scholar] [CrossRef]
- Payne, K.B.; Thompson, M.; Kramer, L. Elephant calling patterns as indicators of group size and composition: The basis for an acoustic monitoring system. Afr. J. Ecol. 2003, 41, 99–107. [Google Scholar] [CrossRef]
- Garstang, M. Long-distance, low-frequency elephant communication. J. Comp. Physiol. A 2004, 190, 791–805. [Google Scholar] [CrossRef]
- O’Connell-Rodwell, C.E. Keeping an “ear” to the ground: Seismic communication in elephants. Physiology 2007, 22, 287–294. [Google Scholar] [CrossRef]
- Brenowitz, E.A.; Wilczynski, W.; Zakon, H.H. Acoustic communication in spring peepers: Environmental and behavioral aspects. J. Comp. Physiol. A 1984, 155, 585–592. [Google Scholar] [CrossRef]
- Wilczynski, W.; Brenowitz, E.A. Acoustic cues mediate inter-male spacing in a Neotropical frog. Anim. Behav. 1988, 36, 1054–1063. [Google Scholar] [CrossRef]
- Brenowitz, E.A. Neighbor call amplitude influences aggressive behavior and inter-male spacing in choruses of the Pacific treefrog (Hyla regilla). Ethology 1989, 83, 69–79. [Google Scholar] [CrossRef]
- Gerhardt, H.C.; Diekamp, B.; Ptacek, M. Inter-male spacing in choruses of the spring peeper, Pseudacris (Hyla) crucifer. Anim. Behav. 1989, 38, 1012–1024. [Google Scholar] [CrossRef]
- Koyama, N.; Ichino, S.; Nakamichi, M.; Takahata, Y. Long-term changes in dominance ranks among ring-tailed lemurs at Berenty Reserve, Madagascar. Primates 2005, 46, 225–234. [Google Scholar] [CrossRef]
- Shannon, G.; McKenna, M.F.; Angeloni, L.M.; Crooks, K.R.; Fristrup, K.M.; Brown, E.; Warner, K.A.; Nelson, M.D.; White, C.; Briggs, J.; et al. A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. 2016, 91, 982–1005. [Google Scholar] [CrossRef]
- Rossing, T.D. Springer Handbook of Acoustics; Springer: New York, NY, USA, 2007. [Google Scholar]
- Finneran, J.J.; Branstetter, B.K. Effects of Noise on Sound Perception in Marine Mammals. In Animal Communication in Noise; Brumm, H., Ed.; Springer: Heidelberg, Germany, 2013; pp. 273–309. [Google Scholar]
- Sivle, L.D.; Kvadsheim, P.H.; Cure, C.; Isojunno, S.; Wensveen, P.J.; Lam, F.-P.A.; Visser, F.; Kleivane, L.; Tyack, P.; Harris, C.M.; et al. Severity of expert-identified behavioural response of humpback whale, minke whale, and northern bottlenose whale to naval sonar. Aquat. Mamm. 2015, 41, 469–502. [Google Scholar] [CrossRef]
- Ketten, D.R. Structure and function in whale ears. Bioacoustics 1997, 8, 103–136. [Google Scholar] [CrossRef]
- Reidenberg, J.S.; Laitman, J.T. Discovery of a low frequency sound source in mysticeti (baleen whales): Anatomical establishment of a vocal fold homolog. Anat. Rec. 2007, 290, 745–759. [Google Scholar] [CrossRef]
- Cazau, D.; Adam, O.; Laitman, J.T.; Reidenberg, J.S. Understanding the intentional acoustic behavior of humpback whales: A production-based approach. J. Acoust. Soc. Am. 2013, 134, 2268–2273. [Google Scholar] [CrossRef]
- Burnham, R.E. Whale geography: Acoustics, biogeography, and whales. Prog. Phys. Geogr. 2017, 41, 676–685. [Google Scholar] [CrossRef]
- Cranford, T.; Krysl, P. Fin Whale Sound Reception Mechanisms: Skull Vibration Enables Low-Frequency Hearing. PLoS ONE 2015, 10, e0116222. [Google Scholar] [CrossRef] [PubMed]
- Stafford, K.M.; Mellinger, D.K.; Moore, S.E.; Fox, C.G. Seasonal variability and detection range modeling of baleen whale calls in the Gulf of Alaska, 1999–2002. J. Acoust. Soc. Am. 2007, 122, 3378–3390. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.W.; Ellison, W.T. Potential use of low-frequency sounds by baleen whales for probing the environment: Evidence from models and empirical measurements. In Advances in the Study of Echolocation in Bats and Dolphins; Thomas, J.A., Moss, C.F., Vater, M., Eds.; University of Chicago Press: Chicago, IL, USA, 2004; pp. 564–589. [Google Scholar]
- Stafford, K.M.; Fox, C.G.; Clark, D.S. Long-range acoustic detection and localization of blue whale calls in the northeast Pacific Ocean. J. Acoust. Soc. Am. 1998, 104, 3616–3625. [Google Scholar] [CrossRef] [PubMed]
- Tyack, P.L.; Clark, C.W. Communication and acoustical behavior in dolphins and whales. In Hearing by Whales and Dolphins: Springer Handbook of Auditory Research; Au, W.W.L., Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 2000; pp. 156–224. [Google Scholar]
- Richardson, W.J.; Greene, C.R., Jr.; Malme, C.I.; Thomson, D. Marine Mammals and Noise; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Jasny, M. Sounding the Depths II: The Rising Toll of Sonar, Shipping and Industrial Ocean Noise on Marine Life; Natural Resource Defense Council: New York, NY, USA, 2005. [Google Scholar]
- Sehgal, A.; Tumar, I.; Schönwälder, J. Effects of Climate Change and Anthropogenic Ocean Acidification on Underwater Acoustic Communications. In Proceedings of the OCEANS’10 Asia-Pacific IEEE Sydney, Sydney, Australia, 24–27 May 2010; IEEE: Piscataway, NJ, USA, 2010. [Google Scholar]
- Ilyina, T.; Zeebe, R.; Brewer, P. Future ocean increasingly transparent to low-frequency sound owing to carbon dioxide emission. Nat. Geosci. Lett. 2009, 3, 18–22. [Google Scholar] [CrossRef]
- Etter, P.C. Advanced applications for underwater acoustic modeling. Adv. Acoust. Vib. 2012, 2012, 214839. [Google Scholar] [CrossRef]
- Burnham, R.; Vagle, S. Potential interference of communication and echolocation of southern resident killer whales resulting from soundscape modification with implications on foraging behaviours. In Proceedings of the Effects of Noise on Aquatic Life Conference, Berlin, Germany, 10–15 July 2022; Aquaticnoise: Copenhagen, Denmark, 2023. [Google Scholar]
- Tervo, O.M.; Christoffersen, M.; Simon, M.; Miller, L.A.; Jensen, F.H.; Parks, S.E.; Madsen, P.T. High Source Levels and Small Active Space of High-Pitched Song in Bowhead Whales (Balaena mysticetus). PLoS ONE 2012, 7, e52072. [Google Scholar] [CrossRef]
- Northrop, J.; Cummings, W.C.; Thompson, P.O. 20 Hz signals observed in the central Pacific. J. Acoust. Soc. Am. 1968, 43, 383–384. [Google Scholar] [CrossRef]
- Spiesberger, J.L.; Fristrup, K.M. Passive localization of calling animals and sensing of their acoustic environment using acoustic tomography. Am. Nat. 1990, 135, 107–153. [Google Scholar] [CrossRef]
- Mellinger, D.K.; Clark, C.W. Methods for automatic detection of Mysticete sounds. Mar. Freshw. Behav. Physiol. 1997, 29, 163–181. [Google Scholar] [CrossRef]
- Watkins, W.A.; Daher, M.A.; Reppucci, G.M.; George, J.E.; Martine, D.L.; DiMarzio, N.A.; Gannon, D.A. Seasonality and distribution of whale calls in the North Pacific. Oceanography 2000, 13, 62–67. [Google Scholar] [CrossRef]
- Tyack, P.L.; Janik, V.M. Effects of noise on acoustic signal production in marine mammals. In Animal Communication in Noise; Brumm, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 249–271. [Google Scholar]
- Au, W.W.L. The Sonar of Dolphins; Springer: New York, NY, USA, 1993. [Google Scholar]
- Johnson, M.; Madsen, P.T.; Zimmer, W.M.X.; Aguilar de Soto, N.; Tyack, P.L. Beaked whales echolocate on prey. Proc. Biol. Sci. R. Soc. Lond. B 2004, 271, S383–S386. [Google Scholar] [CrossRef]
- Morrissey, R.P.; Ward, J.; DiMarzio, N.; Jarvis, S.; Moretti, D.J. Passive acoustic detection and localization of sperm whale (Physeter macrocephalus) in the tongue of the ocean. App. Acoust. 2006, 67, 1091–1105. [Google Scholar] [CrossRef]
- Tyack, P.L. Studying how cetaceans use sound to explore their environment. Persp. Ethol. 1997, 12, 251–297. [Google Scholar]
- Madsen, P.T.; Johnson, M.; Aguilar de Soto, N.; Zimmer, W.M.X.; Tyack, P. Biosonar performance of foraging beaked whales (Mesoplodon densirostris). J. Exp. Biol. 2005, 208, 108–191. [Google Scholar] [CrossRef]
- Zimmer, W.M.X.; Tyack, P.L.; Johnson, P.L.; Johnson, M.P.; Madsen, P.T. Three-dimensional beam pattern of regular sperm whale clicks confirms bent-horn hypothesis. J. Acoust. Soc. Am. 2005, 117, 1473–1485. [Google Scholar] [CrossRef]
- Madsen, P.T.; Wilson, M.; Johnson, M.; Hanlon, R.T.; Bocconcelli, A.; Aguilar de Soto, N.; Tyack, P. Clicking for calamari: Toothed whales can echolocate squid Loligo pealeii. Aquat. Biol. 2007, 1, 141–150. [Google Scholar] [CrossRef]
- Lima, S.L.; Zollner, P.A. Anti-predatory vigilance and the limits to collective detection: Visual and spatial separation between foragers. Behav. Ecol. Sociobiol. 1996, 38, 355–363. [Google Scholar] [CrossRef]
- Marten, K.; Marler, P. Sound transmission and its significance for animal vocalization. Behav. Ecol. Sociobiol. 1977, 2, 271–290. [Google Scholar] [CrossRef]
- Morisaka, T.; Connor, R.C. Predation by killer whales (Orcinus orca) and the evolution of whistle loss and narrow-band high frequency clicks in odontocetes. J. Evol. Biol. 2007, 20, 1439–1458. [Google Scholar] [CrossRef]
- Dooling, R.J.; Blumenrath, S.H. Avian sound perception in noise. In Animal Communication in Noise; Brumm, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 229–250. [Google Scholar]
- Janik, V.M. Source levels and the estimated active space of bottlenose dolphin (Tursiops truncatus) whistles in the Moray Firth, Scotland. J. Comp. Physiol. A 2000, 186, 673–680. [Google Scholar] [CrossRef]
- Urick, R.J. Principles of Underwater Sound, 3rd ed.; McGraw Hill: New York, NY, USA, 1983. [Google Scholar]
- Edds-Walton, P.L. Acoustic Communication Signals of Mysticete Whales. Bioacoustics 1997, 8, 47–60. [Google Scholar] [CrossRef]
- Oleson, E.M.; Calambokidis, J.; Burgess, W.C.; McDonald, M.A.; LeDuc, C.A.; Hildebrand, J.A. Behavioral context of call production by eastern North Pacific blue whales. Mar. Ecol. Prog. Ser. 2007, 330, 269–284. [Google Scholar] [CrossRef]
- Oleson, E.; Wiggins, S.; Hildebrand, J. Temporal separation of blue whale call types on a southern California feeding ground. Anim. Behav. 2007, 74, 881–894. [Google Scholar] [CrossRef]
- Rendell, L.E.; Whitehead, H. Vocal clans in sperm whales (Physeter macrocephalus). Proc. R. Soc. B Biol. Sci. 2003, 270, 225–231. [Google Scholar] [CrossRef]
- Bejder, L.; Samuels, A.; Whitehead, H.; Finn, H.; Allen, S. Impact assessment research: Use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar. Ecol. Prog. Ser. 2009, 395, 177–185. [Google Scholar] [CrossRef]
- Holt, M.M.; Noren, D.P.; Emmons, C.K. Effects of noise levels and call types on the source levels of killer whale calls. J. Acoust. Soc. Am. 2011, 130, 3100–3106. [Google Scholar] [CrossRef]
- Thomsen, F.; McCully, S.R.; Weiss, L.; Wood, D.; Warr, K.; Law, R. Cetacean stock assessment in relation to exploration and production industry activity and other human pressures: Review and data needs. Aquat. Mamm. 2011, 37, 1–92. [Google Scholar] [CrossRef]
- Veirs, S.; Veirs, V.; Wood, J. Ship noise in an urban estuary extends to frequencies used for echolocation by endangered killer whales. PeerJ PrePrints 2016, 3, e955v26. [Google Scholar]
- Ellison, W.T.; Southall, B.L.; Clark, C.W.; Frankel, A.S. A new context-based approach to assess marine mammal behavioral responses to anthropogenic sounds. Conserv. Biol. 2012, 26, 21–28. [Google Scholar] [CrossRef]
- Hatch, L.T.; Clark, C.W.; Van Parijs, S.; Frankel, A.S.; Ponirakis, D.W. Quantifying loss of acoustic communication space for right whales in and around a U.S. National Marine Sanctuary. Conserv. Biol. 2012, 26, 983–994. [Google Scholar] [CrossRef]
- Siemers, B.; Schaub, A. Hunting at the highway: Traffic noise reduces foraging efficiency in acoustic predators. Proc. R. Soc. B Biol. Sci. 2011, 278, 1646–1652. [Google Scholar] [CrossRef]
- Francis, C.; Barber, J. A framework for understanding noise impacts on wildlife: An urgent conservation priority. Front. Ecol. Environ. 2013, 11, 305–313. [Google Scholar] [CrossRef]
- Slabbekoorn, H. Habitat-dependent ambient noise: Consistent spectral profiles in two African forest types. J. Acoust. Soc. Am. 2004, 116, 3727–3733. [Google Scholar] [CrossRef]
- Johnson, M.; Tyack, P. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Ocean Eng. 2003, 28, 3–12. [Google Scholar] [CrossRef]
- Weilgart, L.S. A brief review of known effects of noise on marine mammals. Int. J. Comp. Psych. 2007, 20, 159–168. [Google Scholar] [CrossRef]
- International Whaling Commission (IWC). Report of the Scientific Committee. Annex K. Report of the Standing Working Group on Environmental Concerns. J. Cetacean Res. Manag. 2005, 7, 267–305. [Google Scholar]
- Ross, D. Mechanics of Underwater Noise; Pergamon Press: New York, NY, USA, 1976; p. 120. [Google Scholar]
- Gray, L.M.; Greeley, D.S. Source level model for propeller blade rate radiation for the world’s merchant fleet. J. Acoust. Soc. Am. 1980, 67, 516. [Google Scholar] [CrossRef]
- Arveson, P.T.; Vendittis, D.J. Radiated noise characteristics of a modern cargo ship. J. Acoust. Soc. Am. 2000, 107, 118–129. [Google Scholar] [CrossRef]
- Notarbartolo di Sciara, G.; Gordon, J. Bioacoustics: A tool for the conservation of cetaceans in the Mediterranean Sea. Mar. Freshw. Behav. Physiol. 1997, 30, 125–146. [Google Scholar] [CrossRef]
- Rolland, R.M.; Parks, S.E.; Hunt, K.E.; Castellote, M.; Corkeron, P.J.; Nowacek, D.P.; Wasser, S.K.; Kraus, S.D. Evidence that ship noise increases stress in right whales. Proc. R. Soc. B 2012, 279, 2363–2368. [Google Scholar] [CrossRef]
- Blane, J.M.; Jaakson, R. The impact of ecotourism boats on the St. Lawrence beluga whales. Environ. Conserv. 1996, 21, 267–269. [Google Scholar] [CrossRef]
- Bejder, L.; Dawson, S.M.; Harraway, J.A. Responses by Hector’s dolphins to boats and swimmers in Porpoise Bay, New Zealand. Mar. Mamm. Sci. 1999, 15, 738–750. [Google Scholar] [CrossRef]
- Au, W.W.L.; Green, M. Acoustic interaction of humpback whales and whale-watching boats. Mar. Environ. Res. 2000, 49, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Trites, A.W.; Bain, D.E. Behavioural responses of killer whales (Orcinus orca) to whale-watching boats: Opportunistic observations and experimental approaches. J. Zool. 2002, 256, 255–270. [Google Scholar] [CrossRef]
- Nowacek, D.P.; Thorne, L.H.; Johnston, D.W.; Tyack, P. Response of cetaceans to anthropogenic noise. Mamm. Rev. 2007, 37, 81–115. [Google Scholar] [CrossRef]
- Pirotta, E.; Thompson, P.M.; Cheney, B.; Donovan, C.R.; Lusseau, D. Estimating spatial, temporal and individual variability in dolphin cumulative exposure to boat traffic using spatially explicit capture-recapture methods. Anim. Conserv. 2014, 18, 20–31. [Google Scholar] [CrossRef]
- Corkeron, P.J. Humpback whales (Megaptera novaeangliae) in Hervey Bay, Queensland—Behavior and responses to whale-watching vessels. Can. J. Zool. 1995, 73, 1290–1299. [Google Scholar] [CrossRef]
- Ollervides, F.J. Gray Whales and Boat Traffic: Movement, Vocal, and Behavioral Responses in Bahia Magdalena, Mexico. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2001. [Google Scholar]
- Christiansen, F.; Lusseau, D.; Stensland, E.; Berggren, P. Effects of tourist boats on the behaviour of Indo-Pacific bottlenose dolphins off the south coast of Zanzibar. Endanger. Spec. Res. 2010, 11, 91–99. [Google Scholar] [CrossRef]
- Stamation, K.; Croft, D.; Shaughnessey, P.D.; Waples, K.A.; Briggs, S.V. Behavioral responses of humpback whales (Megaptera novaeangliae) to whale-watching vessels on the southeastern coast of Australia. Mar. Mamm. Sci. 2010, 26, 98–122. [Google Scholar] [CrossRef]
- Matsuda, N.; Shirakihara, M.; Shirakihara, K. Effects of dolphin-watching boats on the behavior of Indo-Pacific bottlenose dolphins off Amakusa-Shimoshima Island, Japan. Nippon Suisan Gakkaishi 2011, 77, 8–14. [Google Scholar] [CrossRef]
- Visser, F.; Hartman, K.; Rood, E.J.J.; Hendricks, A.J.E.; Zult, D.B.; Wolff, W.J. Risso’s dolphins alter daily resting pattern in response to whale watching at the Azores. Mar. Mamm. Sci. 2011, 27, 366–381. [Google Scholar] [CrossRef]
- Bejder, L.; Samuels, A.; Whitehead, H.; Gales, N. Interpreting short-term behavioural responses to disturbance within a longitudinal perspective. Anim. Behav. 2006, 72, 1149–1158. [Google Scholar] [CrossRef]
- Arcangeli, A.; Crosti, R. The short-term impact of dolphin-watching on the behavior of bottlenose dolphins (Tursiops truncatus) in Western Australia. J. Mar. Anim. Ecol. 2009, 2, 3–9. [Google Scholar]
- Parks, S.E.; Clark, C.W.; Tyack, P.L. Short and long-term changes in right whale calling behaviour: The potential effects of noise on acoustic communication. J. Acoust. Soc. Am. 2007, 122, 3725–3731. [Google Scholar] [CrossRef]
- Holt, M.M.; Noren, D.P.; Veirs, V.; Emmons, C.K.; Veirs, S. Killer whales (Orcinus orca) increase their call amplitude in response to vessel noise. J. Acoust. Soc. Am. 2009, 125, EL27–EL32. [Google Scholar] [CrossRef]
- Slabbekoorn, H.; Bouton, N.; van Opzeeland, I.; Coers, A.; ten Cate, C.; Popper, A.N. A noisy spring: The impact of globally rising underwater sound levels on fish. Trends Ecol. Evol. 2010, 25, 419–427. [Google Scholar] [CrossRef]
- Wale, M.A.; Simpson, S.D.; Radford, A.N. Noise negatively affects foraging and antipredator behaviour in shore crabs. Anim. Behav. 2013, 86, 111–118. [Google Scholar] [CrossRef]
- Williams, R.; Clark, C.W.; Ponirakis, D.; Ashe, E. Acoustic quality of critical habitats for three threatened whale populations. Anim. Conserv. 2014, 17, 174–185. [Google Scholar] [CrossRef]
- Malme, C.I.; Miles, P.R.; Clark, C.W.; Tyack, P.; Bird, J.E. Investigations of the Potential Effects of Underwater Noise from Petroleum Industry Activities on Migrating Gray Whale Behavior; BBN Report No. 5366; NTIS PB86-174174; Bolt Beranek and Newman Inc. for US Minerals Management Service: Anchorage, AK, USA, 1983. [Google Scholar]
- Malme, C.I.; Miles, P.R.; Clark, C.W.; Tyack, P.; Bird, J.E. Investigations of the Potential Effects of Underwater Noise from Petroleum Industry Activities on Migrating Gray Whale Behavior. Phase II: Migration; BBN Report No. 5586; NTIS PB86-218377; Bolt Beranek and Newman Inc. for US Minerals Management Service: Anchorage, AK, USA, 1984. [Google Scholar]
- Malme, C.I.; Würsig, B.; Bird, J.E.; Tyack, P. Behavioral Responses of Gray Whales to Industrial Noise: Feeding Observations and Predictive Modeling; Outer Continental Shelf Environmental Assessment Program, Final Report of Principal Investigators; NOAA No. PB-88-249057/XAB; BBN Labs: Cambridge, MA, USA, 1986. [Google Scholar]
- Malme, C.I.; Würsig, B.; Bird, J.E.; Tyack, P. Observations of feeding gray whale responses to controlled industrial noise exposure. In Port and Ocean Engineering Under Arctic Conditions; Sackinger, W.M., Jeffries, M.O., Imm, J.L., Treacy, S.D., Eds.; University of Alaska, Geophysical Institute: Fairbanks, AL, USA, 1988; Volume II, pp. 55–73. [Google Scholar]
- Malme, C.I.; Miles, P.R.; Miller, G.W.; Richardson, W.J.; Roseneau, D.G.; Thomson, D.H.; Greene, C.R., Jr. Analysis and Ranking of the Acoustic Disturbance Potential of Petroleum Industry Activities and Other Sources of Noise in the Environment of Marine Mammals in Alaska; OCS Study MMS 89-0006; Report No. 6945 prepared for U.S. Minerals Management Service; Alaska OCS Region by BBN Systems and Technologies Corp.: Washington, DC, USA, 1989. [Google Scholar]
- Bryant, P.J.; Lafferty, C.M.; Lafferty, S.K. Reoccupation of Laguna Guerrero Negro, Baja California, Mexico, by gray whales. In The Gray Whale Eschrichtius robustus; Jones, M.L., Swartz, S.L., Leatherwood, S., Eds.; Academic Press: New York, NY, USA, 1984; pp. 375–387. [Google Scholar]
- Dahlheim, M.E.; Fisher, H.D.; Schempp, J.D. Sound production by the gray whale and ambient noise levels in Laguna San Ignacio, Baja California Sur, Mexico. In The Gray Whale, Eschrichtius robustus; Jones, M.L., Swartz, S., Leatherwood, S., Eds.; Academic Press: Orlando, FL, USA, 1984; pp. 511–541. [Google Scholar]
- Dahlheim, M.E. Bio-Acoustics of the Gray Whale (Eschrichtius robustus). Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1987. [Google Scholar]
- Jones, M.L.; Swartz, S.L.; Dahlheim, M.E. Census of Gray Whale Abundance in San Ignacio Lagoon: A Follow-Up Study in Response to Low Whale Counts Recorded during an Acoustic Playback Study of Noise Effects on Gray Whales; Report No. NTIS PB94195062; US Marine Mammal Commission: Washington, DC, USA, 1994. [Google Scholar]
- Ollervides, F. Effects of Boat Traffic on the Behavior of Gray Whales, Eschrichtius robustus, in Bahia Magdalena, Baja California Sur, Mexico: A Bioacoustic Assessment. Master’s Thesis, Texas A&M University, College Station, TX, USA, 1997. [Google Scholar]
- Würsig, B.; Weller, D.W.; Burdin, A.M.; Blokhin, S.A.; Reeve, S.Y.; Bradford, A.L.; Brownell, R.L., Jr. Gray Whales Summering off Sakhalin Island, Far East Russia: July–October 1997: A Joint U.S.–Russian Scientific Investigation; Report by Texas A&M University and Kamchatka Institute of Ecology and Nature Management; Sakhalin Energy Investment Company and Exxon Neftegas Limited: Yuzhno-Sakhalinsk, Russia, 1999; 101p. [Google Scholar]
- Moore, S.; Clarke, J. Potential impact of offshore human activities on gray whales (Eschrichtius robustus). Fish. Sci. 2002, 4, 19–25. [Google Scholar]
- Beale, C.M.; Monaghan, P. Human disturbance: People as predation-free predators? J. Appl. Ecol. 2004, 41, 335–343. [Google Scholar] [CrossRef]
- Beale, C.M.; Monaghan, P. Behavioural responses to human disturbance: A matter of choice? Anim. Behav. 2004, 68, 1065–1069. [Google Scholar] [CrossRef]
- Christiansen, F.; Lusseau, D. Understanding the ecological effects of whale-watching on cetaceans. In Whale-Watching: Sustainable Tourism and Ecological Management; Higham, J., Bejder, L., Williams, R., Eds.; Cambridge University Press: New York, NY, USA, 2014; pp. 177–192. [Google Scholar]
- Senigaglia, V.; Christiansen, F.; Bejder, L.; Gendron, D.; Lundquist, D.; Noren, D.; Schaffer, A.; Smith, J.C.; Williams, R.; Martinez, E.; et al. Meta-analyses of whale-watching impact studies: Comparisons of cetacean responses to disturbance. Mar. Ecol. Prog. Ser. 2016, 542, 251–263. [Google Scholar] [CrossRef]
- Berrow, S.D.; Holmes, B. Tour boats and dolphins: A note on quantifying the activities of whale watching boats in the Shannon estuary, Ireland. J. Cetacean Res. Manag. 1999, 1, 199–204. [Google Scholar] [CrossRef]
- Constantine, R.; Baker, C. Monitoring the Commercial Swim-with-Dolphins Operation in the Bay of Island; Department of Conservation: Wellington, New Zealand, 1997. [Google Scholar]
- Heckel, G.; Reilly, S.B.; Sumich, J.L.; Espejel, I. The influence of whale watching on the behaviour of migrating gray whales (Eschrichtius robustus) in Todos Santos Bay and surrounding waters, Baja California, Mexico. J. Cetacean Res. Manag. 2001, 3, 227–237. [Google Scholar]
- Moore, S.; Stafford, K.; Mellinger, D.; Hildebrand, J.A. Listening for large whales in the offshore waters of Alaska. Biol. Toolbox 2006, 56, 49–55. [Google Scholar] [CrossRef]
- Blackwell, S.B.; Nations, C.S.; McDonald, T.L.; Greene, C.R.; Thode, A.M.; Guerra, M.; Macrander, A.M. Effects of airgun sounds on bowhead whale calling rates in the Alaskan Beaufort Sea. Mar. Mamm. Sci. 2013, 29, 342–365. [Google Scholar] [CrossRef]
- Holles, S.; Simpson, S.D.; Radford, A.N.; Berten, L.; Lecchini, D. Boat noise disrupts orientation behaviour in a coral reef fish. Mar. Ecol. Prog. Ser. 2013, 485, 295–300. [Google Scholar] [CrossRef]
- Voellmy, I.K.; Purser, J.; Flynn, D.; Kennedy, P.; Simpson, S.D.; Radford, A.N. Acoustic noise reduces foraging success in two sympatric fish species via different mechanisms. Anim. Behav. 2014, 89, 191–198. [Google Scholar] [CrossRef]
- Bradbury, J.W.; Vehrencamp, S.L. Principle of Animal Communication; Sinauer Associates: Sunderland, MA, USA, 1998. [Google Scholar]
- Velez, A.; Schwartz, J.J.; Bee, M.A. Anuran acoustic signal perception in noisy environments. In Animal Communication in Noise; Brumm, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 133–186. [Google Scholar]
- Wiens, J.A. Population responses to patchy environments. Annu. Rev. Ecol. Syst. 1976, 7, 81–120. [Google Scholar] [CrossRef]
- Erbe, C.; MacGillivray, A.; Williams, R. Mapping cumulative noise from shipping to inform marine spatial planning. J. Acoust. Soc. Am. 2012, 132, EL423–EL428. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration (NOAA). Guidance for Assessing the Effects of Anthropogenic Sound on Marine Mammals—Acoustic Threshold Levels for Onset of Permanent and Temporary Threshold Shifts; National Oceanic and Atmospheric Administration (NOAA): Washington, DC, USA, 2013. [Google Scholar]
- Merchant, N.D.; Faulkner, R.C.; Martinez, R. Marine noise budgets in practice. Conserv. Lett. 2017, 11, e12420. [Google Scholar] [CrossRef]
- Levin, P.S.; Fogarty, M.J.; Murawski, S.A.; Fluharty, D. Integrated Ecosystem Assessments: Developing the Scientific Basis for Ecosystem-Based Management of the Ocean. PLoS Biol. 2009, 7, e1000014. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burnham, R.; Duffus, D. Implication of Altered Acoustic Active Space for Cetacean Species That Result from Soundscape Changes and Noise Additions. Acoustics 2023, 5, 444-461. https://doi.org/10.3390/acoustics5020026
Burnham R, Duffus D. Implication of Altered Acoustic Active Space for Cetacean Species That Result from Soundscape Changes and Noise Additions. Acoustics. 2023; 5(2):444-461. https://doi.org/10.3390/acoustics5020026
Chicago/Turabian StyleBurnham, Rianna, and David Duffus. 2023. "Implication of Altered Acoustic Active Space for Cetacean Species That Result from Soundscape Changes and Noise Additions" Acoustics 5, no. 2: 444-461. https://doi.org/10.3390/acoustics5020026
APA StyleBurnham, R., & Duffus, D. (2023). Implication of Altered Acoustic Active Space for Cetacean Species That Result from Soundscape Changes and Noise Additions. Acoustics, 5(2), 444-461. https://doi.org/10.3390/acoustics5020026




