Global Dynamics of Research on Antibiotic Resistance in Helicobacter pylori: A Bibliometric Analysis
Abstract
1. Introduction
2. Results
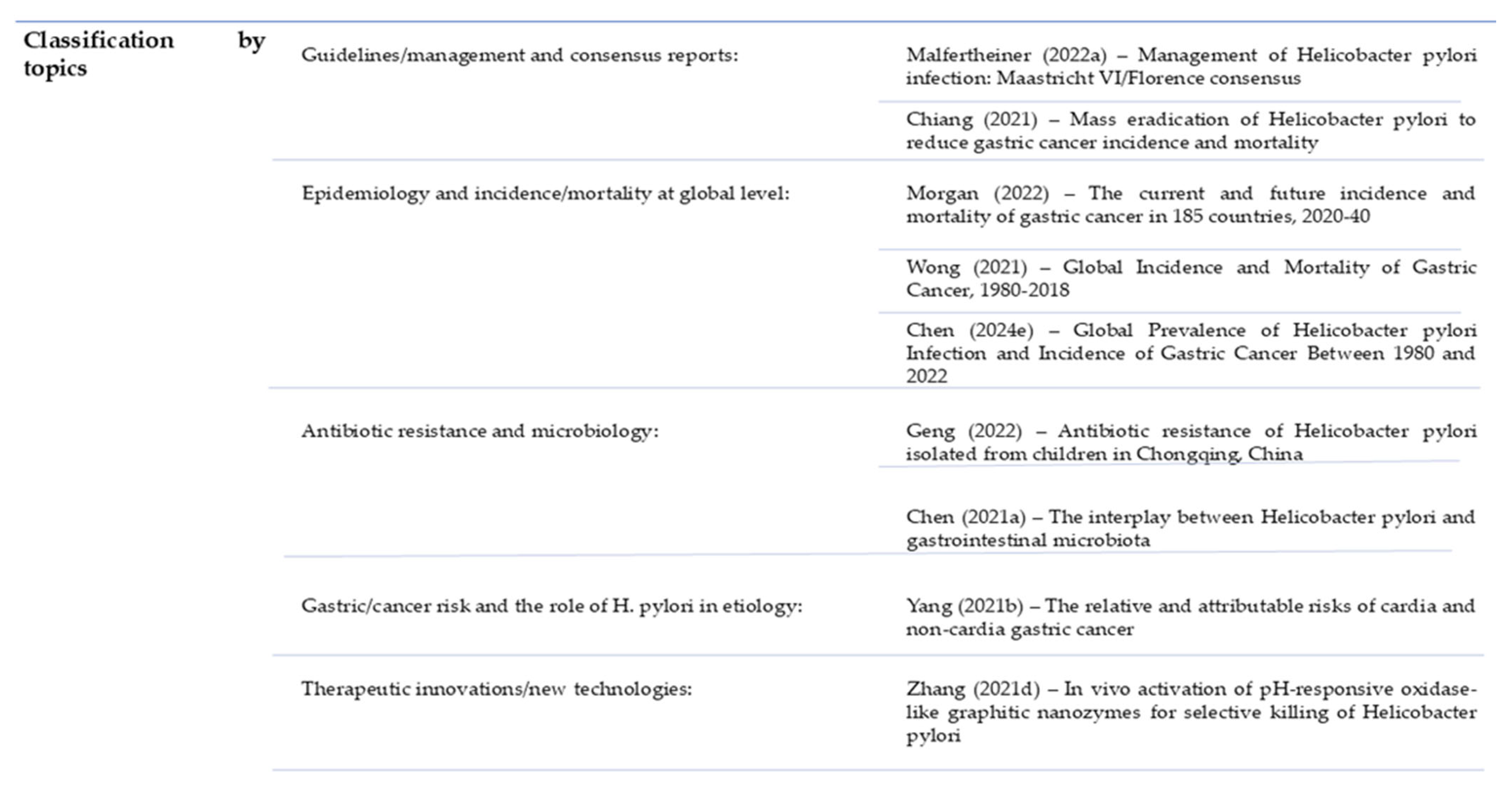
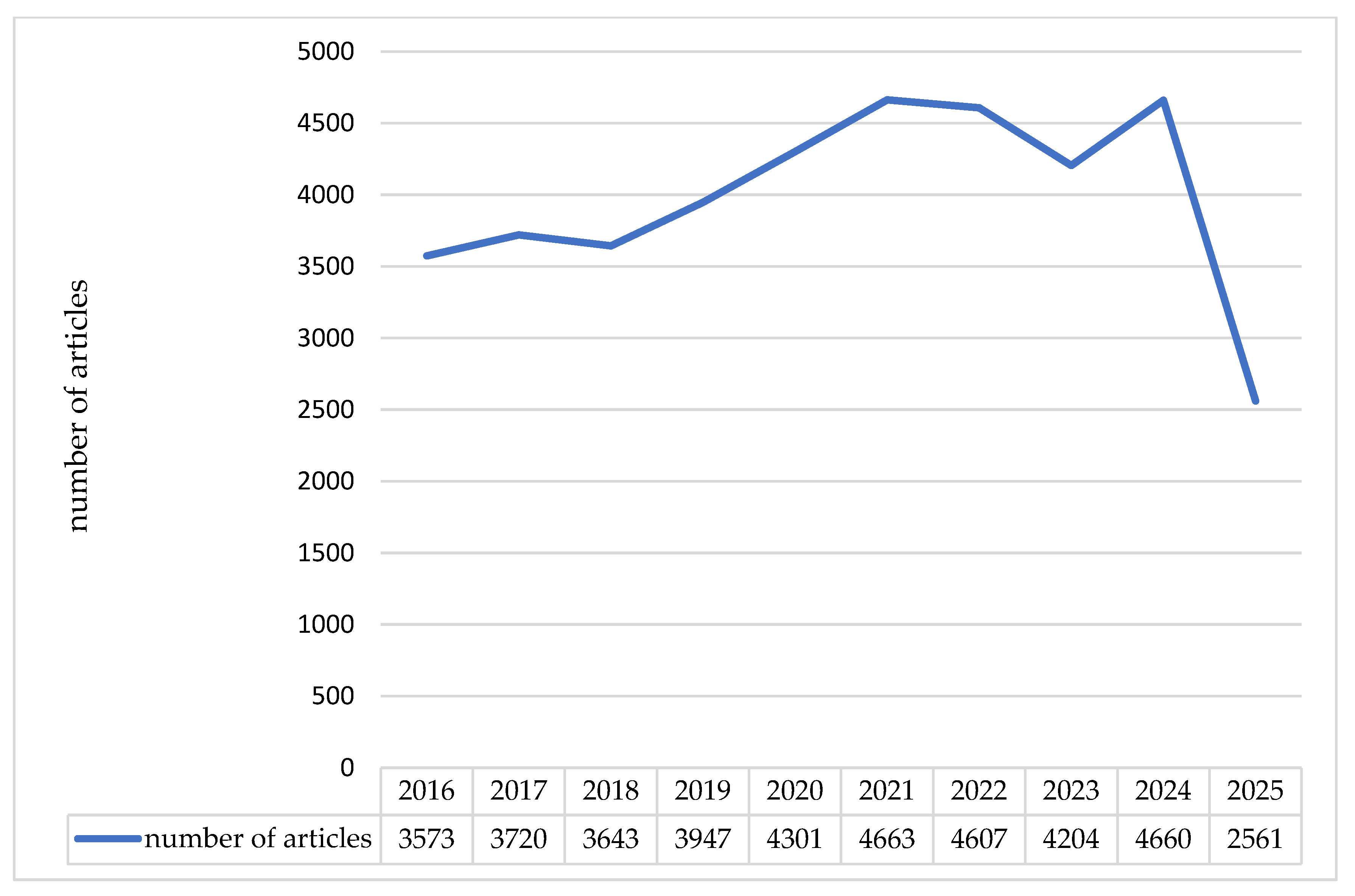
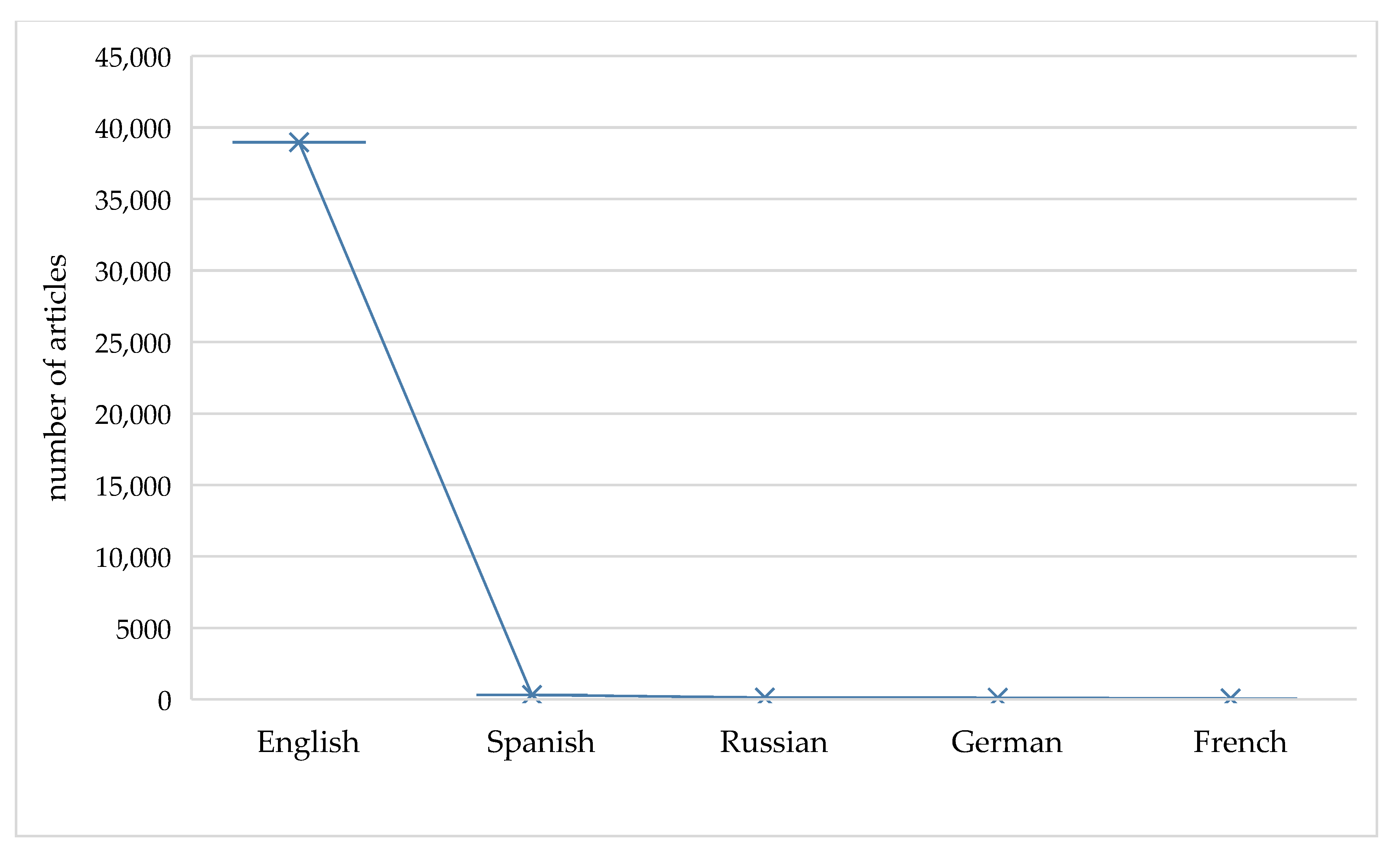
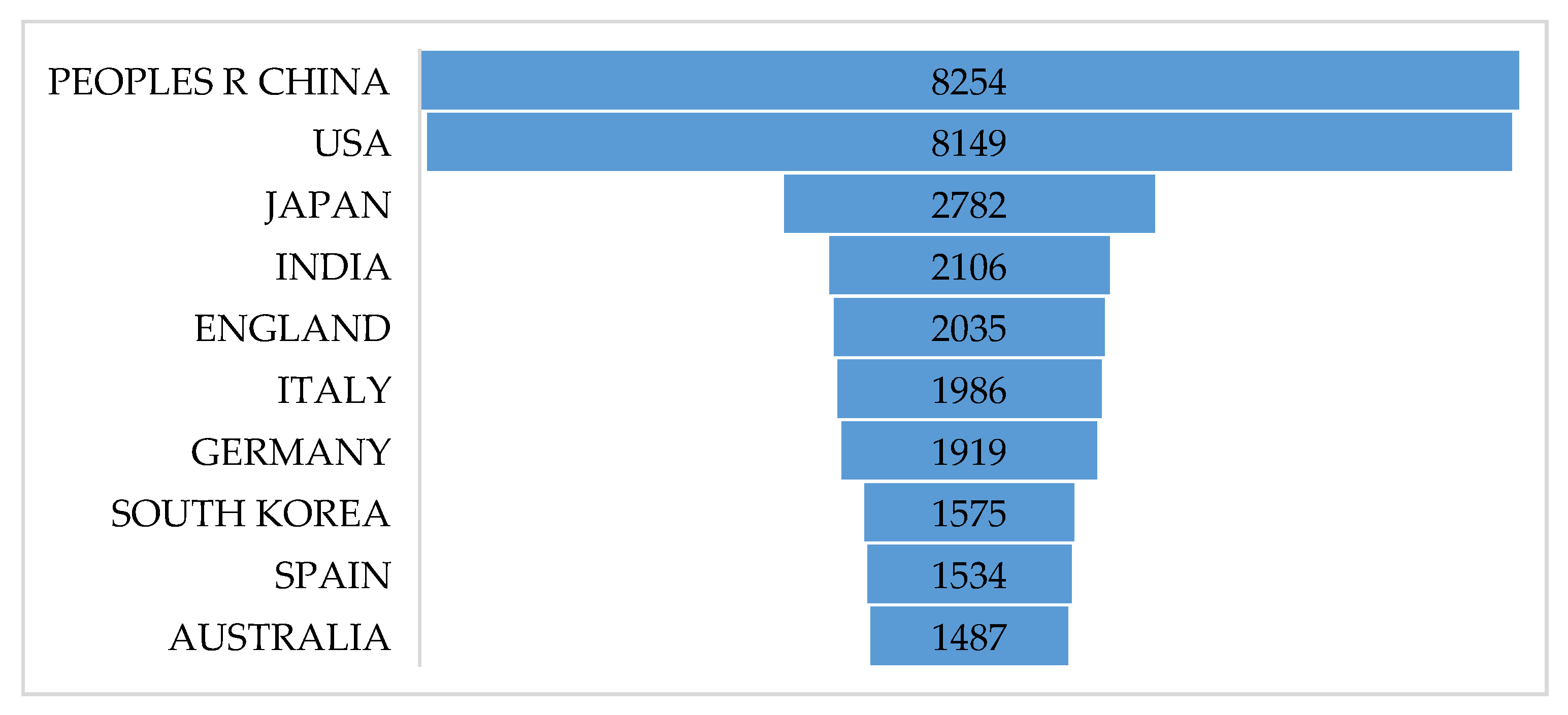
2.1. Literature Overview
2.2. Document Citation Analysis
2.3. Top Cited Authors
2.4. Keyword Analysis
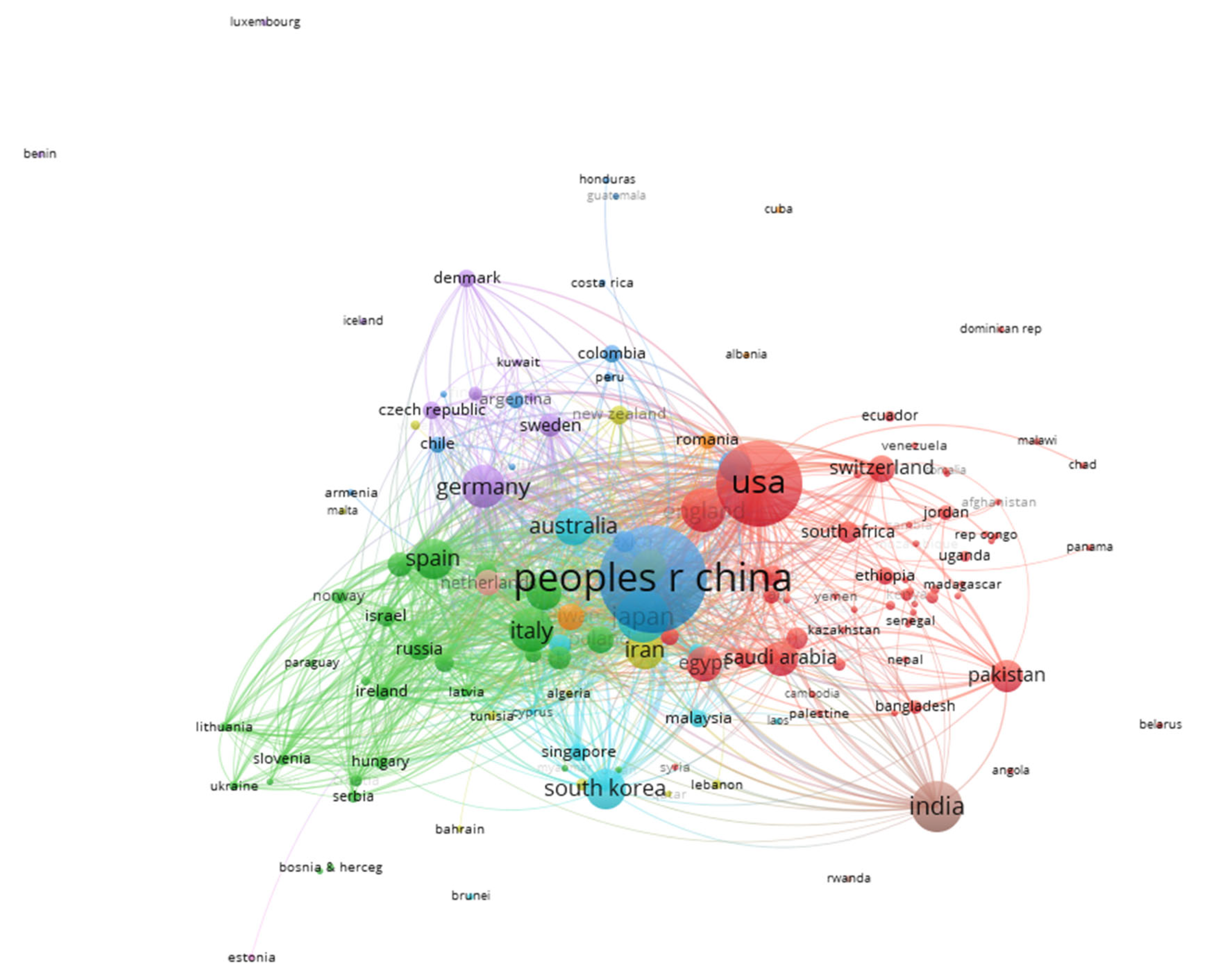
2.5. Scientific Mapping
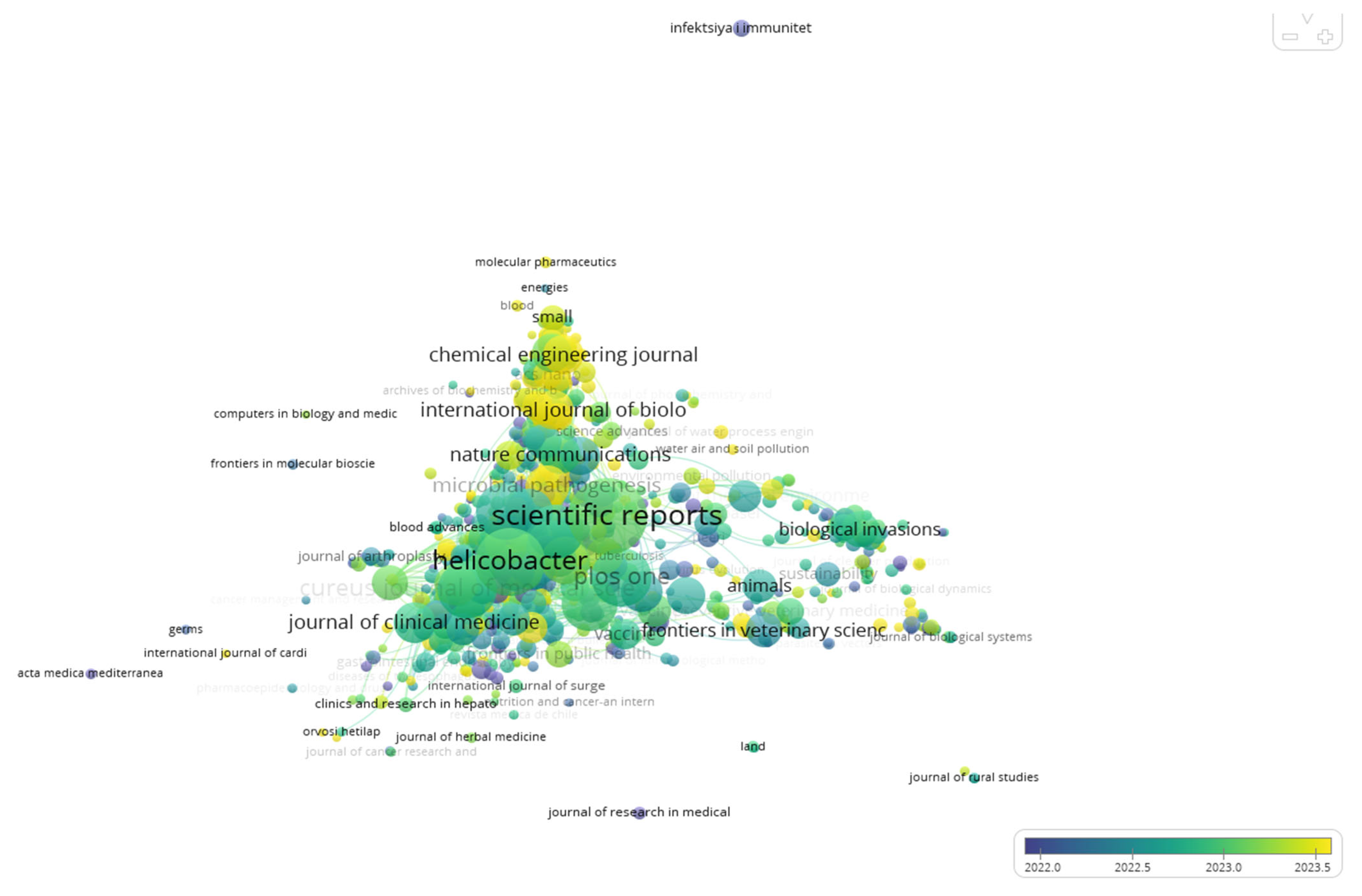
2.6. Distribution by Journals
3. Discussion
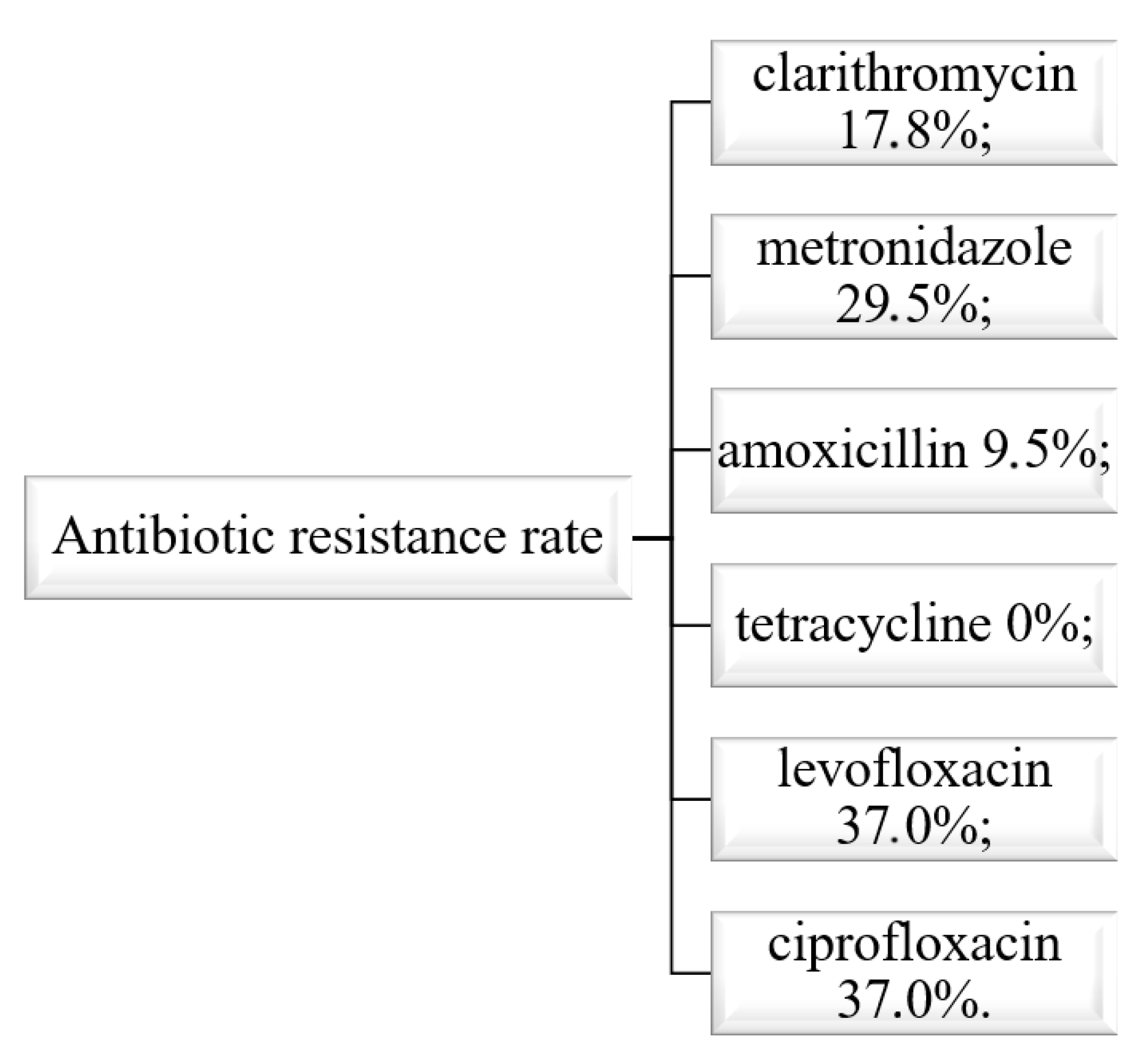
3.1. The Situation in Romania
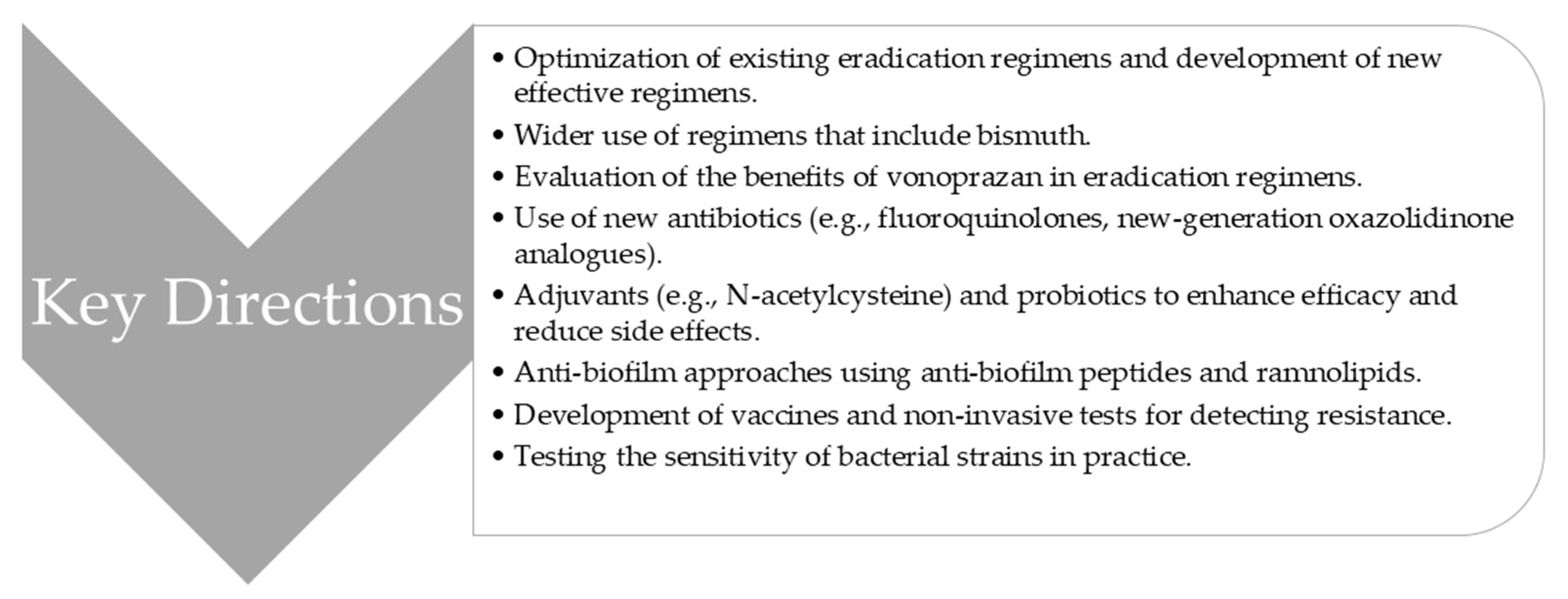
3.2. Future Directions
3.3. Strengths and Limitations of the Study
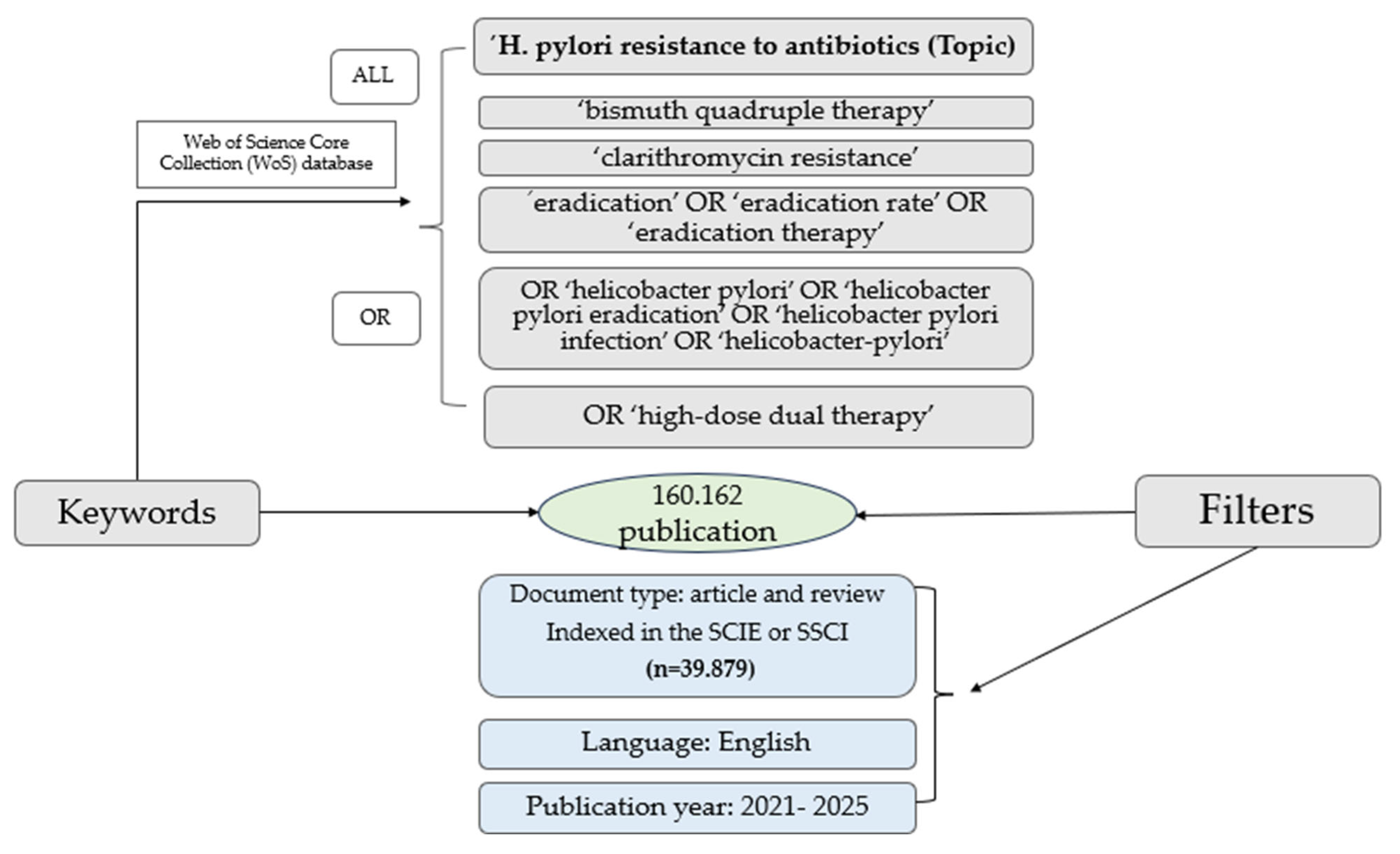
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotilea, K.; Bontems, P.; Touati, E. Epidemiology, Diagnosis and Risk Factors of Helicobacter pylori Infection. Adv. Exp. Med. Biol. 2019, 1149, 17–33. [Google Scholar] [CrossRef]
- Chey, W.D.; Howden, C.W.; Moss, S.F.; Morgan, D.R.; Greer, K.B.; Grover, S.; Shah, S.C. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2024, 119, 1730–1753. [Google Scholar] [CrossRef]
- Violeta Filip, P.; Cuciureanu, D.; Sorina Diaconu, L.; Maria Vladareanu, A.; Silvia Pop, C. MALT lymphoma: Epidemiology, clinical diagnosis and treatment. J. Med. Life 2018, 11, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ailloud, F.; Estibariz, I.; Suerbaum, S. Evolved to vary: Genome and epigenome variation in the human pathogen Helicobacter pylori. FEMS Microbiol. Rev. 2021, 45, fuaa042. [Google Scholar] [CrossRef] [PubMed]
- Aktas, D.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S.; Safarov, T.; Kocazeybek, B.S. Resuscitation of the Helicobacter pylori Coccoid Forms by Resuscitation Promoter Factor Obtained from Micrococcus Luteus. Curr. Microbiol. 2020, 77, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, Y.; Zhang, X.; Fu, B.; Song, Z.; Wang, L.; Fu, R.; Lu, X.; Xing, J.; Lv, J.; et al. Sustained exposure to Helicobacter pylori induces immune tolerance by desensitizing TLR6. Gastric Cancer 2024, 27, 324–342. [Google Scholar] [CrossRef]
- Cammarota, G.; Sanguinetti, M.; Gallo, A.; Posteraro, B. Review article: Biofilm formation by Helicobacter pylori as a target for eradication of resistant infection. Aliment. Pharmacol. Ther. 2012, 36, 222–230. [Google Scholar] [CrossRef]
- Reshetnyak, V.I.; Reshetnyak, T.M. Significance of dormant forms of Helicobacter pyloriin ulcerogenesis. World J. Gastroenterol. 2017, 23, 4867–4878. [Google Scholar] [CrossRef]
- Di Fermo, P.; Di Lodovico, S.; Di Campli, E.; D’arcangelo, S.; Diban, F.; D’ercole, S.; Di Giulio, M.; Cellini, L. Dormant States are Affected by Vitamin C. Int. J. Mol. Sci. 2023, 24, 5776. [Google Scholar] [CrossRef]
- Sarem, M.; Corti, R. Role of Helicobacter pylori coccoid forms in infection and recrudescence. Gastroenterol. Hepatol. 2016, 39, 28–35. [Google Scholar] [CrossRef]
- Zhao, X.; Zhong, J.; Wei, C.; Lin, C.-W.; Ding, T. Current Perspectives on Viable but Non-culturable State in Foodborne Pathogens. Front. Microbiol. 2017, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Hadzhiyski, P.; Gergova, R.; Markovska, R. Evolution of Helicobacter pylori Resistance to Antibiotics: A Topic of Increasing Concern. Antibiotics 2023, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Hadzhiyski, P.; Kandilarov, N.; Markovska, R.; Mitov, I. Multidrug resistance inHelicobacter pylori: Current state and future directions. Expert Rev. Clin. Pharmacol. 2019, 12, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Iovanovici, D.C.; Dogaru, B.G.; Cseppento, C.D.N.; Purza, A.L.; Aur, C.; Mocuta, D.; Behl, T.; Bungau, S.G. Benefits of sacubitril/valsartan administration and physical training in cardiac rehabilitation: Current trends and bibliometric analysis of the years 2015–2024. Balneo PRM Res. J. 2024, 15, 683. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Camargo, M.C.; El-Omar, E.; Liou, J.-M.; Peek, R.; Schulz, C.; Smith, S.I.; Suerbaum, S. Helicobacter pylori infection. Nat. Rev. Dis. Prim. 2023, 9, 19. [Google Scholar] [CrossRef]
- Morgan, E.; Arnold, M.; Camargo, M.C.; Gini, A.; Kunzmann, A.T.; Matsuda, T.; Meheus, F.; Verhoeven, R.H.; Vignat, J.; Laversanne, M.; et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–2040: A population-based modelling study. EClinicalMedicine 2022, 47, 101404. [Google Scholar] [CrossRef]
- Geng, T.; Yu, Z.-S.; Zhou, X.-X.; Liu, B.; Zhang, H.-H.; Li, Z.-Y. Antibiotic resistance of Helicobacter pylori isolated from children in Chongqing, China. Eur. J. Pediatr. 2022, 181, 2715–2722. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.; Chan, P.S.F.; Choi, P.; Lao, X.Q.; Chan, S.M.; Teoh, A.; Liang, P. Global Incidence and Mortality of Gastric Cancer, 1980–2018. JAMA Netw. Open 2021, 4, e2118457. [Google Scholar] [CrossRef]
- Chen, C.-C.; Liou, J.-M.; Lee, Y.-C.; Hong, T.-C.; El-Omar, E.M.; Wu, M.-S. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes 2021, 13, 1909459. [Google Scholar] [CrossRef]
- Yang, L.; Kartsonaki, C.; Yao, P.; de Martel, C.; Plummer, M.; Chapman, D.; Guo, Y.; Clark, S.; Walters, R.G.; Chen, Y.; et al. The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: A case-cohort study. Lancet Public Health 2021, 6, e888–e896. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Malfertheiner, P.; Yu, H.-T.; Kuo, C.-L.; Chang, Y.-Y.; Meng, F.-T.; Wu, Y.-X.; Hsiao, J.-L.; Chen, M.-J.; Lin, K.-P.; et al. Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022. Gastroenterology 2024, 166, 605–619. [Google Scholar] [CrossRef]
- Chiang, T.-H.; Chang, W.-J.; Chen, S.L.-S.; Yen, A.M.-F.; Fann, J.C.-Y.; Chiu, S.Y.-H.; Chen, Y.-R.; Chuang, S.-L.; Shieh, C.-F.; Liu, C.-Y.; et al. Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: A long-term cohort study on Matsu Islands. Gut 2021, 70, 243–250. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Deng, H.; Li, H.; Tang, W.; Guan, L.; Qiu, Y.; Donovan, M.J.; Chen, Z.; Tan, W. In vivo activation of pH-responsive oxidase-like graphitic nanozymes for selective killing of Helicobacter pylori. Nat. Commun. 2021, 12, 2002. [Google Scholar] [CrossRef]
- Yu, C.; Qiu, J.; Xiong, M.; Ou, C.; Zeng, M.; Song, H. Trends in Helicobacter pylori resistance research (2002–2022): A bibliometric analysis. Front. Med. 2022, 9, 1027534. [Google Scholar] [CrossRef]
- Wang, R.; Huang, S.; Gan, P.; Pan, X.; Wang, P.; Zhong, X.; Lü, M.; Zhou, X.; Tang, X. States and hotspots in Helicobacter pylori research from 2002 to 2021: A bibliometric analysis. Helicobacter 2023, 28, e12986. [Google Scholar] [CrossRef]
- Farina, S.; Fevola, G.; Adduci, A.; Maio, A.; Lontano, A.; Ricciardi, W.; Gualano, M.R.; Villani, L. Trends in antimicrobial resistance education research: A bibliometric analysis. One Heal. 2025, 21, 101120. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Aldaz, S.R.; Bonifaz-Díaz, D.R. Revision bibliografica: Prevalencia de la infección por helicobacter pylori drogo-resistente. MQRInvestigar 2024, 8, 3029–3047. [Google Scholar] [CrossRef]
- Yuan, C.; Yu, C.; Sun, Q.; Xiong, M.; Zhou, S.; Zeng, M.; Song, H. Research on antibiotic resistance in Helicobacter pylori: A bibliometric analysis of the past decade. Front. Microbiol. 2023, 14, 1208157. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Zhu, Z.; Huang, L.; Zeng, C.; Zhang, L.; Wu, W.K.; Lu, N.; Xie, C. Research Trends on Clinical Helicobacter pylori Eradication: A Bibliometric Analysis from 1983 to 2020. Helicobacter 2021, 26, e12835. [Google Scholar] [CrossRef]
- Fang, Y.; Jiang, S.; Zhou, X.; Zhou, W.; Jiang, X.; Chen, L.; Wang, M.; Chen, Y.; Li, L. Whole-genome sequencing analyses and antibiotic resistance situation of 48 Helicobacter pylori strains isolated in Zhejiang, China. Gut Pathog. 2024, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, N.; Wang, S.; Guo, Y.; Liu, Z. Bibliometric analysis of Helicobacter pylori resistance—From 2002 to 2022. Helicobacter 2023, 28, e12983. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Bang, C.S.; Gong, E.J. Antibiotic Resistance of Helicobacter pylori: Mechanisms and Clinical Implications. J. Korean Med. Sci. 2024, 39, e44. [Google Scholar] [CrossRef] [PubMed]
- Linz, B.; Balloux, F.; Moodley, Y.; Manica, A.; Liu, H.; Roumagnac, P.; Falush, D.; Stamer, C.; Prugnolle, F.; van der Merwe, S.W.; et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature 2007, 445, 915–918. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, Y.; Xiao, Q.; Zheng, W.; Long, G.; Chen, B.; Shu, X.; Jiang, M. Mutations in the Antibiotic Target Genes Related to Clarithromycin, Metronidazole and Levofloxacin Resistance in Helicobacter pylori Strains from Children in China. Infect. Drug Resist. 2020, 13, 311–322. [Google Scholar] [CrossRef]
- Xu, G.; Shi, Y.; Zhang, X.; Yang, M.; Sun, Y.; Zhang, N.; Li, Z. Correlation Analysis of Pathways and Operons of Helicobacter pylori Resistance Genes Using Bibliometrics. Clin. Lab. 2019, 65, 673–683. [Google Scholar] [CrossRef]
- Jaiswal, N.; Kandpal, M.; Jha, H.C.; Kumar, A. Collective in-silico and in-vitro evaluation indicate natural phenolics as a potential therapeutic candidate targeting antimicrobial-resistant genes of Helicobacter pylori. Int. J. Biol. Macromol. 2025, 307, 142197. [Google Scholar] [CrossRef]
- Yu, Y.; Xue, J.; Lin, F.; Liu, D.; Zhang, W.; Ru, S.; Jiang, F. Global Primary Antibiotic Resistance Rate of Helicobacter pylori in Recent 10 years: A Systematic Review and Meta-Analysis. Helicobacter 2024, 29, e13103. [Google Scholar] [CrossRef]
- Lee, J.H.; Ahn, J.Y.; Choi, K.D.; Jung, H.; Kim, J.M.; Baik, G.H.; Kim, B.; Park, J.C.; Jung, H.; Cho, S.J.; et al. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: A prospective multicenter study. Helicobacter 2019, 24, e12592. [Google Scholar] [CrossRef]
- Kwon, Y.M.; Kim, S.J.; Lee, J.G.; Lee, S.P. Effects of prior antibiotic use on clarithromycin resistance in Helicobacter pylori. Helicobacter 2023, 28, e12974. [Google Scholar] [CrossRef]
- Xu, W.; Yang, B.; Lin, L.; Lin, Q.; Wang, H.; Yang, L.; Li, Z.; Lamm, S.; Chen, Y.; Yang, N.; et al. Antibiotic resistance of Helicobacter pylori in Chinese children: A multicenter study from 2016 to 2023. Helicobacter 2024, 29, e13038. [Google Scholar] [CrossRef]
- Hong, T.-C.; El-Omar, E.M.; Kuo, Y.-T.; Wu, J.-Y.; Chen, M.-J.; Chen, C.-C.; Fang, Y.-J.; Leow, A.H.R.; Lu, H.; Lin, J.-T.; et al. Primary antibiotic resistance of Helicobacter pylori in the Asia-Pacific region between 1990 and 2022: An updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2024, 9, 56–67. [Google Scholar] [CrossRef]
- Mladenova, I. Epidemiology of Helicobacter pylori Resistance to Antibiotics (A Narrative Review). Antibiotics 2023, 12, 1184. [Google Scholar] [CrossRef]
- Costache, C.; Colosi, H.A.; Grad, S.; Paștiu, A.I.; Militaru, M.; Hădărean, A.P.; Țoc, D.A.; Neculicioiu, V.S.; Baciu, A.M.; Opris, R.V.; et al. Antibiotic Resistance in Helicobacter pylori Isolates from Northwestern and Central Romania Detected by Culture-Based and PCR-Based Methods. Antibiotics 2023, 12, 1672. [Google Scholar] [CrossRef] [PubMed]
- Popovici, E.L.; Cojocariu, L.H.; Girleanu, I.; Dimache, M.; Moscalu, A.T. Eradication of Helicobacter Pylori. The Response to Triple Standard Therapy in a Center of North-Eastern Romania. Farmacia 2021, 69, 756–762. [Google Scholar] [CrossRef]
- Albush, A.; Yassine, F.; Abbas, H.; Hanna, A.; Saba, E.; Bilen, M. The impact of Helicobacter pylori infection and eradication therapies on gut microbiota: A systematic review of microbial dysbiosis and its implications in gastric carcinogenesis. Front. Cell. Infect. Microbiol. 2025, 15, 1592977. [Google Scholar] [CrossRef]
- Lai, Y.; Zhang, T.; Yin, X.; Zhu, C.; Du, Y.; Li, Z.; Gao, J. An antibiotic-free platform for eliminating persistent Helicobacter pylori infection without disrupting gut microbiota. Acta Pharm. Sin. B 2024, 14, 3184–3204. [Google Scholar] [CrossRef] [PubMed]

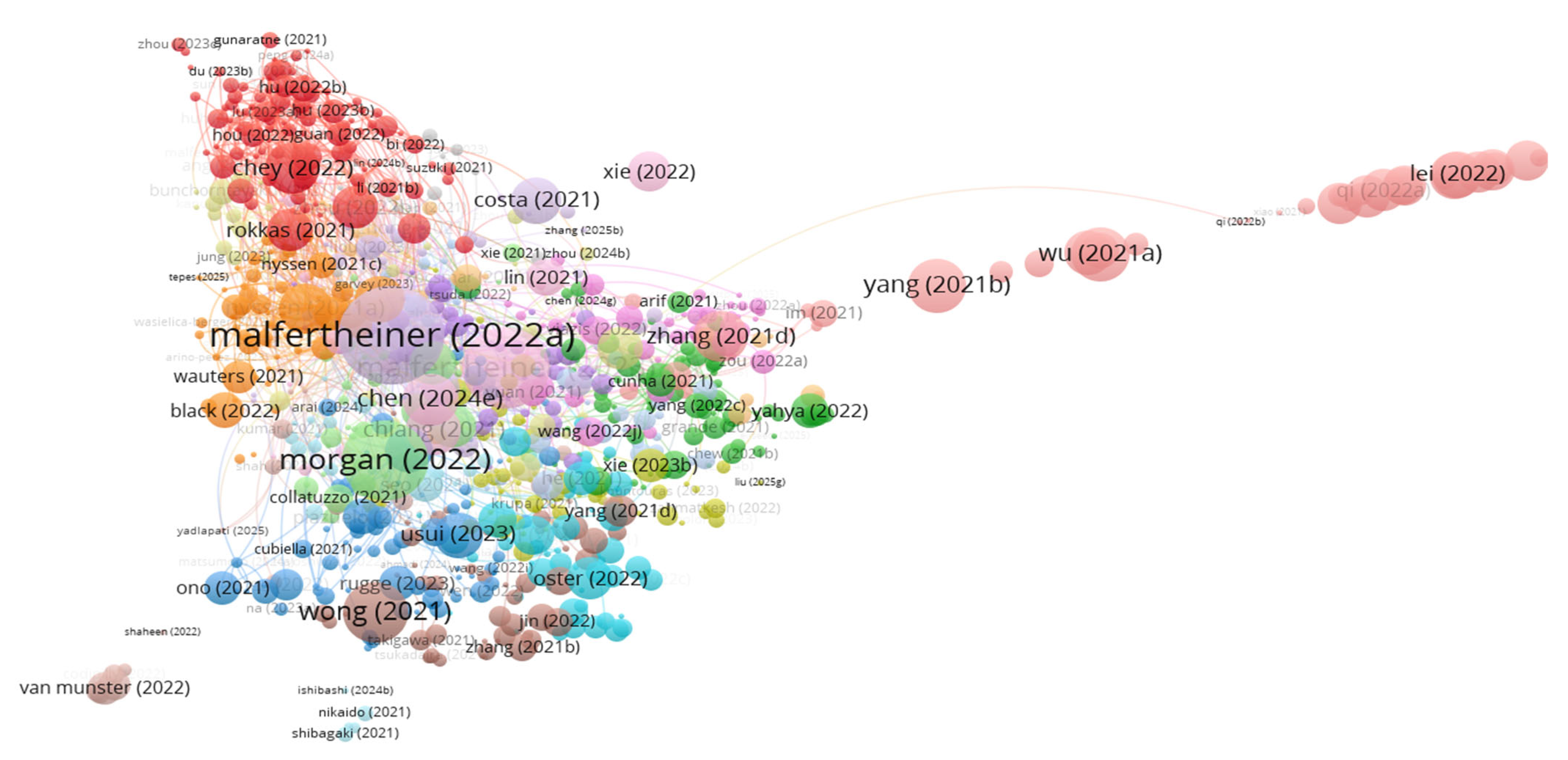
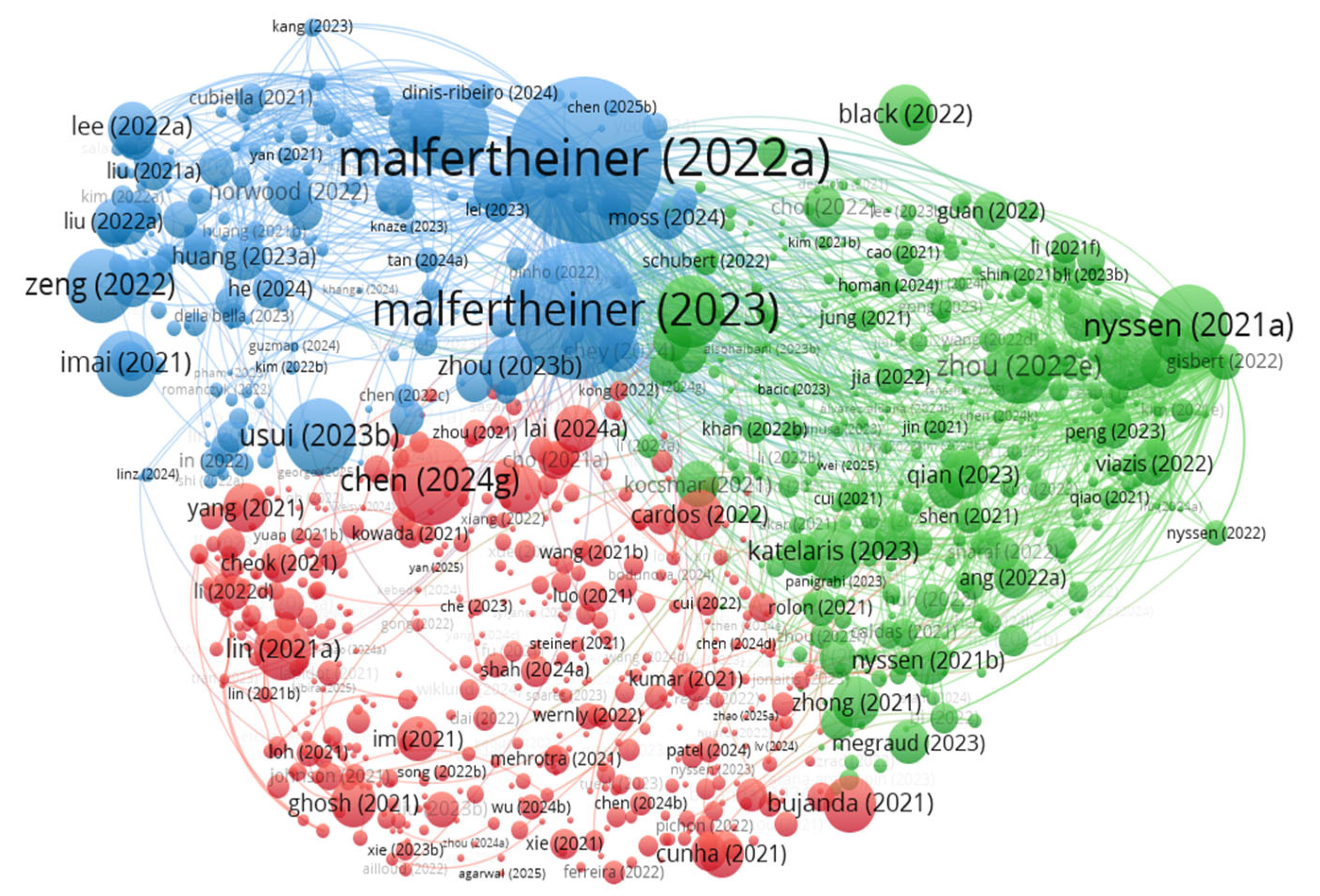
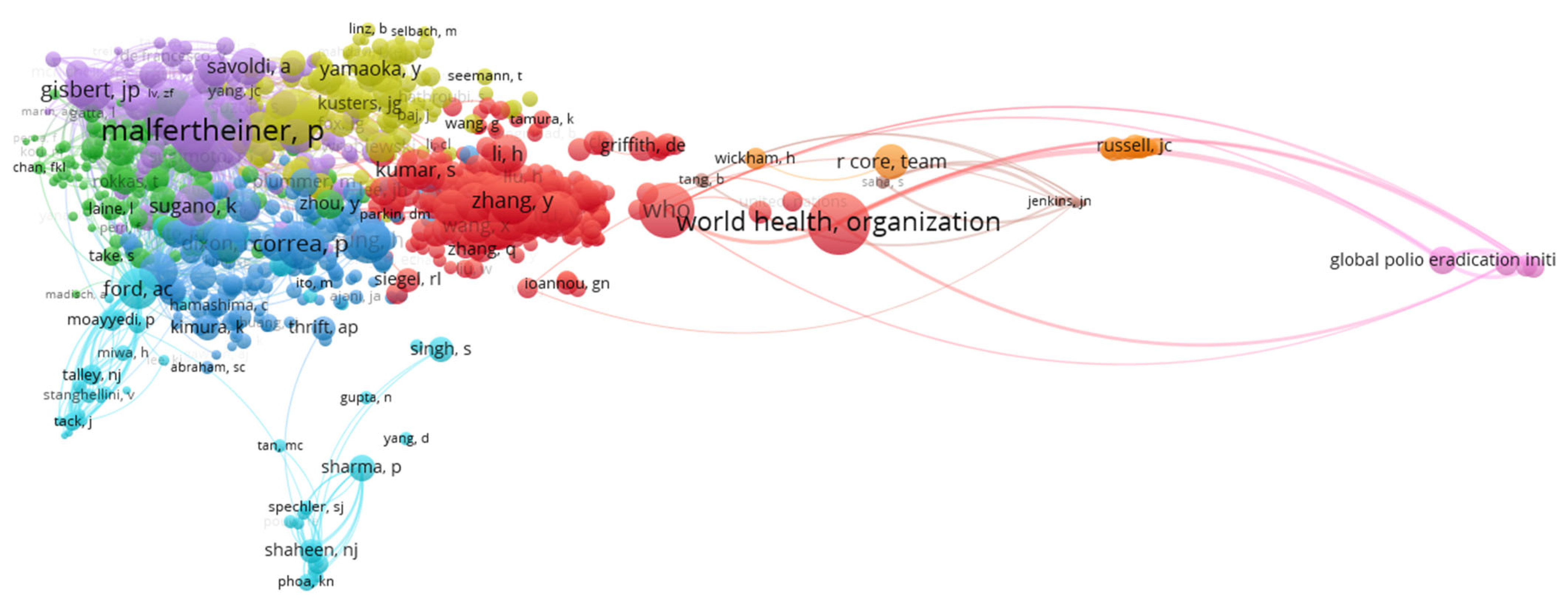
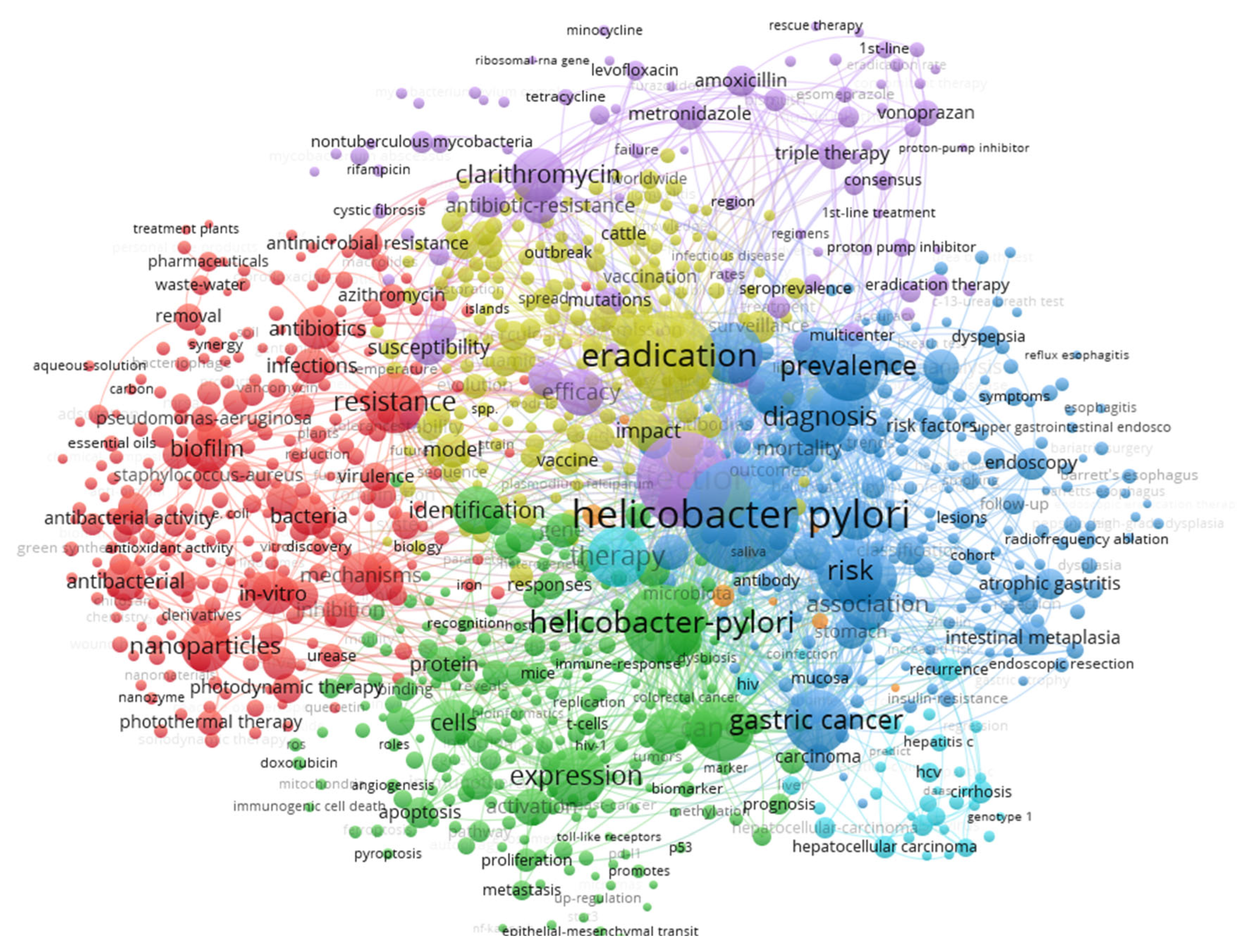
| Author | Article Title | Citations No. | TLS |
|---|---|---|---|
| Malfertheiner (2022a) [15] | Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report | 681 | 207 |
| Malfertheiner (2023) [16] | Helicobacter pylori infection | 464 | 122 |
| Morgan (2022) [17] | The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: A population-based modelling study | 442 | 14 |
| Geng (2022) [18] | Antibiotic resistance of Helicobacter pylori isolated from children in Chongqing, China | 271 | 1 |
| Wong (2021) [19] | Global Incidence and Mortality of Gastric Cancer, 1980–2018 | 254 | 2 |
| Chen (2021) [20] | The interplay between Helicobacter pylori and gastrointestinal microbiota | 204 | 4 |
| Yang (2021b) [21] | The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case–cohort study | 204 | 3 |
| Chen (2024e) [22] | Global Prevalence of Helicobacter pylori Infection and Incidence of Gastric Cancer Between 1980 and 2022 | 186 | 48 |
| Chiang (2021) [23] | Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands | 183 | 53 |
| Zhang (2021d) [24] | In vivo activation of pH-responsive oxidase-like graphitic nanozymes for selective killing of Helicobacter pylori | 183 | 53 |
| Author | N | Citations No. | A C/D | TLS | Co-Citations | Citations No. | TLS |
|---|---|---|---|---|---|---|---|
| P. Malfertheiner | 29 | 1877 | 64.72 | 2505 | P. Malfertheiner | 2075 | 48,447 |
| F. Megraund | 27 | 1785 | 66.11 | 2645 | D. Y. Graham | 1224 | 31,191 |
| J.-M. Liou | 13 | 1463 | 112.53 | 1958 | J.K.Y. Hooi | 1042 | 16,088 |
| S. Seurbaum | 16 | 1242 | 77.62 | 1843 | P. Correa | 765 | 13,872 |
| C. Schulz | 19 | 1226 | 64.53 | 1840 | Y. Zhang | 751 | 8258 |
| C. O´moraim | 15 | 1225 | 81.66 | 1836 | H. Sung | 712 | 9678 |
| M. C. Camargo | 19 | 1212 | 63.78 | 906 | Y. Liu | 698 | 6612 |
| J.P. Gisbert | 21 | 1079 | 51.38 | 1903 | W.D. Chey | 674 | 14,419 |
| A. Gasbarrini | 9 | 1073 | 119.22 | 1503 | J.P. Gibert | 652 | 28,103 |
| T. Rokkas | 9 | 964 | 107.11 | 1513 | Y. Wang | 652 | 6419 |
| Journals | No. of P. | No. of C. | Average No. of C/Article | IF (2024) | Source | Co-Citation | TLS |
|---|---|---|---|---|---|---|---|
| SCIENTIFIC REPORT | 354 | 2779 | 7.85 | 3.9 | PLOS ONE | 11,656 | 544,086 |
| HELICOBACTER | 287 | 2062 | 7.18 | 4.3 | GASTROENTEROLOGY | 11,178 | 593,177 |
| PLOS ONE | 210 | 1084 | 5.16 | 2.6 | HELICOBACTER | 10,493 | 462,524 |
| CUREUS J. OF MEDICAL SCIENCE | 206 | 388 | 1.88 | 1.3 | GUT | 9978 | 535,809 |
| INT.J.OF MEDICAL SCIENCE | 201 | 1538 | 7.65 | 3.2 | SCI REP-UK | 8410 | 401,612 |
| FRONTIERS IN MICROBIOLOGY | 182 | 1394 | 7.65 | 4.5 | P NATL ACAD SCI USA | 7866 | 435 |
| ANTIBIOTICS-BASEL | 181 | 1534 | 8.47 | 4.6 | WORD J. GASTROENTERO | 7690 | 400 |
| MICROORGANISM | 138 | 805 | 5.83 | 4.2 | NATURE | 6651 | 300 |
| MICROBIOLOGY SPECTRUM | 138 | 923 | 6.68 | 3.8 | ANTIMICROB AGENTS CH | 6356 | 230 |
| FRONTIERS IN IMMUNOLOGY | 129 | 1105 | 8.56 | 5.9 | NAT COMMUN | 6001 | 249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, S.D.; Suciu, R.N.; Ilias, T.; Hocopan, C.; Frățilă, O. Global Dynamics of Research on Antibiotic Resistance in Helicobacter pylori: A Bibliometric Analysis. Gastrointest. Disord. 2025, 7, 69. https://doi.org/10.3390/gidisord7040069
Matei SD, Suciu RN, Ilias T, Hocopan C, Frățilă O. Global Dynamics of Research on Antibiotic Resistance in Helicobacter pylori: A Bibliometric Analysis. Gastrointestinal Disorders. 2025; 7(4):69. https://doi.org/10.3390/gidisord7040069
Chicago/Turabian StyleMatei, Sergiu Dorin, Ramona Nicoleta Suciu, Tiberia Ilias, Cristian Hocopan, and Ovidiu Frățilă. 2025. "Global Dynamics of Research on Antibiotic Resistance in Helicobacter pylori: A Bibliometric Analysis" Gastrointestinal Disorders 7, no. 4: 69. https://doi.org/10.3390/gidisord7040069
APA StyleMatei, S. D., Suciu, R. N., Ilias, T., Hocopan, C., & Frățilă, O. (2025). Global Dynamics of Research on Antibiotic Resistance in Helicobacter pylori: A Bibliometric Analysis. Gastrointestinal Disorders, 7(4), 69. https://doi.org/10.3390/gidisord7040069