1. Inflammatory Bowel Diseases: Definition, Epidemiology
1.1. Definition
Inflammatory bowel disease (IBD) encompasses two chronic inflammatory disorders of the gastrointestinal tract: Crohn’s disease and ulcerative colitis. Crohn’s disease primarily affects the terminal ileum and proximal colon [
1,
2], whereas ulcerative colitis is generally confined to the colon and rectum [
3]. Both diseases are marked by alternating phases of relapse and remission. IBD can progress from mild to severe stages [
4].
It is therefore crucial for healthcare providers to deepen their understanding of the pharmacological profiles of IBD therapies, including their indications based on disease pathophysiology and severity. We will also address the role of patient education in promoting treatment adherence, maintaining remission, and reducing hospitalization rates. For these reasons, a multidisciplinary approach among healthcare professionals is urgently needed to address the diverse needs of individuals with IBD, including variations in disease expression and considerations for mental health.
1.2. Epidemiology
IBD currently affects approximately 0.3% of the global population [
1], with projections suggesting this figure could reach 1% by 2030 [
2]. Crohn’s disease is most commonly diagnosed in childhood, whereas ulcerative colitis is primarily diagnosed between the ages of 20 and 40 [
1,
3]. However, diagnoses of ulcerative colitis in individuals over 60 are becoming increasingly common, now representing 20% of the new cases [
1]. Nonetheless, early-onset and very-early-onset pediatric IBD are increasingly recognized, with a concerning rise in incidence. Some clinicians even consider these early forms to be distinct entities from adult-onset IBD due to differences in clinical presentation, pathophysiology, and response to treatment [
5,
6]. The incidence of IBD has risen alongside lifestyle changes, particularly diets high in processed foods, urban living, and industrialization, with childhood exposure being a significant factor [
7,
8]. The incidence of IBD in children ranges from 1.6 to 10.9 cases per 100,000 individuals in Europe and from 11.4 to 13.2 per 100,000 in North America. Among adults, the incidence varies between 10.5 and 46.1 per 100,000 in Europe and between 7.3 and 30.2 per 100,000 in North America. In contrast, incidence rates in Asia remain low across both age groups, with 0.5 to 2.2 per 100,000 in children and 1.4 to 1.5 per 100,000 in adults [
9]. Previously considered a health issue predominantly affecting Western countries, IBD has now become a global health concern [
1,
3,
10].
2. Inflammatory Bowel Diseases: Physiopathology and Risk Factors
2.1. Pathophysiology
IBD are multifactorial diseases. On one hand, genetic predisposition plays a role in its development, with hundreds of loci implicated, including
NOD2 in Crohn’s disease [
11,
12]. On the other hand, an impaired gut epithelial barrier can lead to a pro-inflammatory environment and dysbiosis [
1,
13,
14]. The number of goblet cells, responsible for mucin production—a key component of intestinal and colonic mucus—is reduced in individuals with IBD, contributing to gut barrier dysfunction [
15]. This compromised barrier facilitates microbial translocation, thereby triggering intestinal immune responses in IBD patients [
16,
17]. Due to gut permeability in IBD, innate immunity is profoundly disrupted, with aberrant activated macrophages and neutrophils producing elevated levels of pro-inflammatory cytokines such as Tumor Necrosis Factor α (TNF-α) and interleukins (IL), notably IL-1β, IL-12, and IL-23 [
18]. Additionally, stromal cells within the lamina propria may contribute to inflammation and epithelial damage. Indeed, these cells exacerbate gut impairment by expressing chemotactic molecules, such as sphingosine-1-phosphate (S1p), that drive T cell recruitment to the inflamed tissue [
19,
20,
21]. The infiltration of CD4
+ T cells into intestinal tissue is facilitated by a reduction in regulatory T lymphocyte (Treg) levels. Innate lymphoid cells (ILCs), which are essential for maintaining gut health and functionality, are significantly affected by IBD. A healthy gut requires a balanced distribution of ILC subtypes; however, this equilibrium is disrupted in IBD, leading to the production of pro-inflammatory cytokines and contributing to intestinal inflammation [
22]. The activation of cytokines signalization involves Janus Kinase (JAK) and Signal Transducer and Activator of Transcription (STAT) pathway.
Moreover, studies have shown a reduction in microbial diversity alongside an enrichment in pathogenic bacteria [
1,
3,
15,
16,
17,
19,
20,
21,
23,
24]. This dysbiosis may also contribute to the pathophysiology of IBD [
25,
26]. Food additives such as emulsifiers and preservatives can impair intestinal epithelial cells, promote the production of reactive oxygen species, and contribute to a pro-inflammatory environment, potentially leading to gut dysbiosis [
27].
2.2. Risk Factors and Associated Factors
IBD results from multiple contributing factors, thought to result from detrimental environmental exposures in combination with genetic predisposition.
The incidence increases by a factor of 7.77 (95% confidence interval (CI): 7.05–8.56) in individuals with a first-degree relative affected by Crohn’s disease [
1,
26]. However, genetic factors alone are insufficient for the development of Crohn’s disease, as demonstrated by twin studies [
26].
In a meta-analysis involving 7208 individuals with IBD, antibiotic exposure was found to be associated with an increased risk of Crohn’s disease (odds ratio (OR) 1.74, 95% CI: 1.35–2.23) but not with ulcerative colitis (OR 1.08, 95% CI: 0.91–1.27) [
28]. Childhood exposure to antibiotics (OR 2.75, 95% CI: 1.72–4.38), excluding penicillin, has been identified as a risk factor for developing IBD. Among these, metronidazole (OR 5.01, 95% CI: 1.65–15.25) and fluoroquinolones (OR 1.79, 95% CI: 1.03–3.12) were associated with the highest risk. Antibiotics are highly effective therapeutic agents when used appropriately. However, their overuse—particularly in cases where they are not clinically indicated—can contribute to the development of antibiotic resistance. Conversely, untreated or improperly managed gastrointestinal infections may result in significant intestinal damage, underscoring the importance of accurate diagnosis and targeted antimicrobial therapy.
Cigarette smoking is also a recognized risk factor for the development and exacerbation of Crohn’s disease. In a meta-analysis of 33 studies [
29], To et al. reported a higher odds ratio for disease flare-ups in the smoking group compared to nonsmokers (OR 1.56, 95% CI: 1.21–2.01). Conversely, smoking has been identified as a protective factor against ulcerative colitis [
30,
31]. In a meta-analysis conducted by Mi et al., the odds ratio was estimated at 0.26 (95% CI: 0.21–0.32), indicating a significantly reduced risk among smokers [
32]. In contrast, smoking cessation has been associated with an increase in disease severity [
33].
A diet high in ultra-processed foods, containing additives and preservatives, even at consumption levels of 1 to 4 servings per day (OR 1.67, 95% CI: 1.18–2.37), is associated with an increased risk of developing IBD [
1,
3,
34]. An animal-based diet has been shown to increase the abundance of certain bacterial strains, including
Bacteroidetes,
Bilophila, and
Alistipes, which are associated with gut inflammation and may contribute to the pathogenesis of IBD [
35]. Conversely, breastfeeding appears to be a protective factor against the development of IBD, particularly when its duration exceeds 12 months [
36,
37].
3. Inflammatory Bowel Diseases: Symptoms and Diagnosis
3.1. Symptoms and Severity
Crohn’s disease primarily affects the terminal ileum and proximal colon but can involve the entire gastrointestinal tract, leading to chronic diarrhea and abdominal pain [
1,
38]. These symptoms often generate chronic fatigue and weight loss. The intestinal manifestations are episodic, segmental, irregular, and transmural, potentially resulting in complications such as fistulas and abscesses. Approximately 20% of individuals with Crohn’s disease develop perianal lesions, such as skin tags, stenosis, fissures, and ulcers [
39,
40]. Additionally, extraintestinal symptoms occur in approximately 50% of individuals with Crohn’s disease, including joint arthritis, erythema nodosum, psoriasis, and uveitis [
41]. Classifications have been established to characterize Crohn’s disease based on symptom severity, disease location, and phenotype for adults and children, the Montreal and Paris systems, respectively [
42,
43]. This is why the second encompasses the age at diagnostic.
Ulcerative colitis progresses through phases of relapse and remission. The most common symptom is rectal bleeding, accompanied by an increased stool frequency and decreased stool consistency [
3,
44]. Conversely, individuals with ulcerative colitis and proctitis may experience constipation rather than diarrhea [
39]. Extraintestinal manifestations, such as arthritis, occur similarly to Crohn’s disease but are less frequent, affecting 20–35% of patients [
40]. Individuals with ulcerative colitis also have an elevated risk of venous thromboembolism, which further increases during relapse [
45]. The classification of ulcerative colitis is based on symptom severity: mild-to-moderate, moderate-to-severe, and acute severe [
3].
The presence of extraintestinal manifestations, combined with the diverse locations and symptoms of the disease, complicates the diagnosis and comprehensive understanding of IBD.
3.2. Complications
Complications of IBD include anemia due hemorrhage and to blood loss during flares. Both Crohn’s disease and ulcerative colitis can lead to intestinal stenosis, obstruction, or perforation, as well as the development of anal fistulas or abscesses. Ulcerative colitis may result in toxic megacolon, causing acute colonic dilation during severe phases. Additionally, individuals with IBD have an elevated risk of developing colorectal cancer, with a standardized incidence ratio of 1.9 for Crohn disease and 2.4 for ulcerative colitis [
46,
47,
48].
3.3. Diagnosis
The presence of extraintestinal manifestations can delay the diagnosis of IBD. The diagnosis of IBD is invasive due to the location of the disease. However, the initial diagnostic phase involves blood and stool sampling to detect inflammation and rule out infection. To confirm an IBD diagnosis, visualization of affected intestinal areas via endoscopy, biopsies, and histological analysis is essential [
49]. For Crohn’s disease, patient age is an important factor, with children and young adults representing most new diagnoses [
50]. The Crohn’s Disease Activity Index (CDAI) is the most widely used score to assess the activity of this IBD, based on clinical criteria such as abdominal pain, the number of liquid or very loose stools, current and baseline weight, and biological markers like hematocrit [
51,
52]. Several indices are used to assess ulcerative colitis activity [
53], with the most frequently used being the Mayo endoscopic score [
54,
55]. This score, also known as the Ulcerative Colitis Disease Activity Index (UCDAI), is based on stool frequence, rectal bleeding, endoscopic appearance, and a global evaluation by the physician. The Ulcerative Colitis Endoscopic Index of Severity (UCEIS) [
56,
57], however, specifically evaluates bleeding and ulceration. The Lichtiger score is commonly applied to evaluate the severity of acute disease. In addition to the other criteria, it includes nocturnal bowel movements, abdominal pain, altered general condition, and need for anti-diarrheal medication [
58,
59].
The microscopic examination of IBD lesions unravels histopathological differences supporting the differential diagnosis between Crohn’s disease and ulcerative colitis.
Crohn’s disease lesions appear transmural, i.e., they affect all layers of the intestinal wall in its entire thickness. The lesions tend to be characterized by focal, discontinuous transmural inflammation associated with granuloma formations, lymphoid cells clusters, as well as deep fissuring ulcers. The presence of non-caseating granuloma is considered as a strong marker, although not systematically present or noticed.
Ulcerative colitis, in contrast, is characterized by a continuous inflammation unfolding from the rectum and expanding proximally upward with absence of granuloma. This diffuse inflammatory infiltrate is predominantly composed of a mix of lymphocytes and plasma cells. On this base, lesions affect the intestinal mucosa and submucosa, i.e., they are limited to the intestine top layers without affecting the deepest ones. The presence of crypt abscesses and crypt architectural distortion at the histological level (associated with the previously cited continuous inflammation) combined with the absence of granuloma are strong markers for the diagnosis.
Objectifying these histopathological differences between Crohn’s disease and ulcerative colitis is critical not only for differential diagnosis but also for subsequent therapeutic decision-making, patient follow-up, and therapeutic education [
52,
55].
The differential diagnosis of IBD includes a wide range of conditions that can mimic its clinical and endoscopic presentation [
60]. These include infectious colitis (e.g.,
Clostridioides difficile,
Salmonella,
Yersinia,
Shigella, and
Entamoeba histolytica), ischemic colitis, and drug-induced colitis—particularly from nonsteroidal anti-inflammatory drugs. Sexually transmitted infections such as
Chlamydia trachomatis or
Neisseria gonorrhoeae may also present with gastrointestinal symptoms resembling IBD. Additionally, endometriosis involving the bowel can mimic IBD, especially in women of reproductive age, due to overlapping symptoms such as abdominal pain, diarrhea, and rectal bleeding.
4. Therapeutic Strategies
Diet and lifestyle management, including maintaining a healthy Body Mass Index (BMI) and engaging in regular physical activity, are crucial factors that can modulate the progression of IBD due to the underlying pathophysiology of the disease.
Since tobacco smoking is a known risk factor for the development and progression of Crohn’s disease, smoking cessation is a crucial recommendation for affected individuals [
1,
34]. Quitting smoking not only reduces the risk of developing Crohn’s disease but also lowers the likelihood of disease-related complications [
34].
The goal of treatment is to induce remission and maintain it [
4]. Different treatments are used to manage acute flares and to sustain remission. Additionally, medication choice depends on the severity of the IBD. Corticosteroids are primarily used during acute phases. For mild-to-moderate IBD, topical and anti-inflammatory medications, along with pain relief, are prescribed. For moderate-to-severe phases, immunosuppressants are used, while surgery may be considered in cases of severe IBD.
Surgery remains a last-line therapeutic approach to manage disease progression and severity [
4]. In Crohn’s disease with well-defined, localized lesions, surgery may serve as an alternative to pharmacological therapy. Surgical intervention becomes necessary when drug-based treatments fail to maintain remission or to address complications.
Surgical intervention in Crohn’s disease may be warranted in both emergent and non-emergent situations. Emergencies are characterized by acute symptoms such as bowel obstruction with accompanying vomiting, intestinal perforation, and the absence of stool or flatus via the rectum. Non-emergent situations are defined by complications or disease that is refractory to medical therapy. However, the overall reliance on surgery has been decreasing in Crohn’s disease.
In ulcerative colitis, surgery may be required in 25–30% of patients for whom medical treatments are ineffective or in cases of dysplasia.
Therapeutic strategies for IBD can be challenging for healthcare providers to comprehend, as the underlying pathophysiology and therapeutic targets remain unclear. Treatments primarily focus on inflammatory mediators, with varying degrees of success. Additionally, the disease’s progression through phases of relapse and remission complicates care management. New biotherapeutic treatments also pose difficulties in adherence and understanding of therapeutic regimens. Ongoing research is essential to deepen our understanding of the pathophysiology of inflammatory bowel disease (IBD) and its underlying mechanisms, with the ultimate goal of identifying novel therapeutic targets and developing more effective treatments. This review aims to assist healthcare providers in understanding pharmacotherapeutic approaches to IBD.
5. Management of Pharmacotherapeutics in Crohn’s Disease for Healthcare Providers
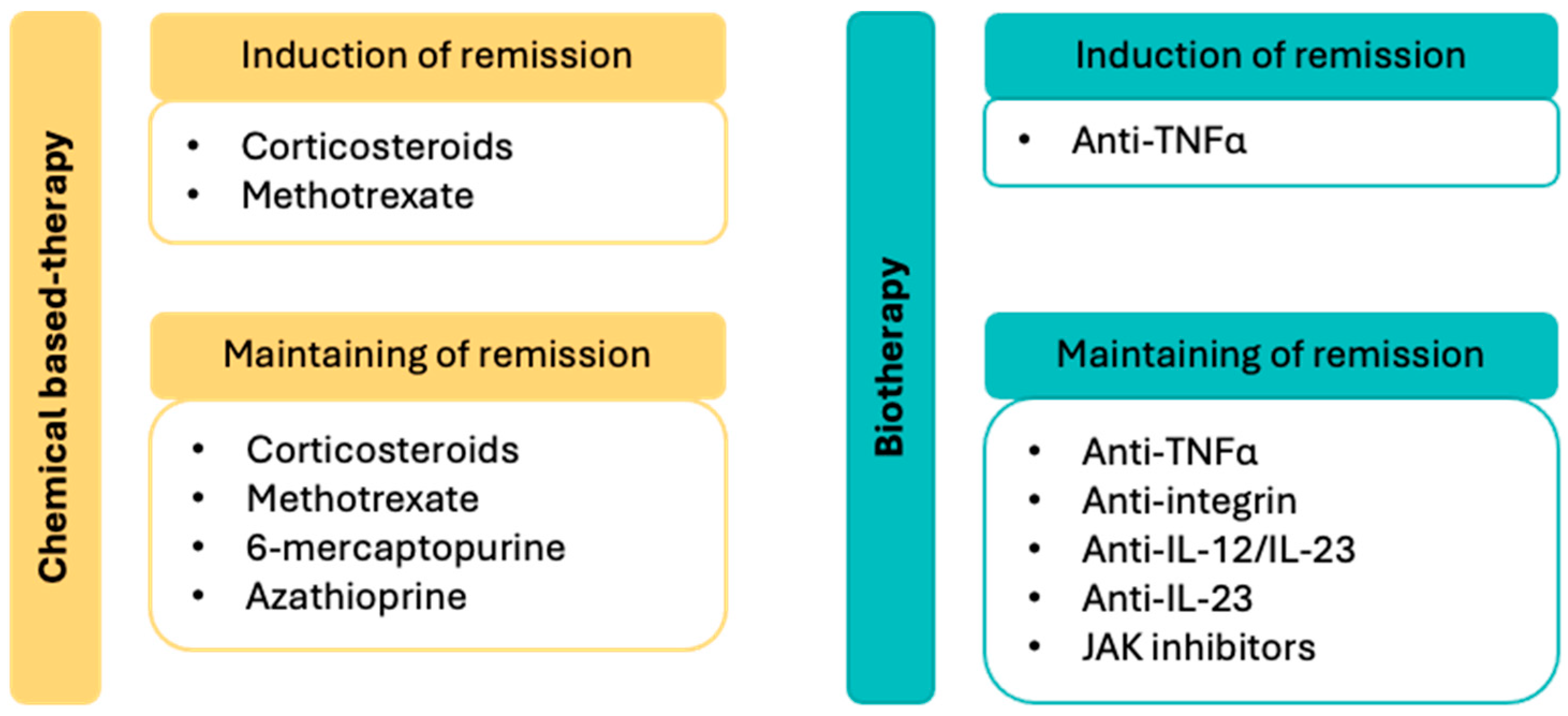
In the management of Crohn’s disease, chemical-based therapies are used to induce and/or maintain remission, while biotherapies are primarily employed for long-term disease control (
Figure 1). Corticosteroids, administered orally or intravenously, are effective in inducing remission. In cases of corticosteroid dependence or resistance, azathioprine or a TNFα-targeting biotherapy may be considered. Methotrexate can also aid in inducing remission and may help manage potential extraintestinal manifestations. Antineoplastic agents such as azathioprine and 6-mercaptopurine are commonly used to reduce disease flare-ups and support long-term disease management [
1,
52].
6. Management of Pharmacotherapeutics in Ulcerative Colitis for Healthcare Providers
In the management of ulcerative colitis, remission induction can be achieved using chemical-based therapies, such as corticosteroids and/or aminosalicylates, or biotherapies targeting TNFα, IL-12/IL-23, and JAK pathways (
Figure 2). Aminosalicylates are also used to maintain remission, alongside biotherapies initially employed to induce remission.
In the acute severe phase, hospitalization is required to manage the crisis with intravenous corticosteroids. In cases of corticosteroid resistance, infliximab, an anti-TNFα monoclonal antibody, or ciclosporin, an immunosuppressant, may be considered. If these therapies fail, surgery may be necessary [
3,
55,
61].
7. Chemical-Based Therapies Used in IBD: Side Effects and Precautions of Use
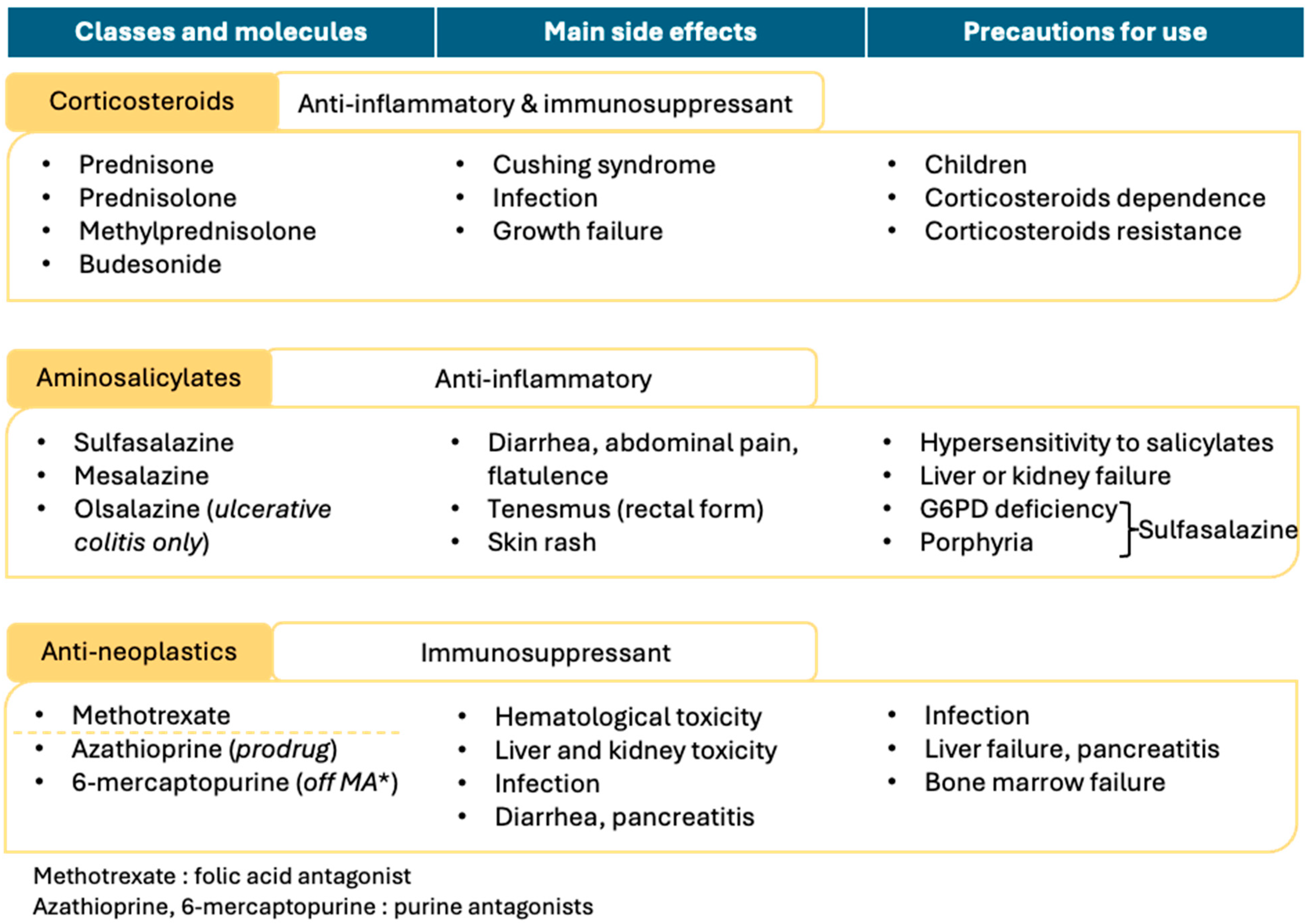
Chemical-based therapies, illustrated in
Figure 3, are primarily used to induce remission and are typically applied in cases of mild to moderate IBD severity.
7.1. Corticosteroids
Corticosteroids (prednisone, prednisolone, methylprednisolone, and budesonide) are primarily used to induce remission in both forms of IBD, though they can also contribute to remission maintenance [
61,
62,
63]. During acute exacerbations, corticosteroids may be administered orally or intravenously. However, corticosteroid dependence—defined as the inability to reduce the dose below a certain threshold without triggering a relapse—is common among IBD patients. To minimize dependence, corticosteroid treatment should be administered at the most appropriate dose for the shortest possible duration.
Corticosteroid resistance, characterized by the lack of therapeutic response after four weeks, may also occur. In cases of corticosteroid dependence or resistance, alternative therapies, such as antineoplastic agents or TNFα-targeted biotherapies, are required. Budesonide, a locally acting corticosteroid in the intestinal tract, can be used as an intermediary treatment for corticosteroid-dependent patients, bridging conventional corticosteroid therapy and alternative options.
The potential for corticosteroids to cause adverse effects, such as Cushing’s syndrome or growth impairment in children, necessitates a thorough assessment of the benefit–risk balance prior to use in pediatric populations. Due to their immunosuppressive effects, corticosteroid therapy also increases the risk of infection, underscoring the importance of close monitoring for infectious complications [
1,
3,
64,
65].
7.2. Aminosalicylates
Aminosalicylates (mesalazine, sulfasalazine, and olsalazine) are primarily used to induce and maintain remission in ulcerative colitis [
55,
65,
66] but may also be utilized in Crohn’s disease management [
1], except for olsalazine, which is exclusively indicated for ulcerative colitis. Mesalazine and sulfasalazine are the two most frequently used agents in this class.
The mechanism of action of aminosalicylates remains unclear but is thought to involve PPARγ activation, which subsequently inhibits the NFκB pathway, resulting in anti-inflammatory effects. The primary side effects of aminosalicylates are local, including diarrhea, abdominal pain, flatulence, and tenesmus, especially when rectal forms are used in ulcerative colitis management. Skin rash has also been reported as a potential adverse effect.
The main contraindications for this therapeutic class are hypersensitivity to salicylates and liver or kidney failure. Sulfasalazine is contraindicated in individuals with G6PD deficiency or porphyria due to its metabolite, sulfapyridine, which may lead to hemolysis of red blood cells
7.3. Antineoplastics
Antineoplastic agents used in IBD management include two purine antagonists, azathioprine and 6-mercaptopurine [
67,
68,
69], as well as the folic acid antagonist methotrexate. Due to their immunosuppressive effects, infection risk and hematologic toxicity are primary adverse effects, making these agents contraindicated in cases of severe infection or bone marrow failure. Hepatic and renal toxicity associated with these drugs necessitate careful assessment prior to use. Additionally, diarrhea and pancreatitis have been reported with this therapeutic class, with pancreatitis warranting particular caution in treatment [
70,
71].
8. Biotherapies Used in IBD: Side Effects and Precautions of Use
Biotherapies used in the management of IBD target five primary pathways: TNFα, integrins, IL-12/IL-23, JAK, and the sphingosine-1 phosphate receptors, as shown in
Figure 4. All these therapeutic classes act as immunosuppressants, increasing the risk of infections, with severe infections such as tuberculosis representing a contraindication for their use.
Among these, only JAK inhibitors and sphingosine-1 phosphate receptor modulators are available orally, while the other three classes require intravenous or subcutaneous administration, which may lead to injection-site reactions, including pain.
8.1. TFNα Inhibitors
TNFα inhibitors are the most commonly used biotherapies in IBD, exerting both anti-inflammatory and immunosuppressive effects. Key drugs in this class include infliximab, adalimumab, and certolizumab. Due to their mechanism of action, the main adverse effects associated with TNFα inhibitors are increased susceptibility to infections and hematological toxicity, such as neutropenia and lymphopenia. Liver toxicity, tachycardia, and headache have also been reported, along with gastrointestinal symptoms like nausea and abdominal pain. Additionally, psychiatric symptoms, including depression and insomnia, have been observed.
Infliximab, a chimeric antibody combining human and mouse protein sequences, may induce hypersensitivity reactions in individuals sensitive to murine proteins, making this a contraindication for its use. Furthermore, heart failure should be carefully evaluated before initiating treatment with TNFα inhibitors [
72,
73].
8.2. Anti-Integrin
Vedolizumab is a monoclonal antibody that specifically targets the α4β7 integrin. This integrin is highly expressed on helper lymphocytes that migrate to the gastrointestinal tract, contributing to inflammation in IBD. Due to its mechanism of action, this biotherapy exerts a localized effect in the management of IBD. Consequently, the adverse effects are primarily local, including dyspepsia and constipation, and can occasionally lead to complications such as anal abscesses. Additionally, systemic effects such as arthralgia and skin rashes have also been reported [
74,
75].
8.3. Anti-IL-12 and/or IL-23
Ustekinumab is a human monoclonal antibody that specifically binds to the p40 protein subunit shared by IL-12 and IL-23, thereby inhibiting these two inflammatory interleukins from binding to their respective receptors. Risankizumab and mirikizumab are humanized monoclonal antibodies that selectively target the p19 protein subunit of IL-23, preventing it from interacting with its receptor [
76]. The primary adverse effects associated with these two monoclonal antibodies include arthralgia, cephalalgia, and skin rash with pruritus.
8.4. JAK Inhibitors
Filgotinib and upadacitinib are JAK inhibitors utilized in the management of IBD, while tofacitinib is specifically indicated for ulcerative colitis. By targeting the JAK signaling pathway, these therapies may pose a risk of hematological toxicity. Additionally, liver toxicity, nausea, dyspepsia, and abdominal pain have been reported as potential side effects. The main adverse effects associated with these agents include arthralgia, headache (cephalalgia), as well as skin rash and pruritus. It is important to note that these medications are contraindicated in individuals with liver failure or during pregnancy [
77,
78,
79,
80,
81].
8.5. S1p Receptors Modulator
Ozanimod, an S1p receptor (S1pR) modulator, was initially approved for multiple sclerosis but is now also available for the treatment of IBD. The activation of sphingosine-1-phosphate (S1p) receptors on lymphocytes signals their migration from lymph nodes to the gastrointestinal tract, where they exert a pro-inflammatory effect. Ozanimod induces the internalization of S1p receptors, thereby preventing lymphocyte migration to the intestine. This modulation produces anti-inflammatory and immunosuppressive effects, though it can also lead to adverse effects, including infections, hematological toxicity, and liver toxicity. Ozanimod is contraindicated in individuals with liver failure, those with a history of cancer within the past five years, and in pregnancy. Bradycardia has been observed as a potential side effect, rendering this therapy unsuitable for patients with heart failure [
82,
83,
84].
9. Future Directions
Enhanced support for the follow-up of individuals living with IBD may significantly improve their quality of life and help prevent relapses. Eltantawy et al. [
82] suggest that a pharmacogenomic approach could be used to select the most effective therapy, monitor treatment responses (including the dosage of inflammatory markers such as TNF-α), and minimize the occurrence of adverse effects.
IBD arises from a combination of factors, including dysbiosis. This phenomenon, marked by a reduction in bacterial strain diversity and an overrepresentation of opportunistic microorganisms, may be mitigated through supplementation with probiotics and/or prebiotics. This leads to the formulation and testing of probiotics and prebiotics in its management. Probiotics are live microorganisms that confer health benefits, while prebiotics are non-digestible ingredients that promote the growth of beneficial microorganisms. The primary probiotics evaluated for IBD management include various strains of
Lactobacillus and
Bifidobacterium, which may exert anti-inflammatory effects and contribute to the repair of the gut epithelium [
83]. Prebiotics are primarily sugar-based compounds designed to stimulate the proliferation of gut bacteria, thereby enhancing microbial diversity [
83]. Although these approaches may aid in maintaining remission, they appear to be less effective in halting active disease flares.
Matsuoka [
84] reviewed four trials evaluating fecal microbiota transplantation (FMT) in the management of ulcerative colitis. Three of these trials concluded that FMT is an effective therapy. However, certain parameters remain undetermined, such as optimal donor selection and the frequency of administration, indicating that FMT protocols still require refinement. Additionally, long-term follow-up is necessary to determine whether FMT provides only a temporary solution or offers sustained improvement over time.
In their review, Sahoo et al. [
85] proposed improved management strategies for inflammatory bowel disease through advancements in treatment formulations. The gastrointestinal tract exhibits a higher pH, which may facilitate localized delivery of therapeutic agents, thereby minimizing systemic adverse effects. Examples of such delivery systems include pH-sensitive polymers and hydrogels. Additionally, a controlled drug delivery system based on pressure has been developed, taking advantage of the increased pressure in the colon resulting from water absorption. This system involves the design of a hydrophobic pill that collapses in the colonic environment, thereby releasing active molecules. This innovative approach holds promise for the treatment of ulcerative colitis and colonic forms of Crohn’s disease.
10. Therapeutic Education of the Patient
The World Health Organization (WHO) defines therapeutic patient education as a process enabling patients to acquire and retain knowledge and skills related to their disease, thereby improving disease management and quality of life. This approach is particularly relevant for chronic diseases, including IBD. Sessions can be conducted individually or in groups, often involving family members. Patient education in IBD involves a multidisciplinary team, including doctors, nurses, pharmacists, nutritionists, and experienced patients. Multidisciplinary collaboration among healthcare providers is essential for the effective management of individuals living with IBD. Dietitians play a key role in modulating dietary intake, recommending appropriate nutritional strategies, and promoting beneficial components such as omega-3 fatty acids and vitamin-D-rich foods [
86,
87,
88]. Dietary interventions play a supportive role in the management of IBD. The Crohn’s Disease Exclusion Diet (CDED) aims to reduce exposure to certain components such as animal fats, emulsifiers, and maltodextrins, which may contribute to intestinal inflammation. In contrast, The Ulcerative Colitis Exclusion Diet (UCED) encourages increased fiber intake while limiting the consumption of proteins, fats, and food additives. This dietary approach aims to protect intestinal epithelial cells, preserve mucosal integrity, and support a healthy gut microbiota [
89]. The IBD Anti-Inflammatory Diet targets dysbiosis by eliminating specific carbohydrates, including refined sugars, gluten, and starches. Additionally, vegetarian, semi-vegetarian, and Mediterranean diets have shown potential in reducing gut inflammation and improving clinical outcomes [
90,
91]. The application of artificial intelligence and machine learning technologies holds promise for the early prediction of disease risk and onset, as well as for the prevention of disease progression.
Therapeutic education programs promote two main skill categories: self-care and adaptive skills. Self-care skills encompass understanding follow-up care, performing technical tasks such as self-administering biotherapies, engaging family and friends for support, adjusting lifestyle as needed, and preventing complications. Adaptive skills include building self-confidence, communicating effectively about the disease, making informed choices, and managing emotions and stress—especially during relapses.
Educational programs have been developed to enhance disease knowledge among individuals living with IBD. Many of these programs involve daily or weekly informational messages [
92], while others promote learning through illustrated books and portfolios facilitated by healthcare professionals [
93]. Although these interventions provide patients with additional information about their condition, studies indicate that they do not significantly increase knowledge beyond what is acquired through standard care without an educational program [
94].
Berding et al. [
95] describes a two-part educational program for patients living with IBD; the first part focuses on enhancing disease knowledge, while the second addresses managing fear associated with the disease. Their study demonstrates that the program improved patients’ understanding of disease-related worries and concerns. However, it does not show a significant impact on other aspects, such as quality of life or IBD activity.
While educational programs for adults show limited impact on IBD progression, pediatric programs—especially those incorporating peer mentoring among young patients—are promising for improving disease management and quality of life for youth with IBD. Additionally, programs designed for family members aim to enhance their understanding of IBD, thereby helping them support disease management and improve the quality of life for children affected by IBD [
96,
97].
11. Conclusions
IBD is a global health issue characterized by a cycle of relapse and remission. Although various therapeutic options exist, they do not provide a cure. Both conventional chemical-based therapies and biotherapies enable individuals with IBD to lead a “normal” lifestyle; however, patients must remain vigilant about potential adverse effects and factors that may contribute to disease flares, such as diet and stress. Surgery is considered a last resort and may introduce additional challenges in social, occupational, and familial contexts.
Improving the quality of life for patients with IBD is a critical objective, necessitating enhanced follow-up care, educational program, consideration of mental health, and the development of new treatment delivery methods aimed at personalized medicine. While healthcare providers are increasingly educated about IBD, there remains a need for greater clarity regarding the available treatment options for their patients. We hope this review will enhance their understanding and improve the quality of care provided to individuals with IBD.
Author Contributions
Conceptualization, C.S. and P.P.; Methodology, C.S., J.A., B.P., and L.F.; Validation, F.B. and P.P.; Writing—Original Draft Preparation, C.S., B.P., and L.F.; Writing—Review and Editing, J.A., F.B., and P.P.; Supervision, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank the students and healthcare providers for their support in developing our didactic and pedagogy research project ISM (Institut Santé et Médicaments)/HDI (Health and Drugs Institute) for Health Care Professionals and Students education.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dolinger, M.; Torres, J.; Vermeire, S. Crohn’s Disease. Lancet 2024, 403, 1177–1191. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.-F.; Peyrin-Biroulet, L. Crohn’s Disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative Colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Eliakim, A.; Fedail, S.; Fried, M.; Gearry, R.; Goh, K.-L.; Hamid, S.; Khan, A.G.; Khalif, I.; Ng, S.C.; et al. World Gastroenterology Organisation Global Guidelines Inflammatory Bowel Disease: Update August 2015. J. Clin. Gastroenterol. 2016, 50, 803–818. [Google Scholar] [CrossRef]
- Rosen, M.J.; Dhawan, A.; Saeed, S.A. Inflammatory Bowel Disease in Children and Adolescents. JAMA Pediatr. 2015, 169, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Ashton, J.J.; Beattie, R.M. Inflammatory Bowel Disease: Recent Developments. Arch. Dis. Child. 2024, 109, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Borowitz, S.M. The Epidemiology of Inflammatory Bowel Disease: Clues to Pathogenesis? Front. Pediatr. 2023, 10, 1103713. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G.; Ng, S.C. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology 2017, 152, 313–321.e2. [Google Scholar] [CrossRef]
- Caron, B.; Honap, S.; Peyrin-Biroulet, L. Epidemiology of Inflammatory Bowel Disease across the Ages in the Era of Advanced Therapies. J. Crohn’s Colitis 2024, 18, ii3–ii15. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases with Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef]
- Ogura, Y.; Bonen, D.K.; Inohara, N.; Nicolae, D.L.; Chen, F.F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.H.; et al. A Frameshift Mutation in NOD2 Associated with Susceptibility to Crohn’s Disease. Nature 2001, 411, 603–606. [Google Scholar] [CrossRef]
- Ashton, J.J.; Seaby, E.G.; Beattie, R.M.; Ennis, S. NOD2 in Crohn’s Disease-Unfinished Business. J. Crohn’s Colitis 2023, 17, 450–458. [Google Scholar] [CrossRef]
- Becker, C.; Neurath, M.F.; Wirtz, S. The Intestinal Microbiota in Inflammatory Bowel Disease. ILAR J. 2015, 56, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Stolzer, I.; Kaden-Volynets, V.; Ruder, B.; Letizia, M.; Bittel, M.; Rausch, P.; Basic, M.; Bleich, A.; Baines, J.F.; Neurath, M.F.; et al. Environmental Microbial Factors Determine the Pattern of Inflammatory Lesions in a Murine Model of Crohn’s Disease–Like Inflammation. Inflamm. Bowel Dis. 2020, 26, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Kurashima, Y.; Kiyono, H. Mucosal Ecological Network of Epithelium and Immune Cells for Gut Homeostasis and Tissue Healing. Annu. Rev. Immunol. 2017, 35, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Pullan, R.D.; Thomas, G.A.; Rhodes, M.; Newcombe, R.G.; Williams, G.T.; Allen, A.; Rhodes, J. Thickness of Adherent Mucus Gel on Colonic Mucosa in Humans and Its Relevance to Colitis. Gut 1994, 35, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef]
- Bamias, G.; Zampeli, E.; Domènech, E. Targeting Neutrophils in Inflammatory Bowel Disease: Revisiting the Role of Adsorptive Granulocyte and Monocyte Apheresis. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 721–735. [Google Scholar] [CrossRef]
- Kinchen, J.; Chen, H.H.; Parikh, K.; Antanaviciute, A.; Jagielowicz, M.; Fawkner-Corbett, D.; Ashley, N.; Cubitt, L.; Mellado-Gomez, E.; Attar, M.; et al. Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 2018, 175, 372–386.e17. [Google Scholar] [CrossRef]
- Karuppuchamy, T.; Tyler, C.J.; Lundborg, L.R.; Pérez-Jeldres, T.; Kimball, A.K.; Clambey, E.T.; Jedlicka, P.; Rivera-Nieves, J. Sphingosine-1-Phosphate Lyase Inhibition Alters the S1P Gradient and Ameliorates Crohn’s-like Ileitis by Suppressing Thymocyte Maturation. Inflamm. Bowel Dis. 2020, 26, 216–228. [Google Scholar] [CrossRef]
- Zou, F.; Wang, S.; Xu, M.; Wu, Z.; Deng, F. The Role of Sphingosine-1-Phosphate in the Gut Mucosal Microenvironment and Inflammatory Bowel Diseases. Front. Physiol. 2023, 14, 1235656. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 7618. [Google Scholar] [CrossRef] [PubMed]
- Schaubeck, M.; Clavel, T.; Calasan, J.; Lagkouvardos, I.; Haange, S.B.; Jehmlich, N.; Basic, M.; Dupont, A.; Hornef, M.; von Bergen, M.; et al. Dysbiotic Gut Microbiota Causes Transmissible Crohn’s Disease-like Ileitis Independent of Failure in Antimi-crobial Defence. Gut 2016, 65, 225–237. [Google Scholar] [CrossRef]
- Glassner, K.L.; Abraham, B.P.; Quigley, E.M.M. The Microbiome and Inflammatory Bowel Disease. J. Allergy Clin. Immunol. 2020, 145, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut Microbiota, Inflammatory Bowel Disease and Colorectal Cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in Inflammatory Bowel Disease: Pathogenic Role and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Besedin, D.; Shah, R.; Brennan, C.; Panzeri, E.; Hao Van, T.T.; Eri, R. Food Additives and Their Implication in Inflammatory Bowel Disease and Metabolic Syndrome. Clin. Nutr. ESPEN 2024, 64, 483–495. [Google Scholar] [CrossRef]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Picarelli, A.; Rossi, M.; Miyamoto, J. Inflammatory Bowel Diseases and Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3817. [Google Scholar] [CrossRef]
- To, N.; Gracie, D.J.; Ford, A.C. Systematic Review with Meta-Analysis: The Adverse Effects of Tobacco Smoking on the Natural History of Crohn’s Disease. Aliment. Pharmacol. Ther. 2016, 43, 549–561. [Google Scholar] [CrossRef]
- Harries, A.D.; Baird, A.; Rhodes, J. Non-Smoking: A Feature of Ulcerative Colitis. Br. Med. J. 1982, 284, 706. [Google Scholar] [CrossRef]
- Mahid, S.S.; Minor, K.S.; Soto, R.E.; Hornung, C.A.; Galandiuk, S. Smoking and Inflammatory Bowel Disease: A Meta-Analysis. Mayo Clin. Proc. 2006, 81, 1462–1471. [Google Scholar] [CrossRef]
- Mi, Y.; Tan, Y.; Yu, Y.; An, D.; Mo, R.; Shi, S.; Li, M. Relationship between Smoking Status and Ulcerative Colitis: A Meta-Analysis Based on a Case–Control Study. Sci. Rep. 2025, 15, 13329. [Google Scholar] [CrossRef]
- Beaugerie, L.; Massot, N.; Carbonnel, F.; Cattan, S.; Gendre, J.-P.; Cosnes, J. Impact of Cessation of Smoking on the Course of Ulcerative Colitis. AJG 2001, 96, 2113–2116. [Google Scholar] [CrossRef]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Machiels, K.; Joossens, M.; Sabino, J.; Preter, V.D.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; Immerseel, F.V.; Verbeke, K.; et al. A Decrease of the Butyrate-Producing Species Roseburia Hominis and Faecalibacterium Prausnitzii Defines Dysbiosis in Patients with Ulcerative Colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-Phylogenetic Characterization of Microbial Community Imbalances in Human Inflammatory Bowel Diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [PubMed]
- Moller, F.T.; Andersen, V.; Wohlfahrt, J.; Jess, T. Familial Risk of Inflammatory Bowel Disease: A Population-Based Cohort Study 1977–2011. Am. J. Gastroenterol. 2015, 110, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Panés, J.; Rimola, J. Perianal Fistulizing Crohn’s Disease: Pathogenesis, Diagnosis and Therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 652–664. [Google Scholar] [CrossRef]
- Tsai, L.; McCurdy, J.D.; Ma, C.; Jairath, V.; Singh, S. Epidemiology and Natural History of Perianal Crohn’s Disease: A Systematic Review and Meta-Analysis of Population-Based Cohorts. Inflamm. Bowel Dis. 2022, 28, 1477–1484. [Google Scholar] [CrossRef]
- Narula, N.; Wong, E.C.L.; Dehghan, M.; Mente, A.; Rangarajan, S.; Lanas, F.; Lopez-Jaramillo, P.; Rohatgi, P.; Lakshmi, P.V.M.; Varma, R.P.; et al. Association of Ultra-Processed Food Intake with Risk of Inflammatory Bowel Disease: Prospective Cohort Study. BMJ 2021, 374, n1554. [Google Scholar] [CrossRef]
- Agrawal, M.; Sabino, J.; Frias-Gomes, C.; Hillenbrand, C.M.; Soudant, C.; Axelrad, J.E.; Shah, S.C.; Ribeiro-Mourão, F.; Lambin, T.; Peter, I.; et al. Early Life Exposures and the Risk of Inflammatory Bowel Disease: Systematic Review and Meta-Analyses. eClinicalMedicine 2021, 36, 100884. [Google Scholar] [CrossRef]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental Risk Factors for Inflammatory Bowel Diseases: An Umbrella Review of Meta-Analyses. Gastroenterology 2019, 157, 647–659.e4. [Google Scholar] [CrossRef]
- Nóbrega, V.G.; de Novais Silva, I.N.; Brito, B.S.; Silva, J.; da Silva, M.C.M.; Santana, G.O. The Onset of Clinical Manifestations in Inflammatory Bowel Disease Patients. Arq. Gastroenterol. 2018, 55, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, M.W.M.D.; Van Oijen, M.G.H.; Van Der Heijden, G.J.M.G.; Vleggaar, F.P.; Siersema, P.D.; Oldenburg, B. Declining Risk of Colorectal Cancer in Inflammatory Bowel Disease: An Updated Meta-Analysis of Population-Based Cohort Studies. Inflamm. Bowel Dis. 2013, 19, 789–799. [Google Scholar] [CrossRef]
- Jess, T.; Rungoe, C.; Peyrin–Biroulet, L. Risk of Colorectal Cancer in Patients with Ulcerative Colitis: A Meta-Analysis of Population-Based Cohort Studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef]
- Silverberg, M.S.; Satsangi, J.; Ahmad, T.; Arnott, I.D.; Bernstein, C.N.; Brant, S.R.; Caprilli, R.; Colombel, J.-F.; Gasche, C.; Geboes, K.; et al. Toward an Integrated Clinical, Molecular and Serological Classification of Inflammatory Bowel Disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can. J. Gastroenterol. Hepatol. 2005, 19, 5A–36A. [Google Scholar] [CrossRef]
- Levine, A.; Griffiths, A.; Markowitz, J.; Wilson, D.C.; Turner, D.; Russell, R.K.; Fell, J.; Ruemmele, F.M.; Walters, T.; Sherlock, M.; et al. Pediatric Modification of the Montreal Classification for Inflammatory Bowel Disease: The Paris Classification. Inflamm. Bowel Dis. 2011, 17, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Holdsworth, C.D.; Read, N.W. Symptoms and Stool Patterns in Patients with Ulcerative Colitis. Gut 1988, 29, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.E. From Symptom to Diagnosis: Clinical Distinctions among Various Forms of Intestinal Inflammation. Gastroenterology 2004, 126, 1518–1532. [Google Scholar] [CrossRef]
- Vavricka, S.R.; Rogler, G.; Gantenbein, C.; Spoerri, M.; Prinz Vavricka, M.; Navarini, A.A.; French, L.E.; Safroneeva, E.; Fournier, N.; Straumann, A.; et al. Chronological Order of Appearance of Extraintestinal Manifestations Relative to the Time of IBD Diagnosis in the Swiss Inflammatory Bowel Disease Cohort. Inflamm. Bowel Dis. 2015, 21, 1794–1800. [Google Scholar] [CrossRef]
- Fumery, M.; Xiaocang, C.; Dauchet, L.; Gower-Rousseau, C.; Peyrin-Biroulet, L.; Colombel, J.-F. Thromboembolic Events and Cardiovascular Mortality in Inflammatory Bowel Diseases: A Meta-Analysis of Observational Studies. J. Crohn’s Colitis 2014, 8, 469–479. [Google Scholar] [CrossRef]
- Flynn, S.; Eisenstein, S. Inflammatory Bowel Disease Presentation and Diagnosis. Surg. Clin. N. Am. 2019, 99, 1051–1062. [Google Scholar] [CrossRef]
- Yu, Y.R.; Rodriguez, J.R. Clinical Presentation of Crohn’s, Ulcerative Colitis, and Indeterminate Colitis: Symptoms, Extraintestinal Manifestations, and Disease Phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef]
- Travis, S.P.L.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.-F.; Feagan, B.G.; Hanauer, S.B.; Lémann, M.; Lichtenstein, G.R.; et al. Developing an Instrument to Assess the Endoscopic Severity of Ulcerative Colitis: The Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012, 61, 535–542. [Google Scholar] [CrossRef]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F. Development of a Crohn’s Disease Activity Index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976, 70, 439–444. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Hashash, J.G. A Comprehensive Review and Update on Crohn’s Disease. Dis.-A-Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef] [PubMed]
- Bakkaloğlu, O.K.; Çelik, A.F. Differential Diagnosis of Inflammatory Bowel Disease. J. Enterocolitis 2025, 4, S20–S27. [Google Scholar] [CrossRef]
- D’Haens, G.; Sandborn, W.J.; Feagan, B.G.; Geboes, K.; Hanauer, S.B.; Irvine, E.J.; Lémann, M.; Marteau, P.; Rutgeerts, P.; Schölmerich, J.; et al. A Review of Activity Indices and Efficacy End Points for Clinical Trials of Medical Therapy in Adults with Ulcerative Colitis. Gastroenterology 2007, 132, 763–786. [Google Scholar] [CrossRef]
- Sieber, A.; Aberra, F.N.; Bonhomme, B.; McKeever, L.; Lewis, J.D. Influence of Corticosteroid Use on Short- and Long-Term Outcomes of Biologic Therapy for Inflammatory Bowel Diseases. Dig. Dis. Sci. 2022, 67, 5168–5176. [Google Scholar] [CrossRef]
- Salice, M.; Rizzello, F.; Calabrese, C.; Calandrini, L.; Gionchetti, P. A Current Overview of Corticosteroid Use in Active Ulcerative Colitis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 557–561. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated Oral 5-Aminosalicylic Acid Therapy for Mildly to Moderately Active Ulcerative Colitis. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Gros, B.; Kaplan, G.G. Ulcerative Colitis in Adults: A Review. JAMA 2023, 330, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Nguyen, T.M.; Parker, C.E.; Feagan, B.G.; MacDonald, J.K. Oral 5-aminosalicylic Acid for Induction of Remission in Ulcerative Colitis. Cochrane Database Syst. Rev. 2020, 8, CD000543. [Google Scholar] [CrossRef] [PubMed]
- Lichtiger, S.; Present, D.H.; Kornbluth, A.; Gelernt, I.; Bauer, J.; Galler, G.; Michelassi, F.; Hanauer, S. Cyclosporine in Severe Ulcerative Colitis Refractory to Steroid Therapy. N. Engl. J. Med. 1994, 330, 1841–1845. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- de Boer, N.K.H.; Peyrin-Biroulet, L.; Jharap, B.; Sanderson, J.D.; Meijer, B.; Atreya, I.; Barclay, M.L.; Colombel, J.-F.; Lopez, A.; Beaugerie, L.; et al. Thiopurines in Inflammatory Bowel Disease: New Findings and Perspectives. J. Crohn’s Colitis 2018, 12, 610–620. [Google Scholar] [CrossRef]
- Singh, A.; Mahajan, R.; Kedia, S.; Dutta, A.K.; Anand, A.; Bernstein, C.N.; Desai, D.; Pai, C.G.; Makharia, G.; Tevethia, H.V.; et al. Use of Thiopurines in Inflammatory Bowel Disease: An Update. Intest. Res. 2021, 20, 11–30. [Google Scholar] [CrossRef]
- Coskun, M.; Steenholdt, C.; de Boer, N.K.; Nielsen, O.H. Pharmacology and Optimization of Thiopurines and Methotrexate in Inflammatory Bowel Disease. Clin. Pharmacokinet. 2016, 55, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Lago, P.; Faria, R.; Torres, T. Safety of Anti-TNF Therapies in Immune-Mediated Inflammatory Diseases: Focus on Infections and Malignancy. Drug Dev. Res. 2015, 76, 419–427. [Google Scholar] [CrossRef]
- Smith, M.A.; Mohammad, R.A. Vedolizumab: An A4β7 Integrin Inhibitor for Inflammatory Bowel Diseases. Ann. Pharmacother. 2014, 48, 1629–1635. [Google Scholar] [CrossRef]
- Rath, T.; Billmeier, U.; Ferrazzi, F.; Vieth, M.; Ekici, A.; Neurath, M.F.; Atreya, R. Effects of Anti-Integrin Treatment with Vedolizumab on Immune Pathways and Cytokines in Inflammatory Bowel Diseases. Front. Immunol. 2018, 9, 1700. [Google Scholar] [CrossRef]
- Vuyyuru, S.K.; Solitano, V.; Hogan, M.; MacDonald, J.K.; Zayadi, A.; Parker, C.E.; Sands, B.E.; Panaccione, R.; Narula, N.; Feagan, B.G.; et al. Efficacy and Safety of IL-12/23 and IL-23 Inhibitors for Crohn’s Disease: Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2023, 68, 3702–3713. [Google Scholar] [CrossRef]
- Herrera-deGuise, C.; Serra-Ruiz, X.; Lastiri, E.; Borruel, N. JAK Inhibitors: A New Dawn for Oral Therapies in Inflammatory Bowel Diseases. Front. Med. 2023, 10, 1089099. [Google Scholar] [CrossRef]
- Núñez, P.; Quera, R.; Yarur, A.J. Safety of Janus Kinase Inhibitors in Inflammatory Bowel Diseases. Drugs 2023, 83, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Becher, N.; Swaminath, A.; Sultan, K. A Literature Review of Ozanimod Therapy in Inflammatory Bowel Disease: From Concept to Practical Application. Ther. Clin. Risk Manag. 2022, 18, 913–927. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; D’Haens, G.; Wolf, D.C.; Jovanovic, I.; Hanauer, S.B.; Ghosh, S.; Petersen, A.; Hua, S.Y.; Lee, J.H.; et al. Ozanimod as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2021, 385, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Sleutjes, J.A.M.; de Vries, A.C.; van der Woude, C.J. Ozanimod in Crohn’s Disease: A Promising New Player. Lancet Gastroenterol. Hepatol. 2020, 5, 791–792. [Google Scholar] [CrossRef]
- Eltantawy, N.; El-Zayyadi, I.A.E.-H.; Elberry, A.A.; Salah, L.M.; Abdelrahim, M.E.A.; Kassem, A.B. A Review Article of Inflammatory Bowel Disease Treatment and Pharmacogenomics. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 35. [Google Scholar] [CrossRef]
- Roy, S.; Dhaneshwar, S. Role of Prebiotics, Probiotics, and Synbiotics in Management of Inflammatory Bowel Disease: Current Perspectives. World J. Gastroenterol. 2023, 29, 2078–2100. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K. Fecal Microbiota Transplantation for Ulcerative Colitis. Immunol. Med. 2021, 44, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, C.K.; Tripathy, S.K.; Mishra, A. Management of Inflammatory Bowel Diseases: A Review. Saudi J. Med. Pharm. Sci. 2022, 8, 622–627. [Google Scholar] [CrossRef]
- Charpentier, C.; Chan, R.; Salameh, E.; Mbodji, K.; Ueno, A.; Coëffier, M.; Guérin, C.; Ghosh, S.; Savoye, G.; Marion-Letellier, R. Dietary N-3 PUFA May Attenuate Experimental Colitis. Mediat. Inflamm. 2018, 2018, 8430614. [Google Scholar] [CrossRef]
- Kikut, J.; Konecka, N.; Ziętek, M.; Kulpa, D.; Szczuko, M. Diet Supporting Therapy for Inflammatory Bowel Diseases. Eur. J. Nutr. 2021, 60, 2275–2291. [Google Scholar] [CrossRef]
- Kikut, J.; Drozd, A.; Mokrzycka, M.; Grzybowska-Chlebowczyk, U.; Ziętek, M.; Szczuko, M. Are EPA and DHA Derivatives Involved in IBD Remission? J. Clin. Med. 2022, 11, 2388. [Google Scholar] [CrossRef]
- Radziszewska, M.; Smarkusz-Zarzecka, J.; Ostrowska, L.; Pogodziński, D. Nutrition and Supplementation in Ulcerative Colitis. Nutrients 2022, 14, 2469. [Google Scholar] [CrossRef]
- Sasson, A.N.; Ananthakrishnan, A.N.; Raman, M. Diet in Treatment of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 425–435.e3. [Google Scholar] [CrossRef]
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and Nutritional Factors in Inflammatory Bowel Diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef]
- Abutaleb, A.; Buchwald, A.; Chudy-Onwugaje, K.; Langenberg, P.; Regueiro, M.; Schwartz, D.A.; Tracy, J.K.; Ghazi, L.; Patil, S.A.; Quezada, S.M.; et al. Inflammatory Bowel Disease Telemedicine Clinical Trial: Impact of Educational Text Messages on Disease-Specific Knowledge over 1 Year. Inflamm. Bowel Dis. 2018, 24, 2191–2197. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.; Hammoudi, N.; Marthey, L.; Trang-Poisson, C.; Nachury, M.; Altwegg, R.; Grimaud, J.C.; Orempuller, S.; Hébuterne, X.; Aubourg, A.; et al. Impact of an Education Programme on IBD Patients’ Skills: Results of a Randomised Controlled Multicentre Study [ECIPE]. J. Crohn’s Colitis 2021, 15, 432–440. [Google Scholar] [CrossRef]
- Gordon, M.; Sinopoulou, V.; Ibrahim, U.; Abdulshafea, M.; Bracewell, K.; Akobeng, A.K. Patient Education Interventions for the Management of Inflammatory Bowel Disease. Cochrane Database Syst. Rev. 2023, 5, CD013854. [Google Scholar] [CrossRef] [PubMed]
- Berding, A.; Witte, C.; Gottschald, M.; Kaltz, B.; Weiland, R.; Gerlich, C.; Reusch, A.; Kruis, W.; Faller, H. Beneficial Effects of Education on Emotional Distress, Self-Management, and Coping in Patients with Inflammatory Bowel Disease: A Prospective Randomized Controlled Study. Inflamm. Intest. Dis. 2017, 1, 182–190. [Google Scholar] [CrossRef]
- Mackner, L.M.; Ruff, J.M.; Vannatta, K. Focus Groups for Developing a Peer Mentoring Program to Improve Self-Management in Pediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 487–492. [Google Scholar] [CrossRef]
- Pollock, M.D.; Brotkin, S.M.; Denio, E.; Dave, S.; Fisher, E.B.; Docherty, S.L.; Maslow, G.R. Clinical Application of a Peer Coaching Intervention to Enhance Self-Management for Adolescents and Young Adults with Inflammatory Bowel Disease. Clin. Pract. Pediatr. Psychol. 2022, 10, 409–427. [Google Scholar] [CrossRef]
- Donegan, A.; Boyle, B.; Crandall, W.; Dotson, J.L.; Lemont, C.; Moon, T.; Kim, S.C. Connecting Families: A Pediatric IBD Center’s Development and Implementation of a Volunteer Parent Mentor Program. Inflamm. Bowel Dis. 2016, 22, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





.png)


