Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases Offers 60% Five-Year Overall Survival for Low-Volume Disease
Abstract
1. Introduction
2. Methods
2.1. Patients and Inclusion Criteria
2.2. CRS and HIPEC
2.3. Data and Statistical Analysis
3. Results
3.1. Demographic and Perioperative Data
3.2. Survival Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Mohamed, F.; Cecil, T.; Moran, B.; Sugarbaker, P.H. A new standard of care for the management of peritoneal surface malignancy. Curr. Oncol. 2011, 18, 84–96. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Kyang, L.S.; Alzahrani, N.A.; Valle, S.J.; Rahman, M.K.; Arrowaili, A.; Liauw, W.; Morris, D.L. Long-term survival outcomes of cytoreductive surgery and perioperative intraperitoneal chemotherapy: Single-institutional experience with 1225 cases. J. Surg. Oncol. 2019, 120, 794–802. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritonectomy procedures. Ann. Surg. 1995, 221, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Farid, S.G.M.; Aldouri, A.F.; Morris-Stiff, G.F.; Khan, A.Z.F.; Toogood, G.J.F.; Lodge, J.P.A.F.; Prasad, K.R.F. Correlation between postoperative infective complications and Long-Term outcomes after hepatic resection for colorectal liver metastasis. Ann. Surg. 2010, 251, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Iusco, D.; Cotsoglou, C.; Guaglio, M.; Battaglia, L.; Virzì, S.; Mazzaferro, V.; Deraco, M. Should a history of extraperitoneal disease be a contraindication to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer peritoneal metastases? Dis. Colon Rectum 2018, 61, 1026–1034. [Google Scholar] [CrossRef]
- Narasimhan, V.; Britto, M.; Pham, T.; Warrier, S.; Naik, A.; Lynch, A.C.; Michael, M.; Tie, J.; Ramsay, R.; Heriot, A. Evolution of Cytoreductive Surgery and Hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: 8-Year Single-Institutional experience. Dis. Colon Rectum 2019, 62, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Hentzen, J.E.K.R.; Rovers, K.P.; Kuipers, H.; van der Plas Bsc, W.Y.; Been, L.B.; Hoogwater, F.J.H.; Van Ginkel, R.J.; Hemmer, P.H.J.; Van Dam, G.M.; De Hingh, I.H.J.T.; et al. Impact of Synchronous Versus Metachronous Onset of Colorectal Peritoneal Metastases on Survival Outcomes After Cytoreductive Surgery (CRS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC): A Multicenter, Retrospective, Observational Study. Ann. Surg. Oncol. 2019, 26, 2210–2221. [Google Scholar] [CrossRef]
- Cascales-Campos, P.A.; López-López, V.; Torres-Melero, J.; Arjona, A.; Muñoz-Casares, F.C.; Barrios, P.; Morales, R.; Pereira, F.; Bretcha-Boix, P.; González-Bayón, L.; et al. Survival outcomes in patients aged 75 years and over with peritoneal colorectal carcinomatosis after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC): Multicenter study of the Spanish Group of Peritoneal Cancer Surgery (GECOP). Clin. Transl. Oncol. 2019, 22, 130–136. [Google Scholar] [CrossRef]
- Karadayi, K.; Bostanci, M.E.; Mollaoglu, M.C.; Karabacak, U.; Yip, C.H. Cytoreductive surgery and perioperative intraperitoneal chemotherapy experience in peritoneal carcinomatosis: Single-Center analysis of 180 cases. Int. J. Surg. Oncol. 2021, 2021, 8851751. [Google Scholar] [CrossRef]
- An, S.L.; Zhang, K.; Ji, Z.H.; Li, X.B.; Yu, Y.; Zhang, Y.B.; Liu, G.; Li, B.; Yan, G.J.; Li, Y. The effect of cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy on peritoneal carcinomatosis from colorectal cancer. Chin. J. Oncol. 2021, 43, 1298–1303. (In Chinese) [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Slooten, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Ihemelandu, C.; Sugarbaker, P.H. Management for peritoneal metastasis of colonic Origin: Role of cytoreductive surgery and perioperative intraperitoneal chemotherapy: A single institution’s experience during two decades. Ann. Surg. Oncol. 2016, 24, 898–905. [Google Scholar] [CrossRef]
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A Multi-Institutional Study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef]
- Huang, C.-Q.; Min, Y.; Wang, S.-Y.; Yang, X.-J.; Liu, Y.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: A systematic review and meta-analysis of current evidence. Oncotarget 2017, 8, 55657–55683. [Google Scholar] [CrossRef] [PubMed]
- Bhandare, M.; Patil, P.; Pai, V.; Bhamre, R.; Engineer, R.; Ostwal, V.; Saklani, A. Peritoneal carcinomatosis in colorectal cancers—Management perspective needs a change. Clin. Color. Cancer 2017, 16, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Liu, S.; Ye, H.; Guo, F.; Jin, X.; Ye, S. Prognostic value of neutrophil-to-lymphocyte ratio and CA 19-9 in overall survival of patients with peritoneal carcinomatosis of colorectal cancer undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J. Surg. Oncol. 2025, 23, 220. [Google Scholar] [CrossRef]
- Taqi, E.; Alhomoud, A.; Al-Otaibi, A. Cytoreductive surgery and HIPEC in colorectal peritoneal carcinomatosis: A retrospective cohort study from a tertiary centre. Curr. Oncol. 2024, 31, 269. [Google Scholar]
- Sarfaty, E.; Khajoueinejad, N.; Yu, A.T.; Hiotis, S.; Golas, B.J.; Sarpel, U.; Labow, D.M.; Cohen, N.A. Actual 5-Year Survival After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Patients with Peritoneal Carcinomatosis of Colorectal Origin. Ann. Surg. Oncol. 2024, 31, 1970–1979. [Google Scholar] [CrossRef]
- Astruc, A.; Seegers, V.; Dumont, F.; Loaec, C.; Thibaudeau, E.; Bourgin, C.; Wernert, R.; Body, N.; De Franco, V. Can inter-observer consistency be achieved in the laparoscopic assessment of the peritoneal carcinomatosis index score in peritoneal metastasis? A pilot study. Pleura Peritoneum 2025, 10, 19–23. [Google Scholar] [CrossRef]
- Angeles, M.A.; Migliorelli, F.; Del, M.; Martínez-Gómez, C.; Daix, M.; Bétrian, S.; Gabiache, E.; Balagué, G.; Leclerc, S.; Mery, E.; et al. Concordance of laparoscopic and laparotomic peritoneal cancer index using a two-step surgical protocol to select patients for cytoreductive surgery in advanced ovarian cancer. Arch. Gynaecol. Obstet. 2021, 303, 1295–1304. [Google Scholar] [CrossRef]
- Chia, C.S.; Wong, L.C.K.; Hennedige, T.P.; Ong, W.S.; Zhu, H.-Y.; Tan, G.H.C.; Kwek, J.W.; Seo, C.J.; Wong, J.S.M.; Ong, C.-A.J.; et al. Prospective Comparison of the Performance of MRI Versus CT in the Detection and Evaluation of Peritoneal Surface Malignancies. Cancers 2022, 14, 3179. [Google Scholar] [CrossRef]
- Gege, Z.; Xueju, W.; Bin, J. Head-To-Head Comparison of 68Ga-FAPI PET/CT and FDG PET/CT for the Detection of Peritoneal Metastases: Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2023, 220, 490–498. [Google Scholar] [CrossRef]
- Wong, J.S.M.; Tan, G.H.C.; Chia, C.S.; Ong, J.; Ng, W.Y.; Teo, M.C. The importance of synchronicity in the management of colorectal peritoneal metastases with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J. Surg. Onc. 2020, 18, 10. [Google Scholar] [CrossRef]
- Schultz, K.S.; Bansal, V.V.; Wach, M.M.; Bhutiani, N.; Godley, F.A.; Wang, J.J.; Waheed, M.T.; Buchheit, J.T.; Papai, E.; Campbell, S.; et al. Peritoneal Surface Malignancies (PSM) Consortium Group. Consensus Guideline for the Management of Colorectal Cancer with Peritoneal Metastases. Ann. Surg. Oncol. 2025, 1–23. [Google Scholar] [CrossRef]
- Georgescu, D.E.; Georgescu, M.T.; Bobircă, F.T.; Georgescu, T.F.; Doran, H.; Pătraşcu, T. Synchronous Locally Advanced Rectal Cancer with Clinical Complete Remission and Important Downstaging after Neoadjuvant Radiochemotherapy—Personalised Therapeutic Approach. Chirurgia 2017, 112, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Barbáchano, A.; Rodríguez-Salas, N.; Viñal, D.; Cortés-Guiral, D.; Muñoz, A.; Fernández-Barral, A. Tailored chemotherapy for colorectal cancer peritoneal metastases based on a drug-screening platform in patient-derived organoids: A case report. J. Gastrointest. Oncol. 2023, 14, 442–449. [Google Scholar] [CrossRef] [PubMed]


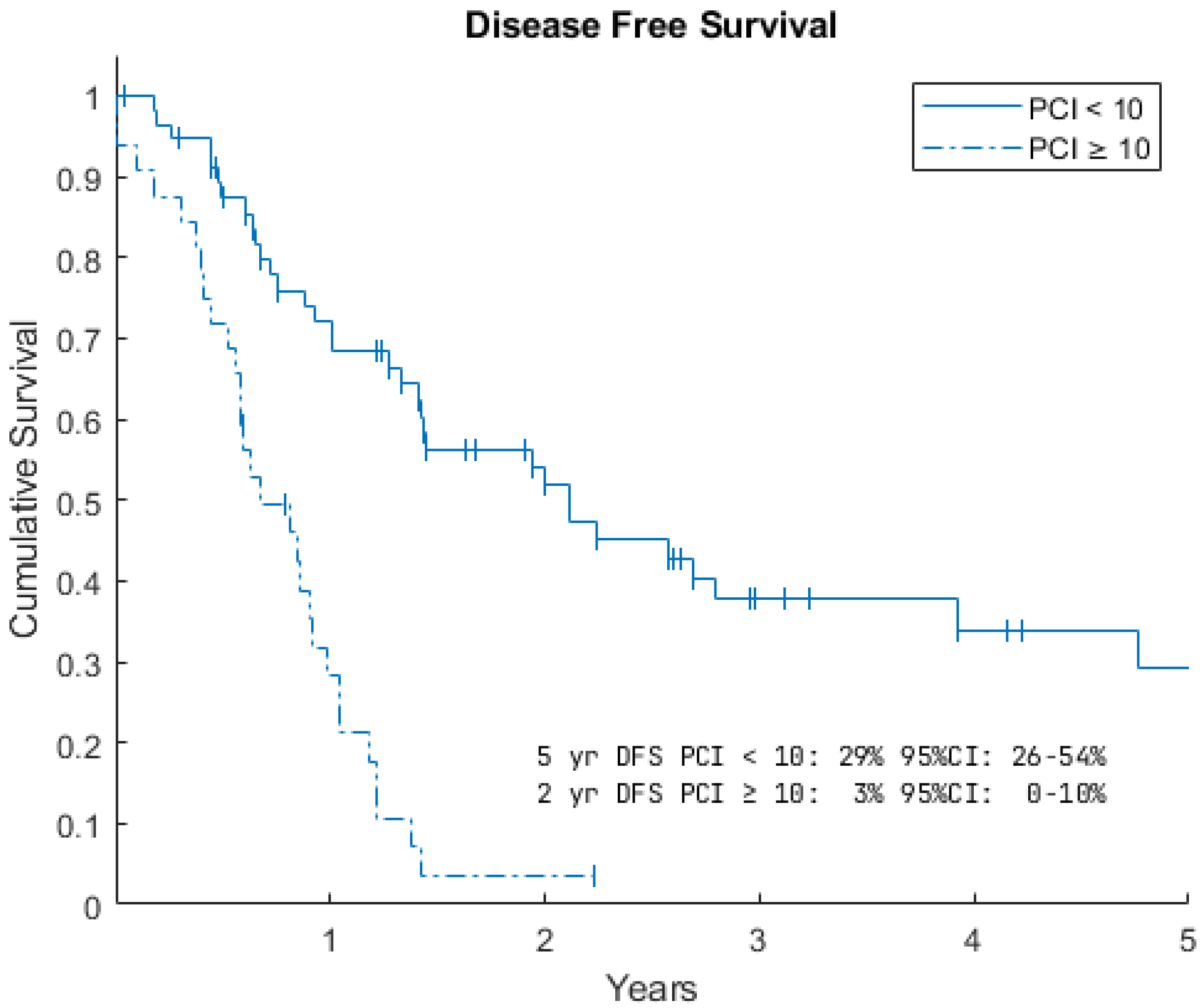
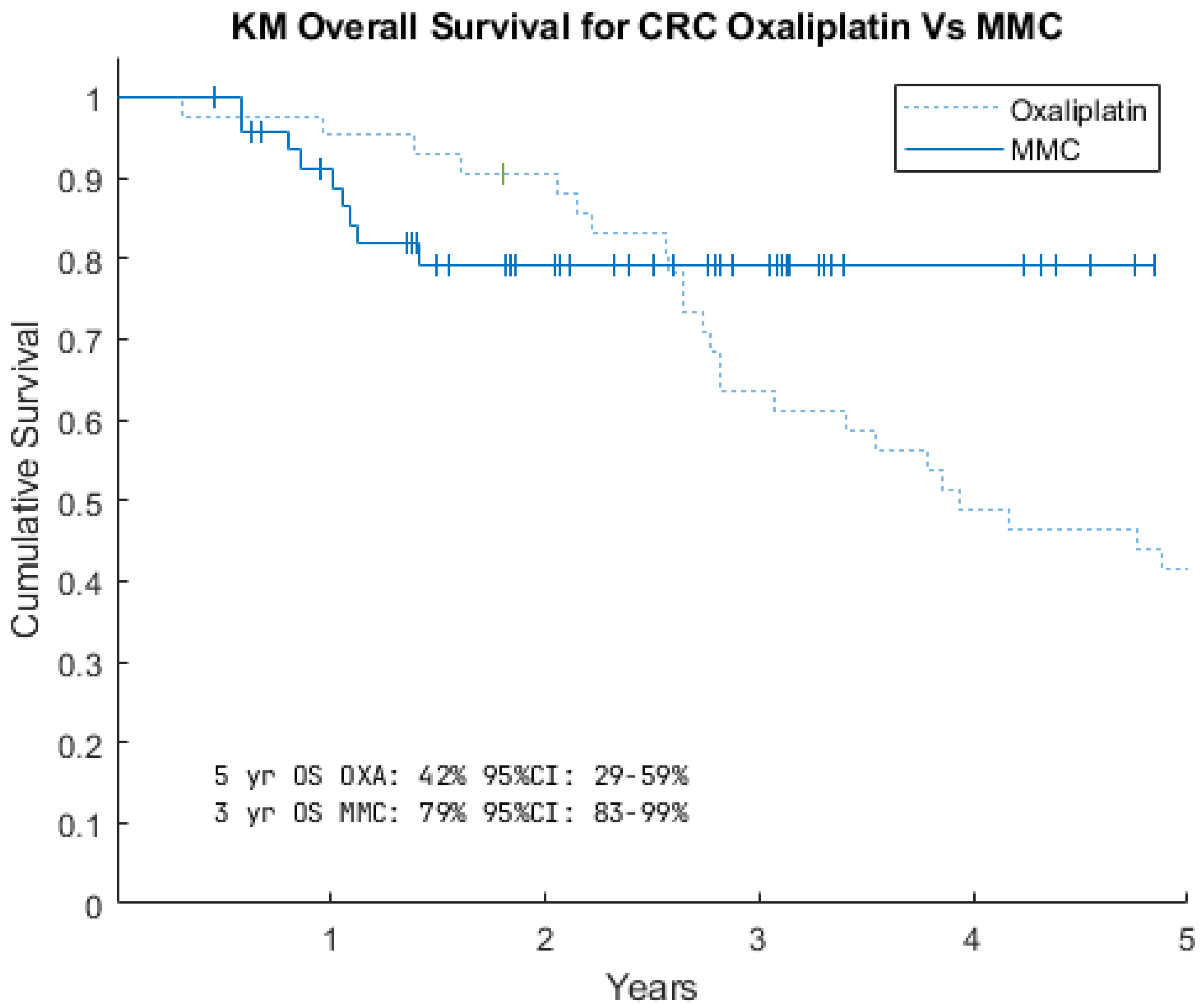
| PCI < 10 | PCI ≥ 10 | All Patients | |
|---|---|---|---|
| Number | 57 | 32 | 89 |
| Age | 58 (23–78) | 57 (34–74) | 58 (23–78) |
| Male/Female | 33:34 | 16:16 | 49:40 |
| PCI | 6 (1–9) | 14 (10–24) | 7 (1–24) |
| Synchronous/Metachronous | 12/45 | 18/14 | 30/59 |
| Op time (hours) | 9 (6–16) | 11 (8–14) | 10 (6–16) |
| Inpatient days | 13 (7–37) | 13 (9–74) | 13 (7–74) |
| Transfusion | 2 (3.5%) | 1 (3%) | 3 (3%) |
| Stoma | 16 (28%) | 16 (52%) | 32 (36%) |
| TPN | 12 (21%) | 11 (34%) | 23 (26%) |
| CD I/II | 15 (26%) | 9 (28%) | 24 (27%) |
| CD III/IV | 3 (5%) | 6 (19%) | 9 (10%) |
| Return theatre | 3 (5%) | 6 (19%) | 9 10% |
| Readmission | 8 (14%) | 6 (19%) | 14 16% |
| HR | CI (95%) | p Value | ||
|---|---|---|---|---|
| Male | 1.46 | 0.72 | 2.94 | 0.29 |
| Age > 40 | 2.42 | 0.57 | 10 | 0.23 |
| PCI ≥ 10 | 2.90 | 1.43 | 5.9 | <0.01 |
| HR | CI (95%) | p Value | ||
|---|---|---|---|---|
| Male | 1.43 | 0.82 | 2.48 | 0.20 |
| Age > 40 | 1.52 | 0.65 | 3.5 | 0.33 |
| PCI ≥ 10 | 3.25 | 1.75 | 6.0 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guirgis, M.; Sala, M.; Palan, R.; Beh, H.; Apikatoa, S.; Zubair, O.; Moroz, P. Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases Offers 60% Five-Year Overall Survival for Low-Volume Disease. Gastrointest. Disord. 2025, 7, 57. https://doi.org/10.3390/gidisord7030057
Guirgis M, Sala M, Palan R, Beh H, Apikatoa S, Zubair O, Moroz P. Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases Offers 60% Five-Year Overall Survival for Low-Volume Disease. Gastrointestinal Disorders. 2025; 7(3):57. https://doi.org/10.3390/gidisord7030057
Chicago/Turabian StyleGuirgis, Mina, Michael Sala, Ranesh Palan, Han Beh, Sharie Apikatoa, Omar Zubair, and Paul Moroz. 2025. "Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases Offers 60% Five-Year Overall Survival for Low-Volume Disease" Gastrointestinal Disorders 7, no. 3: 57. https://doi.org/10.3390/gidisord7030057
APA StyleGuirgis, M., Sala, M., Palan, R., Beh, H., Apikatoa, S., Zubair, O., & Moroz, P. (2025). Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases Offers 60% Five-Year Overall Survival for Low-Volume Disease. Gastrointestinal Disorders, 7(3), 57. https://doi.org/10.3390/gidisord7030057






