Colorectal Cancer Screening in the Middle East and North Africa: Current Practices, Challenges, and Insights from the British Society of Gastroenterology (BSG) International Section
Abstract
1. Introduction
2. Results
2.1. CRC Screening Practices in MENA Region
2.1.1. Saudi Arabia
2.1.2. United Arab Emirates (UAE)
2.1.3. Egypt
2.1.4. Jordan
2.1.5. Turkey
2.1.6. Iraq
2.2. The Increasing Cancer Burden in the MENA Region
2.3. MENA-CRC Screening and Prevention Collaborator Experts’ Feedback
3. Discussion
4. Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer (IARC) TIA for R on. Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 10 December 2024).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancers 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Schreuders, E.H.; Ruco, A.; Rabeneck, L.; Schoen, R.E.; Sung, J.J.Y.; Young, G.P.; Kuipers, E.J. Colorectal Cancer Screening: A Global Overview of Existing Programmes. Gut 2015, 64, 1637–1649. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.M.; Bray, F.; Vaccarella, S.; Soerjomataram, I. Assessing Global Transitions in Human Development and Colorectal Cancer Incidence. Int. J. Cancer 2017, 140, 2709–2715. [Google Scholar] [CrossRef]
- Atkin, W.; Wooldrage, K.; Parkin, D.M.; Kralj-Hans, I.; MacRae, E.; Shah, U.; Duffy, S.; Cross, A.J. Long-Term Effects of Once-Only Flexible Sigmoidoscopy Screening after 17 Years of Follow-Up: The UK Flexible Sigmoidoscopy Screening Randomised Controlled Trial. Lancet 2017, 389, 1299–1311. [Google Scholar] [CrossRef]
- Citarda, F.; Tomaselli, G.; Capocaccia, R.; Barcherini, S.; Crespi, M.; Group TIMS. Efficacy in Standard Clinical Practice of Colonoscopic Polypectomy in Reducing Colorectal Cancer Incidence. Gut 2001, 48, 812–815. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Yoon, M.; Kim, N.; Nam, B.; Joo, J.; Ki, M. Changing Trends in Colorectal Cancer in the Republic of Korea: Contrast with Japan. Epidemiol. Health 2015, 37, e2015038. [Google Scholar] [CrossRef][Green Version]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global Burden of Colorectal Cancer in 2020 and 2040: Incidence and Mortality Estimates from GLOBOCAN. Gut 2022, 72, 338–344. [Google Scholar] [CrossRef]
- Stryker, S.J.; Wolff, B.G.; Culp, C.E.; Libbe, S.D.; Ilstrup, D.M.; MacCarty, R.L. Natural History of Untreated Colonic Polyps. Gastroenterology 1987, 93, 1009–1013. [Google Scholar] [CrossRef]
- Wilson, J.M.; Jungner, Y.G. Principles and Practice of Mass Screening for Disease. Boletín Oficina Sanit. Panam. Pan Am. Sanit. Bur. 1968, 65, 281–393. [Google Scholar]
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Waye, J.D.; Schapiro, M.; Bond, J.H.; Panish, J.F.; et al. Prevention of Colorectal Cancer by Colonoscopic Polypectomy. N. Engl. J. Med. 1993, 329, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Gini, A.; Jansen, E.E.L.; Zielonke, N.; Meester, R.G.S.; Senore, C.; Anttila, A.; Segnan, N.; Mlakar, D.N.; de Koning, H.J.; Lansdorp-Vogelaar, I.; et al. Impact of Colorectal Cancer Screening on Cancer-Specific Mortality in Europe: A Systematic Review. Eur. J. Cancer 2020, 127, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Perdue, L.A.; Henrikson, N.B.; Bean, S.I.; Blasi, P.R. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021, 325, 1978–1998. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, B.; Yang, M.; Yuan, H.; Li, X.; Zheng, X.; Wang, L.; Fan, X.; Zhou, W. A three-plasma miRNA panel predicts the risk of colorectal cancer: A community-based nested case–control study. Sci. Rep. 2023, 13, 4196. [Google Scholar] [CrossRef]
- Takashima, Y.; Shimada, T.; Yokozawa, T. Clinical benefit of measuring both haemoglobin and transferrin concentrations in faeces: Demonstration during a large-scale colorectal cancer screening trial in Japan. Diagnosis 2015, 2, 53–59. [Google Scholar] [CrossRef]
- Dwyer, D.M.; Groves, C.; Hopkins, A.; Lane, D.S.; Ransohoff, D.F. Experience of a Public Health Colorectal Cancer Testing Program in Maryland. Public Health Rep. 2012, 127, 330–339. [Google Scholar] [CrossRef]
- Nishiumi, S.; Kobayashi, T.; Kawana, S.; Yoshida, M.; Azuma, T. Investigations in the possibility of early detection of colorectal cancer by gas chromatography/triple-quadrupole mass spectrometry. Oncotarget 2017, 8, 17115–17126. [Google Scholar] [CrossRef]
- Brenner, H.; Stock, C.; Hoffmeister, M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014, 348, g2467. [Google Scholar] [CrossRef]
- von Karsa, L.; Patnick, J.; Segnan, N. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition—Executive summary. Endoscopy 2012, 44 (Suppl. S3), SE1–SE8. [Google Scholar] [CrossRef]
- Levin, B.; Lieberman, D.A.; McFarland, B.; Smith, R.A.; Brooks, D.; Andrews, K.S.; Dash, C.; Giardiello, F.M.; Glick, S.; Levin, T.R.; et al. Screening and Surveillance for the Early Detection of Colorectal Cancer and Adenomatous Polyps, 2008: A Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J. Clin. 2008, 58, 130–160. [Google Scholar] [CrossRef]
- Sung, J.J.Y.; Ng, S.C.; Chan, F.T.S.; Chiu, H.-M.; Kim, H.-C.; Matsuda, T.; Ng, S.S.M.; Lau, J.; Zheng, S.; Adler, S.N.; et al. An Updated Asia-Pacific Consensus Recommendations on Colorectal Cancer Screening. Gut 2015, 64, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Canadian Task Force on Preventive Health Care. Colorectal cancer screening. Recommendation statement from the Canadian Task Force on Preventive Health Care. CMAJ 2001, 165, 206–208. [Google Scholar]
- Săftoiu, A.; Hassan, C.; Areia, M.; Bhutani, M.S.; Bisschops, R.; Bories, E.; Cazacu, I.M.; Dekker, E.; Deprez, P.H.; Pereira, S.P.; et al. Role of Gastrointestinal Endoscopy in the Screening of Digestive Tract Cancers in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2020, 52, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Bénard, F.; Barkun, A.N.; Martel, M.; Renteln, D.V. Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations. World J. Gastroenterol. 2018, 24, 124–138. [Google Scholar] [CrossRef]
- Granger, S.P.; Preece, R.A.D.; Thomas, M.G.; Dixon, S.W.; Chambers, A.C.; Messenger, D.E. Colorectal cancer incidence trends by tumour location among adults of screening-age in England: A population-based study. Color. Dis. 2023, 25, 1771–1782. [Google Scholar] [CrossRef]
- NHS. Bowel Cancer Screening—NHS. Available online: https://www.nhs.uk/conditions/bowel-cancer-screening/ (accessed on 10 December 2024).
- US Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021, 325, 1965–1977. [Google Scholar] [CrossRef]
- Cancer Control Institute. Colon Cancer. Available online: https://canscreen.ncc.go.jp/guideline/daicyougan.html (accessed on 15 December 2024).
- Dey, S.; Soliman, A.S. Cancer in the global health era: Opportunities for the Middle East and Asia. Asia Pac. J. Public Health 2010, 22 (Suppl. S3), 75S–82S. [Google Scholar] [CrossRef]
- Al-Faluji, A.A.R.; Ali, S.H.; Al-Esawi, A.A.J. Incidence of Cancer in Fallujah above 10 Years Age with Overview of Common Cancers in 2011. Health 2012, 4, 591–596. [Google Scholar] [CrossRef][Green Version]
- Tfaily, M.A.; Naamani, D.; Kassir, A.; Sleiman, S.; Ouattara, M.; Moacdieh, M.P.; Jaffa, M.A. Awareness of Colorectal Cancer and Attitudes Towards Its Screening Guidelines in Lebanon. Ann. Glob. Health 2019, 85, 75. [Google Scholar] [CrossRef]
- Aydogan Gedik, S.; Metintas, S.; Onsuz, M.F. Recognition and Participation of Colorectal Cancer Screening in Turkiye: A Systematic Review and Meta-Analysis Study. North Clin. Istanb. 2023, 10, 819–829. [Google Scholar] [CrossRef]
- Digital, T. ADPHC Revises Recommendations for Early Colorectal Cancer Screening. Available online: https://www.doh.gov.ae/en/news/ADPHC-revises-recommendations-for-early-colorectal-cancer-screening (accessed on 15 December 2024).
- Salimzadeh, H.; Sauvaget, C.; Delavari, A.; Sadeghi, A.; Amani, M.; Salimzadeh, S.; Karimi, A.; Ghanbari Motlagh, A.; Lucas, E.; Basu, P.; et al. Colorectal Cancer Screening Pilot Project in Tehran-Iran, a Feasibility Study. Arch. Iran. Med. 2023, 26, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Abdulelah, Z.A.; Abdulelah, A.A.; Alqaisieh, M.; Khanfar, A.N.; Hammad, N.H.; Al Masoud, E.B.; Alshrouf, M.A.; Abuaisheh, R.M.; Abd-Alrahman, H.M.; Khatib, A.; et al. National Survey of Barriers to Colorectal Cancer Screening in Jordan. East. Mediterr. Health J. 2024, 30, 125–135. Available online: https://www.emro.who.int/emhj-volume-30-2024/volume-30-issue-2/national-survey-of-barriers-to-colorectal-cancer-screen-ing-in-jordan.html#:~:text=Even%20though%20CRC%20ranks%20highly,the%20at%2Drisk%20Jordanian%20population (accessed on 15 December 2024). [CrossRef]
- Michel, A.; Pumpalova, Y. Colorectal Cancer Screening Programs in Middle-Income Countries: A Scoping Review. Med. Res. Arch. 2023, 11. [Google Scholar] [CrossRef]
- Bateman, L.B.; Khamess, S.; Abdelmoneim, S.-E.; Arafat, W.; Fouad, M.N.; Khamis, Y.; Omar, A.; Abdelmoneim, R.S.; Scarinci, I. Designing an Effective Colorectal Cancer Screening Program in Egypt: A Qualitative Study of Perceptions of Egyptian Primary Care Physicians and Specialists. Oncology 2020, 25, e1525–e1531. [Google Scholar] [CrossRef] [PubMed]
- Alessy, S.A.; Alqahtani, S.A.; Vignat, J.; Abuhmaidan, A.A.; Al Lawati, N.; A-Nooh, A.A.; Shelpai, W.; Alhomoud, S.; Al-Zahrani, A.; Bray, F.; et al. The Current and Future Cancer Burden in the Gulf Cooperation Council (GCC) Countries. Cancer Med. 2024, 13, e70141. [Google Scholar] [CrossRef]
- Ghorbanoghli, Z.; Jabari, C.; Sweidan, W.; Hammoudeh, W.; Cortas, G.; Sharara, A.I.; Abedrabbo, A.; Hourani, I.; Mahjoubi, B.; Majidzadeh-A, K.; et al. A New Hereditary Colorectal Cancer Network in the Middle East and Eastern Mediterranean Countries to Improve Care for High-Risk Families. Fam. Cancer 2017, 17, 209–212. [Google Scholar] [CrossRef]
- Sina, M.; Ghorbanoghli, Z.; Abedrabbo, A.; Al-Mulla, F.; Ben Sghaier, R.; Buisine, M.-P.; Cortas, G.; Goshayeshi, L.; Hadjisavvas, A.; Hammoudeh, W.; et al. Identification and Management of Lynch Syndrome in the Middle East and North African Countries: Outcome of a Survey in 12 Countries. Fam. Cancer 2021, 20, 215–221. [Google Scholar] [CrossRef]
- Almadi, M.A.; Basu, P. Doing Things Right and Doing the Right Things: Colorectal Cancer Screening in Saudi Arabia. Saudi J. Gastroenterol. 2023, 29, 67. [Google Scholar] [CrossRef]
- Zacharakis, G.; Almasoud, A.; Arahmaner, O.; Aldossary, K.; Alzahrani, J.; Al-Ghamdi, S.; AlShehri, A.; Nikolaidis, P.; Bawazir, A.; Alfayez, T.; et al. A 5-Year Evaluation of Early- and Late-Onset Sporadic Colorectal Cancer Screening in Central Saudi Arabia. Saudi J. Gastroenterol. 2023, 29, 95–101. [Google Scholar] [CrossRef]
- Almadi, M.A.; Mosli, M.H.; Bohlega, M.S.; Al Essa, M.A.; AlDohan, M.S.; Alabdallatif, T.A.; AlSagri, T.Y.; Algahtani, F.A.; Mandil, A. Effect of Public Knowledge, Attitudes, and Behavior on Willingness to Undergo Colorectal Cancer Screening Using the Health Belief Model. Saudi J. Gastroenterol. 2015, 21, 71–77. [Google Scholar] [CrossRef]
- Alsaad, L.N.; Sreedharan, J. Practice of colorectal cancer screening in the United Arab Emirates and factors associated—A cross-sectional study. BMC Public Health 2023, 23, 2015. [Google Scholar] [CrossRef]
- Allam, A.R.; Elsayed, M.A.; Daghash, I.T.; Abdelaziz, A.M.; Mostafa, O.M.; Sabra, H.K.; Eldaboush, A.M.; Elweza, R.T.; Adwy, E.S.; Hammad, A.E.; et al. Colonoscopy Screening for Colorectal Cancer in Egypt: A Nationwide Cross-Sectional Study. BMC Cancer 2024, 24, 131. [Google Scholar] [CrossRef]
- Jadallah, K.; Khatatbeh, M.; Mazahreh, T.; Sweidan, A.; Ghareeb, R.; Tawalbeh, A.; Masaadeh, A.; Alzubi, B.; Khader, Y. Colorectal Cancer Screening Barriers and Facilitators among Jordanians: A Cross-Sectional Study. Prev. Med. Rep. 2023, 32, 102149. [Google Scholar] [CrossRef] [PubMed]
- Gulten, G.; Memnun, S.; Ayse, K.; Aygul, A.; Gulcin, A. Breast, cervical, and colorectal cancer screening status of a group of Turkish women. Asian Pac. J. Cancer Prev. 2012, 13, 4273–4279. [Google Scholar] [CrossRef]
- Taş, F.; Kocaöz, S.; Çirpan, R. The Effect of Knowledge and Health Beliefs about Colorectal Cancer on Screening Behaviour. J. Clin. Nurs. 2019, 28, 4471–4477. [Google Scholar] [CrossRef] [PubMed]
- Alrubaiy, L.; Al-Rubaye, A.; Alrudainy, W.; Al-Hawaz, M.H.; Mahmoud, R.A.; Saunders, B.P. Colonoscopy Colorectal Cancer Screening Programme in Southern Iraq: Challenges, Knowledge Gaps and Future Potential. J. Pers. Med. 2023, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Alessa, A.M.; Khan, A.S.; Alessa, A.M.; Khan, A.S. Epidemiology of Colorectal Cancer in Saudi Arabia: A Review. Cureus 2024, 16, e64564. [Google Scholar] [CrossRef]
- Alsadhan, N.; Alhurishi, S.A.; Pujades-Rodriguez, M.; Shuweihdi, F.; Brennan, C.; West, R.M. Demographic and Clinical Characteristics Associated with Advanced Stage Colorectal Cancer: A Registry-Based Cohort Study in Saudi Arabia. BMC Cancer 2024, 24, 533. [Google Scholar] [CrossRef]
- Alqarni, S.M.H.; Alamri, M.S.; Pushparaj, P.N.; Rather, I.; Iqbal, Z.; Asif, M.; Rasool, M. Screening, Awareness and Challenges for Colorectal Cancer Treatment in Saudi Arabia: An Update. Bioinformation 2024, 20, 397–403. [Google Scholar] [CrossRef]
- Alabdulkader, A.M.; Mustafa, T.; Almutailiq, D.A.; Al-Maghrabi, R.A.; Alzanadi, R.H.; Almohsen, D.S.; Alkaltham, N.K. Knowledge and Barriers to Screening for Colorectal Cancer among Individuals Aged 40 Years or Older Visiting Primary Healthcare Clinics in Al-Khobar, Eastern Province. J. Fam. Community Med. 2024, 31, 25–35. [Google Scholar] [CrossRef]
- Almadi, M.A.; Alghamdi, F. The gap between knowledge and undergoing colorectal cancer screening using the Health Belief Model: A national survey. Saudi J. Gastroenterol. 2019, 25, 27–39. [Google Scholar] [CrossRef]
- Harbi, A.Z.; Belaila, B.A.B.; Shelpai, W.; Razzak, H.A. UAE National Cancer Registry. In Cancer Care in the United Arab Emirates; Al-Shamsi, H.O., Ed.; Springer Nature: Singapore, 2024; pp. 57–77. [Google Scholar] [CrossRef]
- Almansoori, A.; Alzaabi, M.; Alketbi, L. Colorectal Cancer screening in ambulatory healthcare service clinics in Abu Dhabi, United Arab Emirates in 2015–2016. BMC Cancer 2021, 21, 897. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Abbasi-Kangevari, M.; Abd-Rabu, R.; Abidi, H.; Abu-Gharbieh, E.; Acuna, J.M.; Adhikari, S.; Advani, S.M.; Afzal, M.S.; Meybodi, M.A.; et al. Global, Regional, and National Burden of Colorectal Cancer and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.S.; Bondy, M.L.; Hamilton, S.R.; Levin, B. Colon cancer in young Egyptian patients. Am. J. Gastroenterol. 1999, 94, 1114. [Google Scholar] [CrossRef] [PubMed]
- Egypt Launches Early Cancer Detection Initiative—Health—Egypt—Ahram Online. Available online: https://english.ahram.org.eg/NewsContent/1/1236/502775/Egypt/Health/Egypt-launches-early-cancer-detection-initiative.aspx (accessed on 15 December 2024).
- Sysmex Egypt Goes Hand in Hand with Ministry of Health and Population for National Colorectal Cancer Screening Program. Available online: https://www.sysmex-europe.com/company/news-and-events/news-listings/news-details/sysmex-egypt-goes-hand-in-hand-with-ministry-of-health-and-population-for-national-colorectal-cancer-screening-program/ (accessed on 21 December 2024).
- Damsees, R.; Jaghbir, M.; Salam, M.; Al-Omari, A.; Al-Rawashdeh, N. Unravelling the predictors of late cancer presentation and diagnosis in Jordan: A cross-sectional study of patients with lung and colorectal cancers. BMJ Open 2023, 13, e069529. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- TURKEY CANCER CONTROL PROGRAMME | ICCP Portal. Available online: https://www.iccp-portal.org/plans/turkey-cancer-control-programme (accessed on 19 December 2024).
- Ibrahem, S.; Ahmed, H.; Zangana, S. Trends in Colorectal Cancer in Iraq over Two Decades: Incidence, Mortality, Topography and Morphology. Ann. Saudi Med. 2022, 42, 252–261. [Google Scholar] [CrossRef]
- Hawkes, N.; Al-Rubaiy, L.; Hawkes, B.; Aziz, M. PTH-046 Benchmarking Endoscopy Services in Iraq—Results of a National Survey. Gut 2014, 63 (Suppl. S1), A229. [Google Scholar] [CrossRef]
- Al Hilfi, T.K.; Lafta, R.; Burnham, G. Health Services in Iraq. Lancet 2013, 381, 939–948. [Google Scholar] [CrossRef]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128.9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Alhomoud, S.; Al-Othman, S.; Al-Madouj, A.; Homsi, M.A.; AlSaleh, K.; Balaraj, K.; Alajmi, A.; Basu, P.; Al-Zahrani, A. Progress and Remaining Challenges for Cancer Control in the Gulf Cooperation Council. Lancet Oncol. 2022, 23, e493–e501. [Google Scholar] [CrossRef]
- Alawa, J.; Coutts, A.; Khoshnood, K. Cancer Care in Low- and Middle-Income Countries Affected by Humanitarian Crises. In Handbook of Healthcare in the Arab World; Laher, I., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 539–574. [Google Scholar] [CrossRef]
- Shamseddine, A.; Chehade, L.; Al Mahmasani, L.; Charafeddine, M. Colorectal Cancer Screening in the Middle East: What, Why, Who, When, and How? Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e390520. [Google Scholar] [CrossRef]
- Rawaf, S.; Dubois, E.; Khatib, O.M.N.; Omar, S. Cancer Prevention and Control in Eastern Mediterranean Region. BMJ 2006, 333, 860–861. [Google Scholar] [CrossRef][Green Version]
- Hajjar, R.R.; Atli, T.; Al-Mandhari, Z.; Oudrhiri, M.; Balducci, L.; Silbermann, M. Prevalence of Aging Population in the Middle East and Its Implications on Cancer Incidence and Care. Ann. Oncol. 2013, 24 (Suppl. S7), vii11–vii24. [Google Scholar] [CrossRef] [PubMed]
- World Gastroenterology Organisation (WGO). Available online: https://www.worldgastroenterology.org (accessed on 20 December 2024).
- Dolan, J.G.; Boohaker, E.; Allison, J.; Imperiale, T.F. Patients’ Preferences and Priorities Regarding Colorectal Cancer Screening. Med. Decis. Mak. 2013, 33, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Adefemi, K.; Knight, J.C.; Zhu, Y.; Wang, P.P. Racial and Sociodemographic Distribution of Colorectal Cancer Screening in Canada: A Cross-Sectional Study. Can. J. Public Health 2024, 115, 371–383. [Google Scholar] [CrossRef] [PubMed]
- GOV.UK. Bowel Cancer Screening Annual Report 2021 to 2022. Available online: https://www.gov.uk/government/publications/bowel-cancer-screening-annual-report-2021-to-2022/bowel-cancer-screening-annual-report-2021-to-2022 (accessed on 20 December 2024).
- Sokale, I.O.; Rosales, O.; Montealegre, J.R.; Oluyomi, A.O.; Thrift, A.P. Trends in Up-To-Date Colorectal Cancer Screening Among U.S. Adults Aged 50–75 Years and Variations by Race/Ethnicity and U.S. Census Bureau Divisions. AJPM Focus 2023, 2, 100055. [Google Scholar] [CrossRef]
- Aljumah, A.A.; Aljebreen, A.M. Policy of Screening for Colorectal Cancer in Saudi Arabia: A Prospective Analysis. Saudi J. Gastroenterol. 2017, 23, 161. [Google Scholar] [CrossRef]
- Gimeno Garcia, A.Z.; Hernandez Alvarez Buylla, N.; Nicolas-Perez, D.; Quintero, E. Public Awareness of Colorectal Cancer Screening: Knowledge, Attitudes, and Interventions for Increasing Screening Uptake. ISRN Oncol. 2014, 2014, 425787. [Google Scholar] [CrossRef]
- Arnold, C.L.; Rademaker, A.; Liu, D.; Davis, T.C. Changes in Colorectal Cancer Screening Knowledge, Behavior, Beliefs, Self-Efficacy, and Barriers among Community Health Clinic Patients after a Health Literacy Intervention. J. Community Med. Health Educ. 2017, 7, 497. [Google Scholar] [CrossRef]
- Demyati, E. Knowledge, Attitude, Practice, and Perceived Barriers of Colorectal Cancer Screening among Family Physicians in National Guard Health Affairs, Riyadh. Int. J. Fam. Med. 2014, 2014, 457354. [Google Scholar] [CrossRef]
- Khatib, O. Noncommunicable Diseases: Risk Factors and Regional Strategies for Prevention and Care. East Mediterr. Health J. 2004, 10, 778–788. [Google Scholar] [CrossRef]
- Miles, A.; Cockburn, J.; Smith, R.A.; Wardle, J. A Perspective from Countries Using Organized Screening Programs. Cancer 2004, 101, 1201–1213. [Google Scholar] [CrossRef]
- Canadian Task Force on Preventive Health Care. Colorectal Cancer (2016). Available online: https://canadiantaskforce.ca/guidelines/published-guidelines/colorectal-cancer/ (accessed on 20 December 2024).
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Ran, T.; Cheng, C.Y.; Misselwitz, B.; Brenner, H.; Ubels, J.; Schlander, M. Cost-Effectiveness of Colorectal Cancer Screening Strategies—A Systematic Review. Clin. Gastroenterol. Hepatol. 2019, 17, 1969–1981.e15. [Google Scholar] [CrossRef]
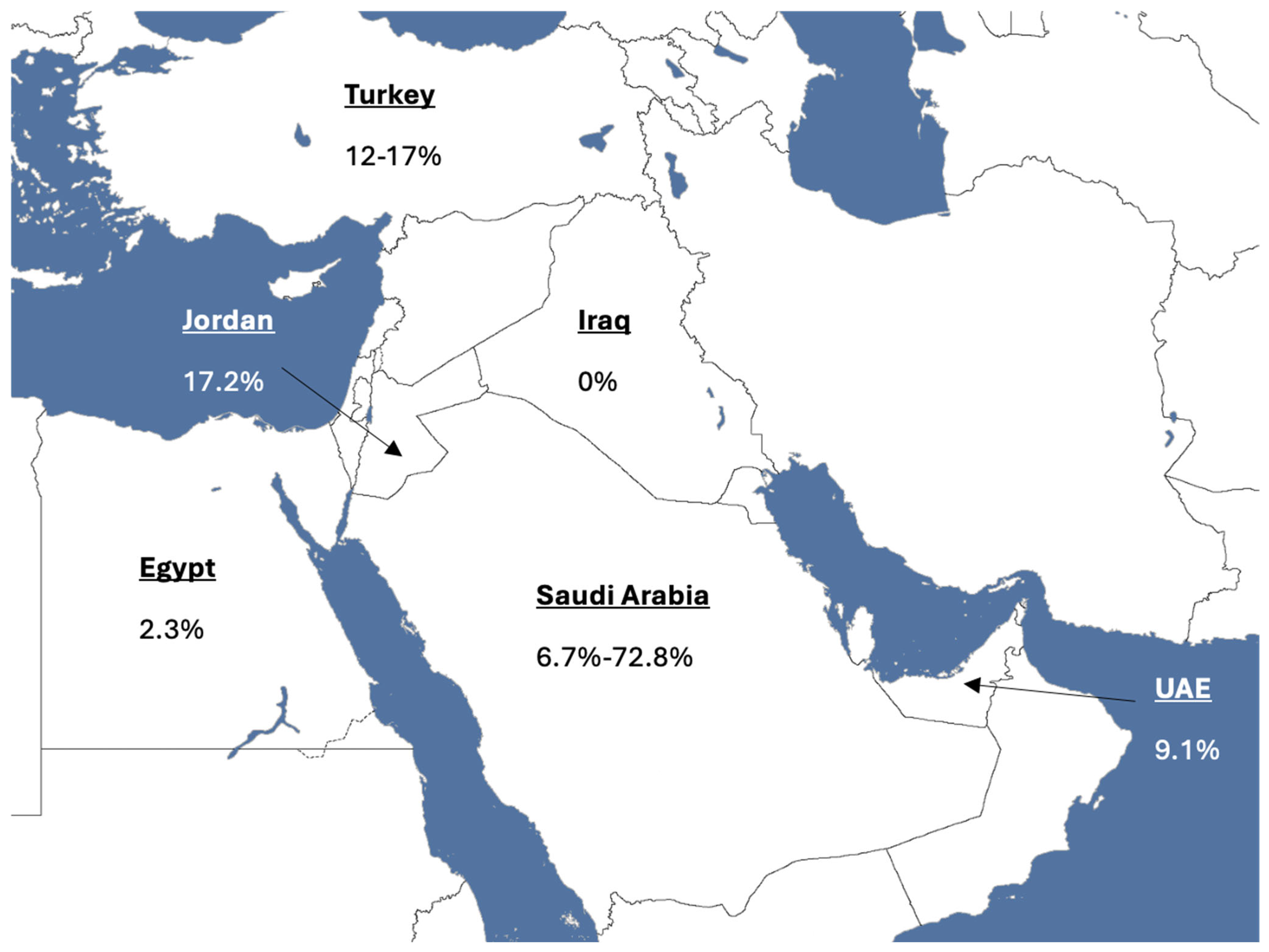
| Country | Age-Standardised Rate (World) [2] | Study | Screening Uptake (%)-Initial Test | Screening Modality |
|---|---|---|---|---|
| Saudi Arabia | 11.7 per 100,000 | MoH Pilot Project (2017) [42] Al-Kharj CRC pilot (2023) [43] Almadi et al. (2015) [44] | 57.6 72.8 6.7% | FIT, Colonoscopy gFOBT, Colonosocpy gFOBT, FIT, Sigmoidoscopy, Colonoscopy |
| UAE | 11.5 per 100,000 | Alsaad et al. (2023) [45] | 9.1 | FOBT, Colonoscopy |
| Egypt | 6.4 per 100,000 | Allam et al. (2024) [46] | 2.3 | Colonoscopy |
| Jordan | 16.5 per 100,000 | Jadallah et al. (2023) [47] | 17.2 | Colonoscopy, Sigmoidoscopy, gFOBT, FIT |
| Turkey | 19.8 per 100,000 | Gulten et al. (2012) [48] Taş et al. (2019) [49] | 12 17 | FOBT, Colonoscopy, Sigmoidosocpy |
| Iraq | 8.9 per 100,000 | Alrubaiy et al. (2023) [50] | 0 | FIT, Colonoscopy |
| Demographics | ||
|---|---|---|
| Question | Answer | No. of Respondents |
| Country of respondent | United Arab Emirates | 7 (41.2%) |
| Egypt | 2 (11.8%) | |
| Saudi Arabia | 2 (11.8) | |
| Iraq | 2 (11.8%) | |
| Morocco | 1 (5.9%) | |
| Lebanon | 1 (5.9%) | |
| Sudan | 1 (5.9%) | |
| Kuwait | 1 (5.9%) | |
| Type of Hospital | Public teaching hospital | 8 (47.1%) |
| Private teaching hospital | 4 (23.5%) | |
| Private non-teaching hospital | 2 (11.8%) | |
| Public non-teaching hospital | 1 (5.9%) | |
| Military hospital | 1 (5.9%) | |
| Private clinic | 1 (5.9%) | |
| Guidelines | ||
| Are there any local or national guidelines which are in use in your country for colorectal cancer screening? | Yes | 8 (47.1%) |
| No | 9 (52.9%) | |
| Do you use international guidelines? | Yes | 12 (70.6%) |
| No | 2 (11.8%) | |
| Left blank | 3 (17.6%) | |
| Which international guidelines do you use? Select all that apply | American Gastroenterological Association | 6 (35.3%) |
| British Society of Gastroenterology | 3 (17.6%) | |
| American College of Gastroenterology | 2 (11.8%) | |
| American Society for Gastrointestinal Endoscopy | 1 (5.9%) | |
| European Society for Medical Oncology | 1 (5.9%) | |
| Endoscopy quality parameters | ||
| Do you maintain a logbook of colonoscopy procedures and outcomes? | Yes | 13 (76.5%) |
| No | 4 (23.5%) | |
| Are there endoscopy key performance indicators (KPI) set for endoscopists in your region/country? | Yes | 10 (58.8%) |
| No | 7 (41.2%) | |
| Do you have dedicated training lists in your unit? | Yes | 6 (35.3%) |
| No | 11 (64.7%) | |
| Is there a regional or national accreditation process for performing endoscopy independently? | Yes | 2 (11.8%) |
| No | 14 (82.4%) | |
| Left blank | 1 (5.9%) | |
| Is there a regional or national accreditation process for endoscopists performing bowel cancer screening/diagnosis? | Yes | 3 (17.6%) |
| No | 13 (76.5%) | |
| Left blank | 1 (5.9%) | |
| Screening | ||
| Do you feel a bowel cancer screening programme would be helpful? | Very helpful | 16 (94.1%) |
| Slightly helpful | 1 (5.9%) | |
| Not helpful or unhelpful | 0 (0%) | |
| Slightly unhelpful | 0 (0%) | |
| Very unhelpful | 0 (0%) | |
| In your practice, is colorectal cancer screening run as an organised national screening programme or is it based on opportunistic screening? | Opportunistic | 14 (82.4%) |
| Organised national programme | 3 (17.6%) | |
| At what age do you stat screening for CRC in asymptomatic average risk patients (healthy adults with no family history of colon cancer)? | 40 | 7 (41.2%) |
| 45 | 6 (35.3%) | |
| 50 | 3 (17.6%) | |
| 60 | 1 (5.9%) | |
| What is the screening frequency for CRC using stool testing, if at all? | Yearly | 8 (47.1%) |
| Every 2 years | 3 (17.6%) | |
| Every 3 years | 1 (5.9%) | |
| Every 5 years | 0 (0%) | |
| If they have symptoms | 2 (11.8%) | |
| Whenever I see my patient | 1 (5.9%) | |
| Never use stool testing | 2 (11.8%) | |
| What is the screening frequency for CRC using colonoscopy, if at all? | Yearly | 0 (0%) |
| Every 2 years | 0 (0%) | |
| Every 3 years | 1 (5.9%) | |
| Every 5 years | 5 (29.4%) | |
| Every 10 years | 8 (47.1%) | |
| If they have symptoms * | 5 (29.4%) | |
| Whenever I see my patient | 0 (0%) | |
| Never use Colonoscopy | 0 (0%) | |
| When receiving referrals for suspected bowel cancer, is there an assessment of patient suitability for colonoscopy? | Yes | 15 (88.2%) |
| No | 2 (11.8%) | |
| Do you have access to CT virtual colonoscopy services at your unit? | Yes | 9 (52.9%) |
| No | 8 (47.1%) | |
| What therapeutic procedures are performed at your unit? | Cold snare polypectomy | 17 (100%) |
| Thermocoagulation | 14 (82.4%) | |
| Endoscopic mucosal resection | 17 (100%) | |
| Endoscopic submucosal dissection | 9 (52.9%) | |
| What are the available options for CRC screening in your practice? | Colonoscopy | 17 (100%) |
| CT colonography | 9 (52.9%) | |
| Stool testing | 13 (76.5%) | |
| Flexible sigmoidoscopy | 10 (58.8%) | |
| Do you have a system for surveillance following initial discovery and removal of colorectal adenomas? | Yes | 10 (58.8%) |
| No | 7 (41.2%) | |
| Do you have access to genetic testing for a suspected inherited susceptibility to colorectal cancer? | Yes | 9 (52.9%) |
| No | 8 (47.1%) | |
| Barriers to screening | ||
| What barriers, if any, do you feel exist to setting up a colorectal cancer screening programme-Patient related? | Fear of finding cancer | 9 (52.9%) |
| Belief that screening is not necessary | 15 (88.2%) | |
| Embarrassment or anxiety about screening tests | 14 (82.4%) | |
| Unaware of screening programmes | 13 (76.5%) | |
| Does not perceive CRC as a serious threat | 5 (29.4%) | |
| Screening programme related to a few centres only | 1 (5.9%) | |
| No barriers | 0 (0%) | |
| What barriers, if any, do you feel exist to setting up a colorectal cancer screening programme-System related? | Costs too much/insurance does not cover it | 12 (70.6%) |
| Primary care physician does not actively recommend screening to their patients | 7 (41.2%) | |
| Shortage of trained providers to conduct screening other than stool testing | 6 (35.3%) | |
| Shortage of trained providers to conduct follow up with invasive procedures | 5 (29.4%) | |
| Administrative issues | 1 (5.9%) | |
| No barriers | 0 (0%) | |
| Items | Specifications |
|---|---|
| Date of search | Initial search: 1 October 2022, most recent update: 10 October 2024 |
| Databases and sources searched | PubMed, MEDLINE, Cochrane Library, and EMBASE databases |
| Search terms | “colorectal cancer screening,” “Middle East,” “North Africa,” “Barriers,” “MENA,” and “colorectal cancer” |
| Time frame | No specific time limitation on publishing date, but recent studies sought |
| Inclusion/exclusion criteria | The inclusion criteria for publications were clinical trials, adult human participants, English language, papers within five years, and seminal papers (no date limit). Papers excluded were those addressing paediatrics and obstetrics |
| Selection process | Author directed |
| Guidelines | National Institute for Health and Care Excellence (NICE), British Society of Gastroenterology (BSG), World Gastroenterology Organisation (WGO), and local societies guidelines |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrubaiy, L.; El-Sayed, A.; Kapila, D.; Akintimehin, A.; Wijeyendram, P. Colorectal Cancer Screening in the Middle East and North Africa: Current Practices, Challenges, and Insights from the British Society of Gastroenterology (BSG) International Section. Gastrointest. Disord. 2025, 7, 56. https://doi.org/10.3390/gidisord7030056
Alrubaiy L, El-Sayed A, Kapila D, Akintimehin A, Wijeyendram P. Colorectal Cancer Screening in the Middle East and North Africa: Current Practices, Challenges, and Insights from the British Society of Gastroenterology (BSG) International Section. Gastrointestinal Disorders. 2025; 7(3):56. https://doi.org/10.3390/gidisord7030056
Chicago/Turabian StyleAlrubaiy, Laith, Ahmed El-Sayed, Diya Kapila, Abisoye Akintimehin, and Papakas Wijeyendram. 2025. "Colorectal Cancer Screening in the Middle East and North Africa: Current Practices, Challenges, and Insights from the British Society of Gastroenterology (BSG) International Section" Gastrointestinal Disorders 7, no. 3: 56. https://doi.org/10.3390/gidisord7030056
APA StyleAlrubaiy, L., El-Sayed, A., Kapila, D., Akintimehin, A., & Wijeyendram, P. (2025). Colorectal Cancer Screening in the Middle East and North Africa: Current Practices, Challenges, and Insights from the British Society of Gastroenterology (BSG) International Section. Gastrointestinal Disorders, 7(3), 56. https://doi.org/10.3390/gidisord7030056







