1. Introduction
Nearly one-third of the worldwide population is classified as overweight or obese, with obesity now listed as a leading cause of preventable morbidity and mortality worldwide [
1,
2]. Addressing this complex epidemic necessitates a comprehensive strategy involving multiple effective treatment options. While bariatric and metabolic surgery (BMS) remains the most effective long-term intervention, its invasiveness and eligibility restrictions, including a body mass index (BMI) of greater than 35 kg per metre squared (kg/m
2), highlight the need for less invasive alternatives for individuals with lower body mass indices [
3]. Intragastric balloons (IGBs) have emerged as a widely utilised, minimally invasive option for weight loss, offering reversibility and a favourable safety profile for patients with a BMI greater than 27 kg/m
2 or who are otherwise not surgical candidates [
4]. IGBs have been approved for treating obesity in one iteration or another since 1985. They are usually kept in situ for a period between 4 and 12 months, stimulating weight loss through restriction, early satiety and reduced gastric emptying [
5,
6,
7,
8]. Typically, patients lose between 10% and 20% of their total body weight (TBW) during this period [
5,
9]. IGBs are typically placed in one of two methods. The first and most common method is placed via endoscopy under direct vision, usually under sedation with all the associated risks of endoscopy. The second and less common involves patients swallowing a deflated capsule attached to an umbilical tubing which allows insufflation once the balloon position has been confirmed with fluoroscopy. This is performed with no sedation or risks associated with endoscopy.
In the broader context of weight loss therapy, multiple modalities offer distinct advantages and disadvantages. Surgical options such as sleeve gastrectomy and gastric bypass provide substantial and durable weight reduction, often achieving ~30% total body weight loss and confer favourable metabolic effects. Sleeve gastrectomy, involving partial gastric resection, is irreversible and carries risks such as leakage and strictures. Gastric bypass, while more complex and associated with higher perioperative risk, offers enhanced weight loss and improved comorbidity resolution but may lead to long-term nutrient malabsorption requiring careful monitoring. Medical therapy, primarily through pharmacological agents like GLP-1 receptor agonists, presents a non-invasive alternative that may achieve moderate weight loss (5–15%) but necessitates long-term adherence and monitoring [
10]. In contrast, IGBs provide a reversible, minimally invasive option with a favourable safety profile but are limited by transient duration, potential gastrointestinal side effects, and the risk of weight regain following removal. Appropriate patient selection and combination approaches remain key to optimising sustained outcomes in obesity management.
Despite their increasing use and general safety, IGBs are linked to rare but serious complications with a morbidity rate reported as 0.2% to 1.4%, while mortality has been reported as 0.08% [
9,
11]. One such concerning complication is spontaneous intragastric balloon hyperinflation (SIBH), characterised by rapid and unpredictable gas expansion of the balloon (
Scheme 1). SIBH can result in severe clinical consequences, including gastric ulceration (0.3%), gastric outlet obstruction (0.8%), pancreatitis, gastric perforation (0.1%) and death (0.05%) [
12,
13,
14,
15,
16].
The underlying mechanisms driving SIBH remain poorly understood, with microbial colonisation and gas-producing pathogens frequently hypothesised but not systematically examined across the literature. Given the growing adoption of IGBs and the potentially life-threatening nature of SIBH, there is a critical need to synthesise existing evidence on its clinical presentation, management, outcomes, and etiological factors. This systematic review addresses the following research question: What are the clinical features, management strategies, complications, and hypothesised mechanisms of spontaneous intragastric balloon hyperinflation as reported in the literature? By consolidating existing knowledge, this review seeks to inform clinical practice and guide future research towards effective prevention and management of this rare yet significant adverse event.
Marques, Lucas & Souza, Thiago & Grecco, Eduardo & Neto, Manoel & Vieira, Felipe & Garcia, Vinícius & Freitas, Carlos. (2015). Proposed Treatment of Adjustable Intragastric Balloon Contaminated with Candida. Bariatric Surgical Practice and Patient Care. 10. 169-172. 10.1089/bari.2015.0029 [
17].
2. Material and Methods
2.1. Protocol and Registration
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The review protocol was not registered.
2.2. Eligibility Criteria
Studies were eligible for inclusion if they reported on cases of SIBH in humans. There were no restrictions on publication year. Non-English language studies were excluded due to language limitations. After exclusions, 17 full-text articles and 1 abstract were included in this review.
2.3. Information Sources
A comprehensive search was performed across the following reputable electronic databases: PubMed, Embase, Google Scholar, MEDLINE, Ebsco, and the Cochrane Database. The last search was conducted on 30 June 2023.
2.4. Search Strategy
The search strategy utilised a combination of medical subject headings (MeSH) and relevant keywords from two domains:
Gastric balloon terms: “gastric balloon”, “intragastric balloon”.
Complication terms: “complication”, “adverse effect”, “hyperinflation”, “hyperinsufflation”, “infection”, “spontaneous”.
The full search strategy for each database is available upon request.
2.5. Selection Process
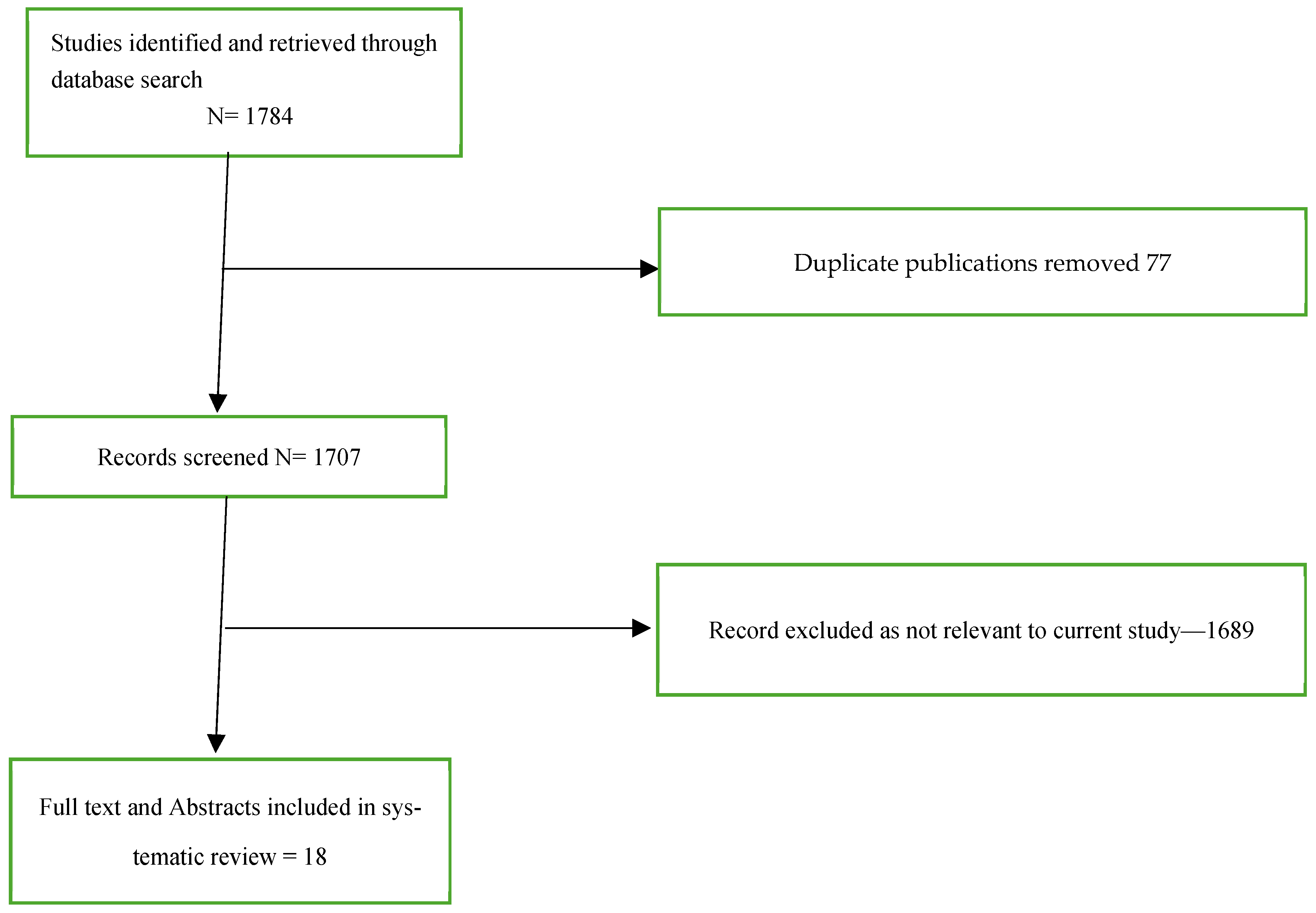
Titles and abstracts obtained from the search were independently screened for relevance by two reviewers (MG and PK). Subsequently, full-text articles of potentially eligible studies were evaluated concurrently by both reviewers (MG and PK) before inclusion. There were no discrepancies regarding article selection requiring external review. The study selection process is depicted in a PRISMA flow diagram (
Figure 1).
2.6. Data Collection Process
Data extraction was performed independently by two reviewers (MG and PK) using a piloted, modified data extraction worksheet. Extracted data points are listed below.
2.7. Data Points
The following data were collected from each study:
- -
Year of publication;
- -
Number of patients;
- -
Participant characteristics (if reported);
- -
Balloon manufacturer and initial fluid volume on placement;
- -
Clinical presentation;
- -
Management strategies;
- -
Complications and outcomes;
- -
Culture of balloon fluid and or surface of balloon;
- -
Hypothesised mechanisms of SIBH.
2.8. Risk of Bias Assessment
Risk of bias was not formally assessed due to the descriptive and case-based nature of the included studies.
2.9. Synthesis of Results
Extracted data was synthesised narratively, with results summarised in tables and text according to the review objectives. Quantitative synthesis was not performed due to heterogeneity in study designs and outcomes.
3. Statistics
Simple descriptive statistics are employed. There were significant gaps in the reported data; hence, continuous data is mentioned as mean (range). Due to the heterogeneous nature of the studies included, no attempt was made to perform a meta-analysis.
4. Results
A comprehensive literature search and screening process was conducted to identify the concerning phenomenon of SIBH. During the initial screen phase, 1784 studies were identified, with 1689 excluded based on irrelevance to the research objectives or being duplicate articles. Following the application of the predefined inclusion and exclusion criteria, 18 studies were deemed eligible for inclusion in this review.
Of the 18 studies included, 14 were individual case reports, while the remaining 4 were case series. Collectively, these studies documented a total of 28 cases of SIBH. Given the varied methodological approaches and reporting, the characteristics and findings of each included study are systematically summarised in
Table 1 to facilitate comparison and further analysis.
Summary of Data Points
Three studies did not provide basic demographic data, and six studies did not report patients’ Body Mass Index (BMI). Of those that did, the mean age was 47.3 years (range: 34–66 years) and the mean BMI was 33.5 (Range: 28–38). Eighteen patients were female, while the sex of ten patients was not reported.
Among the 28 cases, 15 balloons were Orbera (Apollo Endosurgery, Austin, TX, USA), six were from Allurion (Allurion Technologies, Natick, MA, USA), two from Spatz3 (Spatz Medical, Fort Lauderdale, FL, USA), one from Allergan (Allergan, Inc., Irvine, CA, USA), and four cases did not identify the manufacturer. The initial fluid volumes used during balloon placement were reported for only six patients and ranged from 500 mL to 700 mL.
The most common presenting complaints were nausea and/or vomiting in 23 out of 28 cases (82%), followed by abdominal pain in 19 out of 28 cases (62%). Abdominal distension/fullness was a presenting complaint in 12 patients (43%). The duration between insertion of the balloon and onset of symptoms varied from one and a half months to 12 months. 25 out of 28 patients (89%) had their gastric balloon removed endoscopically. One patient had the balloon’s fluid changed (Spatz3 Balloon) at 10 weeks but presented again at 18 weeks post-insertion with persistent symptoms requiring removal of the balloon. Two other patients had their balloon’s fluid changed at 2 months (manufacturer not reported) and 9 months (Spatz3) post-insertion. The balloon fluid was sent for culture in nine cases (32%). Candida was the most common pathogen identified in seven (78%) fluid specimens, followed by Streptococcus in four (44%). One fluid culture showed no growth. There was no long-term morbidity or mortality reported in any cases.
Two complications were identified from 28 published cases: one was superficial mucosal erosions of the stomach, which were minor and did not require further treatment. The other was acute pancreatitis, where cross-sectional imaging revealed compression of the pancreatic body by the hyperinflated balloon and subsequent upstream pancreatic duct dilatation, suggesting this mechanism caused the pancreatitis. This patient required emergency removal and was discharged two days later, indicating a mild pancreatitis episode without significant associated complications.
5. Discussion
The global adoption of intragastric balloon (IGB) systems for weight management has grown substantially, with at least nine distinct proprietary systems currently approved for use. These devices differ in their duration of placement, fill volume, construction materials, insertion and removal techniques, and capacity for adjustability. The primary mechanisms by which IGBS facilitate weight loss include gastric volume displacement, enhanced satiety, and delayed gastric emptying [
31]. Comparative studies consistently demonstrate that IGB therapy achieves superior short- to medium-term weight loss outcomes compared to lifestyle interventions alone [
4,
15]. The minimally invasive nature of IGB placement, coupled with its reversibility and quick procedural time, underpins its clinical and patient appeal. Nevertheless, the reported incidence of adverse events varies widely across studies, ranging from 0% to 28% [
5].
Despite the increasing use of IGBs, there remains a relative scarcity of literature exploring the pathogenesis of SIBH, and perhaps more critically, strategies for its prevention. Among the 18 studies evaluated in this review, 13 (62%) proposed at least one potential aetiological mechanism. The most frequently cited hypothesis involves opportunistic colonisation of the balloon’s external surface by gas-producing microorganisms. This microbial activity is thought to compromise the balloon’s integrity, leading to increased permeability and subsequent translocation of gases into the balloon, resulting in progressive hyperinflation and associated complications.
Additionally, three studies (23%) identified a potential infectious origin linked to contamination of the fill solution during the balloon instillation process. These studies emphasised lapses in aseptic technique, including improper glove handling, non-sterile syringes, or the use of non-sterile saline. In response, manufacturers such as Apollo Endosurgery—producers of the Orbera system—have underscored strict adherence to aseptic protocols as delineated in their Instructions for Use, including sterile handling and filling procedures [
32].
Less commonly discussed mechanisms include structural defects such as faulty valves that permit unidirectional air ingress, and osmotic influx of gas or fluids into the balloon due to a hypertonic intra-balloon environment. Drawing on the cases identified in this review, we consolidate the key proposed mechanisms and provide evidence-informed recommendations aimed at mitigating the risk of SIBH.
5.1. Aetiology
5.1.1. Gas-Producing Microbiological Contamination
Fungus
80% of all cases where IGB material was cultured grew
Candida species, including
glabrata and
tropicalis. One case also cultured another species of fungus,
Saccharomyces cerevisiae.
Candida species and
Saccharomyces are commensal yeasts normally present in small numbers in the human oral flora [
33,
34]. They also produce gas by fermentation. IGB causes delayed gastric emptying, which can create the optimal environment for opportunistic gastric infections [
35]. Additionally, many centres use regular proton pump inhibitor therapy following implantation of the balloon to reduce gastric acidity, as balloon insertion can cause hyperacidity, and the acid can degrade the silicone elastomer wall over time [
27,
36]. Given that obesity is a chronic inflammatory condition and itself produces an immunosuppressive state [
37], it is unsurprising that studies have shown that the fungal gut mycobiome is disturbed in obese patients [
38]. The silicone elastomer structure of the IGB can allow for the fungus to adhere to and penetrate through microscopic surface defects, accounting for the plaque appearances documented on balloon removal in hyperinflation cases, whilst not leaking fluid or air from within the balloon [
35]. As such, one proposed mechanism of hyperinflation is oropharyngeal-oesophageal contamination of the balloon on insertion from gas-producing opportunistic fungal commensals. These subsequently colonise the balloon under the optimal conditions for microbial growth described above, penetrate an imperfect silicone balloon and emit gas within the balloon until a critical balloon mass is reached to cause the adverse effects of SIBH described.
Bacteria
Like the fungal species described above, orogastric bacterial commensals were isolated in 40% of cases. This included the Gram-positive
Viridans streptococcus group, including
salivarius and
oralis,
peptococcus and
lactobacillus and Gram-negative
klebsiella species [
39,
40]. All isolated bacteria are either anaerobes or facultative anaerobes and have the capability of producing gases [
41]. As such, the same circumstances described for the fungal colonisation could allow for anaerobic bacterial commensals to gain access to the internal aspect of the balloon and produce gas, causing unregulated balloon expansion, causing SIBH.
5.1.2. Defective Structural Integrity of the Balloon
Given the high global implantation rate of intragastric balloons (IGBs) and the rarity of SIBH, additional mechanisms beyond microbial colonisation have been proposed, most notably, structural compromise of the balloon itself. Among the cases included in this review, the Orbera system emerged as the most frequently involved, followed by Elipse and Spatz3. These balloon systems are all designed for liquid-only inflation. However, inadvertent air introduction may occur during suboptimal endoscopic visualisation or via contamination of the filling apparatus. In systems like Elipse, which rely solely on plain radiography for confirmation of balloon placement and fill status, the presence of air may go undetected.
Furthermore, intentional overfilling beyond manufacturer recommendations, undertaken to increase satiety and enhance weight loss, may overstress the balloon material, potentially compromising structural integrity. These variations in balloon filling practices have been implicated in contributing to hyperinflation events [
31].
An additional proposed mechanism draws from parallels in other medical devices, such as the spontaneous inflation observed in saline-filled silicone breast implants. Silicone-based balloon shells, like those used in IGBs, may possess semi-permeable properties, allowing the passage of gases, fluids, and macromolecules into the balloon via osmotic gradients, especially given that saline is the standard inflation medium [
5,
42].
All the IGB systems reviewed utilise a valve-based mechanism to control inflation. Once sealed, these valves are designed to prevent both ingress of external air or fluid and egress of the intragastric balloon contents. However, a defective or malfunctioning valve may permit unidirectional air entry, establishing a one-way mechanism whereby air accumulates within the balloon but cannot escape. This process has been proposed as a plausible pathway to unregulated balloon hyperinflation [
12].
6. Recommendations for Prevention, Early Diagnosis and Treatment
Based on the findings and discussion, the leading hypothesis for the development of SIBH is opportunistic colonisation of the balloon by gas-producing fungal and bacterial organisms. Given the rarity of SIBH, it is likely that a unique combination of host, prosthesis, and microbial factors must converge to result in its occurrence.
Pre-Procedural Preparation and Insertion Recommendations
Optimising the management of immunocompromising comorbidities, such as diabetes mellitus, is essential to enhancing host immune function and reducing the risk of opportunistic fungal or bacterial colonisation of the intragastric balloon, thereby potentially lowering the incidence of SIBH.
As the majority of IGBs are placed under direct endoscopic visualisation, we recommend that the proceduralist first perform a diagnostic upper endoscopy. This should include a thorough assessment for oropharyngeal, oesophageal, and gastric candidiasis, as well as an evaluation for structural contraindications such as active ulcerations, varices, or large hiatal hernias [
11]. In cases where candidiasis is identified, balloon placement should be deferred until appropriate antifungal therapy has been completed and resolution is confirmed.
During balloon insertion, meticulous attention must be given to maintaining continuous endoscopic visualisation of the balloon to ensure that neither the endoscope nor the insertion pathway causes trauma to the balloon or its valve. Moreover, strict adherence to the correct inflation protocol is critical to prevent mechanical compromise of the device.
Strict adherence to aseptic technique and avoidance of off-label practices are essential to minimising the risk of microbial contamination during the intragastric balloon (IGB) inflation process. Such measures reduce the likelihood of introducing pathogens into the balloon system. IGBs have historically been marketed as low-risk, minimally invasive, day-procedure devices. This perception has been further reinforced by the advent of “procedureless” systems such as the Ellipse balloon, a swallowable, non-endoscopic IGB that can be administered in an outpatient setting by non-proceduralists, such as obesity clinicians, rather than surgeons or gastroenterologists [
25]. While this convenience improves accessibility, it may also foster environments where strict sterility and aseptic protocols are inconsistently applied. This lapse in technique may facilitate the inadvertent introduction of commensal or pathogenic microorganisms into the balloon system [
30].
We support the adjunctive use of methylene blue (MB) in saline for liquid-filled balloons, where permitted by both device manufacturers and regulatory bodies, in accordance with previous clinical guidelines [
31]. Devices such as the Orbera, Spatz3, and ReShape balloons are compatible with this approach [
7]. The addition of MB serves two key functions. Firstly, in the event of balloon rupture, MB is absorbed and subsequently excreted by the kidneys, leading to a visible blue discolouration of the urine. This acts as an early indicator of rupture, prompting timely balloon removal and preventing potential migration into the small bowel, which may result in obstruction. Secondly, MB and its analogues, particularly when combined with buffering agents such as sodium citrate, exhibit antimicrobial activity against a broad range of organisms. These include pathogens commonly implicated in SIBH cases, such as
Candida spp. and
Klebsiella spp. [
43].
We advocate for further investigation into the minimum inhibitory concentrations (MICs) of MB against relevant microbial species to ensure that the concentration used during balloon inflation confers effective antimicrobial protection. Given its dual diagnostic and antimicrobial benefits, as well as its established safety profile, we recommend the routine use of MB under these conditions.
However, considering current uncertainties regarding the precise aetiology of SIBH, we concur with the Brazilian Intragastric Balloon Consensus Statement, which advises against the routine prophylactic use of antifungal agents in IGB systems [
6].
7. Conclusions
The pathophysiological mechanisms underlying SIBH remain incompletely understood and warrant further investigation. Elucidating these mechanisms is critical to informing both preventive strategies and design improvements by manufacturers, as well as guiding clinical protocols aimed at minimising the risk of this rare but potentially serious complication.
To date, the most extensively studied aetiological pathway involves the opportunistic colonisation of the balloon surface by gas-producing fungal and bacterial organisms. This is thought to result in gas accumulation within the balloon, leading to progressive and unregulated expansion. However, several additional mechanisms have been proposed, including balloon valve malfunction and osmotic shifts, though these remain poorly characterised due to the limited scope of current research.
Given the global rise in obesity and the corresponding increase in the utilisation of intragastric balloon therapies, including newer designs marketed for extended durations, the potential for delayed complications, including SIBH, may increase. As such, clinicians must maintain a high index of suspicion when evaluating patients who present with unexpected or unexplained symptoms following balloon insertion. Early diagnostic imaging or endoscopic evaluation should be promptly employed to facilitate timely diagnosis and removal of the balloon, where appropriate.
Author Contributions
M.G.: Writing of manuscript, data correlation and analysis; P.K.: Writing of manuscript and analysis; J.L.: Review and revision of manuscript; B.M.: Supervision, review and revision of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
Kamal Mahawar, FRCSEd of the Faculty of Health Sciences and Wellbeing, University of Sunderland, Sunderland, United Kingdom and the Bariatric Unit, South Tyneside and Sunderland NHS Trust, Sunderland, United Kingdom for conceptualising this review article and his guidance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Karwi, Q.G.; Zhang, L.; Altamimi, T.R.; Wagg, C.S.; Patel, V.; Uddin, G.M.; Joerg, A.R.; Padwal, R.S.; Johnstone, D.E.; Sharma, A.; et al. Weight loss enhances cardiac energy metabolism and function in heart failure associated with obesity. Diabetes Obes. Metab. 2019, 21, 1944–1955. [Google Scholar] [CrossRef]
- Bazerbachi, F.; Vargas, E.J.; Abu Dayyeh, B.K. Endoscopic Bariatric Therapy: A Guide to the Intragastric Balloon. Off. J. Am. Coll. Gastroenterol. ACG 2019, 114, 1421–1431. [Google Scholar] [CrossRef]
- Saber, A.A.; Shoar, S.; Almadani, M.W.; Zundel, N.; Alkuwari, M.J.; Bashah, M.M.; Rosenthal, R.J. Efficacy of First-Time Intragastric Balloon in Weight Loss: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Obes. Surg. 2017, 27, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Tate, C.M.; Geliebter, A. Intragastric Balloon Treatment for Obesity: Review of Recent Studies. Adv. Ther. 2017, 34, 1859–1875. [Google Scholar] [CrossRef]
- Neto, M.G.; Silva, L.B.; Grecco, E.; de Quadros, L.G.; Teixeira, A.; Souza, T.; Scarparo, J.; Parada, A.A.; Dib, R.; Moon, R.; et al. Brazilian Intragastric Balloon Consensus Statement (BIBC): Practical guidelines based on experience of over 40,000 cases. Surg. Obes. Relat. Dis. 2018, 14, 151–159. [Google Scholar] [CrossRef]
- Galvao Neto, M.; Silva, L.B.; Usuy, E.N., Jr.; Campos, J.M. Intragastric Balloon for Weight Management: A Practical Guide, 1st ed.; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Chow, A.; Mocanu, V.; Verhoeff, K.; Switzer, N.; Birch, D.; Karmali, S. Trends in the Utilization of Intragastric Balloons: A 5-Year Analysis of the MBSAQIP Registry. Obes. Surg. 2022, 32, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Abu Dayyeh, B.K.; Kumar, N.; Edmundowicz, S.A.; Jonnalagadda, S.; Larsen, M.; Sullivan, S.; Thompson, C.C.; Banerjee, S. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest. Endosc. 2015, 82, 425–438.E5. [Google Scholar] [CrossRef]
- Ansari, H.U.H.; Qazi, S.U.; Sajid, F.; Altaf, Z.; Ghazanfar, S.; Naveed, N.; Ashfaq, A.S.; Siddiqui, A.H.; Iqbal, H.; Qazi, S. Efficacy and Safety 450 of Glucagon-Like Peptide-1 Receptor Agonists on Body Weight and Cardiometabolic Parameters in Individuals with Obesity 451 and Without Diabetes: A Systematic Review and Meta-Analysis. Endocr Pract. 2024, 30, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.B.; Neto, M.G. Intragastric balloon. Minim. Invasive Ther. Allied Technol. 2022, 31, 505–514. [Google Scholar] [CrossRef]
- Patel, K.V.; Ooi, J.; Ray, S.; Griffin, N.; Oben, J.A. Abdominal pain following obesity treatment. Gut 2014, 63, 364–365. [Google Scholar] [CrossRef]
- de Quadros, L.G.; Dos Passos Galvão Neto, M.; Grecco, E.; de Souza, T.F.; Kaiser, R.L., Jr.; Campos, J.M.; Teixeira, A.; Filho, A.C.; Macedo, G.; Silva, M. Intragastric Balloon Hyperinsufflation as a Cause of Acute Obstructive Abdomen. ACG Case Rep. J. 2018, 5, e69. [Google Scholar] [CrossRef]
- Barrichello, S.; de Moura, D.T.H.; Hoff, A.C.; Veinert, A.; Thompson, C.C. Acute pancreatitis due to intragastric balloon hyperinflation (with video). Gastrointest. Endosc. 2020, 91, 1207–1209. [Google Scholar] [CrossRef]
- Yorke, E.; Switzer, N.J.; Reso, A.; Shi, X.; de Gara, C.; Birch, D.; Gill, R.; Karmali, S. Intragastric Balloon for Management of Severe Obesity: A Systematic Review. Obes. Surg. 2016, 26, 2248–2254. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA in Brief: FDA Alerts Health Care Providers About 5 Additional Deaths Associated with the Use of Liquid-Filled Intragastric Balloon Devices for Obesity Treatment. 2018. Available online: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-alerts-health-care-providers-about-5-additional-deaths-associated-use-liquid-filled (accessed on 22 May 2023).
- Marques, L.M.; de Souza, T.F.; Grecco, E.; Neto, M.d.P.G.; Ramos, F.M.; Vieira, F.M.; Garcia, V.G.; Freitas, C.E. Proposed Treatment of Adjustable Intragastric Balloon Contaminated with Candida. Bariatr. Surg. Pract. Patient Care 2015, 10, 169–172. [Google Scholar] [CrossRef]
- Madeira, M.; Madeira, E.; Guedes, E.P.; Mafort, T.T.; Lopes, A.J.; Moreira, R.O.; de Farias, M.L.F. Symptomatic bacterial contamination of an intragastric balloon. Gastrointest. Endosc. 2013, 78, 360–361; discussion 1. [Google Scholar] [CrossRef]
- Commey, R.; Lehmann, R. 2019 Scientific Session of the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES), Baltimore, Maryland, USA, 3–6 April 2019: 2019 Posters. Surg. Endosc. 2019, 33, 130–413. [Google Scholar] [CrossRef] [PubMed]
- Barola, S.; Agnihotri, A.; Chang Chiu, A.; Kalloo, A.N.; Kumbhari, V. Spontaneous Hyperinflation of an Intragastric Balloon 5 Months After Insertion. Am. J. Gastroenterol. 2017, 112, 412. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nava, G.; Asokkumar, R.; Bautista, I.; Negi, A. Spontaneous Hyperinflation Intragastric Balloon: What Caused It? Endoscopy 2020, 52, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Quarta, G.; Popov, V.B. Intragastric balloon hyperinflation secondary to polymicrobial overgrowth associated with proton pump inhibitor use. Gastrointest. Endosc. 2019, 90, 311–312. [Google Scholar] [CrossRef]
- Basile, P.; Marre, C.; Le Mouel, J.P. Gastric Obstruction Secondary to an Unexplained Hyperinflation of an Intragastric Balloon. Clin. Gastroenterol. Hepatol. 2020, 18, A16. [Google Scholar] [CrossRef]
- Moore, R.L.; Eaton, L.; Ellner, J. Safety and Effectiveness of an Intragastric Balloon as an Adjunct to Weight Reduction in a Post-Marketing Clinical Setting. Obes. Surg. 2020, 30, 4267–4274. [Google Scholar] [CrossRef] [PubMed]
- Ienca, R.; Al Jarallah, M.; Caballero, A.; Giardiello, C.; Rosa, M.; Kolmer, S.; Sebbag, H.; Hansoulle, J.; Quartararo, G.; Zouaghi, S.A.S.; et al. The Procedureless Elipse Gastric Balloon Program: Multicenter Experience in 1770 Consecutive Patients. Obes. Surg. 2020, 30, 3354–3362. [Google Scholar] [CrossRef]
- Usuy, E.; Silva, M.; Dos Passos Galvão Neto, M.; Grecco, E.; Ferreira de Souza, T.; de Quadros, L.G. Antibiotics to Prevent Relapse of Adjustable Gastric Balloon Hyperinflation: Feasible for Balloon Maintenance? GE Port. J. Gastroenterol. 2020, 28, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.H.; Al-Kanawati, N.; ElAbd, R.; Al-Haddad, M.; AlKhadher, T.; Hamshari, F.; Akrouf, S. A Study Examining the Orbera365 Intragastric Balloon Safety and Effects on Weight Loss. Obes. Surg. 2021, 31, 5342–5347. [Google Scholar] [CrossRef]
- Bomman, S.; Sanders, D.; Larsen, M. Spontaneous Hyperinflation of an Intragastric Balloon Causing Gastric Outlet Obstruction. Cureus 2021, 13, e15962. [Google Scholar] [CrossRef]
- Gaunt, E.A.; Fernandes, R. Dysphagia due to spontaneous hyperinflation of a swallowable intragastric balloon. BMJ Case Rep. 2021, 14, e240669. [Google Scholar] [CrossRef] [PubMed]
- Pontecorvi, V.; Bove, V.; Carlino, G.; Matteo, M.V.; De Siena, M.; Papparella, L.G.; Costamagna, G.; Boškoski, I. Spontaneous Intragastric Balloon Hyperinflation Is Probably Due to Microbial Overgrowth of the Filling Liquid. Obes. Surg. 2022, 32, 1783–1785. [Google Scholar] [CrossRef]
- de Souza, T.F.; Grecco, E.; Usuy, E.N. Hyperinflated Intragastric Balloon. In Intragastric Balloon for Weight Management: A Practical Guide; Galvao Neto, M., Silva, L.B., Usuy, E.N., Jr., Campos, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 171–178. [Google Scholar]
- Orbera TM Intragastric Balloon System (Orbera TM). Directions for Use (DFU); Apollo Endosurgery Inc.: Austin, TX, USA, 2019. [Google Scholar]
- Paterson, M.J.; Oh, S.; Underhill, D.M. Host–microbe interactions: Commensal fungi in the gut. Curr. Opin. Microbiol. 2017, 40, 131–137. [Google Scholar] [CrossRef]
- Cannon, R.D.; Chaffin, W.L. Oral Colonization By Candida Albicans. Crit. Rev. Oral Biol. Med. 1999, 10, 359–383. [Google Scholar] [CrossRef]
- Coskun, H.; Bozkurt, S. A case of asymptomatic fungal and bacterial colonization of an intragastric balloon. World J. Gastroenterol. 2009, 15, 5751–5753. [Google Scholar] [CrossRef]
- Alsabah, S.; Al Haddad, E.; Ekrouf, S.; Almulla, A.; Al-Subaie, S.; Al Kendari, M. The safety and efficacy of the procedureless intragastric balloon. Surg. Obes. Relat. Dis. 2018, 14, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef]
- Mar Rodríguez, M.; Pérez, D.; Javier Chaves, F.; Esteve, E.; Marin-Garcia, P.; Xifra, G.; Vendrell, J.; Jové, M.; Pamplona, R.; Ricart, W.; et al. Obesity changes the human gut mycobiome. Sci. Rep. 2015, 5, 14600. [Google Scholar] [CrossRef]
- Gonzalez-Ferrer, S.; Peñaloza, H.F.; Budnick, J.A.; Bain, W.G.; Nordstrom, H.R.; Lee, J.S.; Van Tyne, D.; Ottemann, K.M. Finding order in the chaos: Outstanding questions in Klebsiella pneumoniae pathogenesis. Infect. Immun. 2021, 89, e00693-20. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Ukaegbu-Obi, K.M.; Ifeanyi, V.O.; Eze, V.C. Microbes-The Key Players in Anaerobic Digestion for Biogas Production. J. Microbiol. Microb. Technol. 2022, 3, 1–5. [Google Scholar] [CrossRef]
- Robinson, O.G.; Jr Benos, D.J.; Mazzochi, C. Spontaneous autoinflation of saline mammary implants: Further studies. Aesthet. Surg. J. 2005, 25, 582–586. [Google Scholar] [CrossRef]
- Thesnaar, L.; Bezuidenhout, J.J.; Petzer, A.; Petzer, J.P.; Cloete, T.T. Methylene blue analogues: In vitro antimicrobial minimum inhibitory concentrations and in silico pharmacophore modelling. Eur. J. Pharm. Sci. 2021, 157, 105603. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).