Updates in Gastroesophageal Reflux Disease Management: From Proton Pump Inhibitors to Dietary and Lifestyle Modifications
Abstract
1. Introduction
1.1. Definition and Prevalence
1.2. Etiology and Pathophysiology
2. Traditional Approach to GERD Treatment
2.1. Overview of Pharmacological Classes
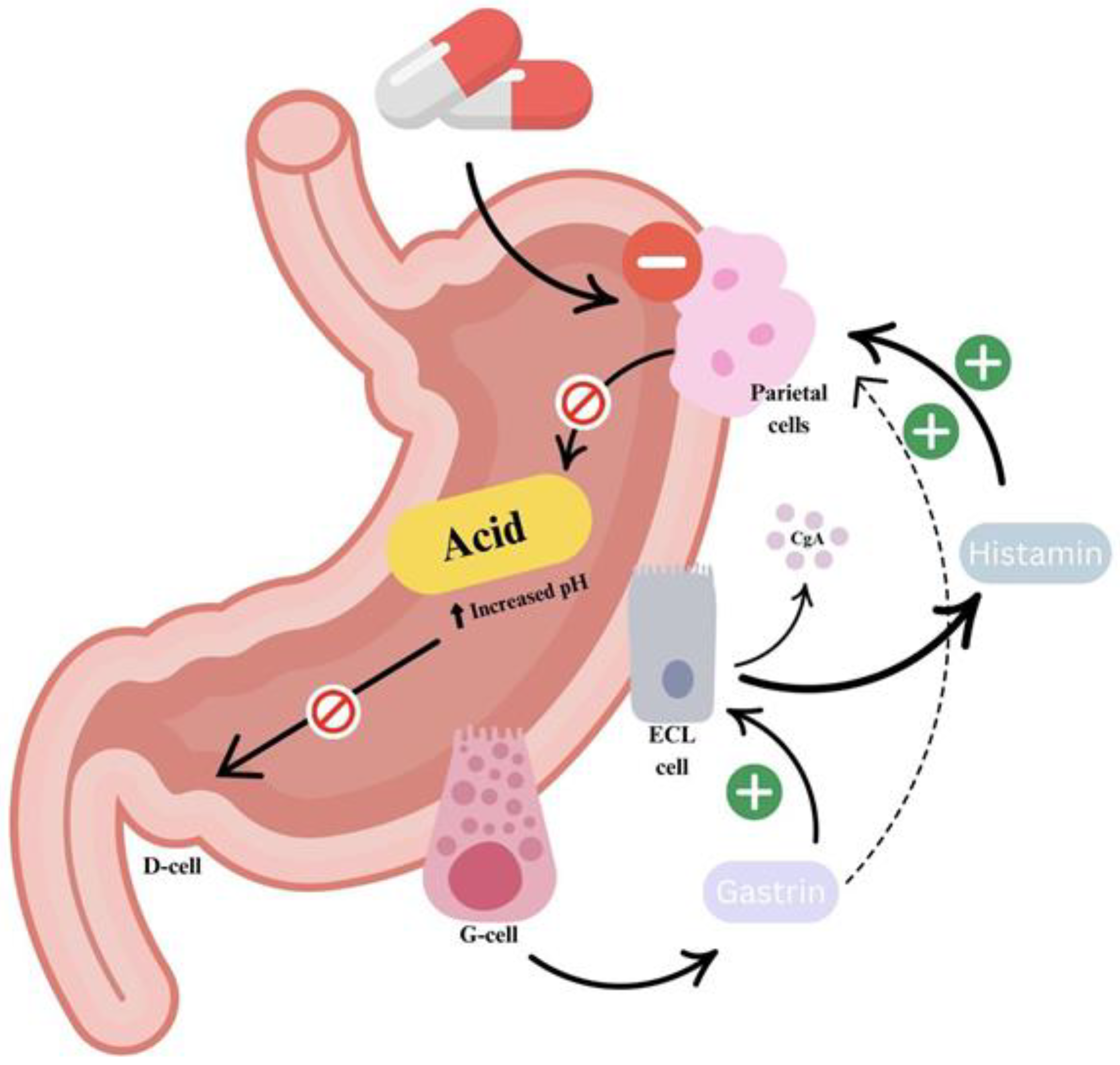
2.1.1. PPIs: The Basis of GERD Pharmacotherapy
2.1.2. Histamine-2 Receptor Antagonists (H2RAs)
2.1.3. Potassium-Competitive Acid Blockers (P-CABs)
2.1.4. Mucosal-Protective Agents: Sucralfate and Alginates
2.2. Therapeutic Strategies and Dosing Approaches
2.3. Adverse Effects of Long-Term Proton Pump Inhibitor Therapy
3. Limitations and Challenges in PPI Therapy
3.1. PPI-Refractory Heartburn Diagnosis: GERD vs. Functional Disorders
3.2. Consequences of PPI Overuse
4. Advances in Understanding Diet and Lifestyle in GERD
4.1. Weight Management and Obesity
4.2. Impact of Meal Timing and Eating Behaviors
4.3. Dietary Triggers and Individualized Food Avoidance
4.3.1. High-Fat and Fried Foods
4.3.2. Coffee and Caffeine
4.3.3. Alcohol
4.3.4. Acidic Foods
4.3.5. Spicy Foods
4.3.6. Chocolate
4.3.7. Carbonated Beverages
4.3.8. Fiber and Carbohydrates
4.3.9. FODMAP
4.4. Bed Elevation and Sleeping Positions
4.5. Smoking Cessation
4.6. Physical Activity
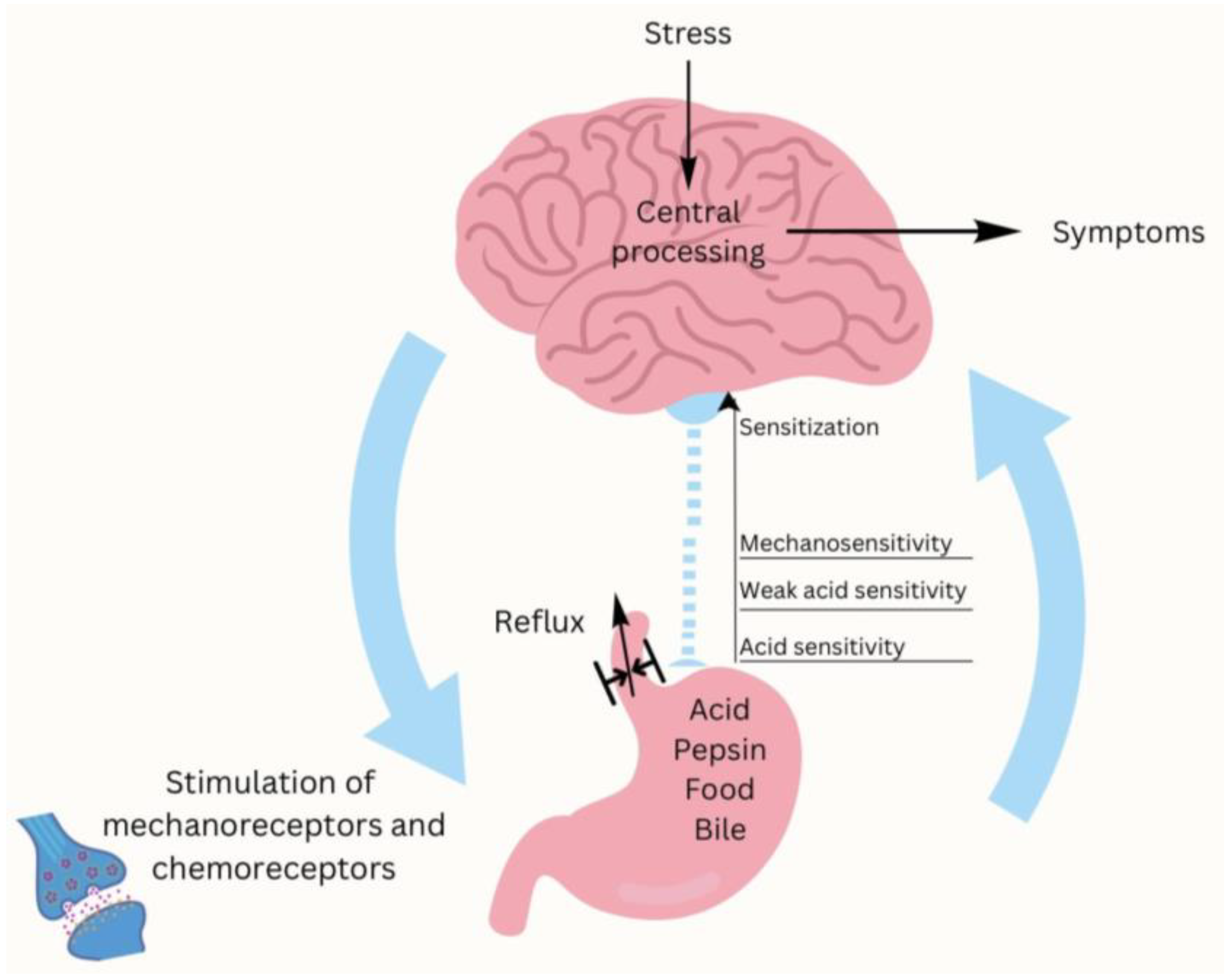
4.7. Stress and the Gut–Brain Axis
5. Future Perspectives and Evolving Trends in GERD Research and Recommendations
5.1. Probiotics and Prebiotics
5.2. Natural Remedies
5.2.1. Ginger
5.2.2. Aloe Vera
5.2.3. Licorice Root
5.2.4. Chamomile
5.2.5. Rose Oil Vera (Rosa Damascena)
5.2.6. Melatonin
5.2.7. Traditional Chinese Herbal Formula
6. Holistic Approach to GERD Treatment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Brady, P. Gastroesophageal Reflux Disease: Pathophysiology, Diagnosis, and Treatment. Gastroenterol. Nurs. 2019, 42, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Eusebi, L.H.; Ratnakumaran, R.; Yuan, Y.; Solaymani-Dodaran, M.; Bazzoli, F.; Ford, A.C. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: A meta-analysis. Gut 2018, 67, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.M.; Lai, K.C.; Lam, K.F.; Hui, W.M.; Hu, W.H.C.; Lam, C.L.K.; Xia, H.H.X.; Huang, J.Q.; Chan, C.K.; Lam, S.K.; et al. Prevalence, clinical spectrum and health care utilization of gastro-oesophageal reflux disease in a Chinese population: A population-based study. Aliment. Pharmacol. Ther. 2003, 18, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Spantideas, N.; Drosou, E.; Bougea, A.; Assimakopoulos, D. Gastroesophageal reflux disease symptoms in the Greek general population: Prevalence and risk factors. Clin. Exp. Gastroenterol. 2016, 9, 143–149. [Google Scholar] [CrossRef]
- Zheng, Z.; Shang, Y.; Wang, N.; Liu, X.; Xin, C.; Yan, X.; Zhang, Y.; Li, M.; Chen, R.; Huang, J.; et al. Current Advancement on the Dynamic Mechanism of Gastroesophageal Reflux Disease. Int. J. Biol. Sci. 2021, 17, 4154–4164. [Google Scholar] [CrossRef]
- Mittal, R.; Vaezi, M.F. Esophageal Motility Disorders and Gastroesophageal Reflux Disease. N. Engl. J. Med. 2020, 383, 1961–1972. [Google Scholar] [CrossRef]
- Zachariah, R.A.; Goo, T.; Lee, R.H. Mechanism and Pathophysiology of Gastroesophageal Reflux Disease. Gastrointest. Endosc. Clin. N. Am. 2020, 30, 209–226. [Google Scholar] [CrossRef]
- Dong, R.; Xu, X.; Yu, L.; Ding, H.; Pan, J.; Yu, Y.; Shi, C.; Chen, Q.; Zhang, M.; Lv, H.; et al. Randomised clinical trial: Gabapentin vs baclofen in the treatment of suspected refractory gastro-oesophageal reflux-induced chronic cough. Aliment. Pharmacol. Ther. 2019, 49, 714–722. [Google Scholar] [CrossRef]
- Diener, U.; Patti, M.G.; Molena, D.; Fisichella, P.M.; Way, L.W. Esophageal dysmotility and gastroesophageal reflux disease. J. Gastrointest. Surg. 2001, 5, 260–265. [Google Scholar] [CrossRef]
- Rettura, F.; Bronzini, F.; Campigotto, M.; Lambiase, C.; Pancetti, A.; Berti, G.; Marchi, S.; de Bortoli, N.; Zerbib, F.; Savarino, E.; et al. Refractory Gastroesophageal Reflux Disease: A Management Update. Front. Med. 2021, 8, 765061. [Google Scholar] [CrossRef]
- Sumi, S.; Ishimura, N.; Mikami, H.; Okimoto, E.; Tamagawa, Y.; Mishiro, T.; Kinoshita, Y.; Ishihara, S. Evaluations of Gastric Acid Pocket Using Novel Vertical 8-Channel pH Monitoring System and Effects of Acid Secretion Inhibitors. J. Neurogastroenterol. Motil. 2021, 27, 370–376. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, B. Management of gastroesophageal reflux disease in adults: A pharmacist’s perspective. Integr. Pharm. Res. Pract. 2018, 7, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M. Gastrointestinal hormones and regulation of gastric emptying. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh-Esfahani, N.; Soleimani, D.; Hajiahmadi, S.; Moradi, S.; Heidarzadeh, N.; Nachvak, S.M. Dietary Intake in Relation to the Risk of Reflux Disease: A Systematic Review. Prev. Nutr. Food Sci. 2021, 26, 367–379. [Google Scholar] [CrossRef]
- Souza, R.F.; Spechler, S.J. Mechanisms and pathophysiology of Barrett oesophagus. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 605–620. [Google Scholar] [CrossRef]
- Herdiana, Y. Functional Food in Relation to Gastroesophageal Reflux Disease (GERD). Nutrients 2023, 15, 3583. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, S.; Liu, L.; Wang, Y. Comparative effectiveness of histamine-2 receptor antagonists as short-term therapy for gastro-esophageal reflux disease: A network meta-analysis. Int. J. Clin. Pharmacol. Ther. 2016, 54, 761–770. [Google Scholar] [CrossRef]
- Wolfe, M.M.; Sachs, G. Acid suppression: Optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome. Gastroenterology 2000, 118 (Suppl. 1), S9–S31. [Google Scholar] [CrossRef]
- Andersson, K.; Carlsson, E. Potassium-competitive acid blockade: A new therapeutic strategy in acid-related diseases. Pharmacol. Ther. 2005, 108, 294–307. [Google Scholar] [CrossRef]
- Weberg, R.; Berstad, A. Symptomatic effect of a low-dose antacid regimen in reflux oesophagitis. Scand. J. Gastroenterol. 1989, 24, 401–406. [Google Scholar] [CrossRef]
- Ahmed, A.; Clarke, J.O. Proton Pump Inhibitors (PPI). In StatPearls Internet; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Al Ghadeer, H.A.; Alabbad, Z.E.; AlShaikh, S.B.; Ahmed, S.U.; Bu-Khamseen, A.A.; Alhashem, A.T. Prevalence of gastroesophageal reflux disease and associated risk factors in the Eastern Region, Saudi Arabia. Cureus 2021, 13, e19599. [Google Scholar] [CrossRef] [PubMed]
- El Rouby, N.; Lima, J.J.; Johnson, J.A. Proton pump inhibitors: From CYP2C19 pharmacogenetics to precision medicine. Expert Opin. Drug Metab. Toxicol. 2018, 14, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Z.; Lu, F.; Xiong, M.; Jiang, L.; Tang, K.; Fu, M.; Wu, Y.; He, B. Effects of CYP2C19 genetic polymorphisms on the cure rates of H. pylori in patients treated with the proton pump inhibitors: An updated meta-analysis. Front. Pharmacol. 2022, 13, 938419. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Tan, Q.; Guo, D.; Shi, Z.; Zhang, C.; Guo, W. Patients carrying CYP2C19 loss of function alleles have a reduced response to clopidogrel therapy and a greater risk of in-stent restenosis after endovascular treatment of lower extremity peripheral arterial disease. J. Vasc. Surg. 2014, 60, 993–1001. [Google Scholar] [CrossRef]
- Kaartinen, T.J.K.; Tornio, A.; Tapaninen, T.; Launiainen, T.; Isoherranen, N.; Niemi, M.; Backman, J.T. Effect of High-Dose Esomeprazole on CYP1A2, CYP2C19, and CYP3A4 Activities in Humans: Evidence for Substantial and Long-lasting Inhibition of CYP2C19. Clin. Pharmacol. Ther. 2020, 108, 1254–1264. [Google Scholar] [CrossRef]
- Jeong, S.H.; Jang, J.H.; Lee, Y.B. Exploring Differences in Pharmacometrics of Rabeprazole between Genders via Population Pharmacokinetic-Pharmacodynamic Modeling. Biomedicines 2023, 11, 3021. [Google Scholar] [CrossRef]
- Weijenborg, P.W.; Cremonini, F.; Smout, A.J.; Bredenoord, A.J. PPI therapy is equally effective in well-defined non-erosive reflux disease and in reflux esophagitis: A meta-analysis. Neurogastroenterol. Motil. 2012, 24, 747–757, e350. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Dulai, G.S.; Fennerty, M.B.; Spiegel, B.M. Esomeprazole versus other proton pump inhibitors in erosive esophagitis: A meta-analysis of randomized clinical trials. Clin. Gastroenterol. Hepatol. 2006, 4, 1452–1458. [Google Scholar] [CrossRef]
- Fass, R. Healing erosive esophagitis with a proton pump inhibitor: The more the merrier? Am. J. Gastroenterol. 2012, 107, 531–533. [Google Scholar] [CrossRef]
- Hammer, J.; Schmidt, B. Effect of Splitting the Dose of Esomeprazole on Gastric Acidity and Nocturnal Acid Breakthrough. Aliment. Pharmacol. Ther. 2004, 19, 1105–1110. [Google Scholar] [CrossRef]
- Wilder-Smith, C.; Röhss, K.; Bokelund Singh, S.; Sagar, M.; Nagy, P. The effects of dose and timing of esomeprazole administration on 24-h, daytime and night-time acid inhibition in healthy volunteers. Aliment. Pharmacol. Ther. 2010, 32, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Nehra, A.K.; Alexander, J.A.; Loftus, C.G.; Nehra, V. Proton Pump Inhibitors: Review of Emerging Concerns. Mayo Clin. Proc. 2018, 93, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Katz, P.O.; Dunbar, K.B.; Schnoll-Sussman, F.H.; Greer, K.B.; Yadlapati, R.; Spechler, S.J. ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2022, 117, 27–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Vela, M.F.; Peterson, K.A.; Carlson, D.A. AGA Clinical Practice Update on the Diagnosis and Management of Extraesophageal Gastroesophageal Reflux Disease: Expert Review. Clin. Gastroenterol. Hepatol. 2023, 21, 1414–1421.e3. [Google Scholar] [CrossRef]
- Fackler, W.K.; Ours, T.M.; Vaezi, M.F.; Richter, J.E. Long-term effect of H2RA therapy on nocturnal gastric acid breakthrough. Gastroenterology 2002, 122, 625–632. [Google Scholar] [CrossRef]
- Sabesin, S.M.; Berlin, R.G.; Humphries, T.J.; Bradstreet, D.C.; Walton-Bowen, K.L.; Zaidi, S. Famotidine relieves symptoms of gastroesophageal reflux disease and heals erosions and ulcerations. Results of a multicenter, placebo-controlled, dose-ranging study. Arch. Intern. Med. 1991, 151, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Inadomi, J.M.; Jamal, R.; Murata, G.H.; Hoffman, R.M.; Lavezo, L.A.; Vigil, J.M.; Swanson, K.M.; Sonnenberg, A. Step-down management of gastroesophageal reflux disease. Gastroenterology 2001, 121, 1095–1100. [Google Scholar] [CrossRef]
- Tougas, G.; Armstrong, D. Efficacy of H2 receptor antagonists in the treatment of gastroesophageal reflux disease and its symptoms. Can. J. Gastroenterol. 1997, 11 (Suppl. B), 51B–54B. [Google Scholar] [PubMed]
- Abdul-Hussein, M.; Freeman, J.; Castell, D. Concomitant Administration of a Histamine2 Receptor Antagonist and Proton Pump Inhibitor Enhances Gastric Acid Suppression. Pharmacotherapy 2015, 35, 1124–1129. [Google Scholar] [CrossRef]
- Mainie, I.; Tutuian, R.; Castell, D.O. Addition of a H2 receptor antagonist to PPI improves acid control and decreases nocturnal acid breakthrough. J. Clin. Gastroenterol. 2008, 42, 676–679. [Google Scholar] [CrossRef]
- Sabesin, S.M. Safety Issues Relating to Long-Term Treatment with Histamine H2-Receptor Antagonists. Aliment. Pharmacol. Ther. 1993, 7 (Suppl. S2), 35–40. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, J.; Tan, X.; Dai, Y.; Xie, C.; Li, X.; Lu, Q.; Kou, F.; Jiang, H.; Li, J. Direct Comparison of the Efficacy and Safety of Vonoprazan Versus Proton-Pump Inhibitors for Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2021, 66, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Igarashi, A.; Takeuchi, T.; Teng, L.; Uda, A.; Deguchi, H.; Higuchi, K.; Tango, T. Vonoprazan versus proton-pump inhibitors for healing gastroesophageal reflux disease: A systematic review. J. Gastroenterol. Hepatol. 2019, 34, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Chen, S.; Zhou, X.; Jia, X.; Zhang, M.; Tan, N.; Chen, F.; Zhang, Z.; Hu, J.; Xiao, Y. Comparative Efficacy of P-CAB vs Proton Pump Inhibitors for Grade C/D Esophagitis: A Systematic Review and Network Meta-analysis. Am. J. Gastroenterol. 2024, 119, 803–813. [Google Scholar] [CrossRef]
- Miwa, H.; Igarashi, A.; Teng, L.; Uda, A.; Deguchi, H.; Tango, T. Systematic review with network meta-analysis: Indirect comparison of the efficacy of vonoprazan and proton-pump inhibitors for maintenance treatment of gastroesophageal reflux disease. J. Gastroenterol. 2019, 54, 718–729. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, S.; Dai, N.; Fei, G.; Goh, K.L.; Chun, H.J.; Sheu, B.S.; Chong, C.F.; Funao, N.; Zhou, W.; et al. Phase III, randomised, double-blind, multicentre study to evaluate the efficacy and safety of vonoprazan compared with lansoprazole in Asian patients with erosive oesophagitis. Gut 2020, 69, 224–230. [Google Scholar] [CrossRef]
- Oshima, T.; Arai, E.; Taki, M.; Kondo, T.; Tomita, T.; Fukui, H.; Watari, J.; Miwa, H. Randomised clinical trial: Vonoprazan versus lansoprazole for the initial relief of heartburn in patients with erosive oesophagitis. Aliment. Pharmacol. Ther. 2019, 49, 140–146. [Google Scholar] [CrossRef]
- Xu, W.; Bai, Z.; Shang, Y.; Wang, J.; Wong, Y.; Qi, X. Incidence and Type of Adverse Events in Patients Taking Vonoprazan: A Systematic Review and Meta-Analysis. Therap. Adv. Gastroenterol. 2023, 16, 17562848231167858. [Google Scholar] [CrossRef]
- Kang, S.H.; Moon, H.S.; Sung, J.K.; Kim, S.M.; Kim, K.B.; Lee, S.W.; Cho, Y.S.; Bang, K.B.; Song, K.H. Assessment of the efficacy of on-demand tegoprazan therapy in gastroesophageal reflux disease through a randomized controlled trial. Sci. Rep. 2025, 15, 168. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simon, B.; Ravelli, G.P.; Goffin, H. Sucralfate gel versus placebo in patients with non-erosive gastro-oesophageal reflux disease. Aliment. Pharmacol. Ther. 1996, 10, 441–446. [Google Scholar] [CrossRef]
- Ali, R.A.R.; Hassan, J.; Egan, L.J. Review of Recent Evidence on the Management of Heartburn in Pregnant and Breastfeeding Women. BMC Gastroenterol. 2022, 22, 219. [Google Scholar] [CrossRef] [PubMed]
- Rohof, W.O.; Bennink, R.J.; Smout, A.J.; Thomas, E.; Boeckxstaens, G.E. An alginate-antacid formulation localizes to the acid pocket to reduce acid reflux in patients with gastroesophageal reflux disease. Clin. Gastroenterol. Hepatol. 2013, 11, 1585–1591. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; de Bortoli, N.; Zentilin, P.; Martinucci, I.; Bruzzone, L.; Furnari, M.; Marchi, S.; Savarino, V. Alginate controls heartburn in patients with erosive and nonerosive reflux disease. World J. Gastroenterol. 2012, 18, 4371–4378. [Google Scholar] [CrossRef] [PubMed]
- Kudaravalli, P.; Patel, P.; John, S. Sucralfate. In StatPearls Internet; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Jung, H.K.; Tae, C.H.; Song, K.H.; Kang, S.J.; Park, J.K.; Gong, E.J.; Shin, J.E.; Lim, H.C.; Lee, S.K.; Jung, D.H.; et al. 2020 Seoul Consensus on the Diagnosis and Management of Gastroesophageal Reflux Disease. J. Neurogastroenterol. Motil. 2021, 27, 453–481. [Google Scholar] [CrossRef]
- Sjöstedt, S.; Befrits, R.; Sylvan, A.; Harthon, C.; Jörgensen, L.; Carling, L.; Modin, S.; Stubberöd, A.; Toth, E.; Lind, T. Daily treatment with esomeprazole is superior to that taken on-demand for maintenance of healed erosive oesophagitis. Aliment. Pharmacol. Ther. 2005, 22, 183–191. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Okada, H.; Kawahara, Y.; Takenaka, R.; Nasu, J.; Ishioka, H.; Fujiwara, A.; Yoshinaga, F.; Yamamoto, K. Proton pump inhibitor step-down therapy for GERD: A multi-center study in Japan. World J. Gastroenterol. 2011, 17, 1480–1487. [Google Scholar] [CrossRef]
- Mine, S.; Iida, T.; Tabata, T.; Kishikawa, H.; Tanaka, Y. Management of symptoms in step-down therapy of gastroesophageal reflux disease. J. Gastroenterol. Hepatol. 2005, 20, 1365–1370. [Google Scholar] [CrossRef]
- Furuta, T.; Sugimoto, M.; Kodaira, C.; Nishino, M.; Yamade, M.; Ikuma, M.; Shirai, N.; Watanabe, H.; Umemura, K.; Kimura, M.; et al. CYP2C19 genotype is associated with symptomatic recurrence of GERD during maintenance therapy with low-dose lansoprazole. Eur. J. Clin. Pharmacol. 2009, 65, 693–698. [Google Scholar] [CrossRef]
- Lauritsen, K.; Devière, J.; Bigard, M.A.; Bayerdörffer, E.; Mózsik, G.; Murray, F.; Kristjánsdóttir, S.; Savarino, V.; Vetvik, K.; De Freitas, D.; et al. Esomeprazole 20 mg and lansoprazole 15 mg in maintaining healed reflux oesophagitis: Metropole study results. Aliment. Pharmacol. Ther. 2003, 17 (Suppl. 1), 24, discussion 25–27. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Hongo, M.; Japan TWICE Study Group. Efficacy of twice-daily rabeprazole for reflux esophagitis patients refractory to standard once-daily administration of PPI: The Japan-based TWICE study. Am. J. Gastroenterol. 2012, 107, 522–530. [Google Scholar] [CrossRef]
- Fass, R.; Sontag, S.J.; Traxler, B.; Sostek, M. Treatment of patients with persistent heartburn symptoms: A double-blind, randomized trial. Clin. Gastroenterol. Hepatol. 2006, 4, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Delchier, J.C.; Cohen, G.; Humphries, T.J. Rabeprazole, 20 mg once daily or 10 mg twice daily, is equivalent to omeprazole, 20 mg once daily, in the healing of erosive gastrooesophageal reflux disease. Scand. J. Gastroenterol. 2000, 35, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Galmiche, J.P.; Zerbib, F.; Ducrottè, P.; Fournet, J.; Rampal, P.; Avasthy, N.; Humphries, T.J. Decreasing oesophageal acid exposure in patients with GERD: A comparison of rabeprazole and omeprazole. Aliment. Pharmacol. Ther. 2001, 15, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Roberts, D.N.; Tierney, W.M. Long-term safety concerns with proton pump inhibitors. Am. J. Med. 2009, 122, 896–903. [Google Scholar] [CrossRef]
- den Elzen, W.P.; Groeneveld, Y.; de Ruijter, W.; Souverijn, J.H.; le Cessie, S.; Assendelft, W.J.; Gussekloo, J. Long-term use of proton pump inhibitors and vitamin B12 status in elderly individuals. Aliment. Pharmacol. Ther. 2008, 27, 491–497. [Google Scholar] [CrossRef]
- Yang, Y.X.; Lewis, J.D.; Epstein, S.; Metz, D.C. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006, 296, 2947–2953. [Google Scholar] [CrossRef]
- Maia, T.F.; de Camargo, B.G.; Pereira, M.E.; de Oliveira, C.S.; Guiloski, I.C. Increased Risk of Fractures and Use of Proton Pump Inhibitors in Menopausal Women: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 13501. [Google Scholar] [CrossRef]
- Cao, F.; Chen, C.X.; Wang, M.; Liao, H.R.; Wang, M.X.; Hua, S.Z.; Huang, B.; Xiong, Y.; Zhang, J.Y.; Xu, Y.L. Updated meta-analysis of controlled observational studies: Proton-pump inhibitors and risk of Clostridium difficile infection. J. Hosp. Infect. 2018, 98, 4–13. [Google Scholar] [CrossRef]
- Moayyedi, P.; Cranney, A. Hip fracture and proton pump inhibitor therapy: Balancing the evidence for benefit and harm. Am. J. Gastroenterol. 2008, 103, 2428–2431. [Google Scholar] [CrossRef]
- Shah, N.H.; LePendu, P.; Bauer-Mehren, A.; Ghebremariam, Y.T.; Iyer, S.V.; Marcus, J.; Nead, K.T.; Cooke, J.P.; Leeper, N.J. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS ONE 2015, 10, e0124653. [Google Scholar] [CrossRef]
- Islam, M.M.; Poly, T.N.; Walther, B.A.; Dubey, N.K.; Anggraini Ningrum, D.N.; Shabbir, S.A.; Jack Li, Y.C. Adverse outcomes of long-term use of proton pump inhibitors: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Poly, T.N.; Islam, M.M.; Yang, H.C.; Wu, C.C.; Li, Y.J. Proton pump inhibitors and risk of hip fracture: A meta-analysis of observational studies. Osteoporos. Int. 2019, 30, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Tleyjeh, I.M.; Bin Abdulhak, A.A.; Riaz, M.; Alasmari, F.A.; Garbati, M.A.; AlGhamdi, M.; Al-Tannir, M.; Alasmari, A.M.; Alshamrani, M.M.; Baddour, L.M.; et al. Association between proton pump inhibitor therapy and Clostridium difficile infection: A contemporary systematic review and meta-analysis. PLoS ONE 2012, 7, e50836. [Google Scholar] [CrossRef]
- Lazarus, B.; Chen, Y.; Wilson, F.P.; Sang, Y.; Chang, A.R.; Coresh, J.; Grams, M.E. Proton Pump Inhibitor Use and the Risk of Chronic Kidney Disease. JAMA Intern. Med. 2016, 176, 238–246. [Google Scholar] [CrossRef]
- Moayyedi, P.; Eikelboom, J.W.; Bosch, J.; Connolly, S.J.; Dyal, L.; Shestakovska, O.; Leong, D.; Anand, S.S.; Störk, S.; Branch, K.R.H.; et al. Safety of Proton Pump Inhibitors Based on a Large, Multi-Year, Randomized Trial of Patients Receiving Rivaroxaban or Aspirin. Gastroenterology 2019, 157, 682–691.e2. [Google Scholar] [CrossRef]
- Abdallah, J.; George, N.; Yamasaki, T.; Ganocy, S.; Fass, R. Most Patients With Gastroesophageal Reflux Disease Who Failed Proton Pump Inhibitor Therapy Also Have Functional Esophageal Disorders. Clin. Gastroenterol. Hepatol. 2019, 17, 1073–1080.e1. [Google Scholar] [CrossRef]
- Yadlapati, R.; DeLay, K. Proton Pump Inhibitor-Refractory Gastroesophageal Reflux Disease. Med. Clin. N. Am. 2019, 103, 15–27. [Google Scholar] [CrossRef]
- Patel, A.; Yadlapati, R. Diagnosis and Management of Refractory Gastroesophageal Reflux Disease. Gastroenterol. Hepatol. 2021, 17, 305–315. [Google Scholar] [PubMed] [PubMed Central]
- Ostovaneh, M.R.; Saeidi, B.; Hajifathalian, K.; Farrokhi-Khajeh-Pasha, Y.; Fotouhi, A.; Mirbagheri, S.S.; Emami, H.; Barzin, G.; Mirbagheri, S.A. Comparing omeprazole with fluoxetine for treatment of patients with heartburn and normal endoscopy who failed once daily proton pump inhibitors: Double-blind placebo-controlled trial. Neurogastroenterol. Motil. 2014, 26, 670–678. [Google Scholar] [CrossRef]
- Gyawali, C.P.; Yadlapati, R.; Fass, R.; Katzka, D.; Pandolfino, J.; Savarino, E.; Sifrim, D.; Spechler, S.; Zerbib, F.; Fox, M.R.; et al. Updates to the Modern Diagnosis of GERD: Lyon Consensus 2.0. Gut 2024, 73, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Visaggi, P.; Del Corso, G.; Gyawali, C.P.; Ghisa, M.; Baiano Svizzero, F.; Stefani Donati, D.; Venturini, A.; Savarino, V.; Penagini, R.; Zeki, S.; et al. Ambulatory pH-Impedance Findings Confirm That Grade B Esophagitis Provides Objective Diagnosis of Gastroesophageal Reflux Disease. Am. J. Gastroenterol. 2023, 118, 794–801. [Google Scholar] [CrossRef]
- Rusu, R.I.; Fox, M.R.; Tucker, E.; Zeki, S.; Dunn, J.M.; Jafari, J.; Warburton, F.; Wong, T. Validation of the Lyon classification for GORD diagnosis: Acid exposure time assessed by prolonged wireless pH monitoring in healthy controls and patients with erosive oesophagitis. Gut 2021, 70, 2230–2237. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, C.P.; Fass, R. Management of Gastroesophageal Reflux Disease. Gastroenterology 2018, 154, 302–318. [Google Scholar] [CrossRef] [PubMed]
- Spechler, S.J.; Hunter, J.G.; Jones, K.M.; Lee, R.; Smith, B.R.; Mashimo, H.; Sanchez, V.M.; Dunbar, K.B.; Pham, T.H.; Murthy, U.K.; et al. Randomized Trial of Medical versus Surgical Treatment for Refractory Heartburn. N. Engl. J. Med. 2019, 381, 1513–1523. [Google Scholar] [CrossRef]
- Savarino, E.; Marabotto, E.; Savarino, V. Recent insights on functional heartburn and reflux hypersensitivity. Curr. Opin. Gastroenterol. 2022, 38, 417–422. [Google Scholar] [CrossRef]
- Aziz, Q.; Fass, R.; Gyawali, C.P.; Miwa, H.; Pandolfino, J.E.; Zerbib, F. Functional Esophageal Disorders. Gastroenterology 2016, 150, 1368–1379. [Google Scholar] [CrossRef]
- Katzka, D.A.; Pandolfino, J.E.; Kahrilas, P.J. Phenotypes of Gastroesophageal Reflux Disease: Where Rome, Lyon, and Montreal Meet. Clin. Gastroenterol. Hepatol. 2020, 18, 767–776. [Google Scholar] [CrossRef]
- Gyawali, C.P.; Kahrilas, P.J.; Savarino, E.; Zerbib, F.; Mion, F.; Smout, A.J.P.M.; Vaezi, M.; Sifrim, D.; Fox, M.R.; Vela, M.F.; et al. Modern diagnosis of GERD: The Lyon Consensus. Gut 2018, 67, 1351–1362. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, X.; Li, R.; Zhang, J.; Zhang, F. Proton pump inhibitor utilisation and potentially inappropriate prescribing analysis: Insights from a single-centred retrospective study. BMJ Open 2020, 10, e040473. [Google Scholar] [CrossRef]
- Heidelbaugh, J.J.; Kim, A.H.; Chang, R.; Walker, P.C. Overutilization of Proton-Pump Inhibitors: What the Clinician Needs to Know. Therap. Adv. Gastroenterol. 2012, 5, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Y.; Sun, L.N.; Zhang, X.H.; Li, Y.Q.; Yu, L.; Yuan, Z.Q.; Meng, L.; Zhang, H.W.; Wang, Y.Q. A Review of the Novel Application and Potential Adverse Effects of Proton Pump Inhibitors. Adv. Ther. 2017, 34, 1070–1086. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Lee, T.; Yang, H.J.; Park, J.H.; Sohn, C.I.; Ryu, S.; Park, D.I. Weight loss and waist reduction is associated with improvement in gastroesophageal disease reflux symptoms: A longitudinal study of 15,295 subjects undergoing health checkups. Neurogastroenterol. Motil. 2017, 29, e13009. [Google Scholar] [CrossRef] [PubMed]
- Pandolfino, J.E.; El-Serag, H.B.; Zhang, Q.; Shah, N.; Ghosh, S.K.; Kahrilas, P.J. Obesity: A challenge to esophagogastric junction integrity. Gastroenterology 2006, 130, 639–649. [Google Scholar] [CrossRef]
- Ahima, R.S.; Flier, J.S. Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 2000, 11, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Piesman, M.; Hwang, I.; Maydonovitch, C.; Wong, R.K. Nocturnal reflux episodes following the administration of a standardized meal. Does timing matter? Am. J. Gastroenterol. 2007, 102, 2128–2134. [Google Scholar] [CrossRef]
- Bor, S.; Erdogan, A.; Bayrakci, B.; Yildirim, E.; Vardar, R. The impact of the speed of food intake on gastroesophageal reflux events in obese female patients. Dis. Esophagus 2017, 30, 1–6. [Google Scholar] [CrossRef]
- Valitova, E.R.; Bayrakçı, B.; Bor, S. The effect of the speed of eating on acid reflux and symptoms of patients with gastroesophageal reflux disease. Turk. J. Gastroenterol. 2013, 24, 379–381. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Satia, J.A.; Rabeneck, L. Dietary intake and the risk of gastro-oesophageal reflux disease: A cross sectional study in volunteers. Gut 2005, 54, 11–17. [Google Scholar] [CrossRef]
- Plaidum, S.; Patcharatrakul, T.; Promjampa, W.; Gonlachanvit, S. The Effect of Fermentable, Oligosaccharides, Disaccharides, Monosaccharides, and Polyols (FODMAP) Meals on Transient Lower Esophageal Relaxations (TLESR) in Gastroesophageal Reflux Disease (GERD) Patients with Overlapping Irritable Bowel Syndrome (IBS). Nutrients 2022, 14, 1755. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.W.; Myung, S.K.; Kwon, H.; Lee, C.; Yun, J.M.; Lee, H.K.; Korean Meta-analysis (KORMA) Study Group. Association between coffee intake and gastroesophageal reflux disease: A meta-analysis. Dis. Esophagus 2014, 27, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Ness-Jensen, E.; Lindam, A.; Lagergren, J.; Hveem, K. Weight loss and reduction in gastroesophageal reflux. A prospective population-based cohort study: The HUNT study. Am. J. Gastroenterol. 2013, 108, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Cen, L.; Chen, W.; Yu, C.; Li, Y.; Shen, Z. Alcohol Consumption and the Risk of Gastroesophageal Reflux Disease: A Systematic Review and Meta-analysis. Alcohol Alcohol. 2019, 54, 62–69. [Google Scholar] [CrossRef] [PubMed]
- López-Colombo, A.; Pacio-Quiterio, M.S.; Jesús-Mejenes, L.Y.; Rodríguez-Aguilar, J.E.; López-Guevara, M.; Montiel-Jarquín, A.J.; López-Alvarenga, J.C.; Morales-Hernández, E.R.; Ortiz-Juárez, V.R.; Ávila-Jiménez, L. Risk factors associated with gastroesophageal reflux disease relapse in primary care patients successfully treated with a proton pump inhibitor. Rev. Gastroenterol. Mex. 2017, 82, 106–114. [Google Scholar] [CrossRef]
- Eslami, O.; Shahraki, M.; Bahari, A.; Shahraki, T. Dietary habits and obesity indices in patients with gastro-esophageal reflux disease: A comparative cross-sectional study. BMC Gastroenterol. 2017, 17, 132. [Google Scholar] [CrossRef]
- Kubo, A.; Block, G.; Quesenberry, C.P., Jr.; Buffler, P.; Corley, D.A. Dietary guideline adherence for gastroesophageal reflux disease. BMC Gastroenterol. 2014, 14, 144. [Google Scholar] [CrossRef]
- Kariri, A.M.; Darraj, M.A.; Wassly, A.; Arishi, H.A.; Lughbi, M.; Kariri, A.; Madkhali, A.M.; Ezzi, M.I.; Khawaji, B. Prevalence and Risk Factors of Gastroesophageal Reflux Disease in Southwestern Saudi Arabia. Cureus 2020, 12, e6626. [Google Scholar] [CrossRef]
- Wright, L.E.; Castell, D.O. The adverse effect of chocolate on lower esophageal sphincter pressure. Am. J. Dig. Dis. 1975, 20, 703–707. [Google Scholar] [CrossRef]
- Murphy, D.W.; Castell, D.O. Chocolate and heartburn: Evidence of increased esophageal acid exposure after chocolate ingestion. Am. J. Gastroenterol. 1988, 83, 633–636. [Google Scholar] [PubMed]
- DiSilvestro, R.A.; Verbruggen, M.A.; Offutt, E.J. Anti-heartburn effects of a fenugreek fiber product. Phytother. Res. 2011, 25, 88–91. [Google Scholar] [CrossRef]
- Morozov, S.; Isakov, V.; Konovalova, M. Fiber-enriched diet helps to control symptoms and improves esophageal motility in patients with non-erosive gastroesophageal reflux disease. World J. Gastroenterol. 2018, 24, 2291–2299. [Google Scholar] [CrossRef] [PubMed]
- Lakananurak, N.; Pitisuttithum, P.; Susantitaphong, P.; Patcharatrakul, T.; Gonlachanvit, S. The Efficacy of Dietary Interventions in Patients with Gastroesophageal Reflux Disease: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2024, 16, 464. [Google Scholar] [CrossRef] [PubMed]
- Rivière, P.; Vauquelin, B.; Rolland, E.; Melchior, C.; Roman, S.; Bruley des Varannes, S.; Mion, F.; Gourcerol, G.; Sacher-Huvelin, S.; Zerbib, F. Low FODMAPs diet or usual dietary advice for the treatment of refractory gastroesophageal reflux disease: An open-labeled randomized trial. Neurogastroenterol. Motil. 2021, 33, e14181. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.C.; Just, R.; Castell, D.O. Body position affects recumbent postprandial reflux. J. Clin. Gastroenterol. 1994, 18, 280–283. [Google Scholar] [CrossRef]
- Allampati, S.; Lopez, R.; Thota, P.N.; Ray, M.; Birgisson, S.; Gabbard, S.L. Use of a positional therapy device significantly improves nocturnal gastroesophageal reflux symptoms. Dis. Esophagus 2017, 30, 1–7. [Google Scholar] [CrossRef]
- Dennish, G.W.; Castell, D.O. Inhibitory effect of smoking on the lower esophageal sphincter. N. Engl. J. Med. 1971, 284, 1136–1137. [Google Scholar] [CrossRef]
- Kahrilas, P.J.; Gupta, R.R. The effect of cigarette smoking on salivation and esophageal acid clearance. J. Lab. Clin. Med. 1989, 114, 431–438. [Google Scholar] [PubMed]
- Ness-Jensen, E.; Lindam, A.; Lagergren, J.; Hveem, K. Tobacco smoking cessation and improved gastroesophageal reflux: A prospective population-based cohort study: The HUNT study. Am. J. Gastroenterol. 2014, 109, 171–177. [Google Scholar] [CrossRef]
- Ness-Jensen, E.; Hveem, K.; El-Serag, H.; Lagergren, J. Lifestyle Intervention in Gastroesophageal Reflux Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 175–182.e1–3. [Google Scholar] [CrossRef]
- Nilsson, M.; Johnsen, R.; Ye, W.; Hveem, K.; Lagergren, J. Lifestyle related risk factors in the aetiology of gastro-oesophageal reflux. Gut 2004, 53, 1730–1735. [Google Scholar] [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Nishida, K.; Kataoka-Kato, A.; Gondo, Y.; Ishikawa, H.; Suda, K.; Kawai, M.; Hoshi, R.; Watanabe, O.; Igarashi, T.; et al. Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models. Neurogastroenterol. Motil. 2016, 28, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Krsek, A.; Baticic, L. Neutrophils in the Focus: Impact on Neuroimmune Dynamics and the Gut–Brain Axis. Gastrointest. Disord. 2024, 6, 557–606. [Google Scholar] [CrossRef]
- Lu, S.; Zhao, Q.; Guan, Y.; Sun, Z.; Li, W.; Guo, S.; Zhang, A. The communication mechanism of the gut-brain axis and its effect on central nervous system diseases: A systematic review. Biomed. Pharmacother. 2024, 178, 117207. [Google Scholar] [CrossRef]
- Lyte, J.M.; Gheorghe, C.E.; Goodson, M.S.; Kelley-Loughnane, N.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Gut-brain axis serotonergic responses to acute stress exposure are microbiome-dependent. Neurogastroenterol. Motil. 2020, 32, e13881. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Wang, G.; Song, X.; Qian, Y.; Liao, Z.; Sui, L.; Ai, L.; Xia, Y. Understanding the Connection between Gut Homeostasis and Psychological Stress. J. Nutr. 2023, 153, 924–939. [Google Scholar] [CrossRef]
- Hungin, A.P.; Yadlapati, R.; Anastasiou, F.; Bredenoord, A.J.; El Serag, H.; Fracasso, P.; Mendive, J.M.; Savarino, E.V.; Sifrim, D.; Udrescu, M.; et al. Management advice for patients with reflux-like symptoms: An evidence-based consensus. Eur. J. Gastroenterol. Hepatol. 2024, 36, 13–25. [Google Scholar] [CrossRef]
- Wickramasinghe, N.; Devanarayana, N.M. Unveiling the intricacies: Insight into gastroesophageal reflux disease. World J. Gastroenterol. 2025, 31, 98479. [Google Scholar] [CrossRef]
- Li, Q.; Duan, H.; Wang, Q.; Dong, P.; Zhou, X.; Sun, K.; Tang, F.; Wang, X.; Lin, L.; Long, Y.; et al. Analyzing the correlation between gastroesophageal reflux disease and anxiety and depression based on ordered logistic regression. Sci. Rep. 2024, 14, 6594. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Raman, R.; Kishor, M.; Nandeesh, H.P. The effectiveness of mindfulness meditation in relief of symptoms of depression and quality of life in patients with gastroesophageal reflux disease. Indian J. Gastroenterol. 2019, 38, 29–38. [Google Scholar] [CrossRef]
- Ma, T.; Jin, H.; Kwok, L.Y.; Sun, Z.; Liong, M.T.; Zhang, H. Probiotic consumption relieved human stress and anxiety symptoms possibly via modulating the neuroactive potential of the gut microbiota. Neurobiol. Stress 2021, 14, 100294. [Google Scholar] [CrossRef] [PubMed]
- McDonald-Haile, J.; Bradley, L.A.; Bailey, M.A.; Schan, C.A.; Richter, J.E. Relaxation training reduces symptom reports and acid exposure in patients with gastroesophageal reflux disease. Gastroenterology 1994, 107, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Ouwehand, A.C. Gastroesophageal Reflux Disease and Probiotics: A Systematic Review. Nutrients 2020, 12, 132. [Google Scholar] [CrossRef]
- Nikkhah Bodagh, M.; Maleki, I.; Hekmatdoost, A. Ginger in gastrointestinal disorders: A systematic review of clinical trials. Food Sci. Nutr. 2018, 7, 96–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anh, N.H.; Kim, S.J.; Long, N.P.; Min, J.E.; Yoon, Y.C.; Lee, E.G.; Kim, M.; Kim, T.J.; Yang, Y.Y.; Son, E.Y.; et al. Ginger on Human Health: A Comprehensive Systematic Review of 109 Randomized Controlled Trials. Nutrients 2020, 12, 157. [Google Scholar] [CrossRef]
- Aregawi, L.G.; Shokrolahi, M.; Gebremeskel, T.G.; Zoltan, C. The Effect of Ginger Supplementation on the Improvement of Dyspeptic Symptoms in Patients With Functional Dyspepsia. Cureus 2023, 15, e46061. [Google Scholar] [CrossRef]
- Palatty, P.L.; Haniadka, R.; Valder, B.; Arora, R.; Baliga, M.S. Ginger in the prevention of nausea and vomiting: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, A.K.; Gupta, A.; Bishayee, A.; Pandey, A.K. Therapeutic potential of Aloe vera-A miracle gift of nature. Phytomedicine 2019, 60, 152996. [Google Scholar] [CrossRef]
- Catalano, A.; Ceramella, J.; Iacopetta, D.; Marra, M.; Conforti, F.; Lupi, F.R.; Gabriele, D.; Borges, F.; Sinicropi, M.S. Aloe vera—An Extensive Review Focused on Recent Studies. Foods 2024, 13, 2155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, M.; Kong, X.Y.; Chen, T.T.; Zou, Z.M. In vivo metabolism combined network pharmacology to identify anti-constipation constituents in Aloe barbadensis Mill. J. Ethnopharmacol. 2024, 319 Pt 1, 117200. [Google Scholar] [CrossRef]
- Keshavarzi, Z.; Rezapour, T.M.; Vatanchian, M.; Zare Hesari, M.; Nabizade Haghighi, H.; Izanlu, M.; Sabaghian, M.; Shahveisi, K. The effects of aqueous extract of Aloe vera leaves on the gastric acid secretion and brain and intestinal water content following acetic acid-induced gastric ulcer in male rats. Avicenna J. Phytomedicine 2014, 4, 137–143. [Google Scholar] [PubMed] [PubMed Central]
- Panahi, Y.; Khedmat, H.; Valizadegan, G.; Mohtashami, R.; Sahebkar, A. Efficacy and safety of Aloe vera syrup for the treatment of gastroesophageal reflux disease: A pilot randomized positive-controlled trial. J. Tradit. Chin. Med. 2015, 35, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Langmead, L.; Feakins, R.M.; Goldthorpe, S.; Holt, H.; Tsironi, E.; De Silva, A.; Jewell, D.P.; Rampton, D.S. Randomized, double-blind, placebo-controlled trial of oral aloe vera gel for active ulcerative colitis. Aliment. Pharmacol. Ther. 2004, 19, 739–747. [Google Scholar] [CrossRef]
- Pastorino, G.; Cornara, L.; Soares, S.; Rodrigues, F.; Oliveira, M.B.P.P. Liquorice (Glycyrrhiza glabra): A phytochemical and pharmacological review. Phytother. Res. 2018, 32, 2323–2339. [Google Scholar] [CrossRef]
- Smedegaard, S.B.; Svart, M.V. Licorice induced pseudohyperaldosteronism, severe hypertension, and long QT. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019, 19-0109. [Google Scholar] [CrossRef]
- Prajapati, S.M.; Patel, B.R. A comparative clinical study of Jethimala (Taverniera nummularia Baker.) and Yashtimadhu (Glycyrrhiza glabra Linn.) in the management of Amlapitta. Ayurveda 2015, 36, 157–162. [Google Scholar] [CrossRef]
- Yeh, A.M.; Golianu, B. Integrative Treatment of Reflux and Functional Dyspepsia in Children. Children 2014, 1, 119–133. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Y.; Huang, T.; Huang, D.; Liu, L.; Shen, C.; Jiang, C.; Wang, Z.; Chen, H.; Liang, P.; et al. Licorice flavonoid alleviates gastric ulcers by producing changes in gut microbiota and promoting mucus cell regeneration. Biomed. Pharmacother. 2023, 169, 115868. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Crotteau, C.A.; Wright, S.T.; Eglash, A. Clinical inquiries. What is the best treatment for infants with colic? J. Fam. Pract. 2006, 55, 634–636. [Google Scholar] [PubMed]
- Baser, K.H.; Demirci, B.; Iscan, G.; Hashimoto, T.; Demirci, F.; Noma, Y.; Asakawa, Y. The essential oil constituents and antimicrobial activity of Anthemis aciphylla BOISS. var. discoidea BOISS. Chem. Pharm. Bull. 2006, 54, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Khayyal, M.T.; Seif-El-Nasr, M.; El-Ghazaly, M.A.; Okpanyi, S.N.; Kelber, O.; Weiser, D. Mechanisms involved in the gastro-protective effect of STW 5 (Iberogast) and its components against ulcers and rebound acidity. Phytomedicine 2006, 13 (Suppl. 5), 56–66. [Google Scholar] [CrossRef]
- Adel Mehraban, M.S.; Shirzad, M.; Ahmadian-Attari, M.M.; Shakeri, R.; Taghizadeh Kashani, L.M.; Tabarrai, M.; Shirbeigi, L. Effect of rose oil on Gastroesophageal Reflux Disease in comparison with omeprazole: A double-blind controlled trial. Complement. Ther. Clin. Pract. 2021, 43, 101361. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.F.; Muhammad, J.S.; Shahryar, S.; Usmanghani, K.; Gilani, A.H.; Jafri, W.; Sugiyama, T. Anti-inflammatory and cytoprotective effects of selected Pakistani medicinal plants in Helicobacter pylori-infected gastric epithelial cells. J. Ethnopharmacol. 2012, 141, 403–410. [Google Scholar] [CrossRef]
- Memariani, Z.; Hajimahmoodi, M.; Minaee, B.; Khodagholi, F.; Yans, A.; Rahimi, R.; Amin, G.; Moghaddam, G.; Toliyat, T.; Sharifzadeh, M. Protective Effect of a Polyherbal Traditional Formula Consisting of Rosa damascena Mill., Glycyrrhiza glabra L. And Nardostachys jatamansi DC., Against Ethanol-induced Gastric Ulcer. Iran J. Pharm. Res. 2017, 16, 694–707. [Google Scholar] [PubMed] [PubMed Central]
- Konturek, S.J.; Konturek, P.C.; Brzozowski, T. Melatonin in gastroprotection against stress-induced acute gastric lesions and in healing of chronic gastric ulcers. J. Physiol. Pharmacol. 2006, 57 (Suppl. 5), 51–66. [Google Scholar] [PubMed]
- Jha, L.K.; Fass, R.; Gadam, R.; Maradey-Romero, C.; Nasrollah, L.; Hershcovici, T.; Quan, S.F.; Dickman, R. The Effect of Ramelteon on Heartburn Symptoms of Patients With Gastroesophageal Reflux Disease and Chronic Insomnia: A Pilot Study. J. Clin. Gastroenterol. 2016, 50, e19–e24. [Google Scholar] [CrossRef] [PubMed]
- Konturek, S.J.; Zayachkivska, O.; Havryluk, X.O.; Brzozowski, T.; Sliwowski, Z.; Pawlik, M.; Konturek, P.C.; Cześnikiewicz-Guzik, M.; Gzhegotsky, M.R.; Pawlik, W.W. Protective influence of melatonin against acute esophageal lesions involves prostaglandins, nitric oxide and sensory nerves. J. Physiol. Pharmacol. 2007, 58, 361–377. [Google Scholar] [PubMed]
- Lin, W.; Huang, G.; Liu, X.; Lin, H.; Zhou, H.; Feng, C.; Wang, T.; Liang, R. Efficacy and safety of traditional Chinese herbal formula combined with western medicine for gastroesophageal reflux disease: A protocol for systematic review and meta-analysis. Medicine 2020, 99, e22454. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.Y.; Xie, C.E.; Wang, Z.B.; Pei, W.J.; Li, X.H.; Shi, L.; Liu, J.L.; Han, Y.F.; Tan, X.; Ding, P.H.; et al. The effect of Heweijiangni-decoction on esophageal morphology in a rat model of OVA-induced visceral hypersensitivity followed by acid exposure. Cell. Mol. Biol. 2019, 65, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Hu, J.L.; Zhao, C.C.; Zhang, J.Q.; Wu, F.; Ma, B.L.; Feng, Y.; Ruan, K.F. Zhujie Hewei Granules Ameliorated Reflux Esophagitis in Rats. Evid.-Based Complement. Altern. Med. 2019, 2019, 1392020. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bucan, J.I.; Braut, T.; Krsek, A.; Sotosek, V.; Baticic, L. Updates in Gastroesophageal Reflux Disease Management: From Proton Pump Inhibitors to Dietary and Lifestyle Modifications. Gastrointest. Disord. 2025, 7, 33. https://doi.org/10.3390/gidisord7020033
Bucan JI, Braut T, Krsek A, Sotosek V, Baticic L. Updates in Gastroesophageal Reflux Disease Management: From Proton Pump Inhibitors to Dietary and Lifestyle Modifications. Gastrointestinal Disorders. 2025; 7(2):33. https://doi.org/10.3390/gidisord7020033
Chicago/Turabian StyleBucan, Jakov Ivan, Tamara Braut, Antea Krsek, Vlatka Sotosek, and Lara Baticic. 2025. "Updates in Gastroesophageal Reflux Disease Management: From Proton Pump Inhibitors to Dietary and Lifestyle Modifications" Gastrointestinal Disorders 7, no. 2: 33. https://doi.org/10.3390/gidisord7020033
APA StyleBucan, J. I., Braut, T., Krsek, A., Sotosek, V., & Baticic, L. (2025). Updates in Gastroesophageal Reflux Disease Management: From Proton Pump Inhibitors to Dietary and Lifestyle Modifications. Gastrointestinal Disorders, 7(2), 33. https://doi.org/10.3390/gidisord7020033






