Effectiveness of Fecal Microbiota Transplantation in Nociplastic Pain Management: A Systematic Review
Abstract
1. Introduction
2. Materials and Method
2.1. Data Sources and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Methodological Quality Assessment
2.4.1. Randomized Controlled Trials
2.4.2. Non-Randomized Clinical Trials
2.4.3. Case Reports
2.4.4. Observational Study
3. Results
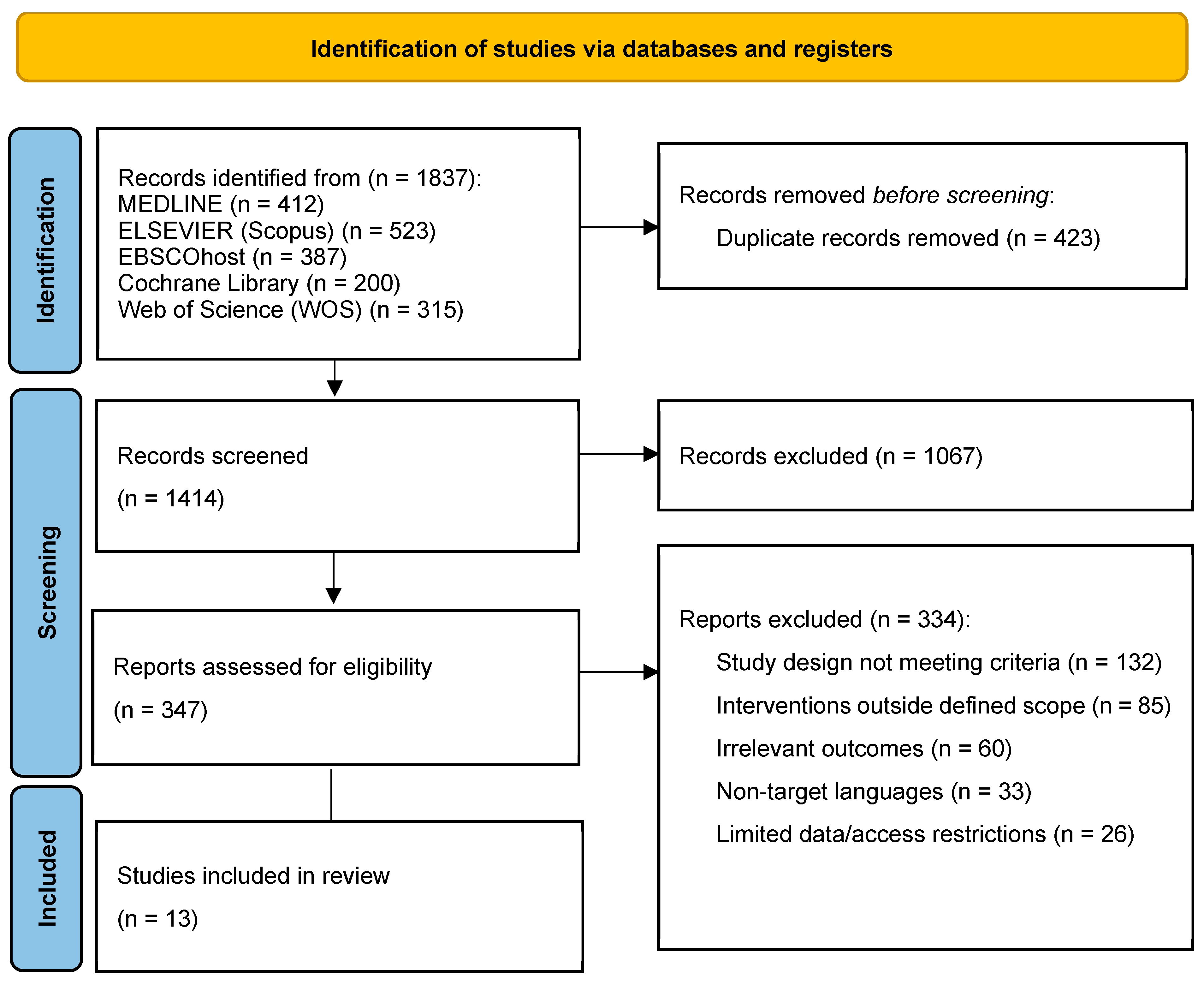
3.1. Study Selection
3.2. Characteristics of Included Studies
3.3. Methodological Quality Assessment
3.3.1. Randomized Controlled Trials
3.3.2. Open-Label Trials
3.3.3. Case Reports
3.3.4. Retrospective Outcome Study
3.4. Main Results
3.4.1. Severity of Nociplastic Pain Following FMT
3.4.2. Functionality and Quality of Life of Nociplastic Pain Following FMT
3.4.3. Other Effects in Nociplastic Pain Following FMT
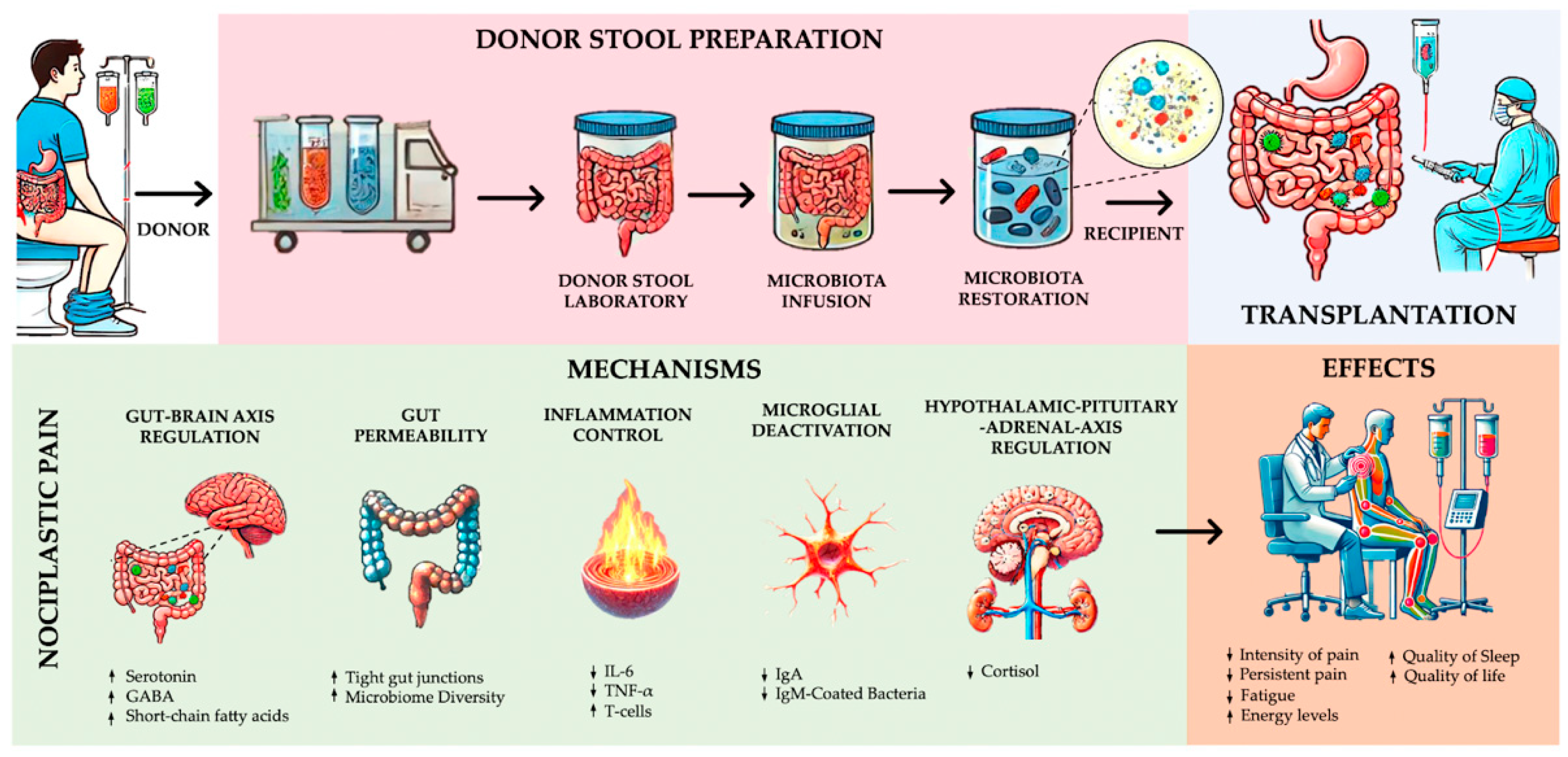
4. Discussion
4.1. Severity of Nociplastic Pain Following FMT
4.2. Functionality and Quality of Life in Nociplastic Pain Following FMT
4.3. Other Physiological and Biochemical Effects of FMT on Nociplastic Pain
4.4. Implications for Practice
4.5. Implications for Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.M.; Kim, K.H. Current understanding of nociplastic pain. Korean J. Pain 2024, 37, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Hausteiner-Wiehle, C.; Henningsen, P. Nociplastic pain is functional pain. Lancet 2022, 399, 1603–1604. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lavín, M. Centralized nociplastic pain causing fibromyalgia: An emperor with no clothes? Rheumatol. 2022, 41, 3915–3917. [Google Scholar] [CrossRef]
- Clauw, D.J. From fibrositis to fibromyalgia to nociplastic pain: How rheumatology helped get us here and where do we go from here? Rheum. Dis. 2024, 83, 1421–1427. [Google Scholar] [CrossRef]
- Murphy, A.E.; Minhas, D.; Clauw, D.J.; Lee, Y.C. Identifying and Managing Nociplastic Pain in Individuals With Rheumatic Diseases: A Narrative Review. Arthritis Care Res. 2023, 75, 2215–2222. [Google Scholar] [CrossRef]
- Kosek, E.; Clauw, D.; Nijs, J.; Baron, R.; Gilron, I.; Harris, R.E.; Mico, J.A.; Rice, A.S.C.; Sterling, M. Chronic nociplastic pain affecting the musculoskeletal system: Clinical criteria and grading system. Pain 2021, 162, 2629–2634. [Google Scholar] [CrossRef]
- Ablin, J.N. Nociplastic Pain: A Critical Paradigm for Multidisciplinary Recognition and Management. J. Clin. Med. 2024, 13, 5741. [Google Scholar] [CrossRef]
- Alcántara Montero, A.; Pacheco de Vasconcelos, S.R.; Castro Arias, A. Contextualization of the concept of nociplastic pain in irritable bowel syndrome. Rev. Esp. Enferm. Dig. 2024, 116, 698–699. [Google Scholar] [CrossRef]
- Midenfjord, I.; Grinsvall, C.; Koj, P.; Carnerup, I.; Törnblom, H.; Simrén, M. Central sensitization and severity of gastrointestinal symptoms in irritable bowel syndrome, chronic pain syndromes, and inflammatory bowel disease. Neurogastroenterol. Motil. 2021, 33, e14156. [Google Scholar] [CrossRef]
- Nijs, J.; Leysen, L.; Vanlauwe, J.; Logghe, T.; Ickmans, K.; Polli, A.; Malfliet, A.; Coppieters, I.; Huysmans, E. Treatment of central sensitization in patients with chronic pain: Time for change? Pharmacother. 2019, 20, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Ryu, H.J.; Bhatt, R.R. The neurobiology of irritable bowel syndrome. Mol. Psychiatry 2023, 28, 1451–1465. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Pratt, M.L.; Plumb, A.N.; Manjrekar, A.; Cardona, L.M.; Chan, C.K.; John, J.M.; Sadler, K.E. Microbiome contributions to pain: A review of the preclinical literature. Pain 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, G.; Ko, G.; Joong Lee, S. Nerve injury-induced gut dysbiosis contributes to spinal cord TNF-α expression and nociceptive sensitization. Brain Behav. Immun. 2023, 110, 155–161. [Google Scholar] [CrossRef]
- Garvey, M. The Association between Dysbiosis and Neurological Conditions Often Manifesting with Chronic Pain. Biomedicines 2023, 11, 748. [Google Scholar] [CrossRef]
- Goudman, L.; Demuyser, T.; Pilitsis, J.G.; Billot, M.; Roulaud, M.; Rigoard, P.; Moens, M. Gut dysbiosis in patients with chronic pain: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1342833. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Donoso, F.; Cryan, J.F.; Olavarría-Ramírez, L.; Nolan, Y.M.; Clarke, G. Inflammation, Lifestyle Factors, and the Microbiome-Gut-Brain Axis: Relevance to Depression and Antidepressant Action. Clin. Pharmacol. Ther. 2023, 113, 246–259. [Google Scholar] [CrossRef]
- Borody, T.J.; Khoruts, A. Fecal microbiota transplantation and emerging applications. Nat. Rev. Gastroenterol. Hepatol. 2011, 9, 88–96. [Google Scholar] [CrossRef]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal microbiota transplantation: In perspective. Therap. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118 (Suppl. S1), S23–S31. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Wang, J.Y.; Kuo, C.H. Current applications of fecal microbiota transplantation in intestinal disorders. Kaohsiung J. Med. Sci. 2019, 35, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.M.; Pestana, L.; Pardi, R.; Pardi, D.S.; Khanna, S. Fecal microbiota transplant via colonoscopy may be preferred due to intraprocedure findings. Intest. Res. 2019, 17, 434–437. [Google Scholar] [CrossRef]
- Ramai, D.; Zakhia, K.; Fields, P.J.; Ofosu, A.; Patel, G.; Shahnazarian, V.; Lai, J.K.; Dhaliwal, A.; Reddy, M.; Chang, S. Fecal Microbiota Transplantation (FMT) with Colonoscopy Is Superior to Enema and Nasogastric Tube While Comparable to Capsule for the Treatment of Recurrent Clostridioides difficile Infection: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2021, 66, 369–380. [Google Scholar] [CrossRef]
- Du, C.; Luo, Y.; Walsh, S.; Grinspan, A. Oral Fecal Microbiota Transplant Capsules Are Safe and Effective for Recurrent Clostridioides difficile Infection: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2021, 55, 300–308. [Google Scholar] [CrossRef]
- Pak, R.; Cho, M.; Pride, K.; Abd-Elsayed, A. The Gut Microbiota and Chronic Pain. Curr. Pain Headache Rep. 2024, 28, 259–269. [Google Scholar] [CrossRef]
- Bartman, C.; Chong, A.S.; Alegre, M.L. The Influence of the Microbiota on the Immune Response to Transplantation. Curr. Opin. Organ Transplant. 2015, 20, 1–7. [Google Scholar] [CrossRef]
- Lucarini, E.; Di Pilato, V.; Parisio, C.; Micheli, L.; Toti, A.; Pacini, A.; Bartolucci, G.; Baldi, S.; Niccolai, E.; Amedei, A.; et al. Visceral Sensitivity Modulation by Faecal Microbiota Transplantation: The Active Role of Gut Bacteria in Pain Persistence. Pain 2022, 163, 861–877. [Google Scholar] [CrossRef]
- de Groot, P.F.; Frissen, M.N.; de Clercq, N.C.; Nieuwdorp, M. Fecal Microbiota Transplantation in Metabolic Syndrome: History, Present and Future. Gut Microbes 2017, 8, 253–267. [Google Scholar] [CrossRef]
- Settanni, C.R.; Ianiro, G.; Bibbò, S.; Cammarota, G.; Gasbarrini, A. Gut Microbiota Alteration and Modulation in Psychiatric Disorders: Current Evidence on Fecal Microbiota Transplantation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110258. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Liu, S.; Crawford, J.; Tao, F. Gut-Brain Crosstalk and the Central Mechanisms of Orofacial Pain. Brain Sci. 2023, 13, 1456. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain Regulation by Gut Microbiota: Molecular Mechanisms and Therapeutic Potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Wang, Y.; Zhang, P.; Yuan, Y.; Zhang, Y.; Chen, G. Gut Microbiota Regulates Neuropathic Pain: Potential Mechanisms and Therapeutic Strategy. J. Headache Pain 2020, 21, 103. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (Updated March 2011); The Cochrane Collaboration: London, UK, 2011; Available online: https://www.handbook.cochrane.org (accessed on 17 November 2024).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomized Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomized Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE Guidelines: Consensus-Based Clinical Case Reporting Guideline Development. J. Clin. Epidemiol. 2014, 67, 46–51. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-randomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 17 November 2024).
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of Faecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome in a Randomised, Double-Blind, Placebo-Controlled Study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef]
- Fretheim, H.; Chung, B.K.; Didriksen, H.; Bækkevold, E.S.; Midtvedt, Ø.; Brunborg, C.; Holm, K.; Valeur, J.; Tennøe, A.H.; Garen, T.; et al. Fecal Microbiota Transplantation in Systemic Sclerosis: A Double-Blind, Placebo-Controlled Randomized Pilot Trial. PLoS ONE 2020, 15, e0232739. [Google Scholar] [CrossRef]
- Holvoet, T.; Joossens, M.; Vázquez-Castellanos, J.F.; Christiaens, E.; Heyerick, L.; Boelens, J.; Verhasselt, B.; van Vlierberghe, H.; De Vos, M.; Raes, J.; et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients With Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-Term Results From a Placebo-Controlled Randomized Trial. Gastroenterology 2021, 160, 145–157.e8. [Google Scholar] [CrossRef]
- Johnsen, P.H.; Hilpüsch, F.; Valle, P.C.; Goll, R. The Effect of Fecal Microbiota Transplantation on IBS-Related Quality of Life and Fatigue in Moderate to Severe Non-Constipated Irritable Bowel: Secondary Endpoints of a Double-Blind, Randomized, Placebo-Controlled Trial. EBioMedicine 2020, 51, 102562. [Google Scholar] [CrossRef] [PubMed]
- Kragsnaes, M.S.; Kjeldsen, J.; Horn, H.C.; Munk, H.L.; Pedersen, J.K.; Just, S.A.; Ahlquist, P.; Pedersen, F.M.; de Wit, M.; Möller, S.; et al. Safety and Efficacy of Faecal Microbiota Transplantation for Active Peripheral Psoriatic Arthritis: An Exploratory Randomised Placebo-Controlled Trial. Ann. Rheum. Dis. 2021, 80, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, P.; Jalanka, J.; Hartikainen, A.; Mattila, E.; Hillilä, M.; Punkkinen, J.; Koskenpato, J.; Anttila, V.J.; Tillonen, J.; Satokari, R.; et al. Randomised Clinical Trial: Faecal Microbiota Transplantation Versus Autologous Placebo Administered via Colonoscopy in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2020, 51, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Salonen, T.; Jokinen, E.; Satokari, R.; Lahtinen, P. Randomized, Double-Blinded, Placebo-Controlled Pilot Study: Efficacy of Faecal Microbiota Transplantation on Chronic Fatigue Syndrome. J. Transl. Med. 2023, 21, 513. [Google Scholar] [CrossRef]
- Fang, H.; Hou, Q.; Zhang, W.; Su, Z.; Zhang, J.; Li, J.; Lin, J.; Wang, Z.; Yu, X.; Yang, Y.; et al. Fecal Microbiota Transplantation Improves Clinical Symptoms of Fibromyalgia: An Open-Label, Randomized, Nonplacebo-Controlled Study. J. Pain 2024, 25, 104535. [Google Scholar] [CrossRef]
- Singh, P.; Alm, E.J.; Kelley, J.M.; Cheng, V.; Smith, M.; Kassam, Z.; Nee, J.; Iturrino, J.; Lembo, A. Effect of Antibiotic Pretreatment on Bacterial Engraftment after Fecal Microbiota Transplant (FMT) in IBS-D. Gut Microbes 2022, 14, 2020067. [Google Scholar] [CrossRef]
- Cai, T.T.; Ye, X.L.; Yong, H.J.; Song, B.; Zheng, X.L.; Cui, B.T.; Zhang, F.M.; Lu, Y.B.; Miao, H.; Ding, D.F. Fecal Microbiota Transplantation Relieve Painful Diabetic Neuropathy: A Case Report. Medicine 2018, 97, e13543. [Google Scholar] [CrossRef]
- Thurm, T.; Ablin, J.N.; Buskila, D.; Maharshak, N. Fecal Microbiota Transplantation for Fibromyalgia: A Case Report and Review of the Literature. Open J. Gastroenterol. 2017, 7, 131–139. [Google Scholar] [CrossRef]
- Zeng, J.; Peng, L.; Zheng, W.; Huang, F.; Zhang, N.; Wu, D.; Yang, Y. Fecal Microbiota Transplantation for Rheumatoid Arthritis: A Case Report. Clin. Case Rep. 2020, 9, 906–909. [Google Scholar] [CrossRef]
- Kenyon, J.N.; Coe, S.; Izadi, H. A Retrospective Outcome Study of 42 Patients with Chronic Fatigue Syndrome, 30 of Whom Had Irritable Bowel Syndrome. Half Were Treated with Oral Approaches, and Half Were Treated with Faecal Microbiome Transplantation. Hum. Microbiome J. 2019, 13, 100061. [Google Scholar] [CrossRef]
- Ustianowska, K.; Ustianowski, Ł.; Machaj, F.; Gorący, A.; Rosik, J.; Szostak, B.; Szostak, J.; Pawlik, A. The Role of the Human Microbiome in the Pathogenesis of Pain. Int. J. Mol. Sci. 2022, 23, 13267. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, Q.; Chen, Y.; Ren, H.; Zhang, Q.; Yang, H.; Zhang, W.; Ding, T.; Wang, S.; Zhang, Y.; et al. Gut Microbiota in Chronic Pain: Novel Insights into Mechanisms and Promising Therapeutic Strategies. Int. Immunopharmacol. 2023, 115, 109685. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Miccini, F.; Battelli, N. Gut Microbiota, Immunity and Pain. Immunol. Lett. 2021, 229, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Zhang, X.J.; Zhang, N.N.; Yan, B.; Xu, K.K.; Peng, L.H.; Pan, F. Fecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome: A Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2022, 9, 890357. [Google Scholar] [CrossRef]
- Matheson, J.T.; Holsinger, R.M.D. The Role of Fecal Microbiota Transplantation in the Treatment of Neurodegenerative Diseases: A Review. Int. J. Mol. Sci. 2023, 24, 1001. [Google Scholar] [CrossRef]
- Vergne-Salle, P.; Bertin, P. Chronic Pain and Neuroinflammation. Jt. Bone Spine 2021, 88, 105222. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Zhang, W.H.; Jin, Z.Y.; Yang, Z.H.; Zhang, J.Y.; Ma, X.H.; Guan, J.; Sun, B.L.; Chen, X. Fecal Microbiota Transplantation Ameliorates Active Ulcerative Colitis by Downregulating Pro-Inflammatory Cytokines in Mucosa and Serum. Front. Microbiol. 2022, 13, 818111. [Google Scholar] [CrossRef]
- Porcari, S.; Benech, N.; Valles-Colomer, M.; Segata, N.; Gasbarrini, A.; Cammarota, G.; Sokol, H.; Ianiro, G. Key Determinants of Success in Fecal Microbiota Transplantation: From Microbiome to Clinic. Cell Host Microbe 2023, 31, 712–733. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Rupprecht, L.E.; Liu, W.W.; Weng, P.; Bohorquez, D.V. Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction. Annu. Rev. Neurosci. 2020, 43, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Szőke, H.; Kovács, Z.; Bókkon, I.; Vagedes, J.; Szabó, A.E.; Hegyi, G.; Sterner, M.G.; Kiss, Á.; Kapócs, G. Gut Dysbiosis and Serotonin: Intestinal 5-HT as a Ubiquitous Membrane Permeability Regulator in Host Tissues, Organs, and the Brain. Rev. Neurosci. 2020, 31, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, G.; Guarnaccia, A.; Fancello, G.; Agrillo, C.; Iannarelli, F.; Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation and Other Gut Microbiota Manipulation Strategies. Microorganisms 2022, 10, 2424. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, A.K.; Jalanka, J.; Lahtinen, P.; Ponsero, A.J.; Mertsalmi, T.; Finnegan, L.; Crispie, F.; Cotter, P.D.; Arkkila, P.; Satokari, R. Fecal Microbiota Transplantation Influences Microbiota without Connection to Symptom Relief in Irritable Bowel Syndrome Patients. NPJ Biofilms Microbiomes 2024, 10, 73. [Google Scholar] [CrossRef]
- Rebeaud, J.; Peter, B.; Pot, C. How Microbiota-Derived Metabolites Link the Gut to the Brain during Neuroinflammation. Int. J. Mol. Sci. 2022, 23, 10128. [Google Scholar] [CrossRef]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-Derived Metabolites as Drivers of Gut-Brain Communication. Gut Microbes 2022, 14, 2102878. [Google Scholar] [CrossRef]
- Albrecht, D.S.; Forsberg, A.; Sandström, A.; Bergan, C.; Kadetoff, D.; Protsenko, E.; Lampa, J.; Lee, Y.C.; Höglund, C.O.; Catana, C.; et al. Brain Glial Activation in Fibromyalgia—A Multi-Site Positron Emission Tomography Investigation. Brain Behav. Immun. 2019, 75, 72–83. [Google Scholar] [CrossRef]
- Mueller, C.; Fang, Y.D.; Jones, C.; McConathy, J.E.; Raman, F.; Lapi, S.E.; Younger, J.W. Evidence of Neuroinflammation in Fibromyalgia Syndrome: A [18F]DPA-714 Positron Emission Tomography Study. Pain 2023, 164, 2285–2295. [Google Scholar] [CrossRef]
- Marcondes Ávila, P.R.; Fiorot, M.; Michels, M.; Dominguini, D.; Abatti, M.; Vieira, A.; de Moura, A.B.; Behenck, J.P.; Borba, L.A.; Botelho, M.E.M.; et al. Effects of Microbiota Transplantation and the Role of the Vagus Nerve in Gut-Brain Axis in Animals Subjected to Chronic Mild Stress. J. Affect. Disord. 2020, 277, 410–416. [Google Scholar] [CrossRef]
- Xu, D.; Ren, L.; Zhang, W.; Wu, S.; Yu, M.; He, X.; Wei, Z. Therapeutic Effects and Mechanisms of Fecal Microbiota Transplantation on EAE Partly through HPA Axis-Mediated Neuroendocrine Regulation. Heliyon 2024, 10, e33214. [Google Scholar] [CrossRef]
- Braga, J.D.; Thongngam, M.; Kumrungsee, T. Gamma-Aminobutyric Acid as a Potential Postbiotic Mediator in the Gut-Brain Axis. NPJ Sci. Food 2024, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Xue, J.; Shao, R.; Yang, Y.; Ning, D.; Mo, C.; Wang, F.; Chen, G. Fecal Microbiota Transplantation and Short-Chain Fatty Acids Reduce Sepsis Mortality by Remodeling Antibiotic-Induced Gut Microbiota Disturbances. Front. Immunol. 2023, 13, 1063543. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dinesh, S.; Sharma, S. Bridging the Mind and Gut: Uncovering the Intricacies of Neurotransmitters, Neuropeptides, and Their Influence on Neuropsychiatric Disorders. Cent. Nerv. Syst. Agents Med. Chem. 2024, 24, 2–21. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-Chain Fatty Acids: Microbial Metabolites That Alleviate Stress-Induced Brain-Gut Axis Alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Marizzoni, M.; Cattaneo, A.; Mirabelli, P.; Festari, C.; Lopizzo, N.; Nicolosi, V.; Mombelli, E.; Mazzelli, M.; Luongo, D.; Naviglio, D.; et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimers Dis. 2020, 78, 683–697. [Google Scholar] [CrossRef]
- Więdłocha, M.; Marcinowicz, P.; Janoska-Jaździk, M.; Szulc, A. Gut Microbiota, Kynurenine Pathway and Mental Disorders—Review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110145. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the Microbiota-Gut-Brain Axis in Chronic Restraint Stress: Disturbances of the Kynurenine Metabolic Pathway in Both the Gut and Brain. Gut Microbes 2021, 13, 1869501. [Google Scholar] [CrossRef]
- Janzon, A.; Goodrich, J.K.; Koren, O.; TEDDY Study Group; Waters, J.L.; Ley, R.E. Interactions Between the Gut Microbiome and Mucosal Immunoglobulins A, M, and G in the Developing Infant Gut. mSystems 2019, 4, e00612-19. [Google Scholar] [CrossRef]
- DuPont, H.L.; Jiang, Z.D.; Alexander, A.S.; DuPont, A.W.; Brown, E.L. Intestinal IgA-Coated Bacteria in Healthy- and Altered-Microbiomes (Dysbiosis) and Predictive Value in Successful Fecal Microbiota Transplantation. Microorganisms 2022, 11, 93. [Google Scholar] [CrossRef]
| Irritable Bowel Syndrome | ||||||
|---|---|---|---|---|---|---|
| Year, Author, and Country | Study Design | Participants | Intervention | Comparison | Outcomes | Conclusion |
| Singh et al. (2022) [48] India | Randomized, placebo-controlled, single-center study | N = 44 (Irritable Bowel Syndrome-d patients mean age ~ 39 years, ~50% female) | Dose: FMT alone or with antibiotic pretreatment. Route: Colonoscopy. Follow-up: 10 weeks. | Placebo | Primary Outcomes (Bacterial Engraftment):
| FMT alone may provide better microbiota engraftment for IBS-D |
| Holvoet et al. (2021) [42] Belgium | Randomized, placebo-controlled trial | N = 62 (Irritable Bowel Syndrome patients with bloating) | Dose: Single nasojejunal administration of 300 mL donor stool. Route: Nasojejunal tube. Follow-up: 12 weeks (up to 1 year). | Autologous stool (placebo) | Primary Outcomes (12 weeks):
| FMT effective for IBS, but long-term effects vary |
| Lahtinen et al. (2020) [45] Finland | Randomized clinical trial | N = 49 (Irritable Bowel Syndrome) | Dose: 30 g donor stool. Route: Colonoscopy. Follow-up: 12 weeks. | Autologous FMT (placebo) | Primary Outcomes (12 weeks)
| FMT provided transient symptom relief, but not a sustained improvement over placebo, limiting its clinical use. |
| El-Salhy et al. (2019) [40] Norway | Randomized, double-blind, placebo-controlled study | N = 165 (Irritable Bowel Syndrome) | Dose: 30 g or 60 g fecal microbiota suspension (from a superdonor). Route: Gastroscope. Follow-up: 12 weeks. | Placebo (own stool) | Primary Outcomes
| FMT is effective for IBS, showing a dose-dependent response with emphasis on donor selection. |
| Johnsen et al. (2019) [43] Norway | Double-blind, randomized, placebo-controlled trial | N = 90 (non-constipated Irritable Bowel Syndrome patients) | Dose: 50–80 g of donor feces (fresh or frozen). Route: colonoscopy. Follow-up: 6 months. | Placebo (own stool) | Primary Outcomes (6 months):
| QoL and fatigue improved in select IBS patients |
| Chronic Fatigue Syndrome | ||||||
| Salonen et al. (2023) [46] Finland | Randomized, double-blinded, placebo-controlled pilot study | N = 11 (Chronic Fatigue Syndrome patients, 10 female, 1 male, mean age 42.2 years) | Dose: Universal donor stool. Route: Colonoscopy. Follow-up: 1 and 6 months. | Placebo (autologous FMT) | Primary Outcomes
| FMT was safe but did not improve QoL for CFS patients |
| Thurm et al. (2017) [50] Germany | Case report and literature review | N = 1 (58-year-old male with Fibromyalgia, Irritable Bowel Syndrome with Chronic Fatigue Syndrome) | Dose: Self-administered FMT (six consecutive enemas using stool from a screened donor). Route: Enema. Follow-up: 9 months. | No formal comparison | Primary Outcomes:
| FMT may help complex functional disorders, though randomized trials are needed |
| Kenyon et al. (2019) [52] United Kingdom | Retrospective outcome study | N = 42 (Chronic Fatigue Syndrome, 30 with Irritable Bowel Syndrome) | Dose: 10 rectal FMTs from different donors. Route: Rectal catheter. Follow-up: Not specified. | Standard oral approaches | Primary Outcomes
| FMT appears less effective for some CFS patients; further trials recommended |
| Fibromyalgia Syndrome | ||||||
| Fang et al. (2024) [47] China | Open-label, randomized, non-placebo-controlled study | N = 60 (Fibromyalgia Syndrome) | FMT vs. continued standard treatment. | Standard treatment | Primary Outcomes:
| FMT may relieve Fibromyalgia symptoms by modulating gut microbiota. Further research is needed for confirmation. |
| Psoriatric arthritis | ||||||
| Kragsnaes et al. (2021) [44] Denmark | Randomized, double-blind, placebo-controlled trial | N = 31 (Psoriatic arthritis) | Dose: Single stool donation (50 g). Route: Gastroscopy (Duodenum). Follow-up: 26 weeks. | Sham (Placebo) | Primary Outcomes
| FMT appears less effective than placebo for PsA symptoms |
| Rheumatoid arthritis | ||||||
| Zeng et al. (2021) [51] China | Case report | N = 1 (20-year-old female with rheumatoid arthritis) | Dose: 300 mL of fecal microbiota suspension (from an 8-year-old healthy donor). Route: Colonoscopy. Follow-up: 4 months. | No comparison | Primary Outcomes
| FMT shows potential for RA but requires further research |
| Systematic sclerosis | ||||||
| Fretheim et al. (2020) [41] Norway | Double-blind, placebo-controlled randomized pilot trial | N = 10 (patients with systemic sclerosis) | Dose: Commercial anerobic human intestinal microbiota (ACHIM). Route: Gastroduodenoscopy. Follow-up: 16 weeks with two FMT sessions in weeks 0 and 2. | Placebo | Primary Outcomes
| FMT reduced GI symptoms but had procedural risks |
| Diabetic neuropathy | ||||||
| Cai et al. (2018) [49] China | Clinical case report | N = 1 (female, diabetic neuropathy) | Dose: Two FMTs over 3 months. Route: Colonoscopy. Follow-up: 3 months. | No explicit comparison group | Primary Outcomes:
| FMT may offer therapeutic benefits for diabetic complications |
| Study | Design | Methodological Quality Tool | Final Score | Observed Biases |
|---|---|---|---|---|
| El-Salhy et al. (2019) [40] | Randomized, double-blind, placebo-controlled | Cochrane RoB 2 | High | Open-label design leading to potential performance and detection biases. |
| Fretheim et al. (2020) [41] | Randomized, double-blind, placebo-controlled | Cochrane RoB 2 | Moderate | Concerns with blinding of outcome assessment and selective reporting. |
| Holvoet et al. (2021) [42] | Randomized, double-blind, placebo-controlled | Cochrane RoB 2 | Low | Open-label nature affecting blinding and performance outcomes. |
| Johnsen et al. (2019) [43] | Randomized, double-blind, placebo-controlled | Cochrane RoB 2 | High | Appropriate randomization and effective blinding; potential unclear risk from missing data handling and selective reporting. |
| Kragsnaes et al. (2021) [44] | Randomized, double-blind, placebo-controlled | Cochrane RoB 2 | Moderate | Issues with selective reporting noted. |
| Lahtinen et al. (2020) [45] | Randomized, double-blind, placebo-controlled | Cochrane RoB 2 | Moderate | Limited by lack of comparative data and generalizability. |
| Salonen et al. (2023) [46] | Randomized, double-blind, placebo-controlled | Cochrane RoB 2 | Moderate | Limited by small sample size and short follow-up. |
| Fang et al. (2024) [47] | Open-label, randomized | ROBINS-I | Moderate | Potential blinding bias. |
| Singh et al. (2022) [48] | Open-label, randomized | ROBINS-I | Moderate | Issues with selection bias and comparability between cohorts. |
| Cai et al. (2018) [49] | Case report | CARE | Good | Minor concerns regarding selective reporting. |
| Thurm et al. (2017) [50] | Case report | CARE | Good | Minimal concerns noted. |
| Zeng et al. (2021) [51] | Case report | CARE | Good | Inherent limitations due to case report’s nature. |
| Kenyon et al. (2019) [52] | Retrospective outcome study | NOS | Moderate | Detailed patient outcomes but limited generalizability and need for controlled trials. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín Pérez, S.E.; Abdel Lah, H.A.L.; García, N.H.; Reyes Carreño, U.A.; Martín Pérez, I.M. Effectiveness of Fecal Microbiota Transplantation in Nociplastic Pain Management: A Systematic Review. Gastrointest. Disord. 2025, 7, 5. https://doi.org/10.3390/gidisord7010005
Martín Pérez SE, Abdel Lah HAL, García NH, Reyes Carreño UA, Martín Pérez IM. Effectiveness of Fecal Microbiota Transplantation in Nociplastic Pain Management: A Systematic Review. Gastrointestinal Disorders. 2025; 7(1):5. https://doi.org/10.3390/gidisord7010005
Chicago/Turabian StyleMartín Pérez, Sebastián Eustaquio, Hakim Al Lal Abdel Lah, Nelson Hernández García, Umabel Aaron Reyes Carreño, and Isidro Miguel Martín Pérez. 2025. "Effectiveness of Fecal Microbiota Transplantation in Nociplastic Pain Management: A Systematic Review" Gastrointestinal Disorders 7, no. 1: 5. https://doi.org/10.3390/gidisord7010005
APA StyleMartín Pérez, S. E., Abdel Lah, H. A. L., García, N. H., Reyes Carreño, U. A., & Martín Pérez, I. M. (2025). Effectiveness of Fecal Microbiota Transplantation in Nociplastic Pain Management: A Systematic Review. Gastrointestinal Disorders, 7(1), 5. https://doi.org/10.3390/gidisord7010005







