Abstract
Nociplastic pain, commonly observed in conditions such as Fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome, arises from altered central pain processing and involves complex mechanisms, including interactions between the gut–brain axis and immune dysregulation. Conventional therapies often fail to address this type of pain effectively, leading to interest in alternative approaches such as fecal microbiota transplantation. This technique has been proposed to restore gut microbial balance and modulate systemic inflammation, neuroinflammation, and neurotransmitter signaling. This systematic review, conducted according to Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines and registered in the International Prospective Register of Systematic Reviews (CRD42024611939), evaluated 13 studies with n = 409 participants, including clinical trials, case reports, and retrospective analyses. A quality assessment was performed using appraisal tools such as Cochrane RoB 2, ROBINS-I, NOS, and CARE. The results suggest that fecal microbiota transplantation may reduce pain intensity and improve fatigue and quality of life, particularly in patients with Fibromyalgia and irritable bowel syndrome. However, outcomes for Chronic Fatigue Syndrome and psoriatic arthritis were inconsistent and limited by methodological flaws, small sample sizes, and variability in protocols and donor selection. Although adverse events were minimal, the current evidence is insufficient to support widespread clinical use. High-quality, standardized studies are needed to confirm the efficacy of fecal microbiota transplantation. Until then, its application should remain experimental and interpreted with caution.
1. Introduction
Nociplastic pain is a complex chronic pain condition characterized by altered central pain processing and increased sensitivity, often in the absence of detectable tissue damage or ongoing inflammation [1,2,3]. This type of pain is prevalent in conditions such as Fibromyalgia [4,5], Chronic Fatigue Syndrome (CFS) [6,7], neuropathy [8], and irritable bowel syndrome (IBS) [9,10], profoundly impacting patients’ quality of life and posing a substantial burden on healthcare systems due to its persistent and treatment-resistant nature. Conventional therapies, often aimed at symptomatic relief, have demonstrated limited success, as they fail to adequately address the underlying pathophysiological drivers of nociplastic pain, including central sensitization, immune dysregulation, and gut–brain axis interactions [11,12].
The gut microbiota, a key modulator of systemic inflammation and neuroimmune pathways, has gained recognition for its role in influencing nociplastic pain [13,14]. Dysbiosis—an imbalance in the gut microbial ecosystem—may worsen nociplastic pain by triggering inflammatory responses and disrupting immune regulation, contributing to both central and peripheral sensitization [15,16,17]. This highlights the gut–brain axis as a promising therapeutic target, with the potential to mitigate chronic inflammation and alter pain perception through the restoration of microbial balance [18,19]. In this context, therapies aimed at modifying the gut microbiota, such as fecal microbiota transplantation (FMT), have garnered growing interest [20,21].
FMT involves transferring processed stool from a healthy donor to a recipient with dysbiosis to restore microbial balance and diversity (eubiosis) [22]. This intervention requires meticulous donor screening, careful preparation and preservation of beneficial microorganisms [23], and precise administration via colonoscopy [24], nasogastric tube [25], or encapsulated formulations [26]. While FMT has shown significant efficacy in treating recurrent Clostridium difficile infections by re-establishing microbial equilibrium, its potential application to manage nociplastic pain remains a novel and emerging area of exploration [27].
Preliminary evidence from human studies suggests that FMT may offer a therapeutic approach for reducing nociplastic pain by directly targeting gut dysbiosis. Restoration of a balanced microbiota has been associated with the modulation of immune responses and reductions in systemic inflammation, both critical contributors to nociceptive and nociciplastic pain [28,29]. For instance, studies on individuals with irritable bowel syndrome and Fibromyalgia have reported decreased levels of pro-inflammatory cytokines, such as interleukin-6 and tumor necrosis factor-alpha, following FMT [30,31].
Additionally, improvements in visceral hypersensitivity have been observed in irritable bowel syndrome, highlighting its potential role in addressing nociplastic pain mechanisms [32]. Changes in neurotransmitter synthesis, including serotonin and dopamine, as well as neuroactive tryptophan metabolites, have also been documented in human studies, further supporting the potential of fecal microbiota transplantation to influence gut–brain axis signaling pathways [33]. This biochemical modulation, mediated by shifts within the gut–brain axis, suggests that FMT can address the root causes of nociplastic pain rather than merely alleviating symptoms [34].
Despite preliminary promising findings, there remains a notable gap in understanding FMT’s full therapeutic potential in managing nociplastic pain, with limited existing evidence necessitating further research into its mechanisms, safety, efficacy, and long-term outcomes. This systematic review aims to synthesize current knowledge on the impact of FMT in modulating nociplastic pain, focusing on its influence on immune and inflammatory responses, pain modulation, microbiota composition, and patient-centered outcomes such as pain reduction and quality of life.
2. Materials and Method
2.1. Data Sources and Search Strategy
A systematic review of the literature was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [35]. The protocol for this systematic review was pre-registered with the PROSPERO database CRD42024611939. The search was performed from 10 November 2024 to 10 December 2024 to identify all available studies examining the efficacy of FMT in addressing nociplastic pain syndrome in various conditions across the following databases: MEDLINE (PubMed), ELSEVIER (Scopus), EBSCOhost, Cochrane Library, and Web of Science (WOS). The strategy used in MEDLINE (PubMed) was as follows: (“faecal microbiota transplant” [Title/Abstract] OR “fecal microbiota transplantation” [Title/Abstract] OR “fecal transplantation” [Title/Abstract] OR “faecal transplant” [Title/Abstract] OR “gut microbiota” [Title/Abstract]) AND (“nociplastic pain” OR “chronic pain” OR “pain sensitization” OR “central sensitization” OR “gut-brain axis” OR “pain modulation”).
Two independent researchers (N.H.G. and H.A.L. conducted the search, and a blinded investigator (U.R.C.) evaluated all retrieved articles by title and abstract, followed by a full-text assessment to determine eligibility. In the event of discrepancies, a fourth author (S.M.P.) acted as an arbitrator. Table S1 outlines the detailed search strategy.
2.2. Inclusion and Exclusion Criteria
This review considered studies that met the following criteria: clinical trials (randomized and non-randomized) and observational studies as case series and case–control studies. Eligible publications were required to be (1) published between 1 January 2014 and 30 November 2024 and (2) available in full-text format in (3) English, Spanish, French, Portuguese, or Arabic. The focus was on (4) adult populations undergoing FMT with an emphasis on its (5) therapeutic impact on nociplastic pain and conditions characterized by central sensitization. These included chronic inflammatory states such as Fibromyalgia, IBS, CFS, neuropathic pain syndromes, and other disorders where nociplastic pain is a critical component. Eligible studies assessed outcomes related to (6) pain modulation, physical and psychological symptoms, immune and inflammatory markers, safety profiles, or quality of life improvements following FMT.
Studies were excluded if they did not match the specified (1) study types or (2) were published outside the defined timeframe. Publications (3) without full-text access or (4) those not available in English, Spanish, French, Portuguese, or Arabic were also excluded. Further exclusions (5) applied to studies involving non-adult populations, such as pediatric cases, or studies that did not directly evaluate the therapeutic impact of FMT on conditions involving central sensitization. (6) Abstract-only publications, commentaries, opinion pieces, and conference proceedings were not considered for inclusion.
2.3. Data Extraction
Data extraction was conducted independently by two authors (N.H.G. and H.A.L.), with a third author (U.R.C.) available to resolve any discrepancies. A standardized data extraction template, structured according to the PICO (Population, Intervention, Comparison, Outcomes) framework, was utilized to collect comprehensive details. This included authorship, year and country of publication, study design, study objectives, results, participant characteristics (e.g., disease characteristics, intervention specifics, sample size, gender distribution), details of the intervention and control groups, measured outcomes, and study conclusions. The data extraction process adhered to the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions v.5.1.0 [35]. The reliability of the data table was evaluated using a representative sample of studies to ensure consistency and accuracy.
2.4. Methodological Quality Assessment
2.4.1. Randomized Controlled Trials
The Cochrane RoB 2 tool was utilized for randomized controlled trials (RCTs), including double-blind and placebo-controlled designs [36]. This instrument assesses potential biases across several key domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. Each domain is rated as “low risk”, “some concerns”, or “high risk” of bias, culminating in an overall risk-of-bias judgment for each study. This tool is widely recognized for its rigor and utility in evaluating the internal validity of RCTs, ensuring the methodological integrity and transparency of study findings.
2.4.2. Non-Randomized Clinical Trials
This tool was employed for assessing non-randomized, open-label trials. The ROBINS-I (Risk of Bias in Non-randomized Studies of Interventions) tool [37] evaluates bias across seven distinct domains, including confounding, participant selection, classification of interventions, deviations from intended interventions, missing data, outcome measurement, and selection of the reported result. Studies are categorized as presenting a “low”, “moderate”, “serious”, or “critical” risk of bias. The tool is specifically designed to address biases that arise from non-randomized intervention designs, highlighting areas where study design may impact the reliability of outcomes.
2.4.3. Case Reports
The Consensus-based Clinical Case Reporting Guideline Development (CARE) guidelines [38] were applied for the assessment of case reports. This instrument ensures comprehensive and structured reporting by evaluating key elements such as patient information, clinical findings, diagnostic assessment, therapeutic intervention, timeline, follow-up, and outcomes. Adherence to these guidelines allows for a detailed and transparent presentation of individual patient cases. While case reports inherently lack generalizability and control groups, adherence to CARE guidelines ensures that they provide valuable, high-quality insights into specific clinical scenarios.
2.4.4. Observational Study
The Newcastle–Ottawa Scale (NOS) [39] was used to evaluate the quality of non-randomized studies, particularly retrospective outcome studies. This tool assesses studies across the following three broad categories: selection of study participants, comparability of cohorts, and ascertainment of exposure or outcomes. Studies are rated based on their adherence to methodological rigor, with a “star” system that identifies strengths and potential sources of bias. The NOS is widely used for assessing the methodological robustness of cohort and case–control studies, providing a reliable framework for evaluating study quality and minimizing the impact of potential biases.
3. Results
3.1. Study Selection
The selection process for publications commenced with an extensive search across multiple academic databases, resulting in the identification of a total of 1837 studies. The contributions from individual databases were as follows: MEDLINE (PubMed) (n = 412 studies), ELSEVIER (Scopus) (n = 523 studies), EBSCOhost (n = 387 studies), Cochrane Library (n = 200 studies), and Web of Science (WOS) (n = 315 studies).
Following the removal of 423 duplicate entries, a total of 1414 unique articles were retained for the screening phase. During this phase, the titles and abstracts of these articles were evaluated for their relevance to the research question, resulting in the exclusion of 1067 articles for reasons such as a lack of relevance to the study’s scope, a focus on non-target populations, or the absence of primary data. Consequently, 347 articles were selected for a detailed full-text eligibility review.
Among the 347 articles assessed at the full-text stage, 335 were excluded for various reasons, including differing study designs that did not fit the inclusion criteria (e.g., systematic or narrative reviews) (n = 131), interventions outside the defined scope of interest (n = 85), the absence of relevant outcome measures (n = 60), publications in non-target languages (n = 33), and articles with access restrictions or limited data (n = 26). Ultimately, 13 studies were selected for qualitative synthesis. Figure 1 illustrates the PRISMA 2020 flowchart for the study selection process.
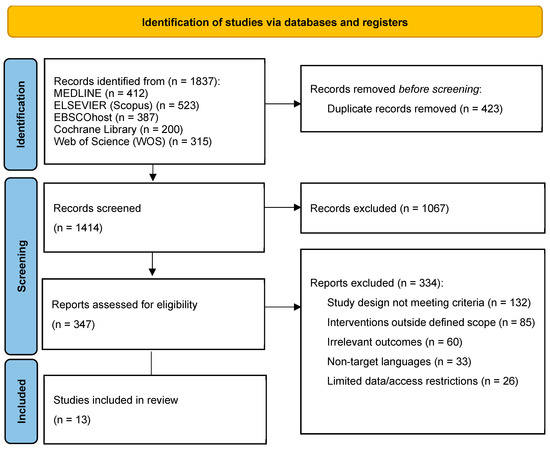
Figure 1.
The PRISMA 2020 flowchart shows the systematic review process. Out of n = 1837 identified records, n = 423 duplicates were removed, leaving n = 1414 articles screened by title and abstract, excluding n = 1067. From n = 347 full-text assessments, n = 334 were excluded due to reasons such as differing study designs, irrelevant interventions, or access issues. A total of 13 studies were included in the final qualitative synthesis, comprising clinical trials, case reports, and retrospective studies. Source: own work.
3.2. Characteristics of Included Studies
The 13 included studies focused on clinical trials involving Fecal Microbiota Transplantation (FMT). Of these, 7 were randomized, double-blind, placebo-controlled trials [40,41,42,43,44,45,46], 2 were open-label, randomized trials [47,48], 3 were case reports [49,50,51], and 1 was a retrospective outcome study [52]. The included studies examined populations with different nociplastic pain conditions such as IBS [40,42,43,45,48,50,52], Fibromyalgia [47,50], Chronic Fatigue Syndrome [46,50,52], psoriatic arthritis [44], rheumatoid arthritis [51], systemic sclerosis [41], and diabetic neuropathy [49], totaling a diverse sample.
Patient groups varied widely, including 60 Fibromyalgia patients in a Chinese open-label trial [40], 11 CFS patients in a Finnish pilot study [41], and smaller cohorts, such as individual case reports for RA and diabetic neuropathy [45,51]. Interventions typically involved FMT delivered via colonoscopy [40,42,45,47,49,50,51], gastroscopy [44,45,50], or rectal catheterization [48], with follow-up periods ranging from 4 months [45] to over a year [43]. Comparators included placebo groups using autologous stool or continued standard treatment. Outcomes varied, encompassing symptom reduction (e.g., pain and fatigue) engraftment rates [42], gut microbiota composition changes [46], quality of life improvements [49,50], and adverse events [44,46].
Notable findings included significant symptom relief in some cases [40,45], while other studies reported limited or no efficacy compared to placebo [41,44], emphasizing the need for further research. Geographically, the studies were conducted in countries such as China [40,45,51], Finland [47,51], Denmark [34], Norway [46,49,50], India [42], Belgium [43], the United Kingdom [48], and Germany [52]. More details in Table 1 below.

Table 1.
Characteristics of the included studies.
3.3. Methodological Quality Assessment
3.3.1. Randomized Controlled Trials
For the randomized controlled trials, including those with double-blinding and placebo controls [40,41,42,43,44,45,46] the Cochrane Risk of Bias (RoB) 2 tool was used. These studies generally exhibited strong methodological rigor, demonstrating robust random sequence generation and allocation concealment. However, concerns were noted in some studies regarding the blinding of outcome assessment and selective reporting, which may have affected the reliability of specific outcomes.
3.3.2. Open-Label Trials
The open-label trials [47,48] were evaluated using the ROBINS-I tool (Risk of Bias in Non-randomized Studies of Interventions). This assessment revealed a moderate risk of bias predominantly arising from the open-label nature of the studies, which may introduce potential performance and detection biases that could affect the interpretation of the results.
3.3.3. Case Reports
Case reports [49,50,51] were evaluated according to the CARE (Case Report) guidelines. All reports adhered well to structured presentation standards and offered detailed patient data. However, they were inherently limited due to the absence of control groups, small sample sizes, and restricted generalizability. Notably, Thurm et al. (2017) [50] presented a unique combination of case reports and literature review, providing insights into complex functional disorders treated with self-administered FMT, though the findings underscore the necessity for randomized trials.
3.3.4. Retrospective Outcome Study
The retrospective outcome study [52] was assessed using the Newcastle–Ottawa Scale (NOS) for non-randomized studies. This evaluation indicated moderate quality, with primary concerns related to cohort selection and comparability, which could influence outcome interpretations. In summary, while many studies demonstrated a sound methodological basis, further rigorous trials with larger, more diverse samples are warranted to validate findings and bolster evidence for the efficacy of FMT across various conditions. For further details, refer to Table 2 below.

Table 2.
Methodological Quality Analysis.
3.4. Main Results
3.4.1. Severity of Nociplastic Pain Following FMT
Fibromyalgia and CFS are conditions characterized by chronic nociplastic pain, often stemming from central sensitization. Fang et al. (2024) [47] conducted an open-label randomized trial on 60 Fibromyalgia patients, demonstrating significant reductions in pain intensity and fatigue following FMT compared to standard treatment, suggesting that FMT may impact nociplastic pain through gut microbiota modulation.
Conversely, Salonen et al. (2023) [46] evaluated 11 CFS patients in a double-blind, placebo-controlled pilot study, finding no significant improvements in fatigue or quality of life. Similarly, Kenyon et al. (2019) [52], in a retrospective study of 42 patients with CFS and IBS, found limited efficacy of FMT compared to standard treatments, highlighting the complexity of nociplastic pain in CFS. Notably, Thurm et al. (2017) [50] reported significant improvement in a patient with Fibromyalgia, IBS, and CFS following self-administered FMTs, pointing to potential benefits of gut microbiota modulation in complex nociplastic pain syndromes, though further randomized trials are needed.
Irritable bowel syndrome is a functional gastrointestinal disorder often associated with nociplastic pain due to disrupted gut–brain communication. In this sense, Singh et al. (2022) [48] conducted a randomized, placebo-controlled study on IBS patients, noting improved microbiota engraftment with FMT, but no significant impact on symptom severity. This suggests that while FMT may modify gut microbiota, its influence on nociplastic pain symptoms in IBS can be limited. Holvoet et al. (2021) [42] and Lahtinen et al. (2020) [45] observed initial symptom relief in IBS patients receiving FMT, but effects diminished over time, indicating challenges in achieving sustained modulation of nociplastic pain pathways. However, Johnsen et al. (2019) [43] and El-Salhy et al. (2019) [40] highlighted improvements in fatigue and quality of life among IBS patients, showing FMT’s potential to impact nociplastic pain-related symptoms, particularly where gut dysbiosis is implicated.
Psoriatic arthritis and rheumatoid arthritis present a combination of inflammatory and nociplastic pain. Kragsnaes et al. (2021) [44] found FMT less effective than a placebo for psoriatic arthritis symptoms in a double-blind, placebo-controlled trial, underscoring the difficulty of modulating nociplastic pain through gut interventions in autoimmune conditions. Conversely, Zeng et al. (2021) [51] reported significant improvements in a young RA patient following FMT, including reduced pain and inflammatory markers, suggesting that gut microbiota may influence nociplastic pain pathways in RA, though more robust evidence is needed.
In systemic sclerosis and diabetic neuropathy, nociplastic pain often coexists with other symptoms. Fretheim et al. (2020) [41] found FMT effective in reducing gastrointestinal symptoms in systemic sclerosis patients, though procedural risks were noted. This underscores the potential role of gut interventions in modulating nociplastic pain within complex chronic conditions. Similarly, Cai et al. (2018) [49] reported pain relief and improved glycemic control in a diabetic neuropathy patient following FMT, indicating potential therapeutic benefits for conditions with nociplastic pain features.
3.4.2. Functionality and Quality of Life of Nociplastic Pain Following FMT
Several studies evaluated the impact of FMT on physical functionality, particularly in conditions with chronic pain or inflammatory components. Fang et al. (2024) [47] examined Fibromyalgia Syndrome patients and observed significant reductions in pain intensity, fatigue, and other physical symptoms, suggesting potential improvements in physical functioning through gut microbiota modulation.
Similarly, Zeng et al. (2021) [51] reported a notable reduction in physical symptoms for a rheumatoid arthritis patient, with the Health Assessment Questionnaire Disability Index (HAQ-DI) score dropping from 1.4 to 0.05 and a reduction in disease activity score (DAS28) from 6.6 to 1.4, indicating enhanced physical functionality and decreased disability.
Kragsnaes et al. (2021) [44] explored FMT’s effects on psoriatic arthritis, finding a lesser improvement in the HAQ-DI score among the FMT group compared to placebo, reflecting limited benefits for physical functionality. In systemic sclerosis patients, Fretheim et al. (2020) [41] found that 80% of those receiving FMT experienced reductions in gastrointestinal symptoms, potentially contributing to overall physical functionality improvements, albeit with risks associated with the procedure.
For IBS patients, physical functionality improvements were reported inconsistently. Holvoet et al. (2021) [42] found symptom improvement, including bloating relief, which could enhance daily functioning. However, effects diminished over time, reflecting variability in long-term outcomes.
Psychological functionality and QoL were also evaluated across several studies. Salonen et al. (2023) [46] examined CFS patients but found no statistically significant improvements in fatigue or QoL after FMT, suggesting limited psychological benefits in this cohort. Conversely, Johnsen et al. (2019) [43] and El-Salhy et al. (2019) [40] observed improvements in QoL and reductions in fatigue in IBS patients, indicating a positive psychological impact from FMT. Moreover, they highlighted a dose-dependent response in QoL improvement, emphasizing the role of donor stool quantity.
Moreover, Kenyon et al. (2019) [52] assessed CFS patients, noting that FMT was less effective compared to standard oral approaches for symptom management and failed to improve psychological outcomes for most patients. In a case study, Thurm et al. (2017) [50] reported significant improvement in psychological functionality, with reduced fatigue and enhanced overall well-being following self-administered FMT for a patient with Fibromyalgia, IBS, and CFS.
In addition, Fang et al. (2024) [47] and Zeng et al. (2021) [51] showed potential combined benefits of FMT on both physical and psychological functionality in Fibromyalgia and rheumatoid arthritis patients, respectively, through reduced symptom severity and improved daily functioning. Furthermore, Fretheim et al. (2020) [41] also suggested potential combined improvements in systemic sclerosis, albeit with procedural risks. Lahtinen et al. (2020) [45] and Singh et al. (2022) [48], however, noted that while there might be initial symptom relief or microbiota changes, the sustained impact on physical and psychological functionality was limited, emphasizing the need for further, larger-scale trials.
3.4.3. Other Effects in Nociplastic Pain Following FMT
In addition to physical and psychological functionality, several studies highlighted other variables influenced by FMT interventions. Microbiota composition changes were a common outcome across studies, with Fretheim et al. (2020) [41] observing quantitative changes in IgA- and IgM-coated bacteria among systemic sclerosis patients, while Singh et al. (2022) [48] reported varying rates of engraftment in IBS-D patients. These findings underscore the role of microbiome alterations in therapeutic outcomes.
Adverse events were also documented, ranging from none in some studies [46,49] to serious events like laryngospasms and duodenal perforation in Fretheim et al. (2020) [41]. Engraftment rates and donor stool characteristics emerged as variables of interest, influencing the degree of symptom relief, as evidenced by El-Salhy et al. (2019) [40], who noted a dose-dependent response in IBS patients.
4. Discussion
4.1. Severity of Nociplastic Pain Following FMT
Nociplastic pain, a hallmark of conditions such as Fibromyalgia and CFS, arises from altered nociceptive processing and heightened central sensitization [1,2,3,53]. The gut–brain axis, a bidirectional communication network linking the gut microbiota with the CNS, is increasingly recognized as pivotal in modulating pain pathways [54]. FMT may influence nociplastic pain through neurophysiological and biochemical mechanisms by restoring gut microbiota balance, potentially modulating systemic inflammation [55], neuroinflammation [56], and neurotransmitter signaling [57].
A primary mechanism involves the modulation of pro-inflammatory cytokines and immune signaling. Chronic nociplastic pain is associated with elevated levels of inflammatory markers such as interleukin-6, tumor necrosis factor-alpha, and C-reactive protein [58,59]. Evidence suggests that FMT may reduce systemic inflammation by promoting the growth of anti-inflammatory commensal bacteria, which can downregulate these mediators [60,61]. For instance, Fang et al. (2024) [47] reported significant reductions in pain intensity among Fibromyalgia patients, potentially attributable to decreased neuroinflammatory signaling that otherwise sustains central sensitization and pain hypersensitivity.
The gut microbiota also plays a relevant role in the production and metabolism of neurotransmitters that regulate pain perception, including serotonin, dopamine, GABA, and tryptophan metabolites. Neuroactive tryptophan metabolites—including xanthurenic acid, quinolinic acid, and 3-indoxyl sulfate—have been recognized for their contribution to the pathogenesis of functional pain [62,63]. These metabolites are key modulators of neuroinflammation and central sensitization, underscoring FMT’s potential to influence these pathways. By restoring a healthy gut microbiome, FMT may enhance serotonin biosynthesis and regulate tryptophan pathways, thereby modulating nociceptive and neuroinflammatory processes [64,65]. More details in Figure 2.
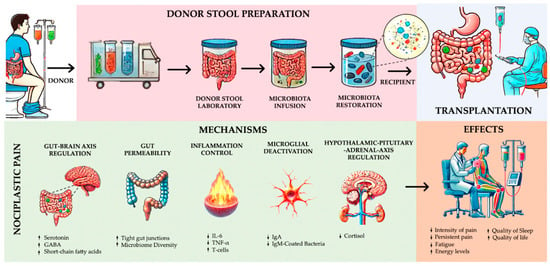
Figure 2.
Mechanisms and analgesic effects of fecal microbiota transplantation in nociplastic pain management. Source: own work.
Despite these promising mechanisms, the efficacy of FMT in relieving symptoms of nociplastic pain remains inconsistent. For example, Salonen et al. (2023) [46] reported no significant improvements in fatigue or quality of life in CFS patients, underscoring the variability in individual microbiota profiles and host-specific factors. As noted by Hartikainen et al. (2024), this variability may reflect the influence of individual microbiota profiles or host-specific factors on neurotransmitter biosynthesis and signaling [66].
Another critical mechanism involves the modulation of microglial cells, the resident immune cells of the CNS. Microglial activation, often driven by systemic inflammation and gut-derived metabolites, contributes to neuroinflammation and heightened pain sensitivity [67]. By altering the gut microbiota, FMT may reduce pro-inflammatory microglial activation, thereby alleviating central sensitization [68]. Evidence from studies on Fibromyalgia patients suggests symptom improvement following FMT [69,70], although further research is needed to confirm the impact of microbiota transplantation on neuroimmune interactions.
4.2. Functionality and Quality of Life in Nociplastic Pain Following FMT
Improvements in physical and psychological functionality following FMT may be attributed to its impact on neuroendocrine signaling pathways and systemic immune modulation. Zeng et al. (2021) [51] reported significant reductions in disease activity and improvements in physical functionality in a rheumatoid arthritis patient post-FMT. These outcomes likely result from a decrease in systemic inflammation mediated by changes in gut microbiota composition, reducing peripheral nociceptive signaling and central sensitization.
That FMT may also influence the hypothalamic–pituitary–adrenal (HPA) axis is also relevant, as dysregulation of the HPA axis is common in nociplastic pain syndromes, leading to altered stress responses, increased cortisol levels, and exacerbated pain perception [71]. By restoring gut microbiome balance, FMT may recalibrate HPA axis activity, mitigating stress-related amplification of pain signals [72]. This mechanism may partly explain improvements in fatigue and quality of life observed in IBS patients [40,43].
On the other hand, psychological improvements following FMT, including enhanced mood and reduced anxiety, can be linked to gut microbiota’s ability to synthesize and modulate neurotransmitters such as GABA, which exerts inhibitory effects on neural excitability and stress responses [73]. Enhanced GABAergic signaling, along with increased production of short-chain fatty acids (SCFAs) like butyrate [74,75], may stabilize the blood–brain barrier (BBB), reduce neuroinflammation, and promote resilience to nociplastic pain triggers [76]. However, inconsistent outcomes, as noted by Kenyon et al. (2019) [52], suggest variability in individual responses based on baseline gut microbiota composition and other host factors.
4.3. Other Physiological and Biochemical Effects of FMT on Nociplastic Pain
Beyond its impact on nociceptive signaling and neurotransmitter modulation, FMT can alter the gut microbiome’s production of key metabolites, such as SCFAs (e.g., butyrate, propionate, and acetate) [77]. SCFAs exert anti-inflammatory and neuroprotective effects by modulating immune cell activity, reducing oxidative stress, and promoting gut barrier integrity [78]. By mitigating systemic inflammation and enhancing intestinal permeability, FMT may decrease the translocation of bacterial endotoxins (e.g., lipopolysaccharides) [79] that trigger neuroinflammation and worsen nociplastic pain.
Microbial metabolites such as tryptophan-derived kynurenine also play a role in pain modulation and neuroinflammation [80]. Dysregulation of the kynurenine pathway can lead to neurotoxic metabolite accumulation, contributing to heightened pain sensitivity and depression in nociplastic pain syndromes [81]. FMT may restore a balanced microbial metabolite profile, reducing neurotoxicity and improving pain outcomes. Fretheim et al. (2020) [41] observed changes in IgA- and IgM-coated bacteria, indicating shifts in mucosal immunity that may further modulate systemic and neuroinflammatory responses [82,83].
While adverse events such as those reported by Fretheim et al. (2020) [41] highlight potential risks associated with FMT, optimizing donor selection, engraftment protocols, and microbiota composition can enhance therapeutic outcomes while minimizing complications. Collectively, these neurophysiological and biochemical pathways underscore FMT’s complex and multifaceted role in modulating nociplastic pain, necessitating a precision-medicine approach to maximize its therapeutic potential.
4.4. Implications for Practice
FMT presents considerable promise in the management of nociplastic pain—a chronic pain state marked by altered pain processing and central sensitization, prevalent in disorders such as Fibromyalgia, IBS, and CFS. Current evidence suggests that FMT can alter gut microbiota composition, thereby modulating systemic inflammation, gut–brain communication, and immune responses implicated in nociplastic pain. This modulation may reduce pain intensity, alleviate fatigue, and improve gastrointestinal symptoms. However, the quality of evidence remains limited, with studies often having small sample sizes and variable methodologies, leading to mixed outcomes.
At present, FMT should be considered a complementary or alternative intervention to conventional pharmacological therapies, which frequently show limited effectiveness and significant side effects. Despite the promising results, the scientific community lacks high-quality, large-scale RCTs to definitively establish FMT’s efficacy and safety in managing nociplastic pain. Thus, the clinical use of FMT in pain management should be approached with caution, especially as more robust evidence is required.
In clinical settings, incorporating FMT into nociplastic pain management strategies could transform treatment paradigms by offering a natural and potentially sustainable means of symptom alleviation through microbiome restoration. By attenuating systemic inflammation and enhancing gut barrier integrity, FMT may reduce reliance on conventional pain medications, thereby mitigating risks such as opioid dependence and associated adverse effects. Furthermore, its potential to modulate neuroinflammatory pathways could lead to significant improvements in both physical and psychological patient well-being.
To achieve consistent therapeutic outcomes, the establishment of standardized protocols for donor selection, preparation, and FMT administration will be essential. Future studies should focus on improving the quality of the data, with larger sample sizes, long-term follow-ups, and rigorous methodological designs. Ultimately, FMT may not only provide symptomatic relief but also target the underlying mechanisms of pain sensitization, offering a transformative opportunity in the care of patients with nociplastic pain.
4.5. Implications for Research
Future research on the role of FMT in managing nociplastic pain presents several critical opportunities. First, refining and standardizing FMT protocols is essential. This includes establishing not only a context for developing more robust study designs but also optimal donor selection criteria, improving preparation techniques, and determining the most effective delivery routes tailored specifically to nociplastic pain conditions. Investigating how FMT influences the gut–brain axis, modulates immune responses, and interacts with pathways involved in central sensitization could provide profound insights into its therapeutic potential and inherent limitations in chronic pain syndromes.
To ensure consistency and comparability across studies, it is imperative to incorporate FDA-approved endpoints in future research. Standardizing these endpoints alongside FMT protocols will not only enhance the reliability of study results but also pave the way for regulatory approval and wider clinical adoption. This alignment with regulatory standards will strengthen the credibility of findings and facilitate the integration of FMT into evidence-based practice for nociplastic pain management.
Furthermore, longitudinal studies are necessary for evaluating the long-term safety and efficacy of FMT across diverse patient populations affected by nociplastic pain. Understanding inter-individual variations in treatment response—shaped by factors such as microbiota composition, genetic predispositions, and other patient-specific variables—is vital for optimizing personalized care strategies. Furthermore, expanding research beyond gastrointestinal disorders to explore the neurobiological and immune-mediated dimensions of nociplastic pain could further elucidate the broader applicability and therapeutic value of FMT.
Additionally, assessing FMT as an adjunctive therapy for conditions like Fibromyalgia, IBS, and related disorders offers an exciting opportunity to develop comprehensive, multi-targeted treatment strategies. By addressing the root causes of nociplastic pain through microbiota modulation, FMT holds great promise for significantly improving clinical outcomes and providing renewed hope for individuals experiencing chronic, refractory pain conditions.
5. Conclusions
This systematic review underscores the potential of FMT in managing nociplastic pain, characterized by altered pain processing and central sensitization. Evidence indicates that FMT may influence systemic inflammation, gut–brain signaling, and neuroimmune pathways, showing promise in conditions such as Fibromyalgia, CFS, IBS, and autoimmune disorders. However, results vary widely, reflecting the complexity of nociplastic pain and the multifactorial factors affecting outcomes. Further research is necessary to standardize protocols, improve study designs, and better define FMT’s role in chronic pain management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/gidisord7010005/s1, Table S1: Search strategy.
Author Contributions
Conceptualization, S.M.P. and I.M.P.; methodology, N.H.G.; software, H.A.L.; validation, N.H.G., H.A.L., and U.R.C.; formal analysis, S.M.P. and I.M.P.; investigation, S.M.P. and I.M.P.; data curation, S.M.P.; writing—original draft preparation, N.H.G., H.A.L., and U.R.C.; writing—review and editing, N.H.G., H.A.L., and U.R.C.; visualization, H.A.L.; supervision, S.M.P. and I.M.P.; project administration, S.M.P. and I.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study is a systematic review and meta-analysis and did not involve human or animal subjects. The review was conducted in accordance with PRISMA guidelines and was prospectively registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42024611939).
Informed Consent Statement
Not Applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Häuser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.M.; Kim, K.H. Current understanding of nociplastic pain. Korean J. Pain 2024, 37, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Hausteiner-Wiehle, C.; Henningsen, P. Nociplastic pain is functional pain. Lancet 2022, 399, 1603–1604. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lavín, M. Centralized nociplastic pain causing fibromyalgia: An emperor with no clothes? Rheumatol. 2022, 41, 3915–3917. [Google Scholar] [CrossRef]
- Clauw, D.J. From fibrositis to fibromyalgia to nociplastic pain: How rheumatology helped get us here and where do we go from here? Rheum. Dis. 2024, 83, 1421–1427. [Google Scholar] [CrossRef]
- Murphy, A.E.; Minhas, D.; Clauw, D.J.; Lee, Y.C. Identifying and Managing Nociplastic Pain in Individuals With Rheumatic Diseases: A Narrative Review. Arthritis Care Res. 2023, 75, 2215–2222. [Google Scholar] [CrossRef]
- Kosek, E.; Clauw, D.; Nijs, J.; Baron, R.; Gilron, I.; Harris, R.E.; Mico, J.A.; Rice, A.S.C.; Sterling, M. Chronic nociplastic pain affecting the musculoskeletal system: Clinical criteria and grading system. Pain 2021, 162, 2629–2634. [Google Scholar] [CrossRef]
- Ablin, J.N. Nociplastic Pain: A Critical Paradigm for Multidisciplinary Recognition and Management. J. Clin. Med. 2024, 13, 5741. [Google Scholar] [CrossRef]
- Alcántara Montero, A.; Pacheco de Vasconcelos, S.R.; Castro Arias, A. Contextualization of the concept of nociplastic pain in irritable bowel syndrome. Rev. Esp. Enferm. Dig. 2024, 116, 698–699. [Google Scholar] [CrossRef]
- Midenfjord, I.; Grinsvall, C.; Koj, P.; Carnerup, I.; Törnblom, H.; Simrén, M. Central sensitization and severity of gastrointestinal symptoms in irritable bowel syndrome, chronic pain syndromes, and inflammatory bowel disease. Neurogastroenterol. Motil. 2021, 33, e14156. [Google Scholar] [CrossRef]
- Nijs, J.; Leysen, L.; Vanlauwe, J.; Logghe, T.; Ickmans, K.; Polli, A.; Malfliet, A.; Coppieters, I.; Huysmans, E. Treatment of central sensitization in patients with chronic pain: Time for change? Pharmacother. 2019, 20, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Ryu, H.J.; Bhatt, R.R. The neurobiology of irritable bowel syndrome. Mol. Psychiatry 2023, 28, 1451–1465. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Pratt, M.L.; Plumb, A.N.; Manjrekar, A.; Cardona, L.M.; Chan, C.K.; John, J.M.; Sadler, K.E. Microbiome contributions to pain: A review of the preclinical literature. Pain 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, G.; Ko, G.; Joong Lee, S. Nerve injury-induced gut dysbiosis contributes to spinal cord TNF-α expression and nociceptive sensitization. Brain Behav. Immun. 2023, 110, 155–161. [Google Scholar] [CrossRef]
- Garvey, M. The Association between Dysbiosis and Neurological Conditions Often Manifesting with Chronic Pain. Biomedicines 2023, 11, 748. [Google Scholar] [CrossRef]
- Goudman, L.; Demuyser, T.; Pilitsis, J.G.; Billot, M.; Roulaud, M.; Rigoard, P.; Moens, M. Gut dysbiosis in patients with chronic pain: A systematic review and meta-analysis. Front. Immunol. 2024, 15, 1342833. [Google Scholar] [CrossRef]
- Agirman, G.; Yu, K.B.; Hsiao, E.Y. Signaling inflammation across the gut-brain axis. Science 2021, 374, 1087–1092. [Google Scholar] [CrossRef]
- Donoso, F.; Cryan, J.F.; Olavarría-Ramírez, L.; Nolan, Y.M.; Clarke, G. Inflammation, Lifestyle Factors, and the Microbiome-Gut-Brain Axis: Relevance to Depression and Antidepressant Action. Clin. Pharmacol. Ther. 2023, 113, 246–259. [Google Scholar] [CrossRef]
- Borody, T.J.; Khoruts, A. Fecal microbiota transplantation and emerging applications. Nat. Rev. Gastroenterol. Hepatol. 2011, 9, 88–96. [Google Scholar] [CrossRef]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal microbiota transplantation: In perspective. Therap. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Kuo, C.H.; Kuo, F.C.; Wang, Y.K.; Hsu, W.H.; Yu, F.J.; Hu, H.M.; Hsu, P.I.; Wang, J.Y.; Wu, D.C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118 (Suppl. S1), S23–S31. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Wang, J.Y.; Kuo, C.H. Current applications of fecal microbiota transplantation in intestinal disorders. Kaohsiung J. Med. Sci. 2019, 35, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.M.; Pestana, L.; Pardi, R.; Pardi, D.S.; Khanna, S. Fecal microbiota transplant via colonoscopy may be preferred due to intraprocedure findings. Intest. Res. 2019, 17, 434–437. [Google Scholar] [CrossRef]
- Ramai, D.; Zakhia, K.; Fields, P.J.; Ofosu, A.; Patel, G.; Shahnazarian, V.; Lai, J.K.; Dhaliwal, A.; Reddy, M.; Chang, S. Fecal Microbiota Transplantation (FMT) with Colonoscopy Is Superior to Enema and Nasogastric Tube While Comparable to Capsule for the Treatment of Recurrent Clostridioides difficile Infection: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2021, 66, 369–380. [Google Scholar] [CrossRef]
- Du, C.; Luo, Y.; Walsh, S.; Grinspan, A. Oral Fecal Microbiota Transplant Capsules Are Safe and Effective for Recurrent Clostridioides difficile Infection: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2021, 55, 300–308. [Google Scholar] [CrossRef]
- Pak, R.; Cho, M.; Pride, K.; Abd-Elsayed, A. The Gut Microbiota and Chronic Pain. Curr. Pain Headache Rep. 2024, 28, 259–269. [Google Scholar] [CrossRef]
- Bartman, C.; Chong, A.S.; Alegre, M.L. The Influence of the Microbiota on the Immune Response to Transplantation. Curr. Opin. Organ Transplant. 2015, 20, 1–7. [Google Scholar] [CrossRef]
- Lucarini, E.; Di Pilato, V.; Parisio, C.; Micheli, L.; Toti, A.; Pacini, A.; Bartolucci, G.; Baldi, S.; Niccolai, E.; Amedei, A.; et al. Visceral Sensitivity Modulation by Faecal Microbiota Transplantation: The Active Role of Gut Bacteria in Pain Persistence. Pain 2022, 163, 861–877. [Google Scholar] [CrossRef]
- de Groot, P.F.; Frissen, M.N.; de Clercq, N.C.; Nieuwdorp, M. Fecal Microbiota Transplantation in Metabolic Syndrome: History, Present and Future. Gut Microbes 2017, 8, 253–267. [Google Scholar] [CrossRef]
- Settanni, C.R.; Ianiro, G.; Bibbò, S.; Cammarota, G.; Gasbarrini, A. Gut Microbiota Alteration and Modulation in Psychiatric Disorders: Current Evidence on Fecal Microbiota Transplantation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 109, 110258. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Liu, S.; Crawford, J.; Tao, F. Gut-Brain Crosstalk and the Central Mechanisms of Orofacial Pain. Brain Sci. 2023, 13, 1456. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, L.H.; Xing, C.; Liu, T. Pain Regulation by Gut Microbiota: Molecular Mechanisms and Therapeutic Potential. Br. J. Anaesth. 2019, 123, 637–654. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Wang, Y.; Zhang, P.; Yuan, Y.; Zhang, Y.; Chen, G. Gut Microbiota Regulates Neuropathic Pain: Potential Mechanisms and Therapeutic Strategy. J. Headache Pain 2020, 21, 103. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (Updated March 2011); The Cochrane Collaboration: London, UK, 2011; Available online: https://www.handbook.cochrane.org (accessed on 17 November 2024).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomized Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomized Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE Guidelines: Consensus-Based Clinical Case Reporting Guideline Development. J. Clin. Epidemiol. 2014, 67, 46–51. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-randomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 17 November 2024).
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of Faecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome in a Randomised, Double-Blind, Placebo-Controlled Study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef]
- Fretheim, H.; Chung, B.K.; Didriksen, H.; Bækkevold, E.S.; Midtvedt, Ø.; Brunborg, C.; Holm, K.; Valeur, J.; Tennøe, A.H.; Garen, T.; et al. Fecal Microbiota Transplantation in Systemic Sclerosis: A Double-Blind, Placebo-Controlled Randomized Pilot Trial. PLoS ONE 2020, 15, e0232739. [Google Scholar] [CrossRef]
- Holvoet, T.; Joossens, M.; Vázquez-Castellanos, J.F.; Christiaens, E.; Heyerick, L.; Boelens, J.; Verhasselt, B.; van Vlierberghe, H.; De Vos, M.; Raes, J.; et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients With Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-Term Results From a Placebo-Controlled Randomized Trial. Gastroenterology 2021, 160, 145–157.e8. [Google Scholar] [CrossRef]
- Johnsen, P.H.; Hilpüsch, F.; Valle, P.C.; Goll, R. The Effect of Fecal Microbiota Transplantation on IBS-Related Quality of Life and Fatigue in Moderate to Severe Non-Constipated Irritable Bowel: Secondary Endpoints of a Double-Blind, Randomized, Placebo-Controlled Trial. EBioMedicine 2020, 51, 102562. [Google Scholar] [CrossRef] [PubMed]
- Kragsnaes, M.S.; Kjeldsen, J.; Horn, H.C.; Munk, H.L.; Pedersen, J.K.; Just, S.A.; Ahlquist, P.; Pedersen, F.M.; de Wit, M.; Möller, S.; et al. Safety and Efficacy of Faecal Microbiota Transplantation for Active Peripheral Psoriatic Arthritis: An Exploratory Randomised Placebo-Controlled Trial. Ann. Rheum. Dis. 2021, 80, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, P.; Jalanka, J.; Hartikainen, A.; Mattila, E.; Hillilä, M.; Punkkinen, J.; Koskenpato, J.; Anttila, V.J.; Tillonen, J.; Satokari, R.; et al. Randomised Clinical Trial: Faecal Microbiota Transplantation Versus Autologous Placebo Administered via Colonoscopy in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2020, 51, 1321–1331. [Google Scholar] [CrossRef] [PubMed]
- Salonen, T.; Jokinen, E.; Satokari, R.; Lahtinen, P. Randomized, Double-Blinded, Placebo-Controlled Pilot Study: Efficacy of Faecal Microbiota Transplantation on Chronic Fatigue Syndrome. J. Transl. Med. 2023, 21, 513. [Google Scholar] [CrossRef]
- Fang, H.; Hou, Q.; Zhang, W.; Su, Z.; Zhang, J.; Li, J.; Lin, J.; Wang, Z.; Yu, X.; Yang, Y.; et al. Fecal Microbiota Transplantation Improves Clinical Symptoms of Fibromyalgia: An Open-Label, Randomized, Nonplacebo-Controlled Study. J. Pain 2024, 25, 104535. [Google Scholar] [CrossRef]
- Singh, P.; Alm, E.J.; Kelley, J.M.; Cheng, V.; Smith, M.; Kassam, Z.; Nee, J.; Iturrino, J.; Lembo, A. Effect of Antibiotic Pretreatment on Bacterial Engraftment after Fecal Microbiota Transplant (FMT) in IBS-D. Gut Microbes 2022, 14, 2020067. [Google Scholar] [CrossRef]
- Cai, T.T.; Ye, X.L.; Yong, H.J.; Song, B.; Zheng, X.L.; Cui, B.T.; Zhang, F.M.; Lu, Y.B.; Miao, H.; Ding, D.F. Fecal Microbiota Transplantation Relieve Painful Diabetic Neuropathy: A Case Report. Medicine 2018, 97, e13543. [Google Scholar] [CrossRef]
- Thurm, T.; Ablin, J.N.; Buskila, D.; Maharshak, N. Fecal Microbiota Transplantation for Fibromyalgia: A Case Report and Review of the Literature. Open J. Gastroenterol. 2017, 7, 131–139. [Google Scholar] [CrossRef]
- Zeng, J.; Peng, L.; Zheng, W.; Huang, F.; Zhang, N.; Wu, D.; Yang, Y. Fecal Microbiota Transplantation for Rheumatoid Arthritis: A Case Report. Clin. Case Rep. 2020, 9, 906–909. [Google Scholar] [CrossRef]
- Kenyon, J.N.; Coe, S.; Izadi, H. A Retrospective Outcome Study of 42 Patients with Chronic Fatigue Syndrome, 30 of Whom Had Irritable Bowel Syndrome. Half Were Treated with Oral Approaches, and Half Were Treated with Faecal Microbiome Transplantation. Hum. Microbiome J. 2019, 13, 100061. [Google Scholar] [CrossRef]
- Ustianowska, K.; Ustianowski, Ł.; Machaj, F.; Gorący, A.; Rosik, J.; Szostak, B.; Szostak, J.; Pawlik, A. The Role of the Human Microbiome in the Pathogenesis of Pain. Int. J. Mol. Sci. 2022, 23, 13267. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, Q.; Chen, Y.; Ren, H.; Zhang, Q.; Yang, H.; Zhang, W.; Ding, T.; Wang, S.; Zhang, Y.; et al. Gut Microbiota in Chronic Pain: Novel Insights into Mechanisms and Promising Therapeutic Strategies. Int. Immunopharmacol. 2023, 115, 109685. [Google Scholar] [CrossRef] [PubMed]
- Santoni, M.; Miccini, F.; Battelli, N. Gut Microbiota, Immunity and Pain. Immunol. Lett. 2021, 229, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Zhang, X.J.; Zhang, N.N.; Yan, B.; Xu, K.K.; Peng, L.H.; Pan, F. Fecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome: A Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2022, 9, 890357. [Google Scholar] [CrossRef]
- Matheson, J.T.; Holsinger, R.M.D. The Role of Fecal Microbiota Transplantation in the Treatment of Neurodegenerative Diseases: A Review. Int. J. Mol. Sci. 2023, 24, 1001. [Google Scholar] [CrossRef]
- Vergne-Salle, P.; Bertin, P. Chronic Pain and Neuroinflammation. Jt. Bone Spine 2021, 88, 105222. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef]
- Zhang, W.H.; Jin, Z.Y.; Yang, Z.H.; Zhang, J.Y.; Ma, X.H.; Guan, J.; Sun, B.L.; Chen, X. Fecal Microbiota Transplantation Ameliorates Active Ulcerative Colitis by Downregulating Pro-Inflammatory Cytokines in Mucosa and Serum. Front. Microbiol. 2022, 13, 818111. [Google Scholar] [CrossRef]
- Porcari, S.; Benech, N.; Valles-Colomer, M.; Segata, N.; Gasbarrini, A.; Cammarota, G.; Sokol, H.; Ianiro, G. Key Determinants of Success in Fecal Microbiota Transplantation: From Microbiome to Clinic. Cell Host Microbe 2023, 31, 712–733. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter Modulation by the Gut Microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Rupprecht, L.E.; Liu, W.W.; Weng, P.; Bohorquez, D.V. Neuropod Cells: The Emerging Biology of Gut-Brain Sensory Transduction. Annu. Rev. Neurosci. 2020, 43, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Szőke, H.; Kovács, Z.; Bókkon, I.; Vagedes, J.; Szabó, A.E.; Hegyi, G.; Sterner, M.G.; Kiss, Á.; Kapócs, G. Gut Dysbiosis and Serotonin: Intestinal 5-HT as a Ubiquitous Membrane Permeability Regulator in Host Tissues, Organs, and the Brain. Rev. Neurosci. 2020, 31, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, G.; Guarnaccia, A.; Fancello, G.; Agrillo, C.; Iannarelli, F.; Sanguinetti, M.; Masucci, L. Fecal Microbiota Transplantation and Other Gut Microbiota Manipulation Strategies. Microorganisms 2022, 10, 2424. [Google Scholar] [CrossRef] [PubMed]
- Hartikainen, A.K.; Jalanka, J.; Lahtinen, P.; Ponsero, A.J.; Mertsalmi, T.; Finnegan, L.; Crispie, F.; Cotter, P.D.; Arkkila, P.; Satokari, R. Fecal Microbiota Transplantation Influences Microbiota without Connection to Symptom Relief in Irritable Bowel Syndrome Patients. NPJ Biofilms Microbiomes 2024, 10, 73. [Google Scholar] [CrossRef]
- Rebeaud, J.; Peter, B.; Pot, C. How Microbiota-Derived Metabolites Link the Gut to the Brain during Neuroinflammation. Int. J. Mol. Sci. 2022, 23, 10128. [Google Scholar] [CrossRef]
- Ahmed, H.; Leyrolle, Q.; Koistinen, V.; Kärkkäinen, O.; Layé, S.; Delzenne, N.; Hanhineva, K. Microbiota-Derived Metabolites as Drivers of Gut-Brain Communication. Gut Microbes 2022, 14, 2102878. [Google Scholar] [CrossRef]
- Albrecht, D.S.; Forsberg, A.; Sandström, A.; Bergan, C.; Kadetoff, D.; Protsenko, E.; Lampa, J.; Lee, Y.C.; Höglund, C.O.; Catana, C.; et al. Brain Glial Activation in Fibromyalgia—A Multi-Site Positron Emission Tomography Investigation. Brain Behav. Immun. 2019, 75, 72–83. [Google Scholar] [CrossRef]
- Mueller, C.; Fang, Y.D.; Jones, C.; McConathy, J.E.; Raman, F.; Lapi, S.E.; Younger, J.W. Evidence of Neuroinflammation in Fibromyalgia Syndrome: A [18F]DPA-714 Positron Emission Tomography Study. Pain 2023, 164, 2285–2295. [Google Scholar] [CrossRef]
- Marcondes Ávila, P.R.; Fiorot, M.; Michels, M.; Dominguini, D.; Abatti, M.; Vieira, A.; de Moura, A.B.; Behenck, J.P.; Borba, L.A.; Botelho, M.E.M.; et al. Effects of Microbiota Transplantation and the Role of the Vagus Nerve in Gut-Brain Axis in Animals Subjected to Chronic Mild Stress. J. Affect. Disord. 2020, 277, 410–416. [Google Scholar] [CrossRef]
- Xu, D.; Ren, L.; Zhang, W.; Wu, S.; Yu, M.; He, X.; Wei, Z. Therapeutic Effects and Mechanisms of Fecal Microbiota Transplantation on EAE Partly through HPA Axis-Mediated Neuroendocrine Regulation. Heliyon 2024, 10, e33214. [Google Scholar] [CrossRef]
- Braga, J.D.; Thongngam, M.; Kumrungsee, T. Gamma-Aminobutyric Acid as a Potential Postbiotic Mediator in the Gut-Brain Axis. NPJ Sci. Food 2024, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Xue, J.; Shao, R.; Yang, Y.; Ning, D.; Mo, C.; Wang, F.; Chen, G. Fecal Microbiota Transplantation and Short-Chain Fatty Acids Reduce Sepsis Mortality by Remodeling Antibiotic-Induced Gut Microbiota Disturbances. Front. Immunol. 2023, 13, 1063543. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Dinesh, S.; Sharma, S. Bridging the Mind and Gut: Uncovering the Intricacies of Neurotransmitters, Neuropeptides, and Their Influence on Neuropsychiatric Disorders. Cent. Nerv. Syst. Agents Med. Chem. 2024, 24, 2–21. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-Chain Fatty Acids: Microbial Metabolites That Alleviate Stress-Induced Brain-Gut Axis Alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Marizzoni, M.; Cattaneo, A.; Mirabelli, P.; Festari, C.; Lopizzo, N.; Nicolosi, V.; Mombelli, E.; Mazzelli, M.; Luongo, D.; Naviglio, D.; et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimers Dis. 2020, 78, 683–697. [Google Scholar] [CrossRef]
- Więdłocha, M.; Marcinowicz, P.; Janoska-Jaździk, M.; Szulc, A. Gut Microbiota, Kynurenine Pathway and Mental Disorders—Review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110145. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the Microbiota-Gut-Brain Axis in Chronic Restraint Stress: Disturbances of the Kynurenine Metabolic Pathway in Both the Gut and Brain. Gut Microbes 2021, 13, 1869501. [Google Scholar] [CrossRef]
- Janzon, A.; Goodrich, J.K.; Koren, O.; TEDDY Study Group; Waters, J.L.; Ley, R.E. Interactions Between the Gut Microbiome and Mucosal Immunoglobulins A, M, and G in the Developing Infant Gut. mSystems 2019, 4, e00612-19. [Google Scholar] [CrossRef]
- DuPont, H.L.; Jiang, Z.D.; Alexander, A.S.; DuPont, A.W.; Brown, E.L. Intestinal IgA-Coated Bacteria in Healthy- and Altered-Microbiomes (Dysbiosis) and Predictive Value in Successful Fecal Microbiota Transplantation. Microorganisms 2022, 11, 93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).