Reliability of Kudo’s Glandular Pit Pattern in Predicting Colorectal Lesion Histology at Routine Colonoscopy with Digital Chromoendoscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
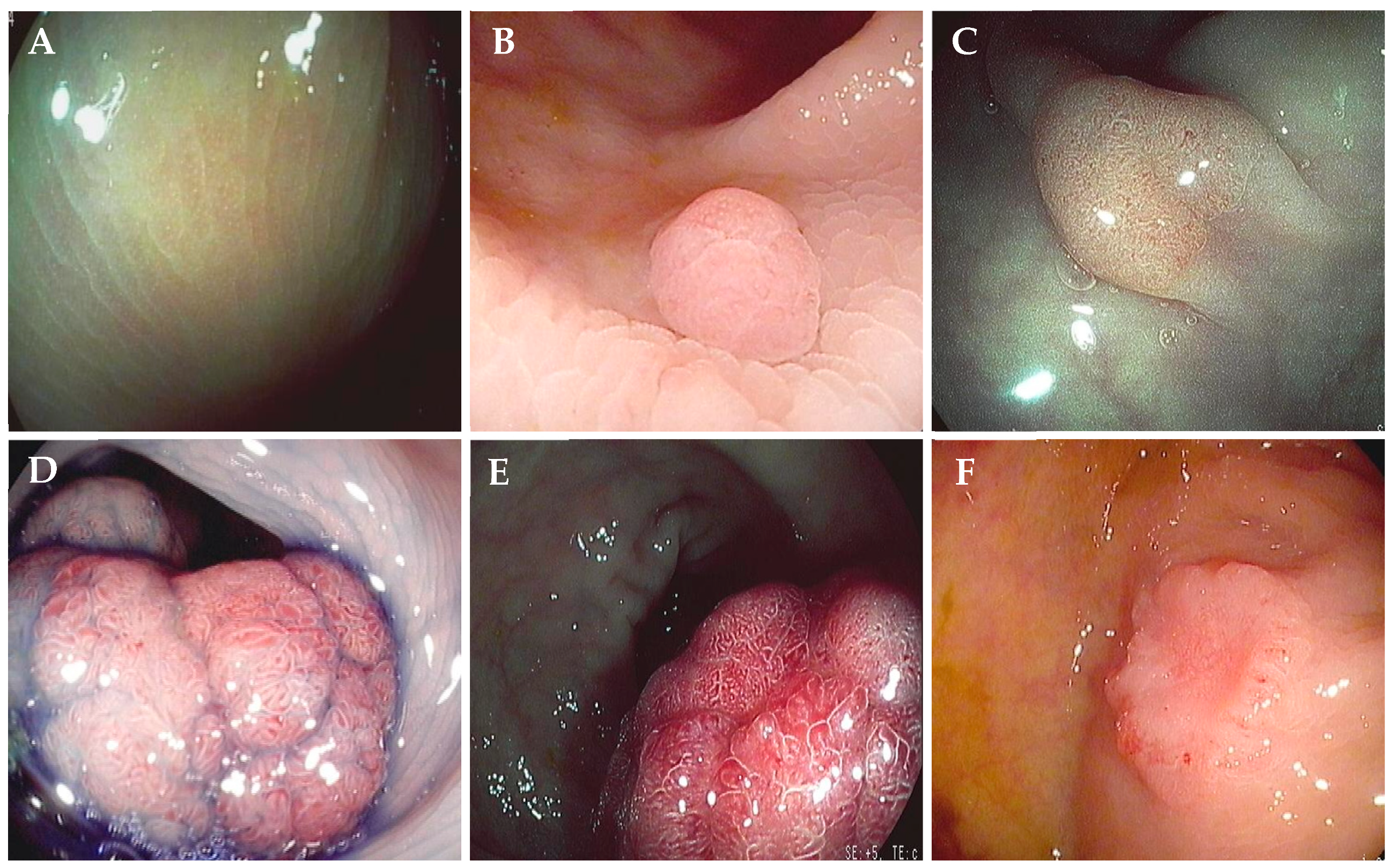
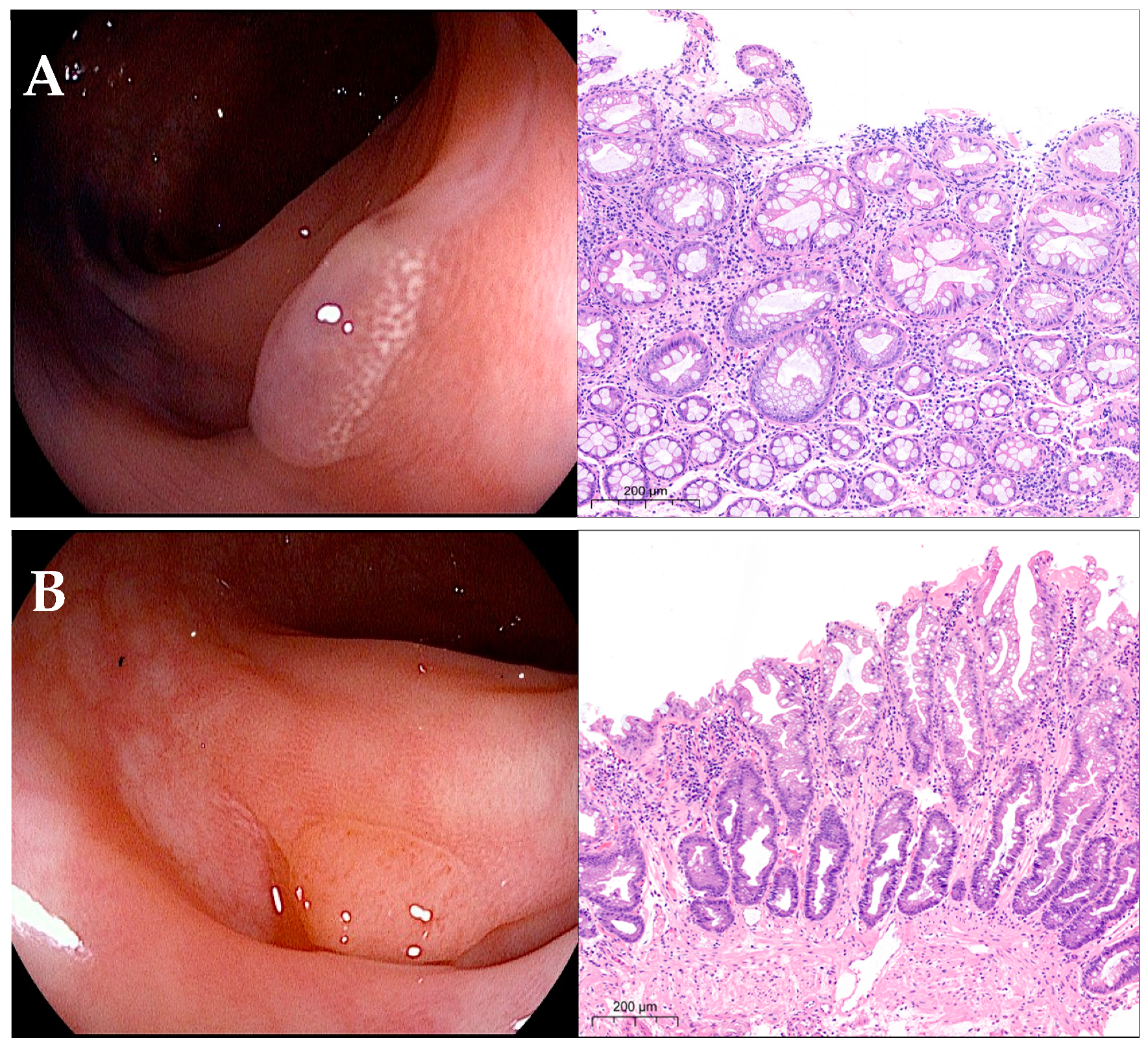
2.2. Endoscopic Procedures and Lesion Characterization
2.3. Study Endpoints and Statistical Analysis
3. Results
3.1. Overview of Colorectal Lesions
3.2. Diagnostic Accuracy of Real-Time Characterization in Diminutive Polyps
3.3. Diagnostic Accuracy of Real-Time Characterization in Small Polyps
3.4. Diagnostic Accuracy of Real-Time Characterization in Large Polyps
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qumseya, B.J.; Wallace, M.B. Advanced colorectal polyp detection techniques. Curr. Gastroenterol. Rep. 2012, 14, 414–420. [Google Scholar] [CrossRef]
- Brenner, H.; Stock, C.; Hoffmeister, M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomized controlled trials and observational studies. BMJ 2014, 348, 2467–2479. [Google Scholar] [CrossRef] [PubMed]
- Gomez, V.; Wallace, M.B. Advances in diagnostic and therapeutic colonoscopy. Curr. Opin. Gastroenterol. 2014, 30, 63–68. [Google Scholar] [CrossRef]
- van Rijn, J.C.; Reitsma, J.B.; Stoker, J.; Bossuyt, P.M.; van Deventer, S.J.; Dekker, E. Polyp miss rate determined by tandem colonoscopy: A systematic review. Am. J. Gastroenterol. 2006, 101, 343–350. [Google Scholar] [CrossRef]
- ASGE Technology Committee; Dayyeh, B.K.A.; Thosani, N.; Konda, V.; Wallace, M.B.; Rex, D.K.; Chauhan, S.S.; Hwang, J.H.; Komanduri, S.; Manfredi, M.; et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest. Endosc. 2015, 81, 501–502. [Google Scholar]
- Hassan, C.; Pickhardt, P.J.; Rex, D.K. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin. Gastroenterol. Hepatol. 2010, 8, 865–869. [Google Scholar] [CrossRef]
- Orlovic, M.; Ahmad, A.; Saunders, B.P. Economic impact of implementing optical diagnosis with a “resect-and-discard” strategy within the English Bowel Cancer Screening Programme: Findings from the DISCARD3 study. Gastrointest. Endosc. 2023, 98, 73–81. [Google Scholar] [CrossRef]
- Kuwai, T.; Yamada, T.; Toyokawa, T.; Iwase, H.; Kudo, T.; Esaka, N.; Ohta, H.; Yamashita, H.; Hosoda, Y.; Watanabe, N.; et al. Local recurrence of diminutive colorectal polyps after cold forceps polypectomy with jumbo forceps followed by magnified narrowband imaging: A multicenter prospective study. Endoscopy 2019, 51, 253–260. [Google Scholar] [PubMed]
- Butterly, L.F.; Chase, M.P.; Pohl, H.; Fiarman, G.S. Prevalence of clinically important histology in small adenomas. Clin. Gastroenterol. Hepatol. 2006, 4, 343–348. [Google Scholar] [CrossRef]
- Gupta, N.; Bansal, A.; Rao, D.; Early, D.S.; Jonnalagadda, S.; Edmundowicz, S.A.; Sharma, P.; Rastogi, A. Accuracy of in vivo optical diagnosis of colon polyp histology by narrow-band imaging in predicting colonoscopy surveillance intervals. Gastrointest. Endosc. 2012, 75, 494–502. [Google Scholar] [CrossRef]
- Meester, R.G.; van Herk, M.M.; Lansdorp-Vogelaar, I.; Ladabaum, U. Prevalence and clinical features of sessile serrated polyps: A systematic review. Gastroenterology 2020, 159, 105–118. [Google Scholar] [CrossRef]
- East, J.E.; Atkin, W.S.; Bateman, A.C.; Clark, S.K.; Dolwani, S.; Ket, S.N.; Leedham, S.J.; Phull, P.S.; Rutter, M.D.; Shepherd, N.A.; et al. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut 2017, 66, 1181–1196. [Google Scholar] [CrossRef]
- Erichsen, R.; Baron, J.A.; Hamilton-Dutoit, S.J.; Snover, D.C.; Torlakovic, E.E.; Pedersen, L.; Frøslev, T.; Vyberg, M.; Hamilton, S.R.; Sørensen, H.; et al. Increased risk of colorectal cancer development among patients with serrated polyps. Gastroenterology 2016, 150, 895–902. [Google Scholar] [CrossRef]
- Kudo, S.; Hirota, S.; Nakajima, T.; Hosobe, S.; Kusaka, H.; Kobayashi, T.; Himori, M.; Yagyuu, A. Colorectal tumors pit pattern. J. Clin. Pathol. 1994, 47, 880–885. [Google Scholar] [CrossRef]
- Tanaka, S.; Haruma, K.; Nagata, S.; Oka, S.; Chayama, K. Diagnosis of invasion depth in early colorectal carcinoma by pit pattern analysis with magnifying endoscopy. Dig. Endosc. 2001, 13, S2–S5. [Google Scholar] [CrossRef]
- Mason, S.E.; Poynter, L.; Takats, Z.; Darzi, A.; Kinross, J.M. Optical technologies for endoscopic real-time histologic assessment of colorectal polyps: A meta-analysis. Am. J. Gastroenterol. 2019, 114, 1219–1230. [Google Scholar] [CrossRef]
- Bhandari, P.; Thayalasekaran, S.; Keisslich, R.; Bisschops, R.; Hoffmann, A.; Haidry, R.; Esteban, J.; Viedma, B.L.; Godzhello, E.; Almadi, M.; et al. Detection and characterization of colorectal polyps using high-definition white light and i-Scan: Evidence-based consensus recommendations using a modified Delphi process. United Eur. Gastroenterol. J. 2018, 6, 748–754. [Google Scholar] [CrossRef]
- Glover, B.; Patel, N.; Ashrafian, H.; Teare, J. Diagnostic accuracy of i-scan image enhancement for real-time endoscopic diagnosis of small colorectal polyps: A meta-analysis. Ther. Adv. Gastroenterol. 2018, 11, 1756284818814948. [Google Scholar] [CrossRef]
- Parsa, N.; Byrne, M.F. Artificial intelligence for identification and characterization of colonic polyps. Ther. Adv. Gastrointest. Endosc. 2021, 14, 26317745211014698. [Google Scholar] [CrossRef]
- Masci, E.; Mangiavillano, B.; Crosta, C.; Fiori, G.; Trovato, C.; Viaggi, P.; Zambelli, A.; Buffoli, F.; Staiano, T.; Manfredi, G.; et al. Interobserver agreement among endoscopists on evaluation of polypoid colorectal lesions visualized with the Pentax i-Scan technique. Dig. Liver Dis. 2013, 45, 207–210. [Google Scholar] [CrossRef]
- Iacucci, M.; Trovato, C.; Daperno, M.; Akinola, O.; Greenwald, D.; Gross, S.A.; Hoffman, A.; Lee, J.; Lethebe, B.C.; Lowerison, M.; et al. SIMPLE classification investigator team. Development and validation of the SIMPLE endoscopic classification of diminutive and small colorectal polyps. Endoscopy 2018, 50, 779–789. [Google Scholar]
- Hoffman, A.; Kagel, C.; Goetz, M.; Tresch, A.; Mudter, J.; Biesterfeld, S.; Galle, P.R.; Neurath, M.F.; Kiesslich, R. Recognition and characterization of small colonic neoplasia with high-definition colonoscopy using i-Scan is as precise as chromoendoscopy. Dig. Liver Dis. 2010, 42, 45–50. [Google Scholar] [CrossRef]
- Mercaldo, N.D.; Lau, K.F.; Zhou, X.H. Confidence intervals for predictive values with an emphasis to case-control studies. Stat. Med. 2007, 26, 2170–2183. [Google Scholar] [CrossRef]
- Cocomazzi, F.; Gentile, M.; Perri, F.; Bossa, F.; Merla, A.; Ippolito, A.; Cubisino, R.; Carparelli, S.; Marra, A.; Mileti, A.; et al. Accuracy and inter-observer agreement of the NICE and Kudo classifications of superficial colonic lesions: A comparative study. Int. J. Color. Dis. 2021, 36, 1561–1568. [Google Scholar] [CrossRef]
- Li, M.; Ali, S.M.; Umm-a-OmarahGilani, S.; Liu, J.; Li, Y.Q.; Zuo, X.L. Kudo’s pit pattern classification for colorectal neoplasm: A meta-analysis. World J. Gastroenterol. 2014, 20, 12649–12656. [Google Scholar] [CrossRef]
- Kim, S.W.; Cha, J.M.; Lee, J.I.; Joo, K.R.; Shin, H.P.; Kim, G.Y.; Lim, S.J. A significant number of sessile serrated adenomas might not be accurately diagnosed in daily practice. Gut Liver 2010, 4, 498–502. [Google Scholar] [CrossRef]
- Khalid, O.; Radaideh, S.; Cummings, O.W.; O’brien, M.J.; Goldblum, J.R.; Rex, D.K. Reinterpretation of histology of proximal colon polyps called hyperplastic in 2001. World J. Gastroenterol. 2009, 15, 3767–3770. [Google Scholar] [CrossRef]
- Singh, H.; Bay, D.; Ip, S.; Bernstein, C.N.; Nugent, Z.; Gheorghe, R.; Wightman, R. Pathological reassessment of hyperplastic colon polyps in a city-wide pathology practice: Implications for polyp surveillance recommendations. Gastrointest. Endosc. 2012, 76, 1003–1008. [Google Scholar] [CrossRef]
- Ijspeert, J.E.G.; Bastiaansen, B.A.J.; E van Leerdam, M.; A Meijer, G.; van Eeden, S.; Sanduleanu, S.; Schoon, E.J.; Bisseling, T.M.; Spaander, M.C.; van Lelyveld, N.; et al. Development and validation of the WASP classification system for optical diagnosis of adenomas, hyperplastic polyps and sessile serrated adenomas/polyps. Dutch Workgroup serrAted polypS & Polyposis (WASP). Gut 2016, 65, 963–970. [Google Scholar]
- Basford, P.J.; Longcroft-Wheaton, G.; Higgins, B.; Bhandari, P. High-definition endoscopy with i-Scan for evaluation of small colon polyps: The HiSCOPE study. Gastrointest. Endosc. 2014, 79, 111–118. [Google Scholar] [CrossRef]
- Klenske, E.; Zopf, S.; Neufert, C.; Nägel, A.; Siebler, J.; Gschossmann, J.; Mühldorfer, S.; Pfeifer, L.; Fischer, S.; Vitali, F.; et al. I-scan optical enhancement for the in vivo prediction of diminutive colorectal polyp histology: Results from a prospective threephased multicentre trial. PLoS ONE 2018, 13, e0197520. [Google Scholar] [CrossRef] [PubMed]
- Soons, E.; Bisseling, T.M.; van der Post, R.S.; Nagtegaal, I.D.; Hazewinkel, Y.; van Kouwen, M.C.A.; Siersema, P.D. The Workgroup Serrated Polyps and Polyposis (WASP) classification for optical diagnosis of colorectal diminutive polyps with iScan and the impact of the revised World Health Organization (WHO) criteria. United Eur. Gastroenterol. J. 2021, 9, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, R.; Samarasena, J.; Luba, D.; Duh, E.; Dao, T.; Requa, J.; Ninh, A.; Karnes, W. Prediction of Polyp Pathology Using Convolutional Neural Networks Achieves “Resect and Discard” Thresholds. Am. J. Gastroenterol. 2020, 115, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Yamamoto, E.; Yamano, H.-O.; Suzuki, H.; Kamimae, S.; Nojima, M.; Sawada, T.; Ashida, M.; Yoshikawa, K.; Takagi, R.; et al. A novel pit pattern identifies the precursor of colorectal cancer derived from sessile serrated adenoma. Am. J. Gastroenterol. 2012, 107, 460–469. [Google Scholar] [CrossRef]
- Nakao, Y.; Saito, S.; Ohya, T.; Aihara, H.; Arihiro, S.; Kato, T.; Ikegami, M.; Tajiri, H. Endoscopic features of colorectal serrated lesions using image-enhanced endoscopy with pathological analysis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 981–988. [Google Scholar] [CrossRef]
| Site of Lesions | Size of Lesions | p-Value | |||||
|---|---|---|---|---|---|---|---|
| ≤5 mm, N. (%) | 6–9 mm, N. (%) | ≥10 mm, N. (%) | Total, N. (%) | Left vs. Right Colon ≤ 5 mm | Left vs. Right Colon 6–9 mm | Left vs. Right Colon ≥ 10 mm | |
| Right colon, N. (%) | 468 (52.12) | 392 (55.68) | 345 (54.94) | 1205 (54.04) | 0.072 | <0.0001 | 0.0005 |
| Left colon, N. (%) | 430 (47.88) | 312 (44.32) | 283 (45.06) | 1025 (45.96) | |||
| Kudo glandular pit pattern | Size of lesions | p-value | |||||
| ≤5 mm, N. (%) | 6–9 mm, N. (%) | ≥10 mm, N. (%) | Total, N. (%) | ≤5 mm vs. 6–9 mm | 6–9 mm vs. ≥10 mm | ≤5 mm vs. ≥10 mm | |
| Type I, N. (%) | 30 (90.9) | 3 (9.09) | 0 | 33 (1.48) | <0.0001 | 0.025 | <0.0001 |
| Type II, N. (%) | 482 (52.45) | 272 (29.6) | 165 (17.95) | 919 (41.21) | <0.0001 | <0.0001 | <0.0001 |
| Type IIIs/IIIL/IV, N. (%) | 386 (32.03) | 424 (35.19) | 395 (32.78) | 1205 (54.04) | <0.0001 | 0.0001 | 0.647 |
| Type Vi/Vn, N. (%) | 0 | 5 (6.85) | 68 (93.15) | 73 (3.27) | 0.016 | <0.0001 | <0.0001 |
| Total, N. (%) | 898 (40.27) | 704 (31.57) | 628 (28.16) | 2230 | |||
| Histology of Lesions | Size of Lesions | p-Value | |||||
|---|---|---|---|---|---|---|---|
| ≤5 mm, N. (%) | 6–9 mm, N. (%) | ≥10 mm, N. (%) | Total, N. (%) | ≤5 mm vs. 6–9 mm | 6–9 mm vs. ≥10 mm | ≤5 mm vs. ≥10 mm | |
| Normal mucosa, N. (%) | 59 (96.72) | 2 (3.28) | 0 | 61 (2.74) | <0.0001 | 0.083 | <0.0001 |
| Serrated non-neoplastic (HPs), N. (%) | 239 (81.29) | 54 (18.37) | 1 (0.34) | 294 (13.18) | <0.0001 | <0.0001 | <0.0001 |
| Serrated adenomas (TSA-SSA/p), N. (%) | 169 (54.69) | 84 (27.18) | 56 (18.12) | 309 (13.86) | <0.0001 | 0.0008 | <0.0001 |
| Conventional adenomas (tubular, villous, tubulo-villous), N. (%) | 431 (28.87) | 559 (37.44) | 503 (33.69) | 1493 (66.95) | <0.0001 | 0.015 | 0.0009 |
| Adenocarcinomas, N. (%) | 0 | 5 (6.85) | 68 (93.15) | 73 (3.27) | 0.003 | <0.0001 | <0.0001 |
| Total, N. (%) | 898 (40.27) | 704 (31.57) | 628 (28.16) | 2.230 | |||
| Size of Lesions | Site of Lesions | Kudo Glandular Pit Pattern | Normal Mucosa, N. (%) | HPs, N. (%) | Adenomas (TSA-SSA/p, Conventional), N. (%) | ADKs, N. (%) | Total, N. (%) | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal Mucosa vs. Lesions | HPs vs. Adenomas | HPs vs. Adenomas-ADKs | Adenomas vs. ADKs | ||||||||
| ≤5 mm | Left colon | Type I, N. (%) | 21 (100) | 0 | 0 | 0 | 21 (4.88) | <0.0001 | − | − | − |
| Type II, N. (%) | 23 (8.69) | 174 (65.66) | 68 (25.66) | 0 | 265 (61.63) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Type IIIs/IIIL/IV, N. (%) | 0 | 8 (5.56) | 136 (94.44) | 0 | 144 (33.49) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Type Vi/Vn, N. (%) | 0 | 0 | 0 | 0 | 0 | − | − | − | − | ||
| Total, N. (%) | 44 (10.23) | 182 (42.33) | 204 (47.44) | 0 | 430 (47.88) | ||||||
| Right colon | Type I, N. (%) | 8 (88.89) | 0 | 1 (11.11) | 0 | 9 (1.92) | 0.001 | 0.317 | 0.317 | 0.317 | |
| Type II, N. (%) | 7 (3.23) | 45 (20.74) | 165 (76.04) | 0 | 217 (46.37) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Type IIIs/IIIL/IV, N. (%) | 0 | 12 (4.96) | 230 (95.04) | 0 | 242 (51.71) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Type Vi/Vn, N. (%) | 0 | 0 | 0 | 0 | 0 | − | − | − | − | ||
| Total, N. (%) | 15 (3.21) | 57 (12.18) | 396 (84.62) | 0 | 468 (52.12) | ||||||
| Grand Total, N. (%) | 59 (6.57) | 239 (26.61) | 600 (66.82) | 0 | 898 | ||||||
| Size of Lesions | Site of Lesions | Kudo Glandular Pit Pattern | Normal Mucosa, N. (%) | HPs, N. (%) | Adenomas (TSA-SSA/p, Conventional), N. (%) | ADKs, N. (%) | Total, N. (%) | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal Mucosa vs. Lesions | HPs vs. Adenomas | HPs vs. Adenomas-ADKs | Adenomas vs. ADKs | ||||||||
| 6–9 mm | Left colon | Type I, N. (%) | 1 (50) | 0 | 1 (50) | 0 | 2 (0.64) | 1.00 | 0.317 | 0.317 | 0.317 |
| Type II, N. (%) | 0 | 14 (14.89) | 79 (84.04) | 1 (1.06) | 94 (30.13) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Type IIIs/IIIL/IV, N. (%) | 0 | 15 (7.01) | 199 (92.99) | 0 | 214 (68.59) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Type Vi/Vn, N. (%) | 0 | 0 | 1 (50) | 1 (50) | 2 (0.64) | 0.083 | 0.317 | 0.083 | 1000 | ||
| Total, N. (%) | 1 (0.32) | 29 (9.29) | 280 (89.74) | 2 (0.64) | 312 (44.32) | ||||||
| Right colon | Type I, N. (%) | 0 | 0 | 1 (100) | 0 | 1 (0.26) | 0.317 | 0.317 | 0.317 | 0.317 | |
| Type II, N. (%) | 1 (0.56) | 7 (3.93) | 170 (95.5) | 0 | 178 (45.41) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Type IIIs/IIIL/IV, N. (%) | 0 | 18 (8.57) | 192 (91.43) | 0 | 210 (53.57) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Type Vi/Vn, N. (%) | 0 | 0 | 0 | 3 (100) | 3 (0.77) | 0.025 | − | 0.025 | 0.025 | ||
| Total, N. (%) | 1 (0.26) | 25 (6.38) | 363 (92.6) | 3 (0.77) | 392 (55.68) | ||||||
| Grand Total, N. (%) | 2 (0.28) | 54 (7.67) | 643 (91.34) | 5 (0.71) | 704 | ||||||
| Size of Lesions | Site of Lesions | Kudo Glandular Pit Pattern | Normal Mucosa, N. (%) | HPs, N. (%) | Adenomas (TSA-SSA/p, Conventional), N. (%) | ADKs, N. (%) | Total, N. (%) | p-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal Mucosa vs. Lesions | HPs vs. Adenomas | HPs vs. Adenomas-ADKs | Adenomas vs. ADKs | ||||||||
| ≥ 10 mm | Left colon | Type I, N. (%) | 0 | 0 | 0 | 0 | 0 | − | − | − | − |
| Type II, N. (%) | 0 | 1 (1.19) | 83 (98.81) | 0 | 84 (29.68) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Type IIIs/IIIL/IV, N. (%) | 0 | 0 | 174 (100) | 0 | 174 (61.48) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Type Vi/Vn, N. (%) | 0 | 0 | 0 | 25 (100) | 25 (8.83) | <0.0001 | − | <0.0001 | <0.0001 | ||
| Total, N. (%) | 0 | 1 (0.35) | 257 (90.81) | 25 (8.83) | 283 (45.06) | ||||||
| Right colon | Type I, N. (%) | 0 | 0 | 0 | 0 | 0 | − | − | − | − | |
| Type II, N. (%) | 0 | 0 | 81 (100) | 0 | 81 (23.48) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Type IIIs/IIIL/IV, N. (%) | 0 | 0 | 221 (100) | 0 | 221 (64.06) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||
| Type Vi/Vn, N. (%) | 0 | 0 | 0 | 43 (100) | 43 (12.46) | <0.0001 | − | <0.0001 | <0.0001 | ||
| Total, N. (%) | 0 | 0 | 302 (87.54) | 43 (12.46) | 345 (54.94) | ||||||
| Grand Total, N. (%) | 0 | 1 (0.16) | 559 (89.01) | 68 (10.83) | 628 | ||||||
| Kudo’s Glandular Pit Pattern | Size of Lesions | |||
|---|---|---|---|---|
| ≤5 mm | 6–9 mm | ≥10 mm | ||
| Type II | Diagnostic accuracy (95% CI) | 62.55% (58.9 to 66.1) | 60.67% (56.91 to 64.34) | 70.69% (66.75 to 74.45) |
| NPV (95% CI) | 84.52% (79.9 to 88.23) | 94.71% (92.94 to 96.06) | 100% (99.41 to 100) | |
| Type IIL/IIIs/IV | Diagnostic accuracy (95% CI) | 71.51% (58.9 to 66.1) | 69.51% (66.43 to 72.47) | 100% (98.43 to 100) |
| NPV (95% CI) | 92.13% (88.33 to 94.76) | 88.42% (88.47 to 91.47) | 100% (99.41 to 100) | |
| Type Vi/Vn | Diagnostic accuracy (95% CI) | - | 99.7% (99.18 to 99.98) | 100% (99.1 to 100) |
| NPV (95% CI) | - | 99.9% (98.97 to 99.97) | 100% (99.22 to 100) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Testoni, S.G.G.; Testoni, P.A.; Notaristefano, C.; Viale, E.; Cavestro, G.M. Reliability of Kudo’s Glandular Pit Pattern in Predicting Colorectal Lesion Histology at Routine Colonoscopy with Digital Chromoendoscopy. Gastrointest. Disord. 2024, 6, 661-674. https://doi.org/10.3390/gidisord6030044
Testoni SGG, Testoni PA, Notaristefano C, Viale E, Cavestro GM. Reliability of Kudo’s Glandular Pit Pattern in Predicting Colorectal Lesion Histology at Routine Colonoscopy with Digital Chromoendoscopy. Gastrointestinal Disorders. 2024; 6(3):661-674. https://doi.org/10.3390/gidisord6030044
Chicago/Turabian StyleTestoni, Sabrina Gloria Giulia, Pier Alberto Testoni, Chiara Notaristefano, Edi Viale, and Giulia Martina Cavestro. 2024. "Reliability of Kudo’s Glandular Pit Pattern in Predicting Colorectal Lesion Histology at Routine Colonoscopy with Digital Chromoendoscopy" Gastrointestinal Disorders 6, no. 3: 661-674. https://doi.org/10.3390/gidisord6030044
APA StyleTestoni, S. G. G., Testoni, P. A., Notaristefano, C., Viale, E., & Cavestro, G. M. (2024). Reliability of Kudo’s Glandular Pit Pattern in Predicting Colorectal Lesion Histology at Routine Colonoscopy with Digital Chromoendoscopy. Gastrointestinal Disorders, 6(3), 661-674. https://doi.org/10.3390/gidisord6030044







