The Impact of a Gluten-Free Diet on Pregnant Women with Celiac Disease: Do We Need a Guideline to Manage Their Health?
Abstract
1. Introduction
2. Celiac Disease, Pregnancy, and Fetal Development
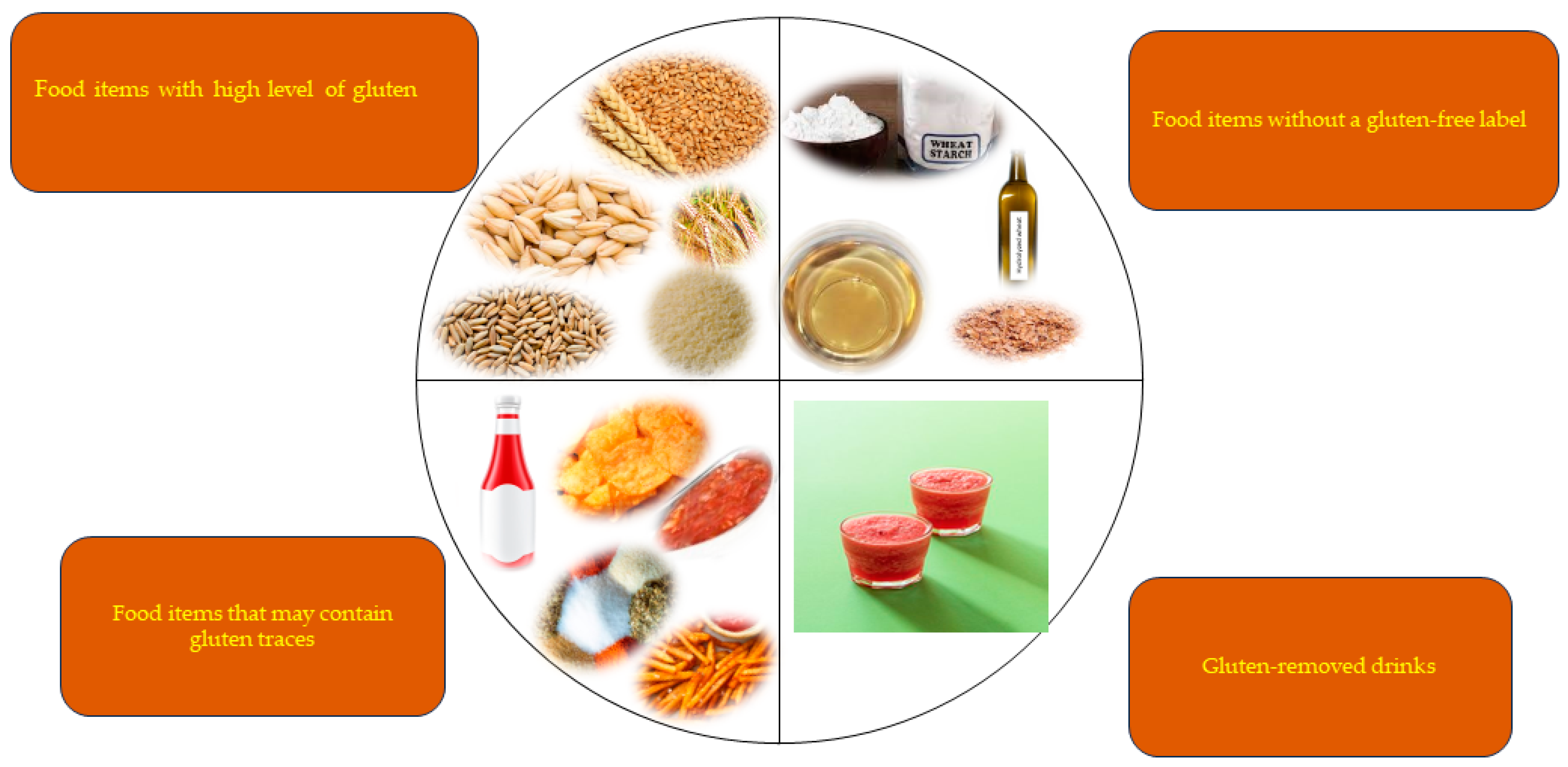
3. What Is a Gluten-Free Diet (GFD)
4. The Importance of GFD Adherence Assessment in Celiac Pregnancy Follow-Up
Nutritional Assessment in CD
5. The Role of GFD in Reproductive Outcomes of Maternal Celiac Pregnancy
6. Nutritional Risk Factors in Maternal Celiac Pregnancy and the Influence on Fetus Development
6.1. Energy Requirements
6.2. Body Mass Index (BMI)
6.3. Carbohydrates
6.4. Folic Acid
6.5. Iron
6.6. Calcium
6.7. Vitamin D
6.8. Vitamin B12
7. Current Status of Nutritional Guidelines for Pregnant Women with Celiac Disease
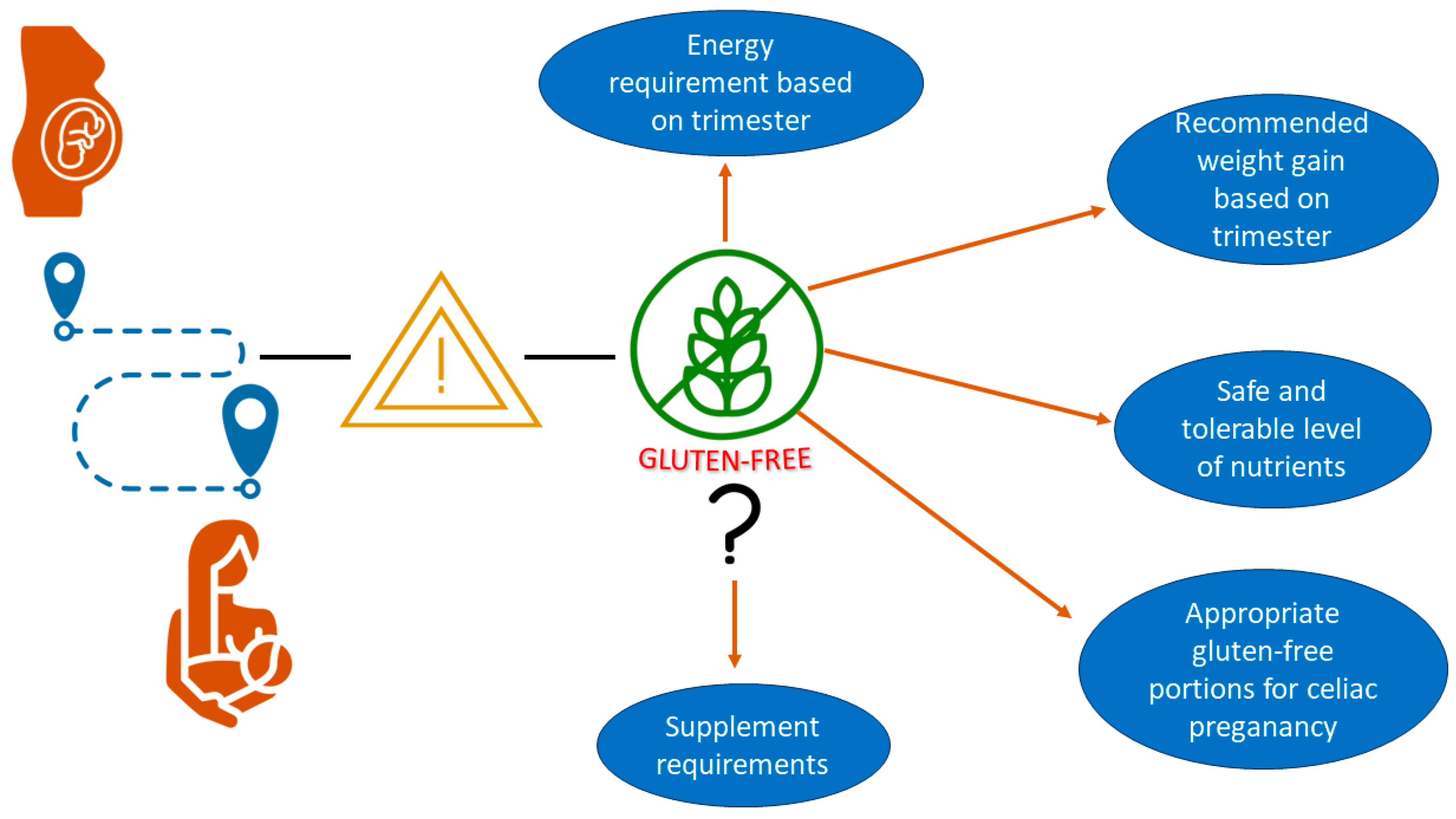
8. Expectations from Guidelines for Pregnant Women with Celiac Disease
9. What Problems Should Guidelines for Pregnant Women with Celiac Disease Solve?
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danowski, L.; Brand, L.G.; Connolly, J. Gluten-free diets, coeliac disease and associated disorders. Fam. Prcat. 2003, 20, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Fasano, A. Clinical practice: Celiac disease. N. Engl. J. Med. 2012, 367, 2419–2426. [Google Scholar]
- Green, P.H.; Cellier, C. Celiac disease. N. Engl. J. Med. 2007, 357, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Bilodeau, D.; Crémieux, P.-Y.; Jaumard, B.; Ouellette, P.; Vovor, T. Measuring hospital performance in the presence of quasi-fixed inputs: An analysis of Québec hospitals. J. Prod. Anal. 2004, 21, 183–199. [Google Scholar] [CrossRef]
- Ashton, J.J.; Smith, R.; Smith, T.; Beattie, R.M. Investigating coeliac disease in adults. BMJ 2020, 369, m2176. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Systemic autoimmune disorders in celiac disease. Curr. Opin. Gastroenterol. 2006, 22, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Ciclitira, P.; Hadjivassiliou, M.; Kaukinen, K.; Ludvigsson, J.F.; McGough, N.; Sanders, D.S.; Woodward, J.; Leonard, J.N.; Swift, G.L. The gluten-free diet and its current application in coeliac disease and dermatitis herpetiformis. United Eur. Gastroenterol. J. 2014, 3, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Aljada, B.; Zohni, A.; El-Matary, W. The gluten-free diet for celiac disease and beyond. Nutrients 2021, 13, 3993. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Adjukiewicz, A.; Read, A. Coeliac infertility: An indication for dietary gluten restriction? Lancet 1970, 295, 213–214. [Google Scholar] [CrossRef]
- Ferguson, R.; Holmes, G.; Cooke, W. Coeliac disease, fertility, and pregnancy. Scand. J. Gastroenterol. 1982, 17, 65–68. [Google Scholar] [CrossRef]
- Sher, K.S.; Mayberry, J.F. Female fertility, obstetric and gynaecological history in coeliac disease. Digestion 1994, 55, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Smecuol, E.; Mauriño, E.; Vazquez, H.; Pedreira, S.; Niveloni, S.; Mazure, R.; Boerr, L.; Bai, J.C. Gynaecological and obstetric disorders in coeliac disease: Frequent clinical onset during pregnancy or the puerperium. Eur. J. Gastroenterol. Hepatol. 1996, 8, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, J.A.; Weir, M.; Gougeon, L. Canadian packaged gluten-free foods are less nutritious than their regular gluten-containing counterparts. PeerJ 2018, 6, e5875. [Google Scholar] [CrossRef]
- Taetzsch, A.; Das, S.K.; Brown, C.; Krauss, A.; Silver, R.E.; Roberts, S.B. Are gluten-free diets more nutritious? An evaluation of self-selected and recommended gluten-free and gluten-containing dietary patterns. Nutrients 2018, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Faliva, M.A.; Gasparri, C.; Peroni, G.; Naso, M.; Picciotto, G.; Riva, A.; Nichetti, M.; Infantino, V.; Alalwan, T.A.; et al. Micronutrients dietary supplementation advices for celiac patients on long-term gluten-free diet with good compliance: A review. Medicina 2019, 55, 337. [Google Scholar] [CrossRef] [PubMed]
- Stazi, A.V.; Mantovani, A. A risk factor for female fertility and pregnancy: Celiac disease. Gynecol. Endocrinol. 2000, 14, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, L.L.; Allen, L. Position of The American dietetic association: Nutrition and lifestyle for a healthy pregnancy outcome. J. Am. Diet. Assoc. 2002, 102, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Mecacci, F.; Biagioni, S.; Ottanelli, S.; Mello, G. Nutrition in pregnancy and lactation: How a healthy infant is born. J. Pediatr. Neonatal Individ. Med. (JPNIM) 2015, 4, e040236. [Google Scholar]
- Ogborn, A. Pregnancy in patients with coeliac disease. BJOG Int. J. Obstet. Gynaecol. 1975, 82, 293–296. [Google Scholar] [CrossRef]
- Saccone, G.; Berghella, V.; Sarno, L.; Maruotti, G.M.; Cetin, I.; Greco, L.; Khashan, A.S.; McCarthy, F.; Martinelli, D.; Fortunato, F.; et al. Celiac disease and obstetric complications: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2016, 214, 225–234. [Google Scholar] [CrossRef]
- Tersigni, C.; Castellani, R.; de Waure, C.; Fattorossi, A.; De Spirito, M.; Gasbarrini, A.; Scambia, G.; Di Simone, N. Celiac disease and reproductive disorders: Meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum. Reprod. Updat. 2014, 20, 582–593. [Google Scholar] [CrossRef]
- Sen, A.; Kushnir, V.A.; Barad, D.H.; Gleicher, N. Endocrine autoimmune diseases and female infertility. Nat. Rev. Endocrinol. 2014, 10, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.; Baker, P.N.; Robinson, N.J.; Aplin, J.D. Maternal celiac disease autoantibodies bind directly to syncytiotrophoblast and inhibit placental tissue transglutaminase activity. Reprod. Biol. Endocrinol. 2009, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Barone, M.V.; Troncone, R.; Auricchio, S. Gliadin Peptides as triggers of the proliferative and stress/innate immune response of the celiac small intestinal mucosa. Int. J. Mol. Sci. 2014, 15, 20518–20537. [Google Scholar] [CrossRef] [PubMed]
- Schuppan, D.; Ciccocioppo, R. Coeliac disease and secondary autoimmunity. Dig. Liver Dis. 2002, 34, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.M.; Hill, I.D. Celiac disease and autoimmunity: Review and controversies. Curr. Allergy Asthma Rep. 2013, 13, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Wambua, S.; Lee, S.I.; Okoth, K.; Wang, Z.; Fazla, F.; Fayaz, A.; Eastwood, K.-A.; Nelson-Piercy, C.; Nirantharakumar, K.; et al. Autoimmune diseases and adverse pregnancy outcomes: An umbrella review. Lancet 2023, 402, S84. [Google Scholar] [CrossRef] [PubMed]
- Ciacci, C.; Cirillo, M.; Auriemma, G.; Di Dato, G.; Sabbatini, F.; Mazzacca, G. Celiac disease and pregnancy outcome. Obstet. Gynecol. Surv. 1996, 51, 643–644. [Google Scholar] [CrossRef]
- Tursi, A.; Giorgetti, G.; Brandimarte, G.; Elisei, W. Effect of gluten-free diet on pregnancy outcome in celiac disease patients with recurrent miscarriages. Dig. Dis. Sci. 2008, 53, 2925–2928. [Google Scholar] [CrossRef]
- Sansotta, N.; Amirikian, K.; Guandalini, S.; Jericho, H. Celiac disease symptom resolution: Effectiveness of the gluten-free diet. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 48–52. [Google Scholar] [CrossRef]
- Saturni, L.; Ferretti, G.; Bacchetti, T. The gluten-free diet: Safety and nutritional quality. Nutrients 2010, 2, 00016–00034. [Google Scholar] [CrossRef]
- Rajpoot, P.; Makharia, G.K. Problems and Challenges to Adaptation of Gluten Free Diet by Indian Patients with Celiac Disease. Nutrients 2013, 5, 4869–4879. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, M.; Szymczak-Tomczak, A.; Mahadea, D.; Eder, P.; Dobrowolska, A.; Krela-Kaźmierczak, I. Multidimensional disadvantages of a gluten-free diet in celiac disease: A narrative review. Nutrients 2021, 13, 643. [Google Scholar] [CrossRef]
- Agarwal, A.; Singh, A.; Mehtab, W.; Gupta, V.; Chauhan, A.; Rajput, M.S.; Singh, N.; Ahuja, V.; Makharia, G.K. Patients with celiac disease are at high risk of developing metabolic syndrome and fatty liver. Intest. Res. 2021, 19, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Akbari, M.; Vanga, R.; Kelly, C.P.; Hansen, J.; Theethira, T.; Tariq, S.; Dennis, M.; Leffler, D.A. Patient perception of treatment burden is high in celiac disease compared with other common conditions. Am. J. Gastroenterol. 2014, 109, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Van Megen, F.; Fossli, M.; Skodje, G.I.; Carlsen, M.H.; Andersen, L.F.; Veierød, M.B.; Lundin, K.E.A.; Henriksen, C. Nutritional assessment of women with celiac disease compared to the general population. Clin. Nutr. ESPEN 2023, 54, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Maniero, D.; Lorenzon, G.; Marsilio, I.; D’odorico, A.; Savarino, E.V.; Zingone, F. Assessment of Nutritional Status by Bioelectrical Impedance in Adult Patients with Celiac Disease: A Prospective Single-Center Study. Nutrients 2023, 15, 2686. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, A.; Olsen, I.E.; Stallings, V. Clinical assessment of Nutritional Status. In Nutrition in Pediatrics: Basic Science and Clinical Applications, 4th ed.; BC Decker: Hamilton, ON, USA, 2008; pp. 6–7. [Google Scholar]
- Soriano-Moreno, D.R.; Dolores-Maldonado, G.; Benites-Bullón, A.; Ccami-Bernal, F.; Fernandez-Guzman, D.; Esparza-Varas, A.L.; Caira-Chuquineyra, B.; Taype-Rondan, A. Recommendations for nutritional assessment across clinical practice guidelines: A scoping review. Clin. Nutr. ESPEN 2022, 49, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.L.; Regnault, T.R.H. Nutrition in Pregnancy: Optimising Maternal Diet and Fetal Adaptations to Altered Nutrient Supply. Nutrients 2016, 8, 342. [Google Scholar] [CrossRef]
- Alecsandru, D.; Lopez-Palacios, N.; Castano, M.; Aparicio, P.; Garcia-Velasco, J.A.; Nunez, C. Exploring undiagnosed celiac disease in women with recurrent reproductive failure: The gluten-free diet could improve reproductive outcomes. Am. J. Reprod. Immunol. 2020, 83, e13209. [Google Scholar] [CrossRef]
- Ayoub, A.; Firwana, M.; Amjahdi, A.; Rahaoui, A.; Benelbarhdadi, I.; Zahra, A.F. Evolution of Reproductive Disorders Related to Celiac Disease under Gluten-free Diet. Int. J. Celiac Dis. 2017, 5, 69–71. [Google Scholar] [CrossRef]
- Kvamme, J.-M.; Sørbye, S.; Florholmen, J.; Halstensen, T.S. Population-based screening for celiac disease reveals that the majority of patients are undiagnosed and improve on a gluten-free diet. Sci. Rep. 2022, 12, 12647. [Google Scholar] [CrossRef] [PubMed]
- Theethira, T.G.; Dennis, M.; Leffler, D.A. Nutritional consequences of celiac disease and the gluten-free diet. Expert. Rev. Gastroenterol. Hepatol. 2014, 8, 123–129. [Google Scholar] [CrossRef]
- Aaron, L.; Patricia, W.; Ajay, R.; Torsten, M. Celiac disease and lactose intolerance. Int. J. Celiac Dis. 2018, 6, 68–70. [Google Scholar]
- Jain, D.; Khuteta, R.; Chaturvedi, V.; Khuteta, S. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies: Observational study. J. Obstet. Gynecol. India 2012, 62, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Brar, P.S.; Lee, A.R.; Green, P.H. Body mass index in celiac disease: Beneficial effect of a gluten-free diet. J. Clin. Gastroenterol. 2010, 44, 267–271. [Google Scholar] [CrossRef]
- Mijatov, M.A.K.; Mičetić-Turk, D. Dietary intake in adult female coeliac disease patients in Slovenia. Slov. J. Public. Health 2016, 55, 96–103. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gessaroli, M.; Frazzoni, L.; Sikandar, U.; Bronzetti, G.; Pession, A.; Zagari, R.M.; Fuccio, L.; Forchielli, M.L. Nutrient intakes in adult and pediatric coeliac disease patients on gluten-free diet: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2023, 77, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Dervis, S.; Haman, F.; Adamo, K.B.; Redman, L.M. Energy Intake Requirements in Pregnancy. Nutrients 2019, 11, 1812. [Google Scholar] [CrossRef]
- Meyers, L.D.; Hellwig, J.P.; Otten, J.J. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Butte, N.F.; King, J.C. Energy requirements during pregnancy and lactation. Public. Health Nutr. 2005, 8, 1010–1027. [Google Scholar] [CrossRef]
- Hytten, F.; Chamberlain, G. Clinical Physiology in Obstetrics; Blackwell Scientific Publications: Oxford, UK, 1991; pp. 150–172. [Google Scholar]
- Capristo, E.; Addolorato, G.; Mingrone, G.; De Gaetano, A.; Greco, A.V.; A Tataranni, P.; Gasbarrini, G. Changes in body composition, substrate oxidation, and resting metabolic rate in adult celiac disease patients after a 1-y gluten-free diet treatment. Am. J. Clin. Nutr. 2000, 72, 76–81. [Google Scholar] [CrossRef]
- Faisal, M.; Russell, L.; Collins, A.W.; Armstrong, D.; I Pinto-Sanchez, M. A255 Increased energy expenditure and reduced exercise capacity in celiac disease patients on a gluten-free diet. J. Can. Assoc. Gastroenterol. 2022, 5 (Suppl. S1), 149–150. [Google Scholar] [CrossRef]
- Bogin, B.; Varela-Silva, M.I. The body mass index: The good, the bad, and the horrid. Bull Soc Suisse Anthr. 2012, 18, 5–11. [Google Scholar]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Kabbani, T.A.; Goldberg, A.; Kelly, C.P.; Pallav, K.; Tariq, S.; Peer, A.; Hansen, J.; Dennis, M.; Leffler, D.A. Body mass index and the risk of obesity in coeliac disease treated with the gluten-free diet. Aliment. Pharmacol. Ther. 2012, 35, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.M.; Yaktine, A.L. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press (US): Washington, DC, USA, 2009. [Google Scholar]
- A Brion, M.-J.; Ness, A.R.; Rogers, I.; Emmett, P.; Cribb, V.; Smith, G.D.; A Lawlor, D. Maternal macronutrient and energy intakes in pregnancy and offspring intake at 10 y: Exploring parental comparisons and prenatal effects. Am. J. Clin. Nutr. 2010, 91, 748–756. [Google Scholar] [CrossRef]
- Lowensohn, R.I.; Stadler, D.D.; Naze, C. Current concepts of maternal nutrition. Obstet. Gynecol. Surv. 2016, 71, 413–426. [Google Scholar] [CrossRef]
- Seal, C.J.; Courtin, C.M.; Venema, K.; de Vries, J. Health benefits of whole grain: Effects on dietary carbohydrate quality, the gut microbiome, and consequences of processing. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2742–2768. [Google Scholar] [CrossRef] [PubMed]
- Niro, S.; D’agostino, A.; Fratianni, A.; Cinquanta, L.; Panfili, G. Gluten-free alternative grains: Nutritional evaluation and bioactive compounds. Foods 2019, 8, 208. [Google Scholar] [CrossRef]
- Martin, J.; Geisel, T.; Maresch, C.; Krieger, K.; Stein, J. Inadequate nutrient intake in patients with celiac disease: Results from a German dietary survey. Digestion 2013, 87, 240–246. [Google Scholar] [CrossRef]
- Serin, Y.; Akbulut, G. Nutritional status and health-related quality of life in adult patients with celiac disease. CBU Int. Conf. Proc. 2018, 6, 952–959. [Google Scholar] [CrossRef]
- Hall, N.J.; Rubin, G.; Charnock, A. Systematic review: Adherence to a gluten-free diet in adult patients with coeliac disease. Aliment. Pharmacol. Ther. 2009, 30, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Clapp, J.F., III. Maternal carbohydrate intake and pregnancy outcome. Proc. Nutr. Soc. 2002, 61, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Sinco, H.; Ramos-Peñafiel, C.; Santoyo-Sánchez, A.; Collazo-Jaloma, J.; Martínez-Murillo, C.; Montaño-Figueroa, E.; Sinco-Ángeles, A. Megaloblastic anaemia: Folic acid and vitamin B12 metabolism. Rev. Médica Del Hosp. Gen. De México 2015, 78, 135–143. [Google Scholar] [CrossRef][Green Version]
- Schrott, R.; Murphy, S.K. Folic acid throughout pregnancy: Too much? Am. J. Clin. Nutr. 2018, 107, 497–498. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, G.; Villa, M.P.; Conti, L.; Ranucci, G.; Pacchiarotti, C.; Principessa, L.; Raucci, U.; Parisi, P. Nutritional deficiencies in children with celiac disease resulting from a gluten-free diet: A systematic review. Nutrients 2019, 11, 1588. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Dietary Reference Values for Nutrients Summary Report; Wiley Online Library: Minneapolis, MN, USA, 2017. [Google Scholar]
- Scholl, T.O.; Reilly, T. Anemia, Iron and pregnancy outcome. J. Nutr. 2000, 130, 443S–447S. [Google Scholar] [CrossRef]
- Dainty, J.R.; Berry, R.; Lynch, S.R.; Harvey, L.J.; Fairweather-Tait, S.J. Estimation of dietary iron bioavailability from food iron intake and iron status. PLoS ONE 2014, 9, e111824. [Google Scholar] [CrossRef]
- Montoro-Huguet, M.A.; Santolaria-Piedrafita, S.; Cañamares-Orbis, P.; García-Erce, J.A. Iron deficiency in celiac disease: Prevalence, health impact, and clinical management. Nutrients 2021, 13, 3437. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Institute of Medicine/Food and Nutrition Board; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Jouanne, M.; Oddoux, S.; Noël, A.; Voisin-Chiret, A.S. Nutrient requirements during pregnancy and lactation. Nutrients 2021, 13, 692. [Google Scholar] [CrossRef] [PubMed]
- Willemse, J.P.M.M.; Meertens, L.J.E.; Scheepers, H.C.J.; Achten, N.M.J.; Eussen, S.J.; van Dongen, M.C.; Smits, L.J.M. Calcium intake from diet and supplement use during early pregnancy: The Expect study I. Eur. J. Nutr. 2020, 59, 167–174. [Google Scholar] [CrossRef] [PubMed]
- World and Health Organization. Guideline: Calcium Supplementation in Pregnant Women; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- FAO/WHO. Human Vitamin and Mineral Requirements: Report of a Joint FAO/WHO Expert Consultation Bangkok, Thailand Food and Nutrition Division; FAO: Rome, Italy, 2001. [Google Scholar]
- Zanchetta, M.B.; Longobardi, V.; Bai, J.C. Bone and celiac disease. Curr. Osteoporos. Rep. 2016, 14, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Grace-Farfaglia, P. Bones of contention: Bone mineral density recovery in celiac disease—A systematic review. Nutrients 2015, 7, 3347–3369. [Google Scholar] [CrossRef]
- Urrutia, R.P.; Thorp, J.M. Vitamin D in pregnancy: Current concepts. Curr. Opin. Obstet. Gynecol. 2012, 24, 57. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Shapira, Y.; Agmon-Levin, N.; Pacht, A.; Shor, D.B.-A.; López, H.M.; Sanchez-Castanon, M.; Shoenfeld, Y. The clinical significance of 25OH-Vitamin D status in celiac disease. Clin. Rev. Allergy Immunol. 2012, 42, 322–330. [Google Scholar] [CrossRef]
- Perichart-Perera, O.; Rodríguez-Cano, A.M. Micronutrient supplementation during pregnancy: Narrative review of systematic reviews and meta-analyses. Ginecol. Y Obstet. De México 2023, 90, 968–994. [Google Scholar]
- Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- O’Leary, F.; Samman, S. Vitamin B12 in health and disease. Nutrients 2010, 2, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Molloy, A.M.; Kirke, P.N.; Troendle, J.F.; Burke, H.; Sutton, M.; Brody, L.C.; Scott, J.M.; Mills, J.L. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics 2009, 123, 917–923. [Google Scholar] [CrossRef]
- Caruso, R.; Pallone, F.; Stasi, E.; Romeo, S.; Monteleone, G. Appropriate nutrient supplementation in celiac disease. Ann. Med. 2013, 45, 522–531. [Google Scholar] [CrossRef]
- Wierdsma, N.J.; van Bokhorst-de van der Schueren, M.A.E.; Berkenpas, M.; Mulder, C.J.J.; van Bodegraven, A.A. Vitamin and mineral deficiencies are highly prevalent in newly diagnosed celiac disease patients. Nutrients 2013, 5, 3975–3992. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.; Wright, C. Safety and efficacy of supplements in pregnancy. Nutr. Rev. 2020, 78, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Garner, C. Nutrition in Pregnancy: Dietary Requirements and Supplements. Available online: https://www.uptodate.com/contents/nutrition-in-pregnancy-dietary-requirements-and-supplements?sectionName=Iron&search=clinical-presentation-anddiagnosis-of-von-willebrand-disease&topicRef=7150&anchor=H717781762&source=see_link (accessed on 19 April 2023).
- U.S. Food and Drug Administration (FDA). Questions and Answers on Dietary Supplements. Available online: https://www.fda.gov/food/information-consumers-using-dietary-supplements/questions-and-answers-dietary-supplements (accessed on 7 July 2024).
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Guidance for establishing andapplying tolerable upper intake levels for vitamins and essential minerals. EFSA J. 2022, 20, 1–27. [Google Scholar]
- Belkacemi, L.; Nelson, D.M.; Desai, M.; Ross, M.G. Maternal undernutrition influences placental-fetal development1. Biol. Reprod. 2010, 83, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.M.; Skillman, H.E.; Irving, S.Y.; Coss-Bu, J.A.; Vermilyea, S.; Farrington, E.A.; McKeever, L.; Hall, A.M.; Goday, P.S.; Braunschweig, C. Guidelines for the provision and assessment of nutrition support therapy in the pediatric critically ill patient: Society of critical care medicine and American society for parenteral and enteral nutrition. J. Parenter. Enter. Nutr. 2017, 41, 706–742. [Google Scholar] [CrossRef] [PubMed]
- Mihatsch, W.A.; Braegger, C.; Bronsky, J.; Cai, W.; Campoy, C.; Carnielli, V.; Darmaun, D.; Desci, T.; Domellof, M.; Embleton, N.; et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition. Clin. Nutr. 2018, 37, 2303–2305. [Google Scholar] [CrossRef] [PubMed]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Mis, N.F.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary feeding: A position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Hill, I.D.; Fasano, A.; Guandalini, S.; Hoffenberg, E.; Levy, J.; Reilly, N.; Verma, R. NASPGHAN clinical report on the diagnosis and treatment of gluten-related disorders. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, N.; Jackson, A.A.; Courtney-Martin, G.; Elango, R.; Ghosh, S.; Hodgkinson, S.; Xipsiti, M.; Lee, W.T.; Kurpad, A.V.; Tomé, D. Protein quality assessment of follow-up formula for young children and ready-to-use therapeutic foods: Recommendations by the FAO expert. working group. in 2017. J. Nutr. 2020, 150, 195–201. [Google Scholar] [CrossRef]
- WHO. The International Code of Marketing of Breast-Milk Substitutes: Frequently Asked Questions; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Hojsak, I.; Chourdakis, M.; Gerasimidis, K.; Hulst, J.; Huysentruyt, K.; Moreno-Villares, J.M.; Joosten, K. What are the new guidelines and position papers in pediatric nutrition: A 2015–2020 overview. Clin. Nutr. ESPEN 2021, 43, 49–63. [Google Scholar] [CrossRef]
- Romano, C.; van Wynckel, M.; Hulst, J.; Broekaert, I.; Bronsky, J.; Dall’Oglio, L.; Mis, N.F.; Hojsak, I.; Orel, R.; Papadopoulou, A.; et al. European society for paediatric gastroenterology, hepatology and nutrition guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological impairment. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 242–264. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, M.; Bronsky, J.; Gupte, G.; Hojsak, I.; Jahnel, J.; Pai, N.; Quiros-Tejeira, R.E.; Wieman, R.; Sundaram, S. Nutrition support of children with chronic liver diseases: A joint position paper of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, A.; Granito, A.; Giamperoli, A.; Catenaro, T.; Negrini, G.; Tovoli, F. Current guidelines for the management of celiac disease: A systematic review with comparative analysis. World J. Gastroenterol. 2022, 28, 154–176. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Kurppa, K.; Mearin, M.L.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; Christensen, R.; Dolinsek, J.; et al. European society paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Mearin, M.L.; Agardh, D.; Antunes, H.; Al-Toma, A.; Auricchio, R.; Castillejo, G.; Catassi, C.; Ciacci, C.; Discepolo, V.; Dolinsek, J.; et al. ESPGHAN position paper on management and follow-up of children and adolescents with celiac disease. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 369–386. [Google Scholar] [CrossRef]

| Author | Year | Study Design | Number of Participants (F) | GFD Effect |
|---|---|---|---|---|
| Alecsandru et al. [41] | 2019 | Retrospective | 690 | GFD improved reproductive outcomes in women that followed a GFD |
| Ayoub et al. [42] | 2017 | Descriptive retrospective | 173 | The reproductive disorders associated with CD respond very well under GFD |
| Tursi et al. [29] | 2008 | Case–control | 13 | GFD adherence helps in conceiving (1–4 years with GFD adherence) in CD women with recurrent miscarriage |
| Ciacci et al. [28] | 1996 | Case–control | 94 | GFD compliance shows incidence of abortion, low birth weight babies, and increased short breastfeeding periods in celiac women. |
| Healthy Women Pregnancy | Total Weight Gain ** | Recommanded Daily Energy Intake ** | Maternal Celiac Pregnancy |
|---|---|---|---|
| Pre-pregnancy BMI (kg/m2) | Range in kg | kcal/kg/day | |
| Underweight (<18.5) | 12.5–18 | 36–40 | N/A * |
| Normal weight (18.5–24.9) | 11.5–16 | 30 | N/A |
| Overweight (25.0–29.9) | 7–11.5 | 24 | N/A |
| Obese (≥30) | 5–9 | 12–18 | N/A |
| Safety Limit (Tolerable Upper Limit) per Day | Recommended Intake Level from Food or Supplements per Day | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Food Sources | Normal Women | Pregnant Women | Celiac Patients | Celiac Pregnant Women | Normal Women | Pregnant Women | Celiac Patients | Celiac Pregnant Women | |
| Folic acid | Legumes, leafy green vegetables, broccoli, asparagus, and avocado | EU and US: 1000 mcg | EU and US: 1000 mcg | N/A | N/A | EU: 330 mcg US: 400 mcg | EU and US: 400–600 mcg | a.800 mcg/day b.1 mg/day of folic acid for 3 months and once diarrhea improves 400–800 mcg/day | N/A |
| Iron | Red meat; plant source such as legumes, nuts, and dark green vegetables is poorly absorbed | EU: No adequate data to derive a tolerable upper limit. US: 45 mg | None set in the EU: although supplementation in the absence of deficiency is not recommended US: 45 mg based on gastrointestinal side effects only | N/A | N/A | EU: 16 mg US: 18 mg | EU: 16 mg US: 27 mg | Iron supplements (325 mg) 1–3 tablets based on initial ferritin level until iron stores are restored. Consider i.v. iron for severe symptomatic iron deficiency anemia or intolerance of oral iron | N/A |
| Vitamin B12 | Animal products such as meat, eggs, dairy, and fish | None set | None set; usual intakes 35 mcg, but 1000 mcg in malabsorption is commonly administered | N/A | N/A | EU: 4 mcg US: 2.4 mcg | EU: 4.5 mcg US: 2.6 mcg | a. 500 mcg/day b. 1000 mcg orally until the level is normal and then consider daily gluten-free multivitamin/mineral supplement | N/A |
| Vitamin D | Sun exposure | EU and US:4000 IU | EU: and US: 4000 IU | N/A | N/A | EU and USA: 600 IU | EU: and US: 600 IU although this is highly conservative and 1500 IU may be better to reach optimal levels. | Vitamin D: 1000 (or more-based serum level) U.I./day or 50.000 IU. weekly if level is <20 ng/mL. | N/A |
| Calcium | Dairy, nuts, tofu, and tinned fish with bones | EU: 2500 mg US: 2000 mg | EU: and US: 2500 mg | N/A | N/A | EU: 950–1000 mg US: 1000 mg | US: and EU: 1000 mg | Calcium recommended intake of calcium, including supplementation, for patients with CD is 1200–1500 mg/day. | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serin, Y.; Manini, C.; Amato, P.; Verma, A.K. The Impact of a Gluten-Free Diet on Pregnant Women with Celiac Disease: Do We Need a Guideline to Manage Their Health? Gastrointest. Disord. 2024, 6, 675-691. https://doi.org/10.3390/gidisord6030045
Serin Y, Manini C, Amato P, Verma AK. The Impact of a Gluten-Free Diet on Pregnant Women with Celiac Disease: Do We Need a Guideline to Manage Their Health? Gastrointestinal Disorders. 2024; 6(3):675-691. https://doi.org/10.3390/gidisord6030045
Chicago/Turabian StyleSerin, Yeliz, Camilla Manini, Pasqualino Amato, and Anil K. Verma. 2024. "The Impact of a Gluten-Free Diet on Pregnant Women with Celiac Disease: Do We Need a Guideline to Manage Their Health?" Gastrointestinal Disorders 6, no. 3: 675-691. https://doi.org/10.3390/gidisord6030045
APA StyleSerin, Y., Manini, C., Amato, P., & Verma, A. K. (2024). The Impact of a Gluten-Free Diet on Pregnant Women with Celiac Disease: Do We Need a Guideline to Manage Their Health? Gastrointestinal Disorders, 6(3), 675-691. https://doi.org/10.3390/gidisord6030045







