Effect of Two Mucoprotectants, Gelatin Tannate and Xyloglucan plus Gelatin, on Cholera Toxin-Induced Water Secretion in Rats
Abstract
1. Introduction
2. Results
2.1. Experimental Set 1
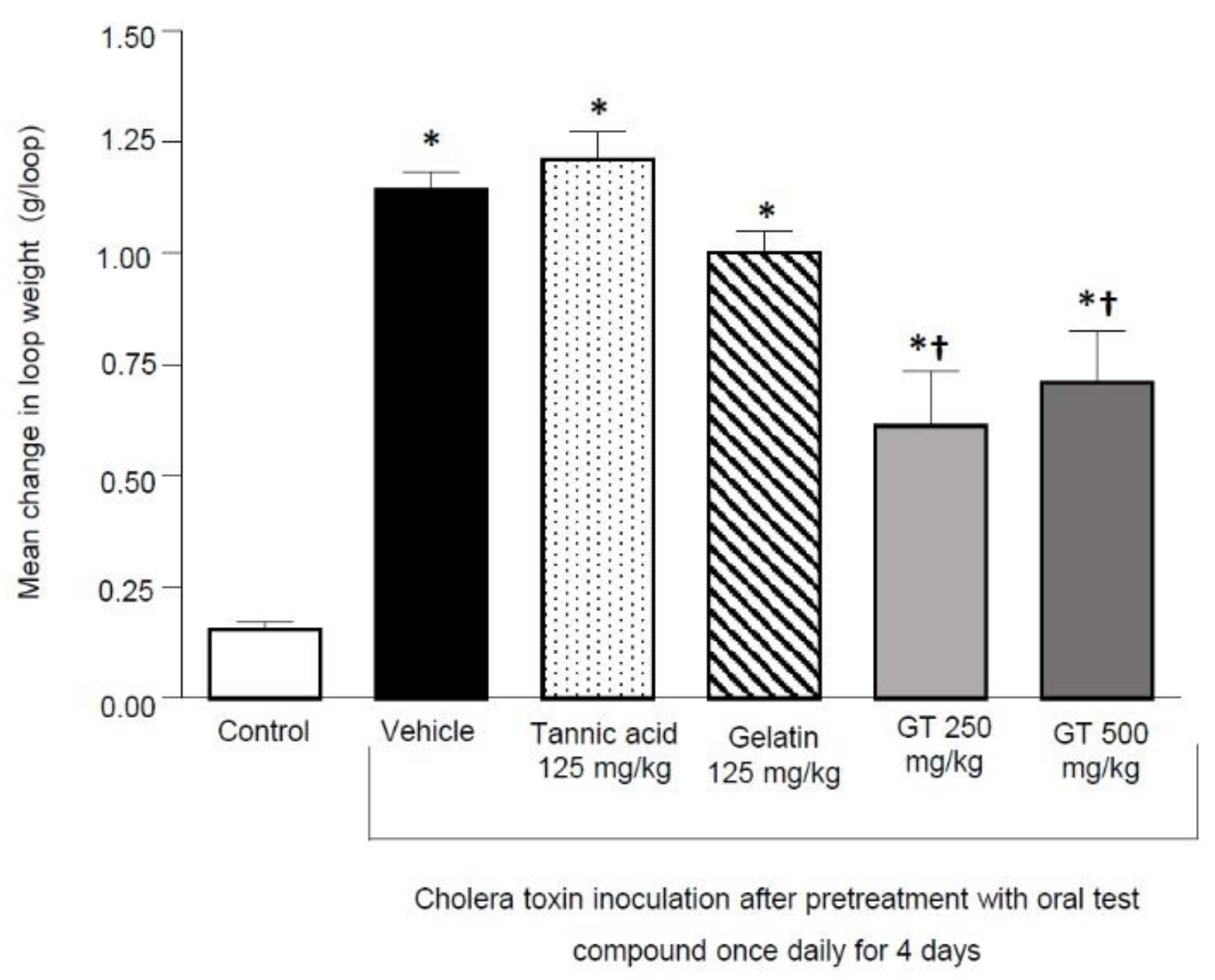
2.1.1. Protective Effect of Gelatin Tannate, Tannic Acid and Gelatin
2.1.2. Local Effect of Gelatin Tannate, Tannic Acid and Gelatin
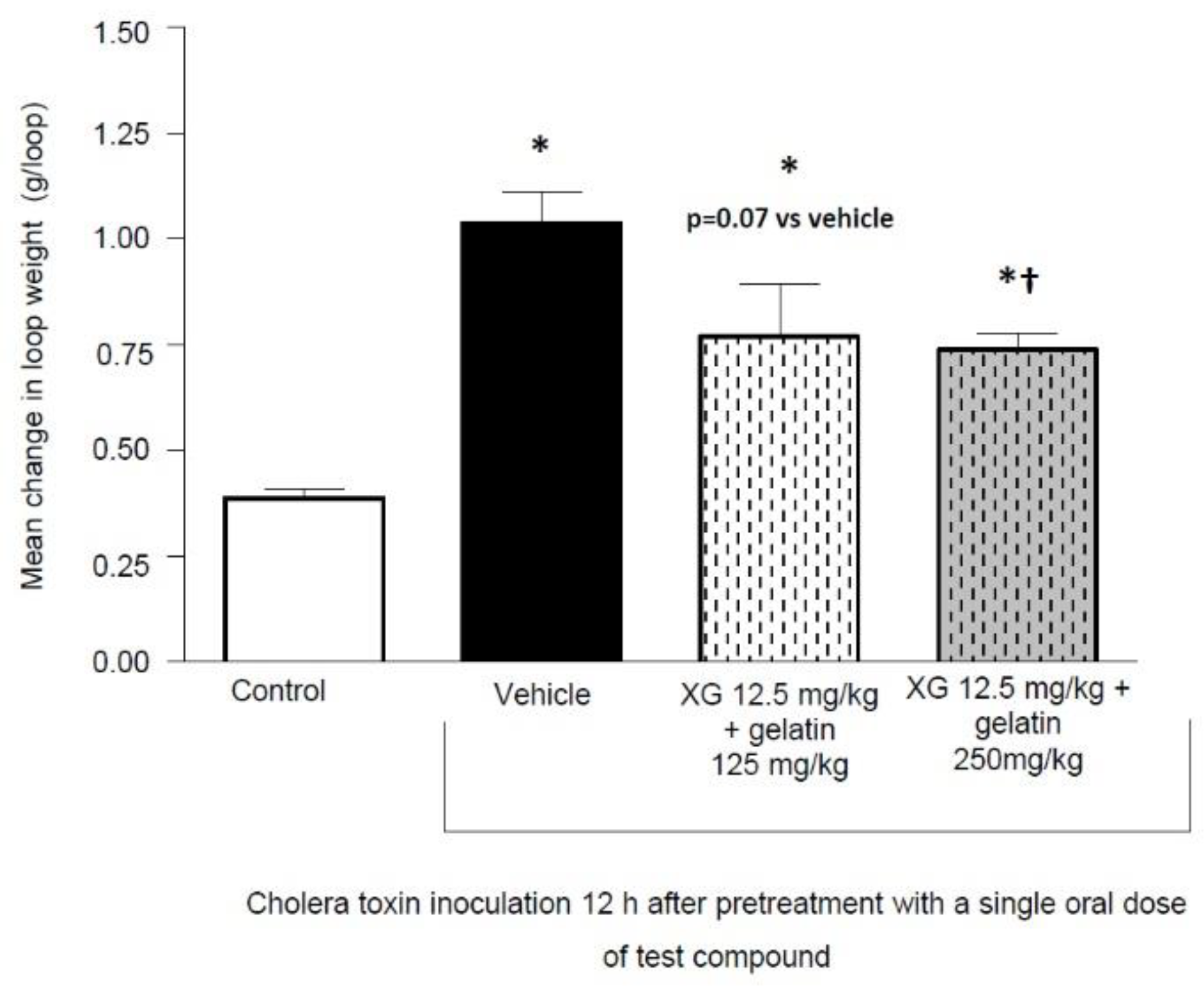
2.2. Experimental Set 2
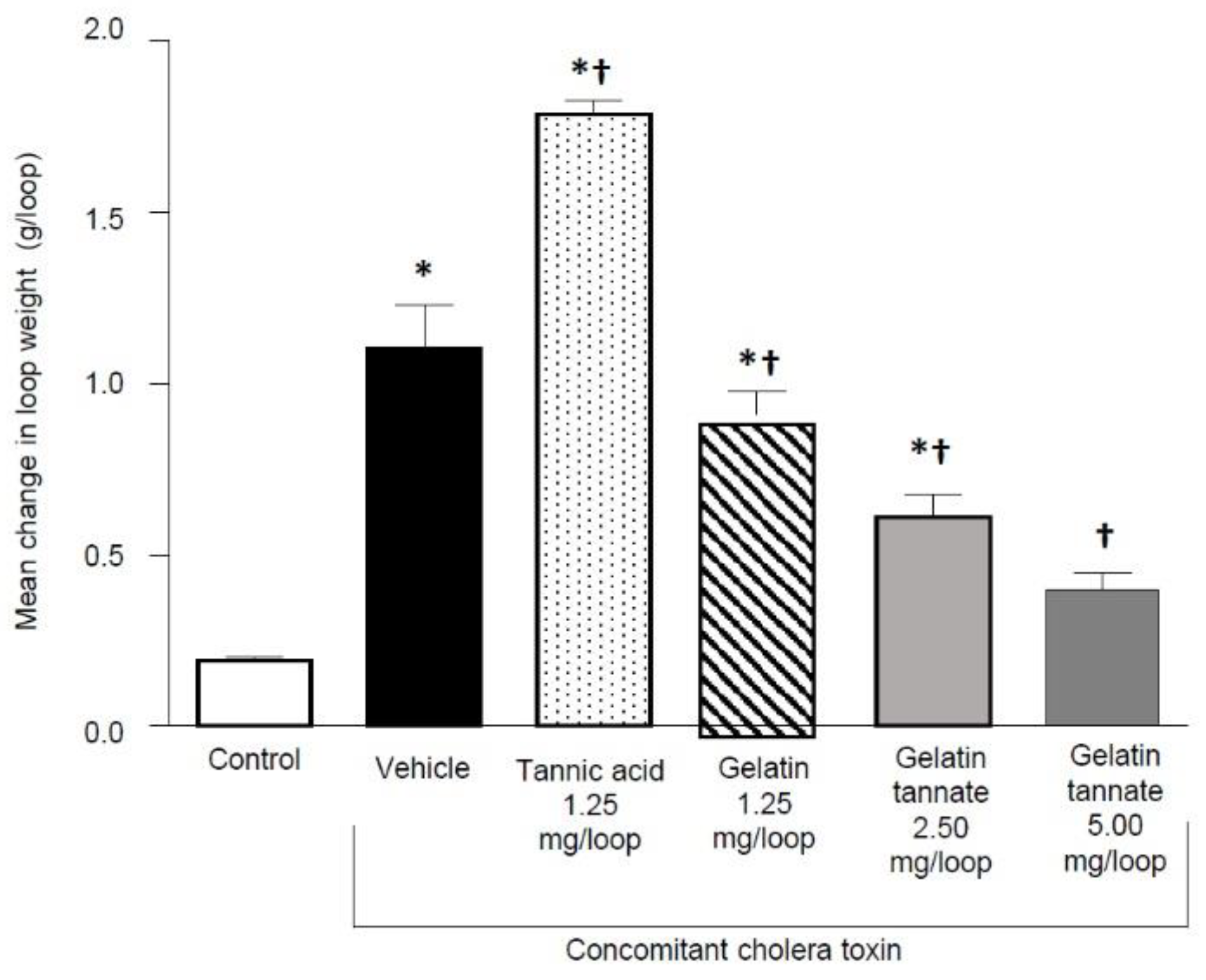
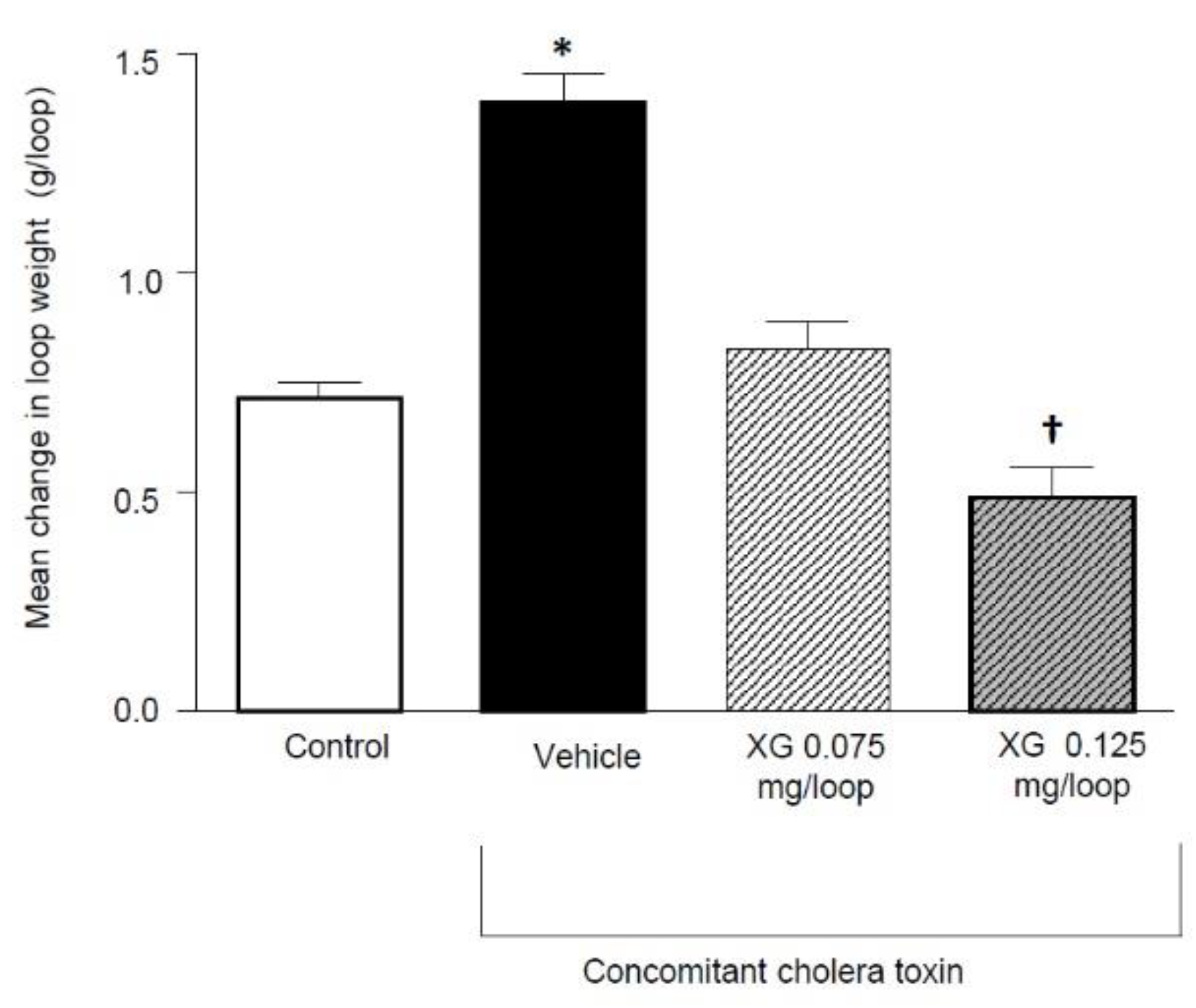
2.2.1. Local Effect of Xyloglucan
2.2.2. Duration of Protective Effect with Gelatin Tannate, Xyloglucan, and Gelatin (Alone or in Combination)
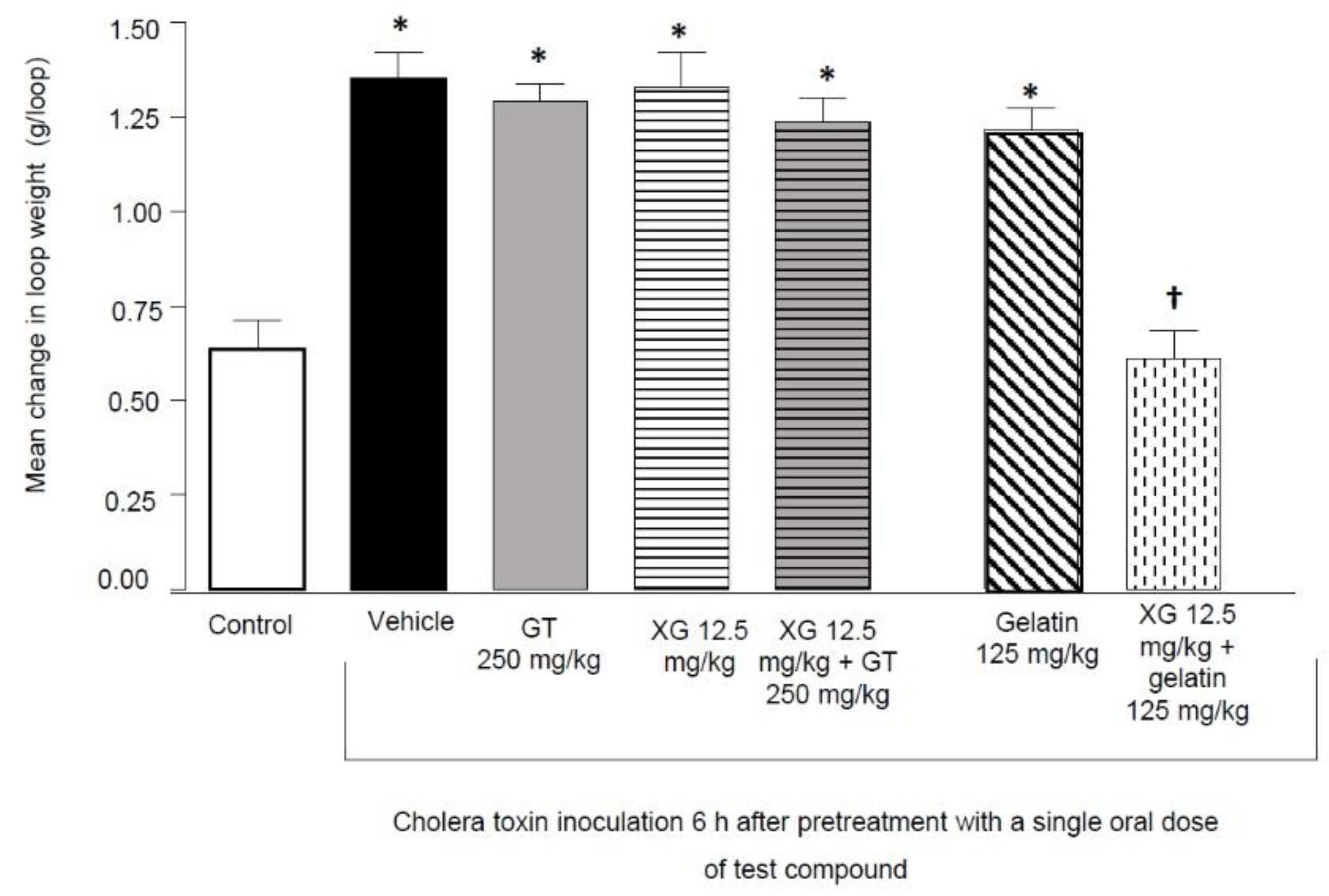
2.3. Experimental Set 3
Influence of Gelatin Dose on Protective Effect of Xyloglucan + Gelatin
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Experimental Set 1
4.3.1. Protective Effect of Gelatin Tannate, Tannic Acid and Gelatin
4.3.2. Local Effect of Gelatin Tannate, Tannic Acid and Gelatin
4.4. Experimental Set 2
4.4.1. Local Effect of Xyloglucan
4.4.2. Duration of Protective Effect with Gelatin Tannate, Xyloglucan, and Gelatin (Alone or in Combination)
4.5. Experimental Set 3
Influence of Gelatin Dose on the Protective Effect of Xyloglucan + Gelatin
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Franceschi, F.; Scaldaferri, F.; Riccioni, M.E.; Casagranda, I.; Forte, E.; Gerardi, V.; Cordischi, C.; Antonini, S.; Tortora, A.; Di Rienzo, T.; et al. Management of acute dyarrhea: Current and future trends. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2065–2069. [Google Scholar] [PubMed]
- Drancourt, M. Chapter 38: Acute diarrhea. In Infectious Diseases, 4th ed.; Cohen, J., Opal, S.M., Powderly, W.G., Eds.; Health Sciences; Elsevier: Amsterdam, The Netherlands, 2016; pp. 335–340.e2. [Google Scholar]
- Sokic-Milutinovic, A.; Pavlovic-Markovic, A.; Tomasevic, R.S.; Lukic, S. Diarrhea as a clinical challenge: General practitioner approach. Dig. Dis. 2022, 40, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Eutamene, H.; Beaufrand, C.; Harkat, C.; Theodorou, V. The role of mucoprotectants in the management of gastrointestinal disorders. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Cotoner, C.; Abril-Gil, M.; Albert-Bayo, M.; Mall, J.G.; Expósito, E.; González-Castro, A.M.; Lobo, B.; Santos, J. The role of purported mucoprotectants in dealing with irritable bowel syndrome, functional diarrhea, and other chronic diarrheal disorders in adults. Adv. Ther. 2021, 38, 2054–2076. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Chávez, F.; Meader, B.T.; Akosman, S.; Koprivica, V.; Mekalanos, J.J. A potent inhibitor of the cystic fibrosis transmembrane conductance regulator blocks disease and morbidity due to toxigenic vibrio cholerae. Toxins 2022, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.; Graziani, C.; Guarino, A.; Lamborghini, A.; Masi, S.; Stanghellini, V. Gelatin tannate and tyndallized probiotics: A novel approach for treatment of diarrhea. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 873–883. [Google Scholar] [PubMed]
- Freli, V.; Da Silva, R.M.; Pescio, P. New insights into the mechanism of action of gelatine tannate for acute diarrhoea. Part 1: Film-forming effect. Arch. Pediatr. 2013, 20, 549. [Google Scholar]
- Scaldaferri, F.; Lopetuso, L.R.; Petito, V.; Cufino, V.; Bilotta, M.; Arena, V.; Stigliano, E.; Maulucci, G.; Papi, M.; Emi-liana, C.M.; et al. Gela-tin tannate ameliorates acute colitis in mice by reinforcing mucus layer and modulating gut microbiota composition: Emerging role for ‘gut barrier protectors’ in IBD? United Eur. Gastroenterol. J. 2014, 2, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, H.; Zhu, Y.; Liu, S.; Yuan, Z.; Wu, J.; Wen, L. Tannic acid induces the mitochondrial pathway of apoptosis and s phase arrest in porcine intestinal IPEC-J2 cells. Toxins 2019, 11, 397. [Google Scholar] [CrossRef] [PubMed]
- Piqué, N.; Gómez-Guillén, M.D.C.; Montero, M.P. Xyloglucan, a plant polymer with barrier protective properties over the mucous membranes: An overview. Int. J. Mol. Sci. 2018, 19, 673. [Google Scholar] [CrossRef] [PubMed]
- De Servi, B.; Ranzini, F.; Piqué, N. Effect of Utipro® (containing gelatin-xyloglucan) against Escherichia coli invasion of intestinal epithelial cells: Results of an in vitro study. Future Microbiol. 2016, 11, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, H.S.; Tyagi, V.K.; Patil, R.R.; Dusunge, S.B. Thiolated xyloglucan: Synthesis, characterization and evaluation as mucoadhesive in situ gelling agent. Carbohydr. Polym. 2013, 91, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Rbii, K.; Violleau, F.; Guedj, S.; Surel, O. Analysis of aged gelatin by AFlFFF-MALS: Identification of high molar mass components and their influence on solubility. Food Hydrocoll. 2009, 23, 1024–1030. [Google Scholar] [CrossRef]
- Çağan, E.; Ceylan, S.; Mengi, Ş.; Çağan, H.H. Evaluation of gelatin tannate against symptoms of acute diarrhea in pediatric patients. Med. Sci. Monit. 2017, 23, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Aloi, M.; Mennini, M. Efficacy of gelatin tannate for acute diarrhea in children: A systematic review and meta-analysis. J. Comp. Eff. Res. 2019, 8, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Gnessi, L.; Bacarea, V.; Marusteri, M.; Piqué, N. Xyloglucan for the treatment of acute diarrhea: Results of a randomized, controlled, open-label, parallel group, multicentre, national clinical trial. BMC Gastroenterol. 2015, 15, 153. [Google Scholar] [CrossRef] [PubMed]
- Condratovici, C.P.; Bacarea, V.; Piqué, N. Xyloglucan for the treatment of acute gastroenteritis in children: Results of a randomized, controlled, clinical trial. Gastroenterol. Res. Pract. 2016, 2016, 6874207. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eutamene, H.; Beaufrand, C.; Harkat, C.; Theodorou, V. Effect of Two Mucoprotectants, Gelatin Tannate and Xyloglucan plus Gelatin, on Cholera Toxin-Induced Water Secretion in Rats. Gastrointest. Disord. 2022, 4, 324-332. https://doi.org/10.3390/gidisord4040030
Eutamene H, Beaufrand C, Harkat C, Theodorou V. Effect of Two Mucoprotectants, Gelatin Tannate and Xyloglucan plus Gelatin, on Cholera Toxin-Induced Water Secretion in Rats. Gastrointestinal Disorders. 2022; 4(4):324-332. https://doi.org/10.3390/gidisord4040030
Chicago/Turabian StyleEutamene, Hélène, Catherine Beaufrand, Cherryl Harkat, and Vassilia Theodorou. 2022. "Effect of Two Mucoprotectants, Gelatin Tannate and Xyloglucan plus Gelatin, on Cholera Toxin-Induced Water Secretion in Rats" Gastrointestinal Disorders 4, no. 4: 324-332. https://doi.org/10.3390/gidisord4040030
APA StyleEutamene, H., Beaufrand, C., Harkat, C., & Theodorou, V. (2022). Effect of Two Mucoprotectants, Gelatin Tannate and Xyloglucan plus Gelatin, on Cholera Toxin-Induced Water Secretion in Rats. Gastrointestinal Disorders, 4(4), 324-332. https://doi.org/10.3390/gidisord4040030




