Microbiome–Gut Dissociation in the Neonate: Autism-Related Developmental Brain Disease and the Origin of the Placebo Effect
Abstract
:1. Introduction: Non-Communicable Disease, the Microbiome, and Symbiosis
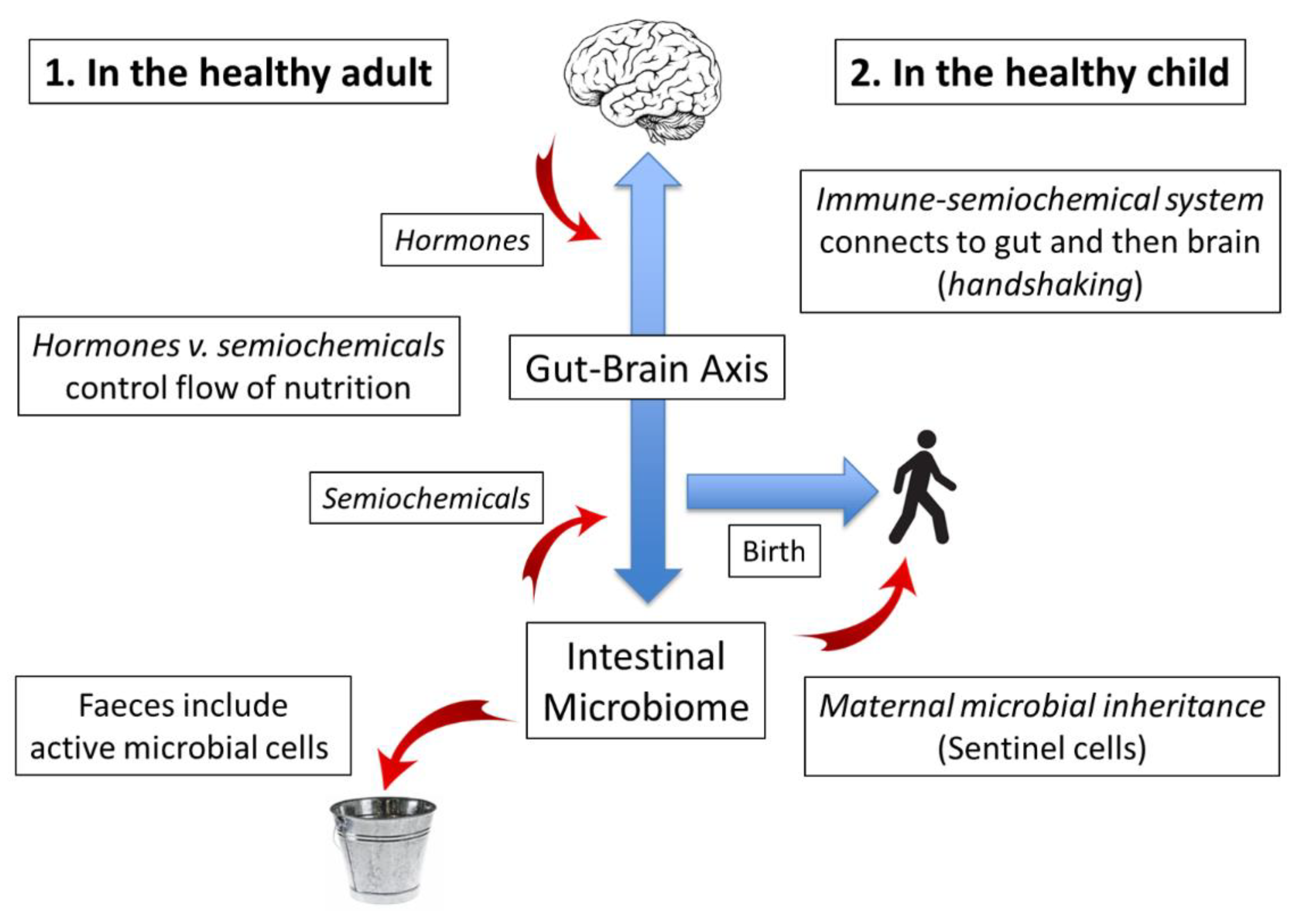
2. Maternal Microbial Inheritance and Disease
2.1. Non-Communicable Disease
2.2. Evolution and Ecology: Maternal Microbial Inheritance
2.3. Genes versus Microbial Environment: The Trouble with Twin Studies
2.4. Research Challenges Posed by Dual Genetic/Microbiome Inheritance
- Ethical dilemmas: As Harald Brüssow reminds us [11], while it is incumbent upon scientists to design definitive experiments under controlled conditions, medicine operates under formidable ethical constraints. Nevertheless, the principles of Bouchard’s experiment [45] could be employed to design an observational study in which the health status of each of a pair of monozygotic twins was followed while noting potential microbiome-degrading factors: the degree of sterility of delivery by caesarean section, for example, or antibiotic use in mother or subject. However, as it is hard to envisage a conclusive outcome from such a study, the most likely result is for a decision on the nature of the microbiome to lie with the perceived overall balance of probability.
- Semiochemicals: Professor Nobuyuki Sudo and his team have been investigating the role of the “commensal” microbiota in the production of amine signalling molecules such as serotonin [12,15]. We have previously suggested that an ingestible sensor be developed to check the flow of such semiochemicals in response to various stimuli [45]. Although suitable for human studies, initial experiments could be carried out in animals, preferably ethically sourced from unpolluted, wild-type environments, rather than laboratory-bred.
- Sentinel cells: The most succinct way to transfer immune information between generations is by the physical transfer of potential antigens along with a method of labelling to distinguish pathogens from harmless environment components. Although no such entity has been described as yet, we have previously suggested that one may have been a eukaryotic evolutionary precursor of antigen-presenting cells [25]. Although similar cells may be present throughout unpolluted populations, they may well be absent from humans or our pets and farmed animals suffering from non-communicable diseases [39].
3. The Functioning Microbiome: Microbiome–Gut Association (A Virtuous Circle)
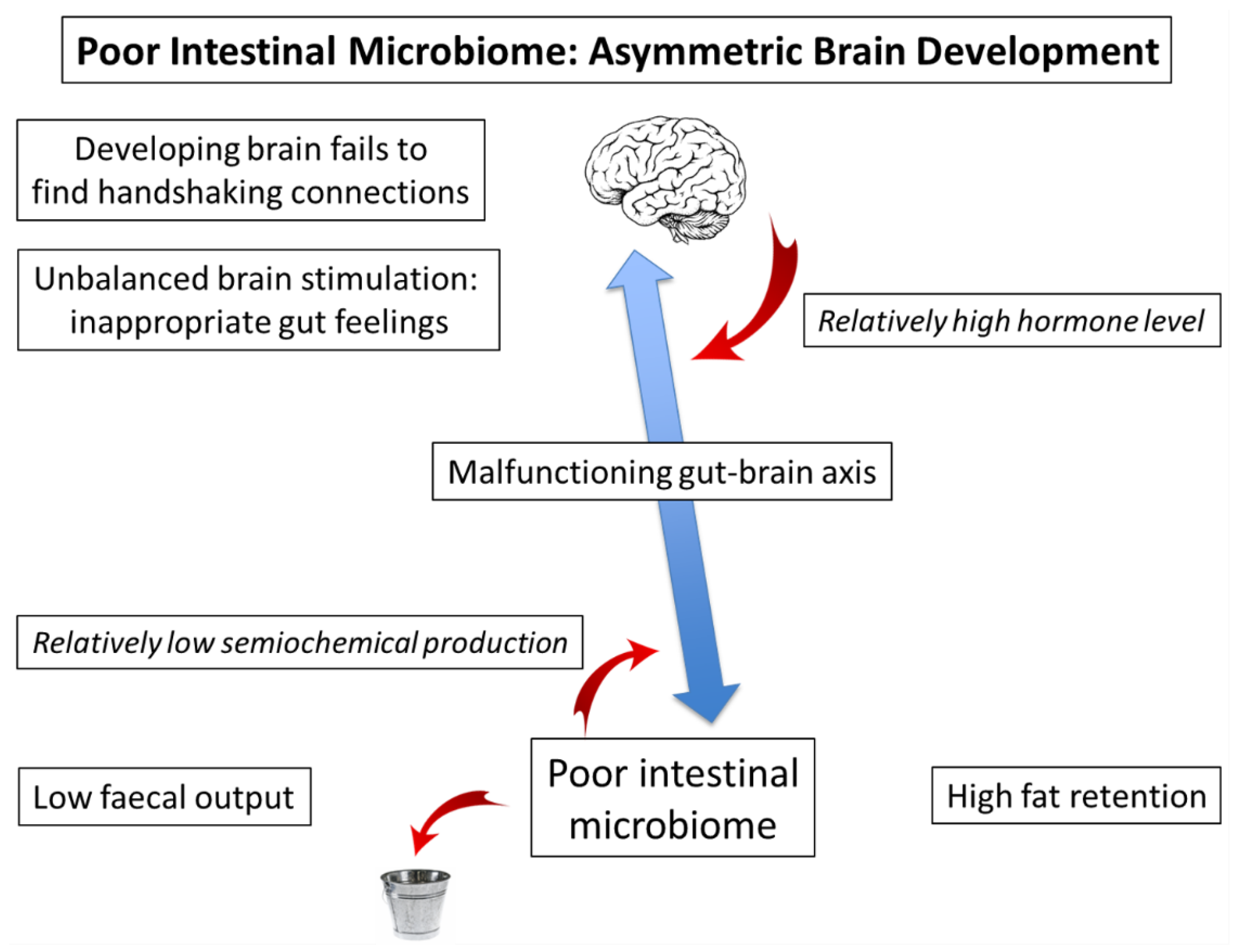
4. Poor Microbial Inheritance: Microbiome–Gut Dissociation (A Vicious Circle)
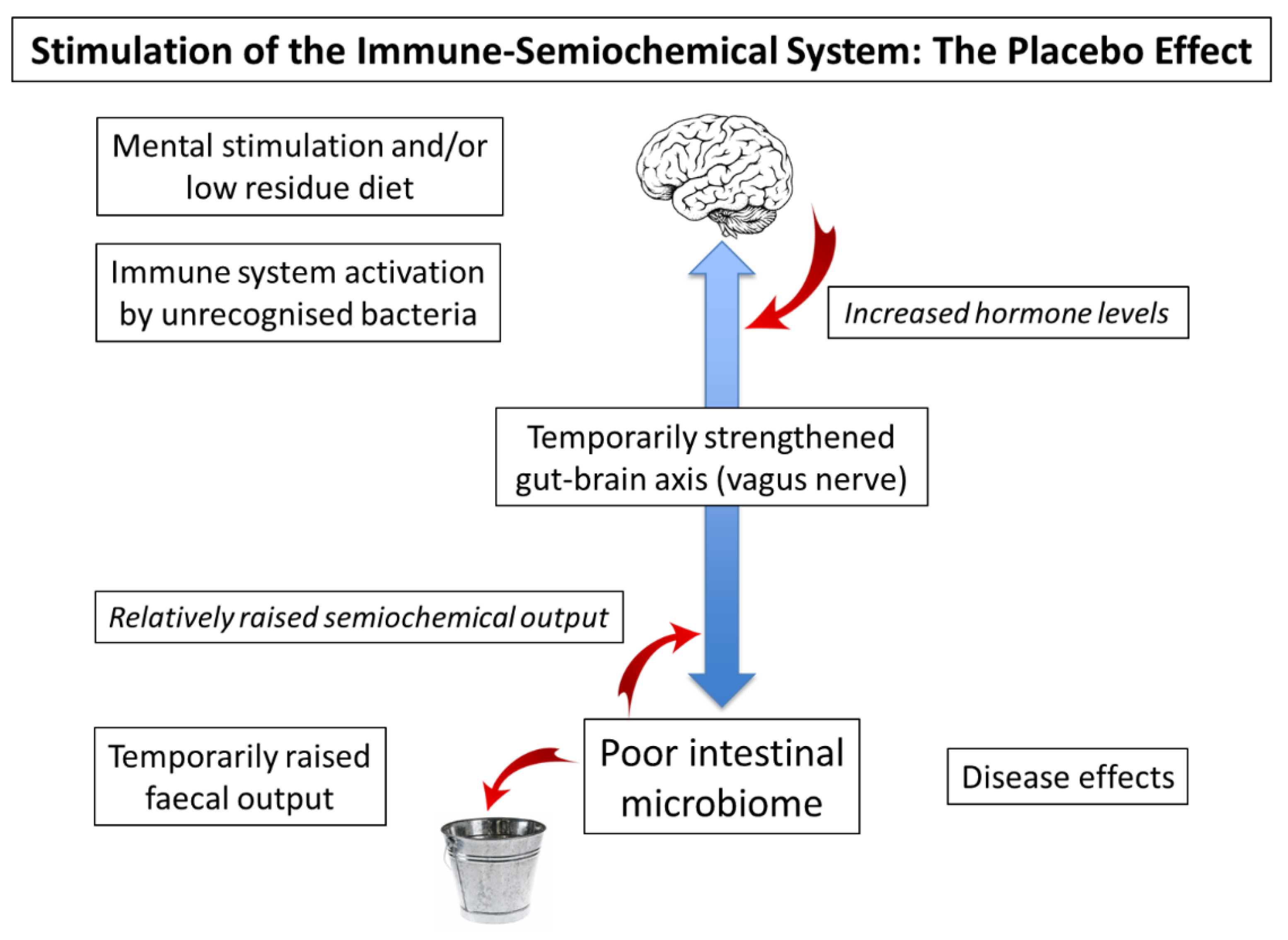
5. Temporary Stimulation of the Gut–Brain Axis: Placebo Effect and Probiotics
6. The Many Variations of Developmental Brain Disease: Do They Have the Same Underlying Cause?
6.1. Autism Spectrum Disorder and Attention Deficit Hyperactivity Disorder
6.2. Dyslexia, Anxiety, Depression, and Other Conditions
6.3. Unexpected Strengths and the Problems of Definition in Psychology
7. Microbiome Measurement: Semiochemicals and Sentinel Cells
7.1. Semiochemical Measurement: An Ingestible Sensor
7.2. Microeukaryotes and Microbial Sentinel Cells
8. Microbiome Degradation and the Development of Society: Where Are We Now?
9. Conclusions and Recommendations
- Search in faecal samples of wild animal populations, preferably primates, looking specifically for any microbial or semiochemical changes in late-stage pregnancy or, indeed, when giving birth. The aim is to detect those microbes, including eukaryotes, which are being transferred to the neonate. Bearing in mind any cultural sensitivities, the same could be asked of humans, comparing populations with and without evidence of non-communicable disease.
- Commence a research programme to discover key semiochemicals in the gut lumen, and their effect on receptors both within and outside the gut wall. In principle, an ingestible sensor could be developed as an aid to research, tracking the rise and fall of these semiochemicals under different circumstances.
- Once key microbes are detected, they would be added to the head of a new-born baby in order to re-introduce functionalities that have been lost through industrialisation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burkitt, D. A sarcoma involving the jaws in African children. Br. J. Surg. 1958, 46, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, D.P. Some diseases characteristic of modern western civilization. Br. Med. J. 1973, 1, 274–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBlanc, J.G.; Chain, F.; Martin, R.; Bermùndez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. Br. Med. J. 2018, 361, k2179. [Google Scholar] [CrossRef] [Green Version]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.A.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced Extinctions in the Gut Microbiota Compound over Generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Reese, E.D.; Chadaideh, K.S.; Diggins, C.E.; Schell, L.D.; Beckel, M.; Callahan, P.; Ryan, R.; Thompson, M.E.; Carmody, R.N. Effects of domestication on the gut microbiota parallel those of human industrialization. eLife 2021, 10, e60197. [Google Scholar] [CrossRef]
- Marsella, R.; De Benedetto, A. Atopic dermatitis in animals and in people: An update and comparative review. Vet. Sci. 2017, 4, 37. [Google Scholar] [CrossRef]
- Burberry, A.; Wells, M.F.; Limone, F.; Couto, A.; Smith, K.S.; Keaney, J.; Gillet, G.; van Gastel, N.; Wang, J.-Y.; Pietilainen, O.; et al. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 2020, 582, 89–94. [Google Scholar] [CrossRef]
- Ganci, M.; Suleyman, E.; Butt, H.; Ball, M. The role of the brain-gut-microbiota in psychology: The importance of considering gut microbiota in the development, perpetuation, and treatment of psychological disorders. Brain Behav. 2019, 9, e01408. [Google Scholar] [CrossRef]
- Brüssow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2019, 13, 423–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudo, N. Biogenic amines: Signals between commensal microbiota and gut physiology. Front. Endocrinol. 2019, 10, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jheeta, S.; Smith, D. Seeing the wood for the trees: A new way to view the human intestinal microbiome and its connection with non-communicable disease. Med. Hypotheses 2019, 125, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Kaelberer, M.M.; Rupprecht, L.E.; Liu, W.W.; Weng, P.; Bohórquez, D.V. Neuropod cells: Emerging biology of the gut-brain sensory transduction. Annu. Rev. Neurosci. 2020, 43, 337–353. [Google Scholar] [CrossRef] [Green Version]
- Hata, T.; Asano, Y.; Yoshihara, K.; Kimura-Todani, T.; Miyata, N.; Zhang, X.-T.; Takakura, S.; Aiba, Y.; Koga, Y.; Sudo, N. Regulation of gut luminal serotonin by commensal microbiota in mice. PLoS ONE 2017, 12, e0180745. [Google Scholar] [CrossRef] [Green Version]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2005, 29, 1395–1403. [Google Scholar] [CrossRef] [Green Version]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Laforest-Lapointe, I.; Arrieta, M.-C. Microbial eukaryotes: A missing link in gut microbiome studies. mSystems 2018, 3, e00201–e00217. [Google Scholar] [CrossRef] [Green Version]
- Scanlan, P.D.; Stensvold, C.R.; Rajilic-Stojanovic, M.; Heilig, H.G.H.J.; De Vos, W.M.; O’Toole, P.W.; Cotter, P.D. The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol. Ecol. 2014, 90, 326–330. [Google Scholar] [CrossRef]
- Ward, T.L.; Dominguez-Bello, M.G.; Heisel, T.; Al-Ghalith, G.; Knights, D.; Gale, C.A. Development of the human mycobiome over the first month of life and across body sites. mSystems 2018, 3, e00140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagier, J.-C.; Million, M.; Hugon, P.; Armougom, F.; Raoult, D. Human gut microbiota: Repertoire and variations. Front. Cell. Infect. Microbiol. 2012, 2, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, J.J.; Auro, R.; Furlan, M.; Whiteson, K.L.; Erb, M.L.; Pogliano, J.; Stotland, A.; Wolkowicz, R.; Cutting, A.S.; Doran, K.S.; et al. Bacteriophage adhering to mucus provide a non–host-derived immunity. Proc. Natl. Acad. Sci. USA 2013, 110, 10771–10776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Smith, D.; Palacios-Pérez, M.; Jheeta, S. The enclosed intestinal microbiome: Semiochemical signals from the Precambrian and their disruption by heavy metal pollution. Life 2022, 12, 287. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Margulis, L. Symbiogenesis and Symbionticism. In Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis; Margulis, L., Fester, R., Eds.; MIT Press: Cambridge, MA, USA, 1991; pp. 49–92. [Google Scholar]
- Rosenburg, E.; Zilber-Rosenburg, I. The hologenome concept of evolution after 10 years. Microbiome 2018, 6, 78. [Google Scholar] [CrossRef]
- Moran, N.; Sloan, D.B. The hologenome concept: Helpful or hollow? PLoS Biol. 2015, 13, e1002311. [Google Scholar] [CrossRef] [Green Version]
- Woese, C. On the evolution of cells. Proc. Natl. Acad. Sci. USA 2002, 99, 8742–8747. [Google Scholar]
- Iyer, L.M.; Aravind, L.; Coon, S.L.; Klein, D.C.; Koonin, E.V. Evolution of cell-cell signaling in animals: Did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004, 20, 292–299. [Google Scholar] [CrossRef]
- ter Horst, K.W.; Lammers, N.M.; Trinko, R.; Opland, D.M.; Figee, M.; Ackermans, M.T.; Booij, J.; van den Munckhof, P.; Schuurman, P.R.; Fliers, E.; et al. Striatal dopamine regulates systemic glucose metabolism in humans and mice. Sci. Transl. Med. 2018, 10, eaar3752. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, H.; Pan, J.; Du, Z.; Zhou, W.; Zhang, Z.; Tian, Z.; Zhou, R.; Bai, L. Peripheral dopamine controlled by gut microbes inhibits invariant natural killer T cell-mediated hepatitis. Front. Immunol. 2018, 9, 2398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipnis, J.; Filiano, A.J. The central nervous system: Privileged by immune connections. Nat. Rev. Immunol. 2018, 18, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Palacios-Pérez, M.; Jheeta, S. Microbiome-gut dissociation in the neonate: Obesity and coeliac disease as examples of microbiome-function deficiency disorder. Gastrointest. Disord. 2022, 4, 108–128. [Google Scholar] [CrossRef]
- Caspi, V.; Houts, R.M.; Belsky, D.W.; Goldman-Mellor, S.J.; Harrington, H.; Israel, S.; Meier, M.H.; Ramraker, S.; Shalev, I.; Poulton, R.; et al. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clin. Psychol. Sci. 2014, 2, 119–137. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal and infant origins of adult disease. Br. Med. J. 1990, 301, 1111. [Google Scholar] [CrossRef] [Green Version]
- Stinson, L.F.; Boyce, M.C.; Payne, M.S.; Keelan, J.A. The not-so-sterile womb: Evidence that the human fetus is exposed to bacteria prior to birth. Front. Microbiol. 2019, 10, 1124. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.; Jheeta, S. The epidemiology of the dysfunctional microbiome in animals and in humans: The propensity for the development of non-communicable disease. EC Gastroenterol. Dig. Syst. 2020, 7, 83–93. [Google Scholar]
- Suzuki, T.A.; Fitzstevens, J.L.; Schmidt, V.T.; Enav, H.; Huus, K.; Mbong, M.; Adegbite, B.R.; Zinsou, J.F.; Esen, M.; Velavan, T.; et al. Codiversification of gut microbiota with humans. Science 2022, 377, 1328–1332. [Google Scholar] [CrossRef]
- Robertson, R.C.; Manges, A.R.; Finlay, B.B.; Prendergast, A.J. The human microbiome and child growth—First 1000 days and beyond. Trends Microbiol. 2019, 27, 131–147. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, C.; Tremblay, A.; Després, J.-P.; Nadeau, A.; Lupien, P.J.; Thériault, G.; Dussault, J.; Moorjani, S.; Pinault, S.; Fournier, G. The response to long-term overfeeding in identical twins. N. Engl. J. Med. 1990, 322, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Forster, S.C.; Tsaliki, E.; Vervier, K.; Strang, A.; Simpson, N.; Kumar, N.; Stares, M.D.; Rodger, A.; Brocklehurst, P.; et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 2019, 574, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Gribble, F.M.; Reimann, F. Enteroendocrine cells: Chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 2016, 78, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Jheeta, S. Measuring microbiome effectiveness: A role for ingestible sensors. Gastrointest. Disord. 2020, 2, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Gutierrez, E.; Narbad, A.; Rodriguez, J.M. Autism spectrum disorder associated with gut microbiota at immune, metabolomic, and neuroactive level. Front. Neurosci. 2020, 14, 578666. [Google Scholar] [CrossRef]
- Hooks, K.B.; O’Malley, M.A. Dysbiosis and its discontents. mBio 2017, 8, e01492-17. [Google Scholar] [CrossRef] [Green Version]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Primers 2019, 5, 3. [Google Scholar] [CrossRef]
- Zingone, F.; Swift, G.L.; Card, T.R.; Sanders, D.S.; Ludvigsson, J.F.; Bai, J.C. Psychological morbidity of celiac disease: A review of the literature. United Eur. Gastroenterol. J. 2015, 3, 136–145. [Google Scholar] [CrossRef]
- Vallgårda, S. Why the concept “lifestyle diseases” should be avoided. Scand. J. Public Health 2011, 39, 773–775. [Google Scholar] [CrossRef]
- Casazza, K.; Brown, A.; Astrup, A.; Bertz, F.; Baum, C.; Brown, B.B.; Dawson, J.; Durant, N.; Dutton, G.; Fields, D.A.; et al. Weighing the evidence of common beliefs in obesity research. Crit. Rev. Food Sci. Nutr. 2015, 55, 2014–2053. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.; Jheeta, S. Microbiome-gut dissociation: Investigating the origins of obesity. Gastrointest. Disord. 2021, 3, 156–172. [Google Scholar] [CrossRef]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elahi, S. New insight into an old concept: Role of immature erythroid cells in immune pathogenesis of neonatal infection. Front. Immunol. 2014, 5, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Wade, P.A. Crosstalk between the microbiome and the epigenome: Messages from bugs. J. Biochem. 2018, 163, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Dick, D.M. Gene-environment interaction in psychological traits and disorders. Annu. Rev. Clin. Psychol. 2011, 7, 383–409. [Google Scholar] [CrossRef] [Green Version]
- Keen, E.C.; Bliskovsky, V.V.; Malagon, F.; Baker, J.D.; Prince, J.S.; Klaus, J.S.; Adhya, S.L.; Groisman, E.A. Novel “superspreader” bacteriophages promote horizontal gene transfer by transformation. mBio 2017, 8, e02115–e02116. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, S.W.; Suttle, C.A. Viruses and nutrient cycles in the sea. BioScience 1999, 49, 781–788. [Google Scholar] [CrossRef] [Green Version]
- Kuzyakov, Y.; Mason-Jones, K. Viruses in soil: Nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biol. Biochem. 2018, 127, 305–317. [Google Scholar] [CrossRef]
- Hehemann, J.H.; Kelly, A.G.; Pudlo, N.A.; Martens, E.C.; Boraston, A.B. Bacteria of the human gut microbiome catabolize red seaweed glycans with carbohydrate-active enzyme updates from extrinsic microbes. Proc. Natl. Acad. Sci. USA 2012, 109, 19786–19791. [Google Scholar] [CrossRef] [Green Version]
- Mesa, D.M.; Loureiro, B.; Iglesia, I.; Gonzalez, S.F.; Olivé, E.L.; Algar, O.G.; Solana, M.J.; Cabero, M.J.; Sainz, T.; Martinez, L.; et al. The evolving microbiome from pregnancy to early infancy: A comprehensive review. Nutrients 2020, 12, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, L.M.; Galley, J.D.; Hade, E.M.; Schoppe-Sullivan, S.; Kamp Dush, C.; Bailey, M.T. Gut microbiome composition is associated with temperament during early childhood. Brain Behav. Immun. 2015, 45, 118–127. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Stilling, R.M.; Dinan, T.G.; Cryan, J.F. The Microbiome and Childhood Diseases: Focus on Brain-Gut Axis. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 296–313. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.G. The fetal origins hypothesis–10 years on. Br. Med. J. 2005, 330, 1096–1097. [Google Scholar] [CrossRef] [PubMed]
- Almond, D.; Currie, J. Killing me softly: The fetal origins hypothesis. J. Econ. Perspect. 2011, 25, 153–172. [Google Scholar] [CrossRef] [Green Version]
- Strachan, D.P. Hay fever, hygiene and household size. Br. Med. J. 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [Green Version]
- Rook, G.A.W.; Lowry, C.A.; Raison, C.L. Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol. Med. Public Health 2013, 1, 46–64. [Google Scholar] [CrossRef] [Green Version]
- Loh, W.; Tang, M.L.K. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef] [Green Version]
- Hill, D.A.; Spergel, J.M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 2018, 120, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Steel, Z.; Mamane, C.; Iranpour, C.; Chey, T.; Jackson, J.W.; Patel, V.; Silove, D. The global prevalence of common mental disorders: A systematic review and meta-analysis 1980–2013. Int. J. Epidemiol. 2014, 43, 476–493. [Google Scholar] [CrossRef] [Green Version]
- Pryce, C.R.; Fontana, A. Depression in autoimmune diseases. Curr. Top. Behav. Neurosci. 2017, 31, 139–154. [Google Scholar] [PubMed] [Green Version]
- Budu-Aggrey, A.; Joyce, S.; Davies, N.M.; Paternoster, L.; Munafò, M.R.; Brown, S.J.; Evans, J.; Sallis, H.M. Investigating the causal relationship between allergic disease and mental health. Clin. Exp. Allergy 2021, 51, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Codagnone, M.G.; Spichak, S.; O’Mahony, S.M.; O’Leary, O.F.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Programming bugs: Microbiota and the developmental origins of brain health and disease. Biol. Psychiatry 2019, 85, 150–163. [Google Scholar] [CrossRef]
- Codagnone, M.G.; Stanton, C.; O’Mahony, S.M.; Dinan, T.G.; Cryan, J.F. Microbiota and neurodevelopmental trajectories: Role of maternal and early-life nutrition. Ann. Nutr. Metab. 2019, 74, 16–27. [Google Scholar] [CrossRef]
- Choy, O.; Raine, A.; Schug, R. Larger striatal volume is associated with increased adult psychopathy. J. Psychiatr. Res. 2022, 149, 185–193. [Google Scholar] [CrossRef]
- Williams, J.A.; Burgess, S.; Suckling, J.; Laloulis, P.A.; Batool, F.; Griffiths, S.L.; Palmer, E.; Karwath, A.; Barsky, A.; Gkoutos, G.V.; et al. Inflammation and brain structure in schizophrenia and other neuropsychiatric disorders: A Mendelian randomisation study. JAMA Psychiatry 2022, 79, 498–507. [Google Scholar] [CrossRef]
- Shapiro, D.J.; Hicks, L.A.; Pavia, A.T.; Hersh, A.L. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–2009. J. Antimicrob. Chemother. 2014, 69, 234–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinidis, T.; Tsigalou, C.; Karvelas, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Effects of antibiotics upon the gut microbiome: A review of the literature. Biomedicines 2020, 8, 502. [Google Scholar] [CrossRef]
- Lepczyńska, M.; Białkowska, J.; Dzika, E.; Piskorz-Ogórek, K.; Korycińska, J. Blastocystis: How do specific diets and human gut microbiota affect its development and pathogenicity? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1531–1540. [Google Scholar] [CrossRef] [Green Version]
- Babakhanova, A.T.; Dzhumabekhov, A.T.; Zhao, A.V.; Kuandykov, Y.K.; Tanabayeva, S.B.; Fakhradiyev, I.R.; Nazarenko, Y.; Saliev, T.M. Impact of appendectomy on gut microbiota. Surg. Infect. 2021, 22, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.M.; Ma, J.; Prince, A.L.; Anthony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatric Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Yi, D.Y. Analysis of the human breast milk microbiome and bacterial extracellular vesicles in healthy mothers. Exp. Mol. Med. 2020, 52, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Bostock, J. Case of a periodical affection of the eyes and chest. Med.-Chir. Trans. 1819, 10, 161–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostock, J. Of the catarrhus æstivus or summer catarrh. Med.-Chir. Trans. 1828, 14, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Corson, R. Fashions in Makeup: From Ancient to Modern Times; Peter Owen Ltd.: London, UK, 1972. [Google Scholar]
- Needleman, H. The removal of lead from gasoline: Historical and personal reflections. Environ. Res. 2000, 84, 20–35. [Google Scholar] [CrossRef] [Green Version]
- Resongles, E.; Dietze, V.; Green, D.C.; Harrison, R.M.; Ochoa-Gonzalez, R.; Tremper, A.H.; Weiss, D.J. Strong evidence for the continued contribution of lead deposited during the 20th century to the atmospheric environment in London of today. Proc. Natl. Acad. Sci. USA 2021, 118, e2102791118. [Google Scholar] [CrossRef]
- Barbante, C.; Veysseyre, A.; Ferrari, C.; van der Velde, C.M.; Capodaglio, G.; Cescon, P.; Scarponi, G.; Boutron, C. Greenland snow evidence of large scale atmospheric contamination for platinum, palladium and rhodium. Environ. Sci. Technol. 2001, 35, 835–839. [Google Scholar] [CrossRef] [Green Version]
- Slobodian, M.R.; Petahtegoose, J.D.; Wallis, A.L.; Levesque, D.C.; Merritt, T.J.S. The effects of essential and non-essential metal toxicity in the Drosophila melanogaster insect model: A review. Toxics 2021, 9, 269. [Google Scholar] [CrossRef]
- de Craen, A.J.; Kaptchuck, T.J.; Tijssen, J.G.; Kleijnen, J. Placebos and placebo effects in medicine: Historical overview. J. R. Soc. Med. 1999, 92, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Hrobjartsson, A.; Kaptchuk, T.J.; Miller, F.G. Placebo effect studies are susceptible to response bias and other types of biases. J. Clin. Epidemiol. 2011, 64, 1223–1229. [Google Scholar] [CrossRef] [Green Version]
- Giddings, S.L.; Stevens, A.M.; Leung, D.T. Traveler’s diarrhea. Med. Clin. N. Am. 2016, 100, 317–330. [Google Scholar] [CrossRef]
- Cohen, S.I. Psychosomatic death: Voodoo death in a modern perspective. Integr. Psychiatry 1985, 3, 46–51. [Google Scholar]
- Barsky, A.J.; Saintfort, R.; Rogers, M.P.; Borus, J.F. Nonspecific medication side effects and the nocebo phenomenon. JAMA 2002, 87, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlesworth, J.E.; Petkovic, G.; Kelley, J.M.; Hunter, M.; Onakpoya, I.; Roberts, N.; Miller, F.G.; Howick, J. Effects of placebos without deception compared with no treatment: A systematic review and meta-analysis. J. Evid.-Based Med. 2017, 10, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.; Jheeta, S.; Fuentes, H.V.; Palacios-Pérez, M. Feeding our microbiota: Stimulation of the immune/semiochemical system and the potential amelioration of non-communicable diseases. Life 2022, 12, 1197. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Li, S.; Li, W.-D.; Wang, Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: A cohort study in the UK Biobank. PLoS Med. 2021, 18, e1003830. [Google Scholar] [CrossRef]
- Ma, G.; Chen, Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Zhou, L.; Foster, J.A. Psychobiotics and the gut-brain axis: In the pursuit of happiness. Neuropsychiatr. Dis. Treat. 2015, 11, 715–723. [Google Scholar]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, D.-W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Kang, D.-W.; Adams, J.B.; Coleman, D.M.; Pollard, E.L.; Maldonado, J.; McDonough-Means, S.; Caporaso, J.G.; Krajmalnik-Brown, R. Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Sci. Rep. 2019, 9, 5821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, A.E.; Andreo-Martínez, P. Prebiotics, probiotics and fecal microbiota transplantation in autism: A systematic review. Rev. De Psiquiatr. Y Salud Ment. (Engl. Ed.) 2020, 13, 150–164. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2019. [Google Scholar]
- Pickersgill, M.D. Debating DSM-5: Diagnosis and the sociology of critique. J. Med. Ethics 2014, 40, 521–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarotti, F.; Venerosi, A. Epidemiology of autism spectrum disorders: A review of worldwide prevalence since 2014. Brain Sci. 2020, 10, 274. [Google Scholar] [CrossRef]
- Pulikkan, J.; Mazumder, A.; Grace, T. Role of the gut microbiome in autism spectrum disorders. Adv. Exp. Med. Biol. 2019, 1118, 253–269. [Google Scholar] [CrossRef]
- Bundgaard-Nielson, C.; Knudson, J.; Leutscher, P.D.C.; Lauritsen, M.B.; Nyegaard, M.; Hagstrøm, S.; Sørensen, S. Gut microbiota profiles of autism spectrum disorder and attention deficit/hyperactivity disorder: A systematic literature review. Gut Microbes 2020, 11, 1172–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; José, J.V.; Nurnberger, J.I.; Torres, E.B. A biomarker characterizing neurodevelopment with applications in autism. Sci. Rep. 2018, 8, 614. [Google Scholar] [CrossRef] [Green Version]
- Sexton, C.C.; Gelhorn, H.L.; Bell, J.A.; Classi, P.M. The co-occurrence of reading disorder and ADHD: Epidemiology, treatment, psychosocial impact, and economic burden. J. Learn. Disabil. 2012, 45, 538–564. [Google Scholar] [CrossRef]
- Taylor, H.; Vestergaard, M.D. Developmental dyslexia: Disorder or specialization in exploration? Front. Psychol. 2022, 13, 889245. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Cler, G.J.; Smith, H.J.; Willis, H.E.; Asaridou, S.S.; Healy, M.P.; Papp, D.; Watkins, K.E. Quantitative MRI revearls differences in striatal myelin in children with DLD. eLife 2022, 11, e74242. [Google Scholar] [CrossRef] [PubMed]
- Espie, J.; Eisler, I. Focus on anorexia nervosa: Modern psychological treatment and guidelines for the adolescent patient. Adolesc. Health Med. Ther. 2015, 6, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Thibaut, F. Anxiety disorders: A review of the current literature. Dialogues Clin. Neurosci. 2017, 19, 87–88. [Google Scholar] [CrossRef]
- Bear, T.L.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.; Gopal, P.K. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miraglia, F.; Colla, E. Microbiome, Parkinson’s disease and Molecular mimicry. Cells 2019, 8, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, B.A.; Sowden, S.; Rybicki, A.J.; Fraser, D.S.; Press, C.; Holland, P.; Cook, J.L. Dopaminergic modulation of dynamic emotion perception. J. Neurosci. 2022, 42, 4394–4400. [Google Scholar] [CrossRef]
- Shah, P.; Hall, R.; Catmur, C.; Bird, G. Alexithymia, not autism, is associated with impaired interoception. Cortex 2016, 81, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Remmers, C.; Michalak, J. Losing your gut feelings. Intuition in depression. Front. Psychol. 2016, 7, 1291. [Google Scholar] [CrossRef] [Green Version]
- Bowe, W.P.; Logan, A.C. Acne vulgaris, probiotics and the gut-brain-skin axis—Back to the future? Gut Pathog. 2011, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Postema, M.C.; van Rooij, D.; Anagnostou, E.; Arango, C.; Auzias, G.; Behrmann, M.; Filho, G.B.; Calderoni, S.; Calvo, R.; Daly, E.; et al. Altered structural brain asymmetry in autism spectrum disorder in a study of 54 datasets. Nat. Communcations 2019, 10, 4958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherkatghanad, Z.; Akhondzadeh, M.; Salari, S.; Zomorodi-Moghadam, M.; Abdar, M.; Acharya, U.R.; Khosrowabadi, R.; Salari, V. Automated detection of autism spectrum disorder using a convolutional neural network. Front. Neurosci. 2020, 13, 1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, L.K. The savant syndrome: Intellectual impairment and exceptional skill. Psychol. Bull. 1999, 125, 31–46. [Google Scholar] [CrossRef]
- Jaarsma, P.; Welin, S. Autism as a natural human variation: Reflections on the claims of the neurodiversity movement. Health Care Anal. 2012, 20, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Zwilling, M.; Levy, B.R. How well environmental design is and can be suited to people with autism spectrum disorder (ASD): A natural language processing analysis. Int. J. Environ. Res. Public Health 2022, 19, 5037. [Google Scholar] [CrossRef]
- Dietrich, A. The mythconception of the mad genius. Front. Psychol. 2014, 5, 79. [Google Scholar] [CrossRef] [Green Version]
- Thys, E.; Sabbe, B.; De Hert, M. Creativity and psychopathology: A systematic review. Psychopathology 2014, 47, 141–147. [Google Scholar] [CrossRef]
- Gritz, E.C.; Bhandari, V. The human neonatal gut microbiome: A brief review. Front. Pediatrics 2015, 3, 17. [Google Scholar]
- Smits, S.A.; Leach, J.; Sonnenburg, E.D.; Gonzalez, C.G.; Lichtman, J.S.; Reid, G.; Knight, R.; Manjurano, A.; Changalucha, J.; Elias, J.E.; et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017, 357, 802–806. [Google Scholar] [CrossRef] [Green Version]
- Irimia, A.; Chaudhari, N.N.; Robles, D.J.; Rostowsky, K.A.; Maher, A.S.; Chowdhury, N.F.; Calvillo, N.F.; Ngo, V.; Gatz, M.; Mack, W.J.; et al. The indigenous South American Tsimane exhibit relatively modest decrease in brain volume with age despite high systemic inflammation. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021, 76, 2147–2155. [Google Scholar] [CrossRef]
- Ryan, C.R. Towards an ethics of reciprocity: Ethnobotanical knowledge and medicinal plants as cancer therapies. Humanities 2014, 3, 624–644. [Google Scholar] [CrossRef] [Green Version]
- Beardslee, L.A.; Banis, G.E.; Chu, S.; Liu, S.; Chapin, A.A.; Stine, J.M.; Pasricha, P.J.; Ghodossi, R. Ingestible sensors and sensing systems for minimally invasive diagnosis and monitoring: The next frontier in minimally invasive screening. ACS Sens. 2020, 5, 891–910. [Google Scholar] [CrossRef] [PubMed]
- Tito, R.Y.; Knights, D.; Metcalf, J.; Obregon-Tito, A.J.; Cleeland, L.; Najar, F.; Roe, B.; Reinhard, K.; Sobolik, K.; Belknap, S.; et al. Insights from characterizing extinct human gut microbiomes. PLoS ONE 2012, 7, e51146. [Google Scholar] [CrossRef] [PubMed]
- McConnell, J.R.; Wilson, A.I.; Stohl, I.; Arienzo, M.M.; Chellman, N.J.; Eckhardt, S.; Thompson, E.M.; Pollard, A.M.; Steffensen, J.P. Lead pollution recorded in Greenland ice indicates European emissions tracked plagues, wars, and imperial expansion during antiquity. Proc. Natl. Acad. Sci. USA 2018, 115, 5726–5731. [Google Scholar] [CrossRef] [Green Version]
- Thompson, R.C.; Allam, A.H.; Lombardi, G.G.; Wann, L.S.; Sutherland, M.L.; Sutherland, J.D.; Soliman, M.A.; Frohlich, B.; Mininberg, D.T.; Monge, J.M.; et al. Atherosclerosis across 4000 years of human history: The Horus study of four ancient populations. Lancet 2013, 381, 1211–1222. [Google Scholar] [CrossRef]
- Gasbarrini, G.; Rickards, O.; Martínez-Labarga, C.; Pacciani, E.; Chilleri, F.; Laterza, L.; Marangi, G.; Scaldaferri, F.; Gasbarrini, A. Origin of celiac disease: How old are predisposing haplotypes? World J. Gastroenterol. 2012, 18, 5300–5304. [Google Scholar]
- Dixson, A.F.; Dixson, B.J. Venus figurines of the early paleolithic: Symbols of fertility or attractiveness? J. Anthropol. 2011, 2011, 569120. [Google Scholar] [CrossRef] [Green Version]
- Borch-Jacobsen, M.; Shamdasani, S. The Freud Files: An Inquiry into the History of Psychoanalysis; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Jones, D.S.; Podolsky, S.H.; Greene, J.A. The burden of disease and the changing task of medicine. N. Engl. J. Med. 2012, 366, 2333–2338. [Google Scholar] [CrossRef] [Green Version]
- Protsiv, M.; Ley, C.; Lankester, J.; Hastie, T.; Parsonnet, J. Decreasing human body temperature in the United States since the Industrial Revolution. eLife 2020, 9, e49555. [Google Scholar] [CrossRef]
- Halden, R.U. Plastics and health risks. Annu. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Cobbina, S.J.; Mao, G.; Xu, H.; Zhang, Z.; Yang, L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ. Sci. Pollut. Res. Int. 2016, 23, 8244–8259. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Zhu, Y. Long term metal exposure changes gut microbiota of residents surrounding a mining and smelting area. Sci. Rep. 2020, 10, 4453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, J.J.; El-Hamdouchi, A.; Diouf, A.; Monyeki, A.; Somda, S.A. Determining the world-wide prevalence of obesity. Lancet 2018, 39, 1773–1774. [Google Scholar] [CrossRef]
- Taylor, R.; Holman, R.R. Normal weight individuals who develop type 2 diabetes: The personal fat threshold. Clin. Sci. 2015, 128, 405–410. [Google Scholar] [CrossRef] [Green Version]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yakov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized nutrition by prediction of glycaemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [Green Version]
- Wolever, T. Personalized nutrition by prediction of glycaemic responses: Fact or fantasy? Eur. J. Clin. Nutr. 2016, 70, 411–413. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Schellenkens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet. Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Honda, H.; Simizu, Y.; Rutter, M. No effect of MMR withdrawal on the incidence of autism: A total population study. J. Child Psychol. Psychiatry 2005, 46, 572–579. [Google Scholar] [CrossRef]
| Appendicitis | Coeliac Disease | Coronary Heart Disease |
|---|---|---|
| Deep vein thrombosis | Diabetes, type 2 | Diverticular disease |
| Gall stones | Haemorrhoids | Hiatus hernia |
| Multiple sclerosis | Obesity | Pernicious anaemia |
| Pulmonary embolism | Rheumatoid arthritis | Thyrotoxicosis |
| Tumours of the bowel | Ulcerative colitis | Varicose veins |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, D.; Jheeta, S.; Fuentes, H.V.; Street, B.; Palacios-Pérez, M. Microbiome–Gut Dissociation in the Neonate: Autism-Related Developmental Brain Disease and the Origin of the Placebo Effect. Gastrointest. Disord. 2022, 4, 291-311. https://doi.org/10.3390/gidisord4040028
Smith D, Jheeta S, Fuentes HV, Street B, Palacios-Pérez M. Microbiome–Gut Dissociation in the Neonate: Autism-Related Developmental Brain Disease and the Origin of the Placebo Effect. Gastrointestinal Disorders. 2022; 4(4):291-311. https://doi.org/10.3390/gidisord4040028
Chicago/Turabian StyleSmith, David, Sohan Jheeta, Hannya V. Fuentes, Bernadette Street, and Miryam Palacios-Pérez. 2022. "Microbiome–Gut Dissociation in the Neonate: Autism-Related Developmental Brain Disease and the Origin of the Placebo Effect" Gastrointestinal Disorders 4, no. 4: 291-311. https://doi.org/10.3390/gidisord4040028
APA StyleSmith, D., Jheeta, S., Fuentes, H. V., Street, B., & Palacios-Pérez, M. (2022). Microbiome–Gut Dissociation in the Neonate: Autism-Related Developmental Brain Disease and the Origin of the Placebo Effect. Gastrointestinal Disorders, 4(4), 291-311. https://doi.org/10.3390/gidisord4040028