1. Introduction
The Hepatitis D virus (HDV) globally affects nearly 5% of people with chronic infection with the hepatitis B virus (HBV) [
1]. Although HDV is one of the smallest viruses that may attack humans and is entirely dependent on HBV, HBV/HDV co-infection is a very severe form of viral hepatitis. Usually, the simultaneous co-infection with HBV and HDV in adults results in the clearance of both viruses. In contrast, the superinfection of an HBV-infected patient with HDV typically results in the development of persistent HBV/HDV co-infection. In addition, it may lead to a more rapid progression toward liver cirrhosis and hepatocellular carcinoma (HCC) [
2].
Although HDV infection can be prevented by HBV immunization, treatment success rates are generally low. The treatment of chronic hepatitis delta (CHD) infection was suboptimal, with an overall response rate of 25–30% with the off-label use of IFNa or pegylated (peg)-IFNa even after a prolonged treatment course of twenty-four months [
3]. Moreover, IFN-based treatment has limited use in clinical practice since is contraindicated in patients with advanced or decompensated liver disease. Bulevirtide (BLV) is a synthetic lipopeptide consisting of 47 amino acids of the pre-S1 domain of the HBV large surface protein, and it inhibits the sodium taurocholate co-transporting polypeptide (NTCP), which is a bile-salt liver transporter that allows the entry of HDV and HBV into hepatocytes [
4,
5]. Based on preliminary presented data, BLV at a dose of 2 mg/day subcutaneously was approved in July 2020 in the European Union (EU) for treating chronic HDV in HDV RNA positive adult patients with compensated liver disease [
5,
6,
7]. In addition, BLV led to a reduction in HDV viral load and improved liver enzyme levels in studies presented at the International Liver Congress (ILC)-European Association for the Study of the Liver (EASL) 2021 and at the American Association for the Study of Liver Diseases (AASLD) 2021 Liver Meetings [
8,
9].
Therefore, we present BLV’s safety and effectiveness in a young female patient with compensated liver cirrhosis and high-risk esophageal varices.
2. Case Report
A 39-year-old Caucasian HBsAg (+)/HBeAg (−) female has been referred to our liver unit due to liver cirrhosis. The patient was married with no family history of liver disease. On admission, the patient was afebrile and hemodynamically stable. Physical examination revealed hepato-splenomegaly and spiders naevi but no signs of ascites or hepatic encephalopathy. Laboratory evaluation revealed aspartate aminotransferase (AST) levels of 56 U/L, alanine aminotransferase (ALT) levels of 53 U/L, gamma-glutamyltransferase (GGT) levels of 34 U/L, alkaline phosphatase (ALP) levels of 63 U/L, total bilirubin levels of 1.33 mg/dL, international normalized ratio (INR) of 1.35, albumin levels of 4.2 g/dL, creatinine levels of 0.7 mg/dL, Hb levels of 13.5 g/dL, white blood count of 3570/μL and platelet count of 88.000/μL. Before treatment initiation, the serum HDV RNA level was 9300 IU/mL, and the HBV DNA level was 12,200 IU/mL (
Table 1). HDV RNA and HBV DNA were quantified by real-time polymerase chain reaction (PCR)-based assays (HDV: QIAamp Viral RNA kit, QIAgen assay, lower limit of detection (LLD) 575 IU/mL; HBV: QIAamp DSP Virus Spin kit, QIAGEN, LLD 26 IU/mL).
Ultrasound revealed surface nodularity with increased echogenicity, hypertrophy of the caudate lobe, and lateral segments of the left lobe with concomitant atrophy of the posterior segments. A liver stiffness measurement (LSM) using transient elastography evaluation revealed 34.3 kPa (IQR 0.3).
Upper gastrointestinal endoscopy revealed four esophageal varices (three small and one large with red spots), and endoscopic variceal ligation (EVL) sessions were scheduled. In the next 24 weeks, she underwent three sessions of EVL with complete eradication of varices.
The patient was classified as compensated cirrhosis with clinically significant portal hypertension (CSPH); Child-Pugh (CP) score was 5, indicating class A and the Model for End-Stage Liver Disease (MELD) score was 11.
She was eligible for BLV (2 mg/day in sub-cutaneous injection according to EMA approval) treatment. Moreover, tenofovir disoproxil fumarate (TDF) at the standard dose of 245 mg/day has also been prescribed since the patient had detectable HBV DNA. She planned for close clinical and laboratory monitoring, including HDV RNA and HBV DNA detection. The entire follow-up time after treatment initiation was 12 months.
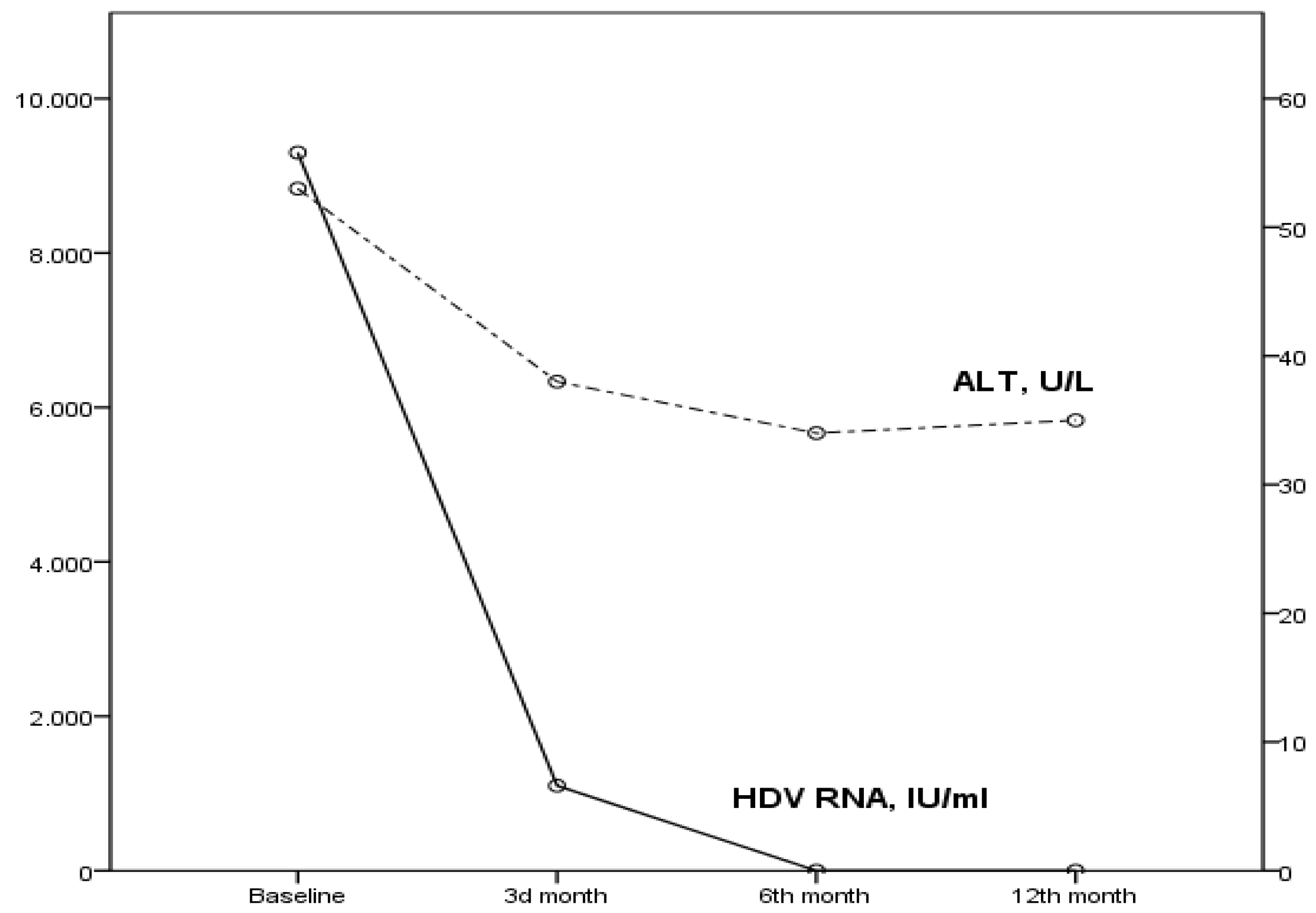
The patient achieved a virological (suppression of HDV RNA below the lower limit of detection) and biochemical (ALT normalization) response in the 6th month of treatment (
Figure 1). In addition, HBV DNA was undetectable since the 3rd month of treatment. Moreover, a significant amelioration of MELD score and LSM have been recorded, indicating improved liver function (
Table 1). Furthermore, compliance to treatment was excellent, and no clinical adverse events related to BLV were reported.
3. Discussion
We present the safety and efficacy of the approved BLV dose of 2 mg/day in combination with TDF for 48 weeks in a real-world case with HDV/HBV-related cirrhosis.
Recent data indicated that BLV’s high-dose co-administration with peginterferon alfa-2a (pegIFNα) or TDF for 48 weeks was safe and well tolerated [
7]. HDV RNA and ALT normalization were documented in 40% of this cohort’s BLV (5 mg per day) plus TDF arm. Data concerning benefits for such a prolonged duration in a real-life matter is minimal. Herta et al. recently published data concerning seven HDV/HBV patients (four with CP A cirrhosis) with the co-administration of BLV (2 mg per day) plus TDF as in our patient for at least 24 weeks [
10]. Extended data > 48 weeks were available in three cases: all patients achieved a virological response concerning both HDV and HBV, while one experienced a biochemical response. Interestingly, one patient showed a breakthrough of HDV RNA after initial complete suppression with normal ALT levels. Nevertheless, real-world published data indicates that BLV 2 mg per day administered for 24 or 48 weeks as monotherapy or combined with pegIFNα reduces HDV viremia and normalizes ALT levels in a significant proportion of patients [
11,
12,
13,
14]. Moreover, as in our patient, BLV has been well tolerated in clinical trials and real-world studies [
15,
16].
However, data about the effectiveness and the safety of BLV-based treatment in patients with liver cirrhosis and indications of clinically significant portal hypertension (CSPH), such as the presence of gastro-esophageal varices, is limited. In a recently published single-center study from Italy concerning eighteen patients with CSPH, the virological response was 78%, ALT normalization was observed in 83%, and four patients improved from CP-A score 6 to CP-A score 5. At the same time, a statistically significant LSM decline in patients who achieved virological response has been observed [
17]. Our case report study further confirms these results and highlights the impact of BLV-based treatment on liver function. We present a significant amelioration in several liver function tests and scores, including the INR, bilirubin, MELD score, and LSM. This promising finding may reflect the decrease in hepatic inflammation and the potential regenerative capacity of the damaged liver with BLV-based antiviral treatment.
Furthermore, as TDF directly ameliorates liver fibrosis by downregulating the PI3K/Akt/mTOR signaling pathway, BLV could be implicated by the exact mechanism and play a significant role in the regression of hepatic fibrosis [
18]. Moreover, our patient’s varices regressed after endoscopic prophylaxis (banding), while no signs of liver decompensation, including ascites, encephalopathy, or bleeding, occurred during the treatment so far. Thus, based on our beneficial clinical results, we decided to prolong therapy beyond week 48.
Although we did not perform a bile acids evaluation, in accordance with previous reports about the safety and tolerability of the long-term administration of BLV, our patient was asymptomatic despite the severity of chronic liver disease [
17]. Since our patient was not decompensated and the HDV genotype was not assessed, our results cannot be extrapolated in all clinical circumstances, including liver decompensation.
Since BLV treatment seems to be a reasonable approach as a ‘suppressive’ strategy for many HDV-positive patients, data regarding its impact on liver function is limited. Our study indicates that prolonged BLV-based treatment leads, beyond the virological response, to both liver function and LSM amelioration. However, a large cohort study must further establish this encouraging result.
Author Contributions
Conceptualization, N.P. and P.A.; methodology, P.T. and I.B.; resources, I.B.; data curation, N.P.; writing—original draft preparation, P.A.; writing—review and editing, P.T.; visualization, N.P.; supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from the subject involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stockdale, A.J.; Kreuels, B.; Henrion, M.Y.; Giorgi, E.; Kyomuhangi, I.; de Martel, C.; Hutin, Y.; Geretti, A.M. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J. Hepatol. 2020, 73, 523–532. [Google Scholar] [CrossRef]
- Hughes, S.A.; Wedemeyer, H.; Harrison, P.M. Hepatitis delta virus. Lancet 2011, 378, 73–85. [Google Scholar] [CrossRef]
- Yurdaydin, C.; Keskin, O.; Kalkan, Ç.; Karakaya, F.; Çalişkan, A.; Kabaçam, G.; Onder, F.O.; Karatayli, S.; Karatayli, E.; Deda, X.; et al. Interferon Treatment Duration in Patients With Chronic Delta Hepatitis and its Effect on the Natural Course of the Disease. J. Infect. Dis. 2018, 217, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Blank, A.; Markert, C.; Hohmann, N.; Carls, A.; Mikus, G.; Lehr, T.; Alexandrov, A.; Haag, M.; Schwab, M.; Urban, S.; et al. First-in-human application of the novel hepatitis B and hepatitis D virus entry inhibitor myrcludex B. J. Hepatol. 2016, 65, 483–489. [Google Scholar] [CrossRef]
- Kang, C.; Syed, Y.Y. Bulevirtide: First Approval. Drugs 2020, 80, 1601–1605. [Google Scholar] [CrossRef]
- European Medicines Agency. Hepcludex: EPAR. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/hepcludex (accessed on 28 April 2022).
- Wedemeyer, H.; Schöneweis, K.; Bogomolov, P.O.; Chulanov, V.; Stepanova, T.; Viacheslav, M.; Allweiss, L.; Dandri, M.; Ciesek, S.; Dittmer, U.; et al. 48 weeks of high dose (10 mg) bulevirtide as monotherapy or with peginterferon alfa-2a in patients with chronic HBV/HDV co-infection. J. Hepatol. 2020, 73, S52–S53. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Aleman, S.; Andreone, P.; Blank, A.; Brunetto, M.; Bogomolov, P.; Chulanov, V.; Geyvandova, N.; Hilgard, G.; Mamonova, N. Bulevirtide monotherapy at low and high dose in patients with chronic hepatitis delta: 24 weeks interim data of the phase 3 MYR301 study. J. Hepatol. 2021, 75, S294–S803. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Aleman, S.; Chulanov, V.; Hilgard, G.; Antonova, J.; Kaushik, A.M.; Lloyd, A.; Manuilov, D.; Suri, V.; Tran, T.T.; et al. Treatment with bulevirtide improves patient reported outcomes in patients with chronic hepatitis delta (CHD): An interim exploratory analysis at week 24. Hepatology 2021, 74, S413A. [Google Scholar]
- Herta, T.; Hahn, M.; Maier, M.; Fischer, J.; Niemeyer, J.; Hönemann, M.; Böhlig, A.; Gerhardt, F.; Schindler, A.; Schumacher, J.; et al. Efficacy and Safety of Bulevirtide plus Tenofovir Disoproxil Fumarate in Real-World Patients with Chronic Hepatitis B and D Co-Infection. Pathogens 2022, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Zoellner, C.; Hofmann, J.; Lutz, K.; Tacke, F.; Demir, M.M. Real-life experiences with bulevirtide for the treatment of hepatitis delta-48 weeks data from a German centre. Liver Int. 2022, 42, 2403–2407. [Google Scholar] [CrossRef] [PubMed]
- De Ledinghen, V.; Guyader, D.; Métivier, S.; Hilleret, M.-N.; Fontaine, H.; Roche, B. Safety and Efficacy of 2 mg Bulevirtide in Patients with Chronic HBV/HDV Co-Infection: First Real-World Results (French Early Access Program). Hepatology 2021, 74, S16A. [Google Scholar]
- Loglio, A.; Uceda Renteria, S.C.; Sambarino, D.; Borghi, M.; Perbellini, R.; Scholtes, C.; Facchetti, F.; Fraquelli, M.; Costantino, A.; Orsini, C.; et al. Early clinical and virological changes in HDV patients with advanced cirrhosis treated with bulevirtide monotherapy in a real-life setting. Hepatology 2021, 74, S1413A. [Google Scholar]
- Binter, T.; Jachs, M.; Hartl, L.; Aberle, S.; Zoller, H.; Aigner, E.; Staettermayer, A.F.; Kozbial, K.; Steindl-Munda, P.E.; Bauer, D.; et al. Efficacy of long-term treatment of chronic hepatitis D patients with bulevirtide–results of a “real world” study. Hepatology 2021, 74, S420A. [Google Scholar]
- Lampertico, P.; Roulot, D.; Wedemeyer, H. Bulevirtide with or without pegIFNα for patients with compensated chronic hepatitis delta: From clinical trials to real-world studies. J. Hepatol. 2022, in press. [CrossRef] [PubMed]
- Degasperi, E.; Anolli, M.P.; Lampertico, P. Bulevirtide for patients with compensated chronic hepatitis delta: A review. Liver Int. 2022, in press. [CrossRef] [PubMed]
- Degasperi, E.; Anolli, M.P.; Renteria, S.C.U.; Sambarino, D.; Borghi, M.; Perbellini, R.; Scholtes, C.; Facchetti, F.; Loglio, A.; Monico, S.; et al. Bulevirtide monotherapy for 48 weeks in patients with HDV-related compensated cirrhosis and clinically significant portal hypertension. J. Hepatol. 2022, in press. [CrossRef] [PubMed]
- Lee, S.W.; Kim, S.M.; Hur, W.; Kang, B.-Y.; Lee, H.L.; Nam, H.; Yoo, S.H.; Sung, P.S.; Kwon, J.H.; Jang, J.W.; et al. Tenofovir disoproxil fumarate directly ameliorates liver fibrosis by inducing hepatic stellate cell apoptosis via downregulation of PI3K/Akt/mTOR signaling pathway. PLoS ONE 2021, 16, e0261067. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).