Neutrophils in Intestinal Inflammation: What We Know and What We Could Expect for the Near Future
Abstract
:1. Introduction
2. Inflammatory Bowel Disease and a Lack of Treatment Options
3. Neutrophils in IBD Pathophysiology
4. Neutrophils in Intestinal Inflammation
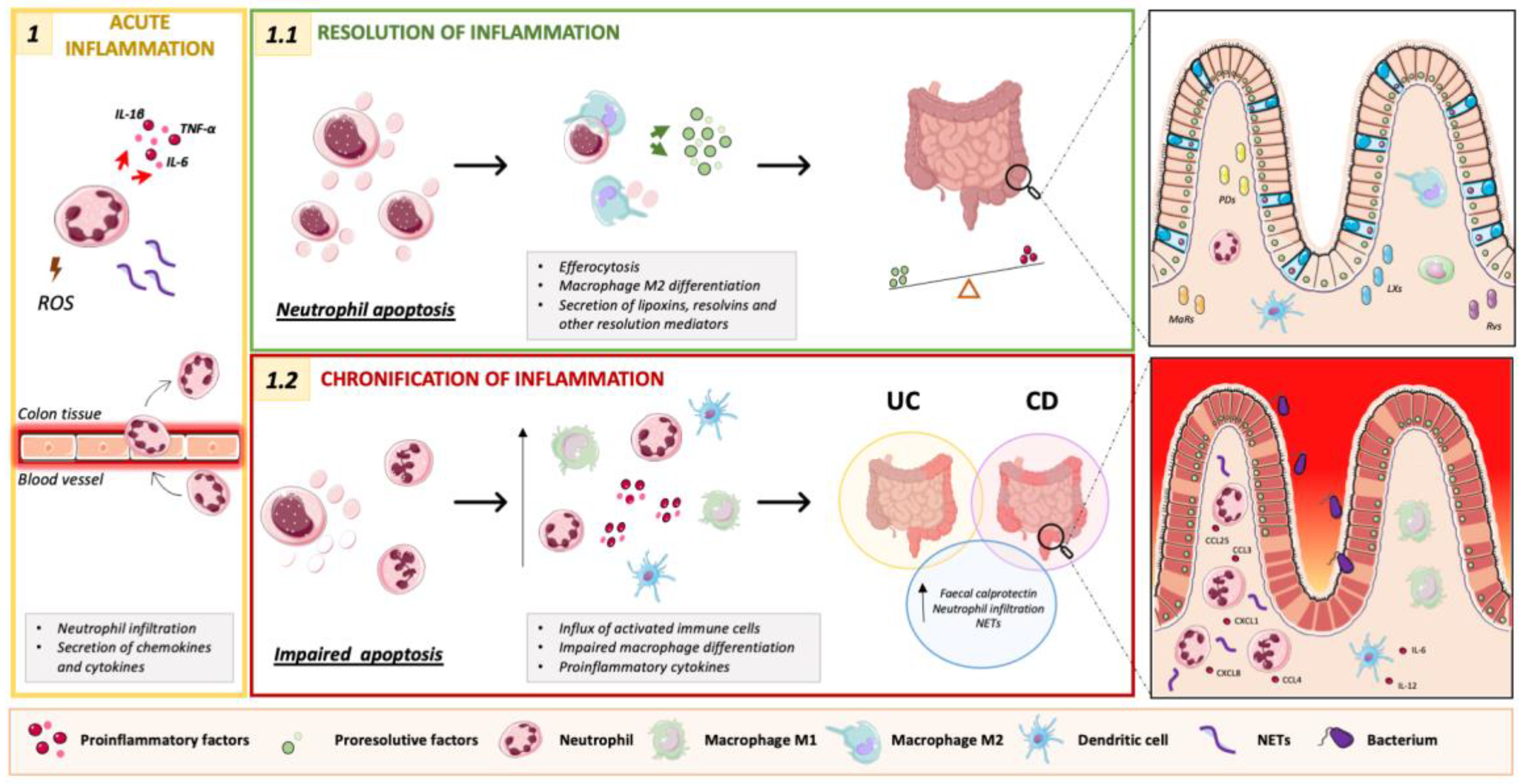
4.1. Neutrophils Participate in the Initiation of Intestinal Inflammation
4.2. Neutrophils Participate in the Resolution of Intestinal Inflammation
4.3. Targeting Neutrophils to Resolve Intestinal Inflammation
4.3.1. Neutrophil Chemotaxis Blockade
4.3.2. Neutrophil Apoptosis
4.3.3. Neutrophil and Specialised Resolving Lipid Mediators
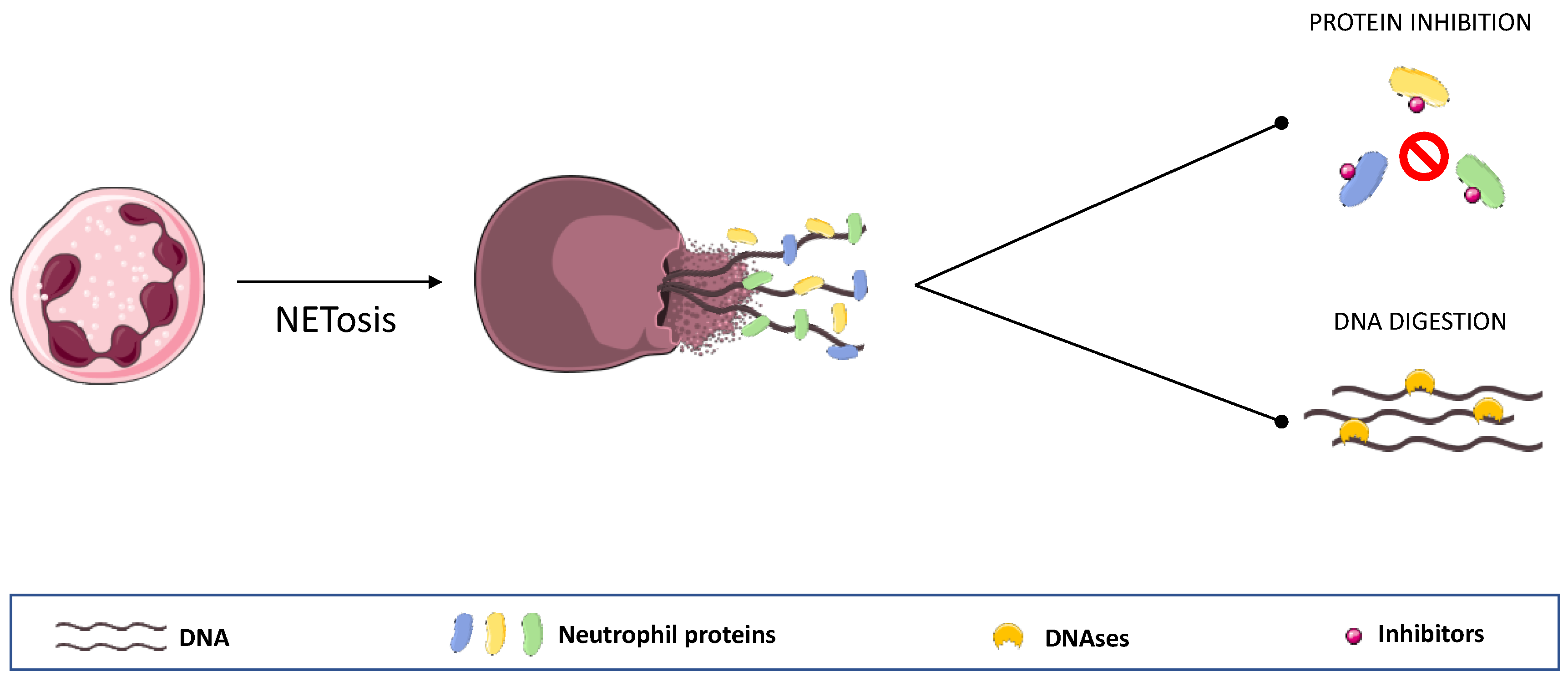
5. NETs Overview
5.1. NETs and IBD
5.1.1. NET Components and IBD
5.1.2. Targeting NET’ Components in IBD
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.X.; Liu, Z.J. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J. Dig. Dis. 2017, 18, 495–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosales, C.; Lowell, C.A.; Schnoor, M.; Uribe-Querol, E. Neutrophils: Their role in innate and adaptive immunity 2017. J. Immunol. Res. 2017, 2017, 9748345. [Google Scholar] [CrossRef]
- Wang, J.; Hossain, M.; Thanabalasuriar, A.; Gunzer, M.; Meininger, C.; Kubes, P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017, 358, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasenberg, A.; Hasenberg, M.; Männ, L.; Neumann, F.; Borkenstein, L.; Stecher, M.; Kraus, A.; Engel, D.R.; Klingberg, A.; Seddigh, P.; et al. Catchup: A mouse model for imaging-based tracking and modulation of neutrophil granulocytes. Nat. Methods 2015, 12, 445–452. [Google Scholar] [CrossRef]
- De Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef] [Green Version]
- Phillipson, M.; Kubes, P. The Healing Power of Neutrophils. Trends Immunol. 2019, 40, 635–647. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A cell with many roles in inflammation or several cell types? Front. Physiol. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Chowers, Y. Review article: Loss of response to anti-TNF treatments in Crohn’s disease. Aliment. Pharmacol. Ther. 2011, 33, 987–995. [Google Scholar] [CrossRef]
- Altajar, S.; Moss, A. Inflammatory Bowel Disease Environmental Risk Factors: Diet and Gut Microbiota. Curr. Gastroenterol. Rep. 2020, 22, 57. [Google Scholar] [CrossRef]
- de Souza, H.; Fiocchi, C.; Iliopoulos, D. The IBD interactome: An integrated view of aetiology, pathogenesis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 739–749. [Google Scholar] [CrossRef] [PubMed]
- De Souza, H.S.P.; Fiocchi, C. Immunopathogenesis of IBD: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Ding, S.; Jiang, H.; Liu, G. Roles of Macrophages in the Development and Treatment of Gut Inflammation. Front. Cell Dev. Biol. 2021, 9, 625423. [Google Scholar] [CrossRef] [PubMed]
- Drury, B.; Hardisty, G.; Gray, R.D.; Ho, G.T. Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Wéra, O.; Lancellotti, P.; Oury, C. The dual role of neutrophils in inflammatory bowel diseases. J. Clin. Med. 2016, 5, 118. [Google Scholar] [CrossRef]
- Biasi, F.; Leonarduzzi, G.; Oteiza, P.I.; Poli, G. Inflammatory bowel disease: Mechanisms, redox considerations, and therapeutic targets. Antioxidants Redox Signal. 2013, 19, 1711–1747. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Jiang, J.; Liu, J.; Xu, L.; Duan, S.; Sun, L.; Zhao, W.; Qian, F. MK2 Is Required for Neutrophil-Derived ROS Production and Inflammatory Bowel Disease. Front. Med. 2020, 7, 207. [Google Scholar] [CrossRef] [PubMed]
- Shea-Donohue, T.; Thomas, K.; Cody, M.J.; Zhao, A.; Detolla, L.J.; Kopydlowski, K.M.; Fukata, M.; Lira, S.A.; Vogel, S.N. Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-α), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun. 2008, 14, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tecchio, C.; Cassatella, M.A. Neutrophil-derived chemokines on the road to immunity. Semin. Immunol. 2016, 28, 119–128. [Google Scholar] [CrossRef]
- Wiendl, M.; Becker, E.; Müller, T.M.; Voskens, C.J.; Neurath, M.F.; Zundler, S. Targeting Immune Cell Trafficking—Insights From Research Models and Implications for Future IBD Therapy. Front. Immunol. 2021, 12, 656452. [Google Scholar] [CrossRef]
- Costa, F.; Mumolo, M.G.; Ceccarelli, L.; Bellini, M.; Romano, M.R.; Sterpi, C.; Ricchiuti, A.; Marchi, S.; Bottai, M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut 2005, 54, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Muthas, D.; Reznichenko, A.; Balendran, C.A.; Böttcher, G.; Clausen, I.G.; Kärrman Mårdh, C.; Ottosson, T.; Uddin, M.; MacDonald, T.T.; Danese, S.; et al. Neutrophils in ulcerative colitis: A review of selected biomarkers and their potential therapeutic implications. Scand. J. Gastroenterol. 2017, 52, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.; Ruano-Gallego, D.; Radhakrishnan, S.T.; Lovell, S.; Yu, L.; Kotik, O.; Glegola-Madejska, I.; Tate, E.W.; Choudhary, J.S.; Williams, H.R.T.; et al. Faecal neutrophil elastase-antiprotease balance reflects colitis severity. Mucosal Immunol. 2020, 13, 322–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortensen, J.H.; Sinkeviciute, D.; Manon-Jensen, T.; Domislović, V.; McCall, K.; Thudium, C.S.; Brinar, M.; Önnerfjord, P.; Goodyear, C.S.; Krznarić, Ž.; et al. A Specific Calprotectin Neo-epitope [CPa9-HNE] in Serum from Inflammatory Bowel Disease Patients Is Associated with Neutrophil Activity and Endoscopic Severity. J. Crohn’s Colitis 2022, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Şimşek-Onat, P.; Hizarcioglu-Gulsen, H.; Ergen, Y.M.; Gumus, E.; Özen, H.; Demir, H.; Özen, S.; Saltık-Temizel, İ.N. Neutrophil-to-Lymphocyte Ratio: An Easy Marker for the Diagnosis and Monitoring of Inflammatory Bowel Disease in Children. Dig. Dis. Sci. 2022. [Google Scholar] [CrossRef]
- Akpinar, M.Y.; Ozin, Y.O.; Kaplan, M.; Ates, I.; Kalkan, I.H.; Kilic, Z.M.Y.; Yuksel, M.; Kayacetin, E. Platelet-to-Lymphocyte Ratio and Neutrophil-to-Lymphocyte Ratio Predict Mucosal Disease Severity in Ulcerative Colitis. J. Med. Biochem. 2018, 37, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Therrien, A.; Chapuy, L.; Bsat, M.; Rubio, M.; Bernard, G.; Arslanian, E.; Orlicka, K.; Weber, A.; Panzini, B.P.; Dorais, J.; et al. Recruitment of activated neutrophils correlates with disease severity in adult Crohn’s disease. Clin. Exp. Immunol. 2019, 195, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Dinallo, V.; Marafini, I.; Di Fusco, D.; Laudisi, F.; Franzè, E.; Di Grazia, A.; Figliuzzi, M.M.; Caprioli, F.; Stolfi, C.; Monteleone, I.; et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J. Crohn’s Colitis 2019, 13, 772–784. [Google Scholar] [CrossRef]
- Li, T.; Wang, C.; Liu, Y.; Li, B.; Zhang, W.; Wang, L.; Yu, M.; Zhao, X.; Du, J.; Zhang, J.; et al. Neutrophil Extracellular Traps Induce Intestinal Damage and Thrombotic Tendency in Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, 240–253. [Google Scholar] [CrossRef]
- Smith, A.M.; Rahman, F.Z.; Hayee, B.; Graham, S.J.; Marks, D.J.B.; Sewell, G.W.; Palmer, C.D.; Wilde, J.; Foxwell, B.M.J.; Gloger, I.S.; et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J. Exp. Med. 2009, 206, 1883–1897. [Google Scholar] [CrossRef]
- Fournier, B.M.; Parkos, C.A. The role of neutrophils during intestinal inflammation. Mucosal Immunol. 2012, 5, 354–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camba-Gómez, M.; Gualillo, O.; Conde-Aranda, J. New perspectives in the study of intestinal inflammation: Focus on the resolution of inflammation. Int. J. Mol. Sci. 2021, 22, 2605. [Google Scholar] [CrossRef]
- Ina, K.; Kusugami, K.; Yamaguchi, T.; Imada, A.; Hosokawa, T.; Ohsuga, M.; Shinoda, M.; Ando, T.; Ito, K.; Yokoyama, Y. Mucosal interleukin-8 is involved in neutrophil migration and binding to extracellular matrix in inflammatory bowel disease. Am. J. Gastroenterol. 1997, 92, 1342–1346. [Google Scholar] [PubMed]
- Johswich, K.; Martin, M.; Bleich, A.; Kracht, M.; Dittrich-Breiholz, O.; Gessner, J.E.; Suerbaum, S.; Wende, E.; Rheinheimer, C.; Klos, A. Role of the C5a receptor (C5aR) in acute and chronic dextran sulfate-induced models of inflammatory bowel disease. Inflamm. Bowel Dis. 2009, 15, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Jupp, J.; Hillier, K.; Elliott, D.H.; Fine, D.R.; Bateman, A.C.; Johnson, P.A.; Cazaly, A.M.; Penrose, J.F.; Sampson, A.P. Colonic expression of leukotriene-pathway enzymes in inflammatory bowel diseases. Inflamm. Bowel Dis. 2007, 13, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Mrsny, R.J.; Gewirtz, A.T.; Siccardi, D.; Savidge, T.; Hurley, B.P.; Madara, J.L.; McCormick, B.A. Identification of hepoxilin A3 in inflammatory events: A required role in neutrophil migration across intestinal epithelia. Proc. Natl. Acad. Sci. USA. 2004, 101, 7421–7426. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, L.; Jiang, S.; Qian, D.; Duan, J. Excessive Apoptosis in Ulcerative Colitis: Crosstalk Between Apoptosis, ROS, ER Stress, and Intestinal Homeostasis. Inflamm. Bowel Dis. 2022, 28, 639–648. [Google Scholar] [CrossRef]
- Ozaka, S.; Sonoda, A.; Ariki, S.; Kamiyama, N.; Hidano, S.; Sachi, N.; Ito, K.; Kudo, Y.; Minata, M.; Saechue, B.; et al. Protease inhibitory activity of secretory leukocyte protease inhibitor ameliorates murine experimental colitis by protecting the intestinal epithelial barrier. Genes to Cells 2021, 26, 807–822. [Google Scholar] [CrossRef]
- Nighot, M.; Ganapathy, A.S.; Saha, K.; Suchanec, E.; Castillo, E.F.; Gregory, A.; Shapiro, S.; Ma, T.; Nighot, P. Matrix Metalloproteinase MMP-12 Promotes Macrophage Transmigration across Intestinal Epithelial Tight Junctions and Increases Severity of Experimental Colitis. J. Crohn’s Colitis 2021, 15, 1751–1765. [Google Scholar] [CrossRef]
- Lin, E.Y.H.; Lai, H.J.; Cheng, Y.K.; Leong, K.Q.; Cheng, L.C.; Chou, Y.C.; Peng, Y.C.; Hsu, Y.H.; Chiang, H. Sen Neutrophil extracellular traps impair intestinal barrier function during experimental colitis. Biomedicines 2020, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Kühl, A.A.; Kakirman, H.; Janotta, M.; Dreher, S.; Cremer, P.; Pawlowski, N.N.; Loddenkemper, C.; Heimesaat, M.M.; Grollich, K.; Zeitz, M.; et al. Aggravation of Different Types of Experimental Colitis by Depletion or Adhesion Blockade of Neutrophils. Gastroenterology 2007, 133, 1882–1892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ito, S.; Nishio, N.; Cheng, Z.; Suzuki, H.; Isobe, K. Up-regulation of Gr1+CD11b+ population in spleen of dextran sulfate sodium administered mice works to repair colitis. Inflamm. Allergy Drug Targets 2011, 10, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Natsui, M.; Kawasaki, K.; Takizawa, H.; Hayashi, S.I.; Matsuda, Y.; Sugimura, K.; Seki, K.; Narisawa, R.; Sendo, F.; Asakura, H. Selective depletion of neutrophils by a monoclonal antibody, RP-3, suppresses dextran sulphate sodium-induced colitis in rats. J. Gastroenterol. Hepatol. 1997, 12, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Qualls, J.E.; Kaplan, A.M.; Van Rooijen, N.; Cohen, D.A. Suppression of experimental colitis by intestinal mononuclear phagocytes. J. Leukoc. Biol. 2006, 80, 802–815. [Google Scholar] [CrossRef]
- Zhu, F.; He, H.; Fan, L.; Ma, C.; Xu, Z.; Xue, Y.; Wang, Y.; Zhang, C.; Zhou, G. Blockade of CXCR2 suppresses proinflammatory activities of neutrophils in ulcerative colitis. Am. J. Transl. Res. 2020, 12, 5237–5251. [Google Scholar]
- Chang, Y.; Kim, J.W.; Yang, S.; Chung, D.H.; Ko, J.S.; Moon, J.S.; Kim, H.Y. Increased GM-CSF-producing NCR- ILC3s and neutrophils in the intestinal mucosa exacerbate inflammatory bowel disease. Clin. Transl. Immunol. 2021, 10, 1–16. [Google Scholar] [CrossRef]
- Na, Y.R.; Stakenborg, M.; Seok, S.H.; Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 531–543. [Google Scholar] [CrossRef]
- Onali, S.; Favale, A.; Fantini, M.C. The Resolution of Intestinal Inflammation: The Peace-Keeper’s Perspective. Cells 2019, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Ho, G.T.; Cartwright, J.A.; Thompson, E.J.; Bain, C.C.; Rossi, A.G. Resolution of inflammation and gut repair in IBD: Translational steps towards complete mucosal healing. Inflamm. Bowel Dis. 2020, 26, 1131–1143. [Google Scholar] [CrossRef]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef] [PubMed]
- Kruidenier, L.; Macdonald, T.T.; Collins, J.E.; Pender, S.L.F.; Sanderson, I.R. Myofibroblast matrix metalloproteinases activate the neutrophil chemoattractant CXCL7 from intestinal epithelial cells. Gastroenterology 2006, 130, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Swee, M.; Wilson, C.L.; Wang, Y.; McGuire, J.K.; Parks, W.C. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J. Leukoc. Biol. 2008, 83, 1404. [Google Scholar] [CrossRef] [Green Version]
- Castaneda, F.E.; Walia, B.; Vijay-Kumar, M.; Patel, N.R.; Roser, S.; Kolachala, V.L.; Rojas, M.; Wang, L.; Oprea, G.; Garg, P.; et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: Central role of epithelial-derived MMP. Gastroenterology 2005, 129, 1991–2008. [Google Scholar] [CrossRef]
- Garg, P.; Rojas, M.; Ravi, A.; Bockbrader, K.; Epstein, S.; Vijay-Kumar, M.; Gewirtz, A.T.; Merlin, D.; Sitaraman, S.V. Selective Ablation of Matrix Metalloproteinase-2 Exacerbates Experimental Colitis: Contrasting Role of Gelatinases in the Pathogenesis of Colitis. J. Immunol. 2006, 177, 4103–4112. [Google Scholar] [CrossRef] [Green Version]
- Koller, F.L.; Dozier, E.A.; Nam, K.T.; Swee, M.; Birkland, T.P.; Parks, W.C.; Fingleton, B. Lack of MMP10 exacerbates experimental colitis and promotes development of inflammation-associated colonic dysplasia. Lab. Investig. 2012, 92, 1749–1759. [Google Scholar] [CrossRef] [Green Version]
- Brauer, R.; Tureckova, J.; Kanchev, I.; Khoylou, M.; Skarda, J.; Prochazka, J.; Spoutil, F.; Beck, I.M.; Zbodakova, O.; Kasparek, P.; et al. MMP-19 deficiency causes aggravation of colitis due to defects in innate immune cell function. Mucosal Immunol. 2016, 9, 974–985. [Google Scholar] [CrossRef] [Green Version]
- Brostjan, C.; Oehler, R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discov. 2020, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Figueroa, E.; Álvarez-Carrasco, P.; Ortega, E.; Maldonado-Bernal, C. Neutrophils: Many Ways to Die. Front. Immunol. 2021, 12, 631821. [Google Scholar] [CrossRef]
- Lampinen, M.; Sangfelt, P.; Taha, Y.; Carlson, M. Accumulation, activation, and survival of neutrophils in ulcerative colitis: Regulation by locally produced factors in the colon and impact of steroid treatment. Int. J. Colorectal Dis. 2008, 23, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Brannigan, A.E.; O’Connell, P.R.; Hurley, H.; O’Neill, A.; Brady, H.R.; Fitzpatrick, J.M.; Watson, R.W.G. Neutrophil apoptosis is delayed in patients with inflammatory bowel disease. Shock 2000, 13, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L.; Singh, B. Neutrophil apoptosis is delayed in an equine model of colitis: Implications for the development of systemic inflammatory response syndrome. Equine Vet. J. 2017, 49, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, L.; Shi, J.; Liu, S.; Liu, Y.; Zheng, D. TRAIL receptor deficiency sensitizes mice to dextran sodium sulphate-induced colitis and colitis-associated carcinogenesis. Immunology 2014, 141, 211–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wicki, S.; Gurzeler, U.; Corazza, N.; Genitsch, V.; Wong, W.W.L.; Kaufmann, T. Loss of BID delays FASL-induced cell death of mouse neutrophils and aggravates DSS-induced weight loss. Int. J. Mol. Sci. 2018, 19, 684. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, C.; Homann, J.; Ball, A.K.; Blöcher, R.; Kleinschmidt, T.K.; Basavarajappa, D.; Angioni, C.; Ferreirós, N.; Häfner, A.K.; Rådmark, O.; et al. Lipoxin and resolvin biosynthesis is dependent on 5-lipoxygenase activating protein. FASEB J. 2015, 29, 5029–5043. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Abdulnour, R.E.E.; Dalli, J.; Colby, J.K.; Krishnamoorthy, N.; Timmons, J.Y.; Tan, S.H.; Colas, R.A.; Petasis, N.A.; Serhan, C.N.; Levy, B.D. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. USA. 2014, 111, 16526–16531. [Google Scholar] [CrossRef] [Green Version]
- Gewirtz, A.T.; Collier-Hyams, L.S.; Young, A.N.; Kucharzik, T.; Guilford, W.J.; Parkinson, J.F.; Williams, I.R.; Neish, A.S.; Madara, J.L. Lipoxin a4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J. Immunol. 2002, 168, 5260–5267. [Google Scholar] [CrossRef] [Green Version]
- Marcon, R.; Bento, A.F.; Dutra, R.C.; Bicca, M.A.; Leite, D.F.P.; Calixto, J.B. Maresin 1, a proresolving lipid mediator derived from omega-3 polyunsaturated fatty acids, exerts protective actions in murine models of colitis. J. Immunol. 2013, 191, 4288–4298. [Google Scholar] [CrossRef] [Green Version]
- Ariyoshi, T.; Hagihara, M.; Eguchi, S.; Fukuda, A.; Iwasaki, K.; Oka, K.; Takahashi, M.; Yamagishi, Y.; Mikamo, H. Clostridium butyricum MIYAIRI 588-Induced Protectin D1 Has an Anti-inflammatory Effect on Antibiotic-Induced Intestinal Disorder. Front. Microbiol. 2020, 11, 587725. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Li, P.; Zhao, H.; Li, X. Maresin 1 alleviates dextran sulfate sodium-induced ulcerative colitis by regulating NRF2 and TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2020, 78, 106018. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Yoshida, M.; Hong, S.; Tjonahen, E.; Glickman, J.N.; Petasis, N.A.; Blumberg, R.S.; Serhan, C.N. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. USA 2005, 102, 7671–7676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masterson, J.C.; McNamee, E.N.; Fillon, S.A.; Hosford, L.; Harris, R.; Fernando, S.D.; Jedlicka, P.; Iwamoto, R.; Jacobsen, E.; Protheroe, C.; et al. Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut 2015, 64, 1236–1247. [Google Scholar] [CrossRef] [Green Version]
- Trilleaud, C.; Gauttier, V.; Biteau, K.; Girault, I.; Belarif, L.; Mary, C.; Pengam, S.; Teppaz, G.; Thepenier, V.; Danger, R.; et al. Agonist anti-ChemR23 mAb reduces tissue neutrophil accumulation and triggers chronic inflammation resolution. Sci. Adv. 2021, 7, eabd1453. [Google Scholar] [CrossRef]
- Gobbetti, T.; Dalli, J.; Colas, R.A.; Canova, D.F.; Aursnes, M.; Bonnet, D.; Alric, L.; Vergnolle, N.; Deraison, C.; Hansen, T.V.; et al. Protectin D1n-3 DPA and resolvin D5n-3 DPA are effectors of intestinal protection. Proc. Natl. Acad. Sci. USA 2017, 114, 3963–3968. [Google Scholar] [CrossRef] [Green Version]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Neumann, A.; Brogden, G.; von Köckritz-Blickwede, M. Extracellular traps: An ancient weapon of multiple kingdoms. Biology (Basel). 2020, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Robb, C.T.; Dyrynda, E.A.; Gray, R.D.; Rossi, A.G.; Smith, V.J. Invertebrate extracellular phagocyte traps show that chromatin is an ancient defence weapon. Nat. Commun. 2014, 5, 4627. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, A.; Libby, P.; Soehnlein, O.; Aramburu, I.V.; Papayannopoulos, V.; Silvestre-Roig, C. Neutrophil extracellular traps: From physiology to pathology. Cardiovasc. Res. 2021, cvab329. [Google Scholar] [CrossRef]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.U.S.; O’Sullivan, K.M. The Expanding Role of Extracellular Traps in Inflammation and Autoimmunity: The New Players in Casting Dark Webs. Int. J. Mol. Sci. 2022, 23, 3793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jiang, P.; Guo, S.; Schrodi, S.J.; He, D. Apoptosis, Autophagy, NETosis, Necroptosis, and Pyroptosis Mediated Programmed Cell Death as Targets for Innovative Therapy in Rheumatoid Arthritis. Front. Immunol. 2021, 12, 809806. [Google Scholar] [CrossRef] [PubMed]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, T.; Schallert, K.; Vilchez-Vargas, R.; Benndorf, D.; Püttker, S.; Sydor, S.; Schulz, C.; Bechmann, L.; Canbay, A.; Heidrich, B.; et al. Metaproteomics of fecal samples of Crohn’s disease and Ulcerative Colitis. J. Proteomics 2019, 201, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Metzler, K.D.; Fuchs, T.A.; Nauseef, W.M.; Reumaux, D.; Roesler, J.; Schulze, I.; Wahn, V.; Papayannopoulos, V.; Zychlinsky, A. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood 2011, 117, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Palmer, L.J.; Cooper, P.R.; Ling, M.R.; Wright, H.J.; Huissoon, A.; Chapple, I.L.C. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin. Exp. Immunol. 2012, 167, 261–268. [Google Scholar] [CrossRef]
- Hansberry, D.R.; Shah, K.; Agarwal, P.; Agarwal, N. Fecal Myeloperoxidase as a Biomarker for Inflammatory Bowel Disease. Cureus 2017, 9, e1004. [Google Scholar] [CrossRef] [Green Version]
- Chami, B.; Martin, N.J.J.; Dennis, J.M.; Witting, P.K. Myeloperoxidase in the inflamed colon: A novel target for treating inflammatory bowel disease. Arch. Biochem. Biophys. 2018, 645, 61–71. [Google Scholar] [CrossRef]
- Saiki, T. Myeloperoxidase concentrations in the stool as a new parameter of inflammatory bowel disease. Kurume Med. J. 1998, 45, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Chami, B.; San Gabriel, P.T.; Kum-Jew, S.; Wang, X.S.; Dickerhof, N.; Dennis, J.M.; Witting, P.K. The nitroxide 4-methoxy-tempo inhibits the pathogenesis of dextran sodium sulfate-stimulated experimental colitis. Redox Biol. 2020, 28, 101333. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, G.; Chami, B.; Liu, Y.; Schroder, A.L.; San Gabriel, P.T.; Gao, A.; Fong, G.; Wang, X.S.; Witting, P.K. The Synthetic Myeloperoxidase Inhibitor AZD3241 Ameliorates Dextran Sodium Sulfate Stimulated Experimental Colitis. Front. Pharmacol. 2020, 11, 556020. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Stadler, S.; Correll, S.; Li, P.; Wang, D.; Hayama, R.; Leonelli, L.; Han, H.; Grigoryev, S.A.; et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 2009, 184, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Li, M.; Lindberg, M.R.; Kennett, M.J.; Xiong, N.; Wang, Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010, 207, 1853–1862. [Google Scholar] [CrossRef]
- You, Q.; Shen, Y.; Wu, Y.; Li, Y.; Liu, C.; Huang, F.; Gu, H.F.; Wu, J. Neutrophil Extracellular Traps Caused by Gut Leakage Trigger the Autoimmune Response in Nonobese Diabetic Mice. Front. Immunol. 2022, 12, 711423. [Google Scholar] [CrossRef]
- Leppkes, M.; Lindemann, A.; Gößwein, S.; Paulus, S.; Roth, D.; Hartung, A.; Liebing, E.; Zundler, S.; Gonzalez-Acera, M.; Patankar, J.V.; et al. Neutrophils prevent rectal bleeding in ulcerative colitis by peptidyl-arginine deiminase-4-dependent immunothrombosis. Gut 2021, gutjnl-2021-324725. [Google Scholar] [CrossRef]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef] [Green Version]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [Green Version]
- Kirov, S.; Sasson, A.; Zhang, C.; Chasalow, S.; Dongre, A.; Steen, H.; Stensballe, A.; Andersen, V.; Birkelund, S.; Bennike, T.B. Degradation of the extracellular matrix is part of the pathology of ulcerative colitis. Mol. Omi. 2019, 15, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Schroder, A.L.; Chami, B.; Liu, Y.; Doyle, C.M.; El Kazzi, M.; Ahlenstiel, G.; Ahmad, G.; Pathma-Nathan, N.; Collins, G.; Toh, J.; et al. Neutrophil Extracellular Trap Density Increases With Increasing Histopathological Severity of Crohn’s Disease. Inflamm. Bowel Dis. 2022, 28, 586–598. [Google Scholar] [CrossRef]
- Curciarello, R.; Sobande, T.; Jones, S.; Giuffrida, P.; Di Sabatino, A.; Docena, G.H.; Macdonald, T.T.; Kok, K. Human neutrophil elastase proteolytic activity in ulcerative colitis favors the loss of function of therapeutic monoclonal antibodies. J. Inflamm. Res. 2020, 13, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Jorch, S.K.; Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 2017, 23, 279–287. [Google Scholar] [CrossRef]
- Angelidou, I.; Chrysanthopoulou, A.; Mitsios, A.; Arelaki, S.; Arampatzioglou, A.; Kambas, K.; Ritis, D.; Tsironidou, V.; Moschos, I.; Dalla, V.; et al. REDD1/Autophagy Pathway Is Associated with Neutrophil-Driven IL-1β Inflammatory Response in Active Ulcerative Colitis. J. Immunol. 2018, 200, 3950–3961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maronek, M.; Gromova, B.; Liptak, R.; Konecna, B.; Pastorek, M.; Cechova, B.; Harsanyova, M.; Budis, J.; Smolak, D.; Radvanszky, J.; et al. Extracellular dna correlates with intestinal inflammation in chemically induced colitis in mice. Cells 2021, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Mei, Y.; Dong, W.; Wang, J.; Huang, F.; Wu, J. Evaluation of protein arginine deiminase-4 inhibitor in TNBS- induced colitis in mice. Int. Immunopharmacol. 2020, 84, 106583. [Google Scholar] [CrossRef]
- Witalison, E.E.; Cui, X.; Hofseth, A.B.; Subramanian, V.; Causey, C.P.; Thompson, P.R.; Hofseth, L.J. Inhibiting protein arginine deiminases has antioxidant consequences. J. Pharmacol. Exp. Ther. 2015, 353, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Knuckley, B.; Jones, J.E.; Bachovchin, D.A.; Slack, J.; Causey, C.P.; Brown, S.J.; Rosen, H.; Cravatt, B.F.; Thompson, P.R. A fluopol-ABPP HTS assay to identify PAD inhibitors. Chem. Commun. 2010, 46, 7175–7177. [Google Scholar] [CrossRef]
- Collins, C.B.; Aherne, C.M.; Ehrentraut, S.F.; Gerich, M.E.; McNamee, E.N.; McManus, M.C.; Lebsack, M.D.P.; Jedlicka, P.; Azam, T.; De Zoeten, E.F.; et al. Alpha-1-antitrypsin therapy ameliorates acute colitis and chronic murine ileitis. Inflamm. Bowel Dis. 2013, 19, 1964–1973. [Google Scholar] [CrossRef] [Green Version]
- Motta, J.; Magne, L.; Descamps, D.; Rolland, C.; Squarzoni-Dale, C.; Rousset, P.; Martin, L.; Cenac, N.; Balloy, V.; Huerre, M.; et al. Modifying the protease, antiprotease pattern by Elafin overexpression protects mice from colitis. Gastroenterology 2011, 140, 1272–1282. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arosa, L.; Camba-Gómez, M.; Conde-Aranda, J. Neutrophils in Intestinal Inflammation: What We Know and What We Could Expect for the Near Future. Gastrointest. Disord. 2022, 4, 263-276. https://doi.org/10.3390/gidisord4040025
Arosa L, Camba-Gómez M, Conde-Aranda J. Neutrophils in Intestinal Inflammation: What We Know and What We Could Expect for the Near Future. Gastrointestinal Disorders. 2022; 4(4):263-276. https://doi.org/10.3390/gidisord4040025
Chicago/Turabian StyleArosa, Laura, Miguel Camba-Gómez, and Javier Conde-Aranda. 2022. "Neutrophils in Intestinal Inflammation: What We Know and What We Could Expect for the Near Future" Gastrointestinal Disorders 4, no. 4: 263-276. https://doi.org/10.3390/gidisord4040025
APA StyleArosa, L., Camba-Gómez, M., & Conde-Aranda, J. (2022). Neutrophils in Intestinal Inflammation: What We Know and What We Could Expect for the Near Future. Gastrointestinal Disorders, 4(4), 263-276. https://doi.org/10.3390/gidisord4040025






