Abstract
Background: The antibody response to coronavirus disease 2019 (COVID-19) vaccination in patients with inflammatory bowel disease (IBD) on biological drugs is still unclear. Aim: To determine the anti-SARS-CoV-2 spike 1 (anti-S1-IgG) response rate and antibody levels following a complete COVID-19 vaccination cycle in patients with IBD on biological treatment. Methods: We assessed antibody response to COVID-19 in consecutive patients with IBD on biological drugs and without prior exposure to COVID-19. Sera were prospectively collected at baseline and at 21 days (T1), 42 days (T2), and 3 months (T3) after the first vaccine dose. Results: Among the 42 patients included in the study, the overall response rate at T3 was 97.6%, with no difference across the various biological drugs. After the first dose (T1), the response rate was higher in patients receiving anti-tumour necrosis factor (TNF) compared to patients treated with other biologics (p = 0.031). Among the responders, the anti-S1 levels were not significantly different among the various biological drugs at all study timepoints. Concomitant corticosteroids and disease activity had no impact on the response rate at all study timepoints. No unexpected side events were observed. Discussion: The antibody response to vaccination against COVID-19 in patients with IBD on biological drugs is optimal, independently of their mechanism of action. Patients treated with anti-TNF seem to have an earlier response to vaccination, while concomitant low-dose corticosteroids and disease activity does not seem to impact response. This information can be used to program vaccination and inform patients.
1. Introduction
Inflammatory bowel disease (IBD) is a chronic, recurrent disease with alternating periods of latency and phases of relapse [1]. Considering the progressive course of IBD and the young age at onset, the use of biological treatment increased during the last decades, and patients with IBD treated with biologics have a higher risk of infection as compared to the general population, which is further increased in those concomitantly treated with steroids and/or immunosuppressive drugs [2,3]. Furthermore, several studies suggested that patients with IBD have an attenuated response to vaccination if treated with immunosuppressive or biological drugs [4,5,6,7]. For example, it has been shown that immunological response to inactivated quadrivalent influenza vaccine is lower in patients with IBD treated with infliximab [8]. Similarly, the response rate to Hepatitis B Virus (HBV) standard vaccination seems to be lower in patients with IBD on infliximab, with or without concomitant azathioprine [9].
Vaccination against coronavirus disease 2019 (COVID-19) for patients with IBD on biological therapy is currently recommended, although few data are available on the response rate to vaccination [10,11]. Indeed, while one study demonstrated that infliximab, as compared to vedolizumab, was associated with attenuated response to a single dose of a COVID-19 vaccine, another study found that patients with IBD treated with either anti-tumour necrosis factor (TNF) or vedolizumab had a blunted vaccination response as compared to healthy controls [12,13]. Thus, the antibody response to COVID-19 vaccination among patients with IBD treated with biological drugs is still unclear, and pragmatic studies detailing the rate of response and the dynamics of antibodies development in this setting are urgently needed.
In this study, our aim was to assess the rate of anti-SARS-CoV 2 spike 1 (anti-S1 IgG) seroconversion following a complete cycle of COVID-19 BNT162b2 vaccination in patients with IBD on biological drugs. We also assessed the impact of concomitant steroid therapy and of active disease in developing a response to vaccination, besides assessing the safety profile of anti-COVID-19 vaccination in these patients.
2. Patients and Methods
2.1. Inclusion and Exclusion Criteria
In this prospective study we included adult patients with IBD (Crohn’s disease and with ulcerative colitis) on stable biological therapy for at least 6 months who underwent a complete vaccination cycle against COVID-19 at our Unit. Criteria for inclusion were diagnosis of Crohn’s disease or ulcerative colitis (confirmed by endoscopic, radiologic, and histologic evaluation) and stable biological treatment initiated at least 6 months before study entry without concomitant use of immunosuppressive drugs (i.e., azathioprine/6-mercaptopurine, methotrexate). An exclusion criterion was prior COVID-19 infection, as assessed by positivity for anti-S1 IgG, or previous presence of symptoms and positivity for COVID-19 PCR.
2.2. Ethics
All patients provided written informed consent before inclusion. The study was performed according to the Declaration of Helsinki, and the protocol was approved by the local Ethical Committee.
2.3. Study Protocol
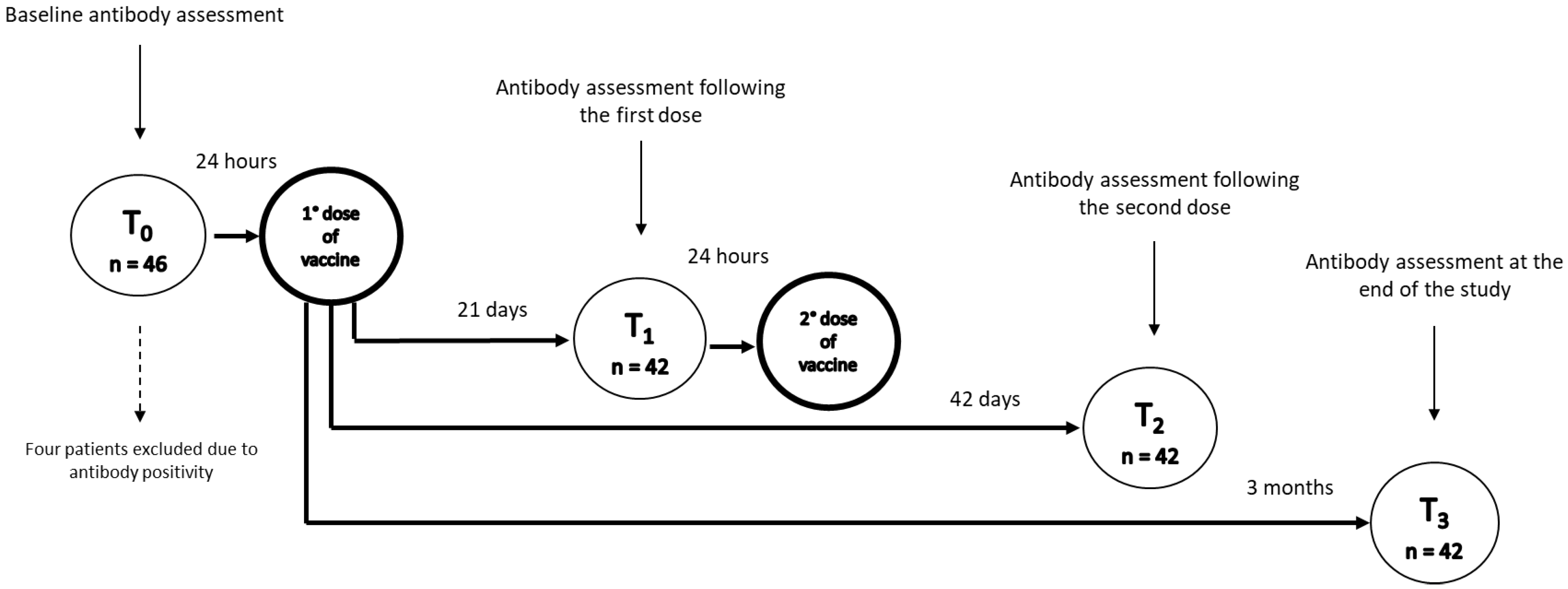
Patients underwent blood sampling at baseline (T0, the day before administration of the first dose of vaccine), which was also used to screen sera for the evidence of previous asymptomatic exposure to COVID-19. Following the baseline assessment, an intramuscular dose of the BNT162b2 m-RNA COVID-19 vaccine (Pfizer-BioNTech) was administered twice, 21 days apart. Patients’ serum was collected at 21 days from baseline (T1), just before the administration of the second dose of the vaccine, at 42 days (T2), and at 3 months from the baseline (T3) (Figure 1).
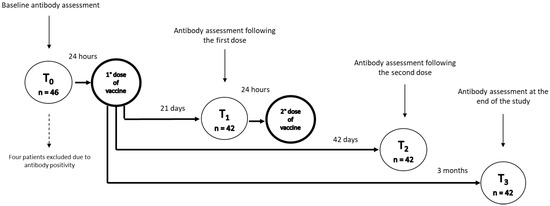
Figure 1.
Vaccination protocol and timepoints of assessment of response. T0 represents the day before administration of the first dose of vaccine, T1 just before the administration of the second dose of vaccine, T2 at 42 days, and T3 at 3 months from baseline.
Anti-SARS-CoV 2 spike 1 (anti-S1 IgG) antibodies were measured with a two-step immunoassay employing fluoromagnetic dyed beads coated with Spike protein S1 subunit, using commercial kits (BioPlex2200, Bio-Rad Laboratories, Redmond, WA, USA). An anti-S1 IgG antibody level >10 U/mL was deemed compatible with response to vaccination.
Side effects were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0, and severe side effects were considered as at least a Grade 3 event, according to the CTCAE scale [14].
2.4. Statistics
Continuous data were compared using the Mann–Whitney U-test and analysis of variance (ANOVA), and categorical data were compared using the Fisher’s exact test or Chi-square test. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Main Characteristics of the Study Population
Among the 46 patients with IBD evaluated for COVID-19 vaccination (30 with Crohn’s disease and 16 with ulcerative colitis), at baseline (T0) we found anti-S1 IgG positivity in four patients (8.7%), all asymptomatic: one patient treated with adalimumab, two patients treated with ustekinumab, and one patient treated with vedolizumab, who, according to the study’s exclusion criterion, were not further included in the analyses. Therefore, our study included 42 patients, 28 with Crohn’s disease (66.6%) and 14 with ulcerative colitis (33.3%), whose baseline characteristics are summarised in Table 1. Twenty-one patients were on adalimumab (50.0%), eight on vedolizumab (19.0%), seven on infliximab (16.7%), and six on ustekinumab (14.3%).

Table 1.
Patients’ characteristics subdivided according to inflammatory bowel disease category.
3.2. Response Rate to COVID-19 Vaccination
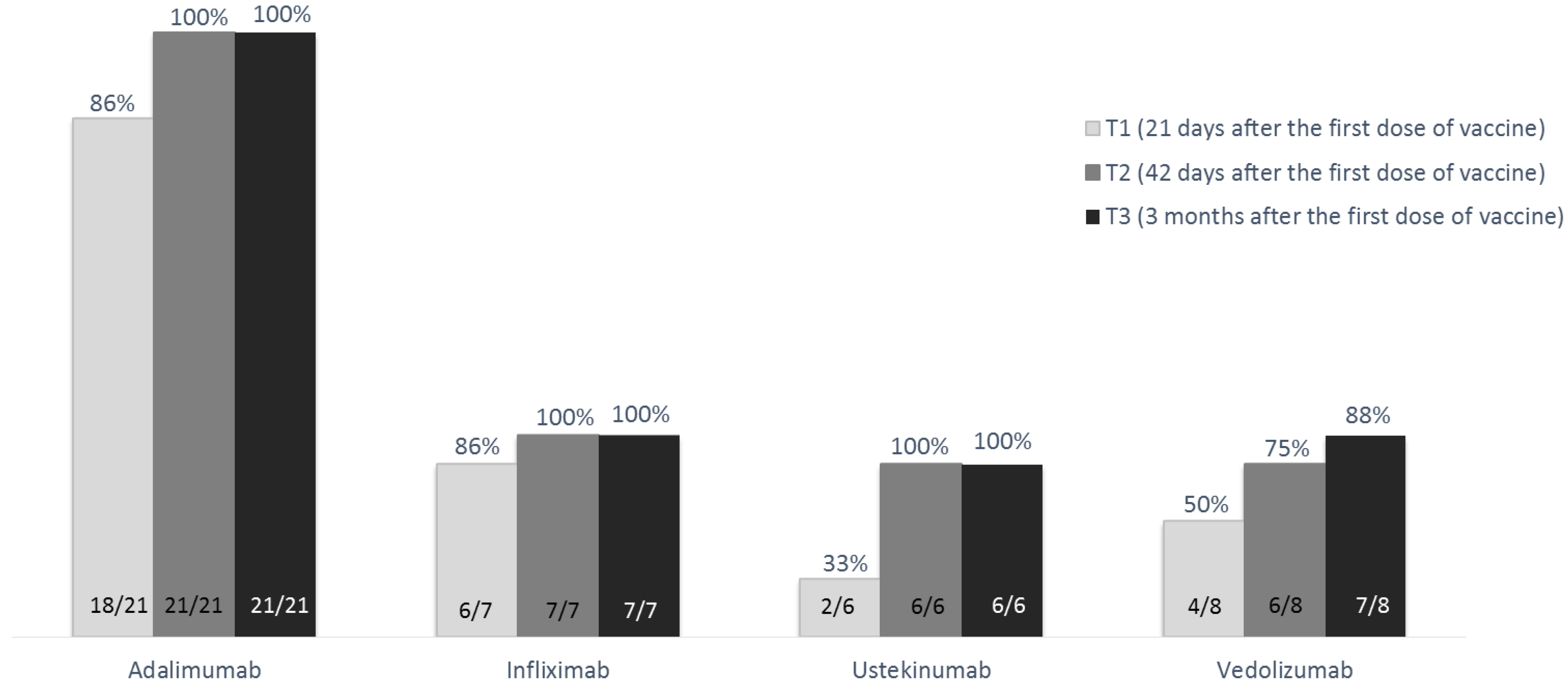
The overall response rate to COVID-19 vaccination was 97.6% (41/42). At T1 the response rate was 85.7% (18/21) in patients on adalimumab, 85.7% (6/7) in those on infliximab, 50.0% (4/8) in those on vedolizumab, and 33.3% (2/6) in those on ustekinumab (p = 0.031). At this timepoint, patients treated with anti-TNF drugs showed a significantly higher response rate than those treated with other biological drugs (85.7% versus 42.9%; p = 0.009). At T2, the response rate increased, and all patients on adalimumab, infliximab, and ustekinumab developed an antibody level compatible with response to vaccination (i.e., anti-S1 IgG >10 U/mL), while this threshold was reached by 6/8 patients on vedolizumab (75.0%, p = 0.03). At T3, the response rate was maintained in patients on adalimumab, infliximab, and ustekinumab, and increased to 87.5% (7/8 patients) in those on vedolizumab (p = 0.226) (Figure 2).
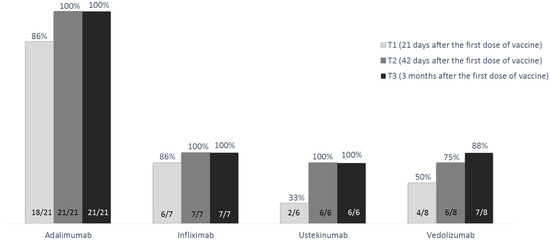
Figure 2.
Rate of response to the BNT162b2 m-RNA COVID-19 vaccine (Pfizer-BioNTech), subdivided according to biological drugs, at the various study time-points in the 42 patients without previous COVID-19 exposure.
At all timepoints, considering only patients who had a response to vaccination, we observed no statistically significant difference in the anti-S1 antibody levels among the various biological drugs (T1 p = 0.107; T2 p = 0.836, T3 p = 0.434).
At the time of vaccination, and during the study, seven patients (16.7%) were on concomitant steroids (median daily dose, 5 mg), with no influence on the rate of response at all timepoints as compared to patients not on steroids (T1, p = 0.647; T2, p = 0.746; T3, p = 0.365). During the study, nine patients (21.4%) had a mildly active disease that did not require treatment modification: at T1 (p = 0.953), T2 (p = 0.900), and T3 (p = 0.481), these patients did not have a different response rate to vaccination as compared to those in complete remission. Lastly, 46.4% of patients treated with anti-TNF, 100% of patients treated with ustekinumab, and 37.5% of patients treated with vedolizumab had been treated with immunosuppressive drugs (i.e., azathioprine/6-mercaptopurine, methotrexate) in the past, and in a pooled analysis we observed no significant difference in vaccination response between patients who had or had not received immunosuppressive drugs in their history.
3.3. Safety
The overall incidence of self-reported side effects—all mild-to-moderate in degree—following vaccine administration was 37.0%, with no severe side effects (Table 2). Noteworthy, none of the patients reported an IBD flare during the study, and no difference rate in side effects was reported among the various biological drugs (p = 0.140).

Table 2.
Main side effects following COVID-19 vaccination in patients with inflammatory bowel disease.
4. Discussion
Patients with IBD often need to be treated with biological drugs in the course of their disease, and either anti-TNF or other biological drugs are commonly used to obtain remission of disease [15]. In the course of the COVID-19 pandemic, patients with IBD treated with biological drugs were the focus of attention due to the fear of a potentially more severe disease course, and vaccination against COVID-19 was recommended by scientific societies and expert consensus [10,11]. Indeed, despite both the willingness to get vaccinated and the actual uptake of vaccines among patients with IBD being elevated, the rate of response to a full-course of COVID-19 vaccination has not been completely described in these patients [12,13,16,17,18]. Therefore, we deemed it of interest to prospectively assess the antibody response to COVID-19 vaccination among patients with IBD treated with biologics, with the aim to provide a relevant piece of information to clinicians managing these patients and to help inform vaccination programs.
In this study, we observed that following a full-course of COVID-19 mRNA vaccination, 97.6% of patients with IBD on stable biological drug treatment developed a complete antibody response. In particular, an earlier, complete response was observed among patients on anti-TNF drugs, either adalimumab or infliximab, as compared to patients on ustekinumab or vedolizumab, where the response rate lagged behind, although it was ultimately reached by almost all patients following completion of the vaccination cycle. Among the responders, the antibodies levels were not different across the various biological drugs at all study timepoints. Our results are somehow at odds with those obtained in the CLARITY study, as we observed that patients treated with anti-TNF drugs were more likely to achieve a response as compared to patients treated with other biological drugs, while in the CLARITY study patients on anti-TNF had a blunted response following a single dose of the BNT162b2 vaccine, although we have to emphasise that in our study we considered not only the initial response alone—as was done in the CLARITY study—but also the response rate following a complete vaccination cycle [12].
We found no association between concomitant steroid treatment and response to vaccination, although it must be emphasised that the median daily steroid dose in our population was quite low, and systemic corticosteroids have a dose-dependent effect on vaccine response [19]. Furthermore, we also assessed whether disease activity might have influenced the response to COVID-19 vaccination and observed no different response between patients with mildly active disease and those in complete remission. Lastly, although treatment with immunosuppressive drugs was not concomitant, we found no difference in antibody response between patients who had, or had not, received immunosuppressive drugs in their medical history. We feel that these findings may help counselling and programming vaccination in these patients.
Lastly, as far as the safety profile of the COVID-19 vaccination in patients with IBD is concerned, the rate and degree of side effects to vaccination were consistent with those reported in the literature in subjects without IBD, and no new or unexpected side effects were observed [20]. Noteworthy, no flares of IBD were observed during the vaccination cycle, and no patient required any adjustment in the dose of the biological drug.
This study undoubtedly has some limitations: first of all, our study population was heterogeneous regarding the biological drug used, and the majority of patients was treated with anti-TNF. This finding is mainly related to the national guideline indications for the use of biological drugs in Italy, although we feel that providing vaccination response data in patients on different biological drugs may be a strength of our study rather than a weak point. Secondly, we assessed antibody response and not more sophisticated means of assessing immune response to vaccination, although also in this case we feel that assessing antibody response might provide a tool available in everyday clinical practice, although we are well aware of the fact that both the level of protection of specific anti-SARS-CoV-2 antibodies and their duration are still largely unknown; lastly, the follow-up was 3 months and we were not able to elaborate on the persistence of the antibody response beyond this period or whether these patients were actually protected from severe forms of COVID-19. This study also has some strengths, such as its prospective design, the use of several timepoints for the assessment of response in the course of the vaccination cycle, and the use of biological monotherapy that allowed to evaluate data avoiding the confounding factor of combination therapy with thiopurine. Due to the high response observed we were unable to sub-categorise patients according to age, although we feel that larger studies on this issue will be able to provide an answer regarding the response to COVID-19 vaccination among elderly patients with IBD.
In conclusion, the rate of complete response to a full cycle of BNT162b2 vaccine in patients with IBD on biological treatment seems optimal, without a significant difference in the rate of overall response among the various biological drugs. Although patients on anti-TNF demonstrate a complete response earlier than patients treated with biological drugs with different modalities of action; this latter group of patients also seemed to obtain full vaccine coverage at the end of the vaccination cycle.
We are aware that our results need to be confirmed in larger and even more heterogeneous populations, and that the duration of response should be assessed in the long-term. However, we feel that these encouraging results should be used to further support vaccination against COVID-19 in patients with IBD on biological treatment, independently of the drug used, and hope they might help counsel these patients and increase the adherence to vaccination in this fragile population.
Author Contributions
G.B.—study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, and critical revision of manuscript; E.G.G.—analysis and interpretation of data, drafting of the manuscript, and critical revision of manuscript; G.D.R., G.I.—critical revision of manuscript; E.G., I.G., G.G., F.B., E.F., M.G.D.—acquisition of data and critical revision of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Regione Liguria (CER) (protocol code 164/2021, approved on 11 March 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are not publicly available due to privacy reasons.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fiocchi, C. Inflammatory bowel disease pathogenesis: Where are we? J. Gastroenterol. Hepatol. 2015, 30 (Suppl. S1), 12–18. [Google Scholar] [CrossRef] [PubMed]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L. ECCO -EpiCom. The burden of inflammatory bowel disease in Europe. J. Crohn’s Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohn’s Colitis 2014, 8, 443–468. [Google Scholar] [CrossRef] [PubMed]
- deBruyn, J.; Fonseca, K.; Ghosh, S.; Panaccione, R.; Gasia, M.F.; Ueno, A.; Kaplan, G.G.; Seow, C.H.; Wrobel, I. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance infliximab therapy: A randomized trial. Inflamm. Bowel Dis. 2016, 22, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Wasan, S.K.; Zullow, S.; Berg, A.; Cheifetz, A.S.; Ganley-Leal, L.; Farraye, F.A. Herpes zoster vaccine response in inflammatory bowel disease patients on low-dose immunosuppression. Inflamm. Bowel Dis. 2016, 22, 1391–1396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marín, A.C.; Gisbert, J.P.; Chaparro, M. Immunogenicity and mechanisms impairing the response to vaccines in inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 11273–11281. [Google Scholar] [CrossRef] [PubMed]
- Andrisani, G.; Frasca, D.; Romero, M.; Armuzzi, A.; Felice, C.; Marzo, M.; Pugliese, D.; Papa, A.; Mocci, G.; De Vitis, I.; et al. Immune response to influenza A/H1N1 vaccine in inflammatory bowel disease patients treated with anti TNF-α agents: Effects of combined therapy with immunosuppressants. J. Crohn's Colitis 2013, 7, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Shirai, S.; Hara, M.; Sakata, Y.; Tsuruoka, N.; Yamamoto, K.; Shimoda, R.; Gomi, Y.; Yoshii, H.; Fujimoto, K.; Iwakiri, R. Immunogenicity of Quadrivalent Influenza Vaccine for Patients with Inflammatory Bowel Disease Undergoing Immunosuppressive Therapy. Inflamm. Bowel Dis. 2018, 24, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Santos-Antunes, J.; Rodrigues, S.; Lopes, S.; Macedo, G. Treatment with infliximab or azathioprine negatively impact the efficacy of hepatitis B vaccine in inflammatory bowel disease patients. J. Gastroenterol. Hepatol. 2015, 30, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.A.; Melmed, G.Y.; McGovern, D.P.B.; Rai, V.; Krammer, F.; Rubin, D.T.; Abreu, M.T.; Dubinsky, M.C. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: Recommendations from an international consensus meeting. Gut 2021, 70, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Moran, G.W.; Gaya, D.R.; Raine, T.; Hart, A.; Kennedy, N.A.; Lindsay, J.O.; MacDonald, J.; Segal, J.P.; Sebastian, S.; et al. SARS-CoV-2 vaccination for patients with inflammatory bowel disease: A British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement. Lancet Gastroenterol. Hepatol. 2021, 6, 218–224. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Lin, S.; Goodhand, J.R.; Chanchlani, N.; Hamilton, B.; Bewshea, C.; Nice, R.; Chee, D.; Cummings, J.R.F.; Fraser, A.; et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021, 70, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-Y.; Dixon, R.; Pazos, V.M.; Gnjatic, S.; Colombel, J.-F.; Cadwell, K.; Gold, S.; Helmus, D.; Neil, J.A.; Sota, S.; et al. Serologic Response to Messenger RNA Coronavirus Disease 2019 Vaccines in Inflammatory Bowel Disease Patients Receiving Biologic Therapies. Gastroenterology 2021, 161, 715–718.e4. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 4 May 2022).
- Mao, R.; Hu, P.J. The Future of IBD Therapy: Where Are We and Where Should We Go Next? Dig. Dis. 2016, 34, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Bodini, G.; DeMarzo, M.G.; Casagrande, E.; De Maria, C.; Kayali, S.; Ziola, S.; Giannini, E.G. Concerns related to COVID-19 pandemic among patients with inflammatory bowel disease and its influence on patient management. Eur. J. Clin. Investig. 2020, 50, e13233. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Demarzo, M.G.; Bodini, G. Elevated adherence to vaccination against SARS-CoV-2 among patients with Inflammatory Bowel Disease. J. Crohn’s Colitis 2021, 15, 2142–2143. [Google Scholar] [CrossRef] [PubMed]
- Schell, T.L.; Richard, L.J.; Tippins, K.; Russ, R.K.; Hayney, M.S.; Caldera, F. High but Inequitable COVID-19 Vaccine Uptake Among Patients with Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization—Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2011, 60, 1–64. [Google Scholar]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).