Hospital-Based Preliminary Observations of Dietary Intake and Physical Activity in Saudi Patients with Colorectal Polyps: A Call for Nutrition Care Integration after Polypectomy Procedure
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
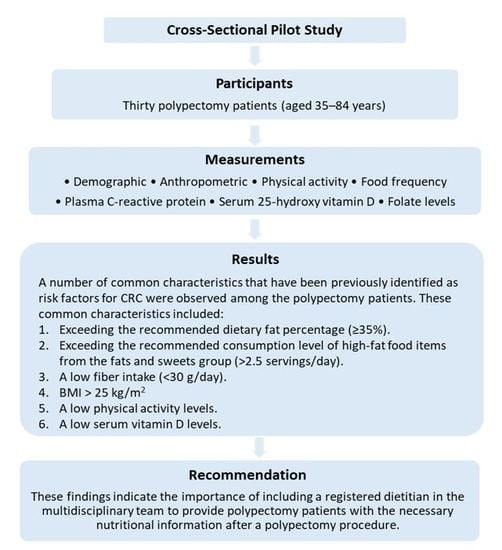
2.2. Subjects and Recruitment
2.3. Study Instruments and Data Collection
2.4. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Dietary Assessment
3.2.1. Estimation of Energy and Macronutrient Intakes Using the FFQ:
3.2.2. Estimation of Food Intake Using the FFQ:
3.3. Physical Activity Assessment
3.4. Measurements of Serum Vitamin D, Folate, and CRP
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Alsanea, N.; Abduljabbar, A.S.; Alhomoud, S.; Ashari, L.H.; Hibbert, D.; Bazarbashi, S. Colorectal cancer in Saudi Arabia: Incidence, survival, demographics and implications for national policies. Ann. Saudi Med. 2015, 35, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Winawer, S.J.; Zauber, A.G.; Ho, M.N.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Waye, J.D.; Schapiro, M.; Bond, J.H.; Panish, J.F. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl. J. Med. 1993, 329, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Macrae, F. Colorectal Cancer: Epidemiology, Risk Factors, and Protective Factors. Available online: https://www.uptodate.com/contents/colorectal-cancer-epidemiology-risk-factors-and-protective-factors (accessed on 2 August 2018).
- Wong, M.C.; Ding, H.; Wang, J.; Chan, P.S.; Huang, J. Prevalence and risk factors of colorectal cancer in Asia. Intest. Res. 2019, 17, 317–329. [Google Scholar] [CrossRef]
- Zauber, A.G.; Winawer, S.J. Initial management and follow-up surveillance of patients with colorectal adenomas. Gastroenterol. Clin. 1997, 26, 85–101. [Google Scholar] [CrossRef]
- Yood, M.U.; Oliveria, S.; Boyer, J.G.; Wells, K.; Stang, P.; Johnson, C.C. Colon polyp recurrence in a managed care population. Arch. Intern. Med. 2003, 163, 422–426. [Google Scholar] [CrossRef]
- Joshu, C.E.; Parmigiani, G.; Colditz, G.A.; Platz, E.A. Opportunities for the primary prevention of colorectal cancer in the United States. Cancer Prev. Res. 2012, 5, 138–145. [Google Scholar] [CrossRef]
- Oruç, Z.; Kaplan, M.A. Effect of exercise on colorectal cancer prevention and treatment. World J. Gastrointest. Oncol. 2019, 11, 348–366. [Google Scholar] [CrossRef]
- Gingras, D.; Béliveau, R. Colorectal cancer prevention through dietary and lifestyle modifications. Cancer Microenviron. 2011, 4, 133–139. [Google Scholar] [CrossRef]
- Hughes, L.A.E.; Simons, C.C.; van den Brandt, P.A.; van Engeland, M.; Weijenberg, M.P. Lifestyle, diet, and colorectal cancer risk according to (epi)genetic instability: Current evidence and future directions of molecular pathological epidemiology. Curr. Colorectal Cancer Rep. 2017, 13, 455–469. [Google Scholar] [CrossRef]
- Ahmed, F. Effect of diet, life style, and other environmental/chemopreventive factors on colorectal cancer development, and assessment of the risks. J. Environ. Sci. Health Part C 2004, 22, 91–147. [Google Scholar] [CrossRef] [PubMed]
- Helander, S.; Heinavaara, S.; Sarkeala, T.; Malila, N. Lifestyle in population-based colorectal cancer screening over 2-year follow-up. Eur. J. Public Health 2018, 28, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.B.; Misyak, S.A.; Hontecillas, R.; Bassaganya-Riera, J. Dietary modulation of inflammation-induced colorectal cancer through PPAR gamma. PPAR Res. 2009. [Google Scholar] [CrossRef] [PubMed]
- Tsilidis, K.K.; Branchini, C.; Guallar, E.; Helzlsouer, K.J.; Erlinger, T.P.; Platz, E.A. C-reactive protein and colorectal cancer risk: A systematic review of prospective studies. Int. J. Cancer 2008, 123, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Toriola, A.T.; Cheng, T.Y.; Neuhouser, M.L.; Wener, M.H.; Zheng, Y.; Brown, E.; Miller, J.W.; Song, X.; Beresford, S.A.; Gunter, M.J.; et al. Biomarkers of inflammation are associated with colorectal cancer risk in women but are not suitable as early detection markers. Int. J. Cancer 2013, 132, 2648–2658. [Google Scholar] [CrossRef]
- Lai, C.C.; You, J.F.; Yeh, C.Y.; Chen, J.S.; Tang, R.; Wang, J.Y.; Chin, C.C. Low preoperative serum albumin in colon cancer: A risk factor for poor outcome. Int. J. Colorectal Dis. 2011, 26, 473–481. [Google Scholar] [CrossRef]
- Abar, L.; Vieira, A.R.; Aune, D.; Sobiecki, J.G.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.C.; Chan, D.S.M.; Schlesinger, S.; et al. Height and body fatness and colorectal cancer risk: An update of the WCRF-AICR systematic review of published prospective studies. Eur. J. Nutr. 2018, 57, 1701–1720. [Google Scholar] [CrossRef]
- Hughes, L.A.E.; Simons, C.C.; van den Brandt, P.A.; Goldbohm, R.A.; van Engeland, M.; Weijenberg, M.P. Body size and colorectal cancer risk after 16.3 years of follow-up: An analysis from the Netherlands cohort study. Am. J. Epidemiol. 2011, 174, 1127–1139. [Google Scholar] [CrossRef]
- Kabat, G.C.; Heo, M.; Wactawski-Wende, J.; Messina, C.; Thomson, C.A.; Wassertheil-Smoller, S.; Rohan, T.E. Body fat and risk of colorectal cancer among postmenopausal women. Cancer Causes Control 2013, 24, 1197–1205. [Google Scholar] [CrossRef]
- Matsuo, K.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Sugawara, Y.; Sasazuki, S.; Nagata, C.; Tamakoshi, A.; Wakai, K.; Inoue, M.; et al. Association between body mass index and the colorectal cancer risk in Japan: Pooled analysis of population-based cohort studies in Japan. Ann. Oncol. 2012, 23, 479–490. [Google Scholar] [CrossRef]
- Shaw, E.; Farris, M.S.; Stone, C.R.; Derksen, J.W.G.; Johnson, R.; Hilsden, R.J.; Friedenreich, C.M.; Brenner, D.R. Effects of physical activity on colorectal cancer risk among family history and body mass index subgroups: A systematic review and meta-analysis. BMC Cancer 2018, 18, 71. [Google Scholar] [CrossRef] [PubMed]
- Van Blarigan, E.L.; Meyerhardt, J.A. Role of physical activity and diet after colorectal cancer diagnosis. J. Clin. Oncol. 2015, 33, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Scragg, R.; Camargo, C.A. Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: Results from the Third National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2008, 168, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Skender, S.; Bohm, J.; Schrotz-King, P.; Chang-Claude, J.; Siegel, E.M.; Steindorf, K.; Owen, R.W.; Ose, J.; Hoffmeister, M.; Brenner, H.; et al. Plasma 25-hydroxyvitamin D-3 levels in colorectal cancer patients and associations with physical activity. Nutr. Cancer 2017, 69, 229–237. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization—Noncommunicable Diseases (NCD) Country Profiles. 2018. Available online: https://www.who.int/nmh/countries/sau_en.pdf?ua=1 (accessed on 4 February 2019).
- Moradi-Lakeh, M.; El Bcheraoui, C.; Afshin, A.; Daoud, F.; AlMazroa, M.A.; Al Saeedi, M.; Basulaiman, M.; Memish, Z.A.; Al Rabeeah, A.A.; Mokdad, A.H. Diet in Saudi Arabia: Findings from a nationally representative survey. Public Health Nutr. 2017, 20, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Al-Hazzaa, H.M. Physical inactivity in Saudi Arabia revisited: A systematic review of inactivity prevalence and perceived barriers to active living. Int. J. Health Sci. 2018, 12, 50–64. [Google Scholar]
- Lohman, T.; Roche, A.; Martorell, R. Anthropometric Standardization Reference Manual; Human Kinetics: Champaign, IL, USA, 1988. [Google Scholar]
- Alissa, E.M.; Bahjri, S.M.; Al-Ama, N.; Ahmed, W.H.; Starkey, B.; Ferns, G.A.A. Dietary vitamin A may be a cardiovascular risk factor in a Saudi population. Asia Pac. J. Clin. Nutr. 2005, 14, 137–144. [Google Scholar]
- Zello, G.A. Dietary Reference Intakes for the macronutrients and energy: Considerations for physical activity. Appl. Physiol. Nutr. Metab. 2006, 31, 74–79. [Google Scholar] [CrossRef]
- Ministry of Health. The Dietary Guidelines for Saudis. Available online: https://www.moh.gov.sa/en/Ministry/MediaCenter/Publications/Pages/Publications-2013-01-15.aspx (accessed on 16 March 2019).
- Alkhaldy, A. Inter-Individual Variability of Polyphenol Metabolism and Colonic Health. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 2014. [Google Scholar]
- Alkhaldy, A.A.; Rizq, N.K.; Del Jaylan, S.A.; Alkendi, E.A.; Alghamdi, W.M.; Alfaraidi, S.M. Dietary Intake and Physical Activity in Relation to Insulin Resistance in Young Overweight Saudi Females: An Exploratory Pilot Study. Prev. Nutr. Food Sci. 2019, 24, 373–380. [Google Scholar] [CrossRef]
- Al-Hazzaa HM, A.-A.M. A Self-reported questionnaire for the assessment of physical activity in youth 15–25 years: Development, reliability and construct validity. Arab J. Food Nutr. 2003, 4, 279–291. [Google Scholar]
- Zhang, Q.L.; Zhao, L.G.; Li, H.L.; Gao, J.; Yang, G.; Wang, J.; Zheng, W.; Shu, X.O.; Xiang, Y.B. The joint effects of major lifestyle factors on colorectal cancer risk among Chinese men: A prospective cohort study. Int. J. Cancer 2018, 142, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.; Holubec, H.; Bhattacharyya, A.K.; Nguyen, H.; Payne, C.M.; Zaitlin, B.; Bernstein, H. Carcinogenicity of deoxycholate, a secondary bile acid. Arch. Toxicol. 2011, 85, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Øines, M.; Helsingen, L.M.; Bretthauer, M.; Emilsson, L. Epidemiology and risk factors of colorectal polyps. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Encarnacao, J.C.; Abrantes, A.M.; Pires, A.S.; Botelho, M.F. Revisit dietary fiber on colorectal cancer: Butyrate and its role on prevention and treatment. Cancer Metastasis Rev. 2015, 34, 465–478. [Google Scholar] [CrossRef]
- Tuan, J.; Chen, Y.X. Dietary and Lifestyle Factors Associated with Colorectal Cancer Risk and Interactions with Microbiota: Fiber, Red or Processed Meat and Alcoholic Drinks. Gastrointest. Tumors 2016, 3, 17–24. [Google Scholar] [CrossRef]
- Yao, Y.; Suo, T.; Andersson, R.; Cao, Y.; Wang, C.; Lu, J.; Chui, E. Dietary fibre for the prevention of recurrent colorectal adenomas and carcinomas. Cochrane Database Syst. Rev. 2017, 1. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Berndt, S.I.; de González, A.B.; Coleman, H.G.; Schoen, R.E.; Hayes, R.B.; Huang, W.Y. Prospective investigation of body mass index, colorectal adenoma, and colorectal cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. J. Clin. Oncol. 2013, 31, 2450–2459. [Google Scholar] [CrossRef]
- Ben, Q.; An, W.; Jiang, Y.; Zhan, X.; Du, Y.; Cai, Q.C.; Gao, J.; Li, Z. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology 2012, 142, 762–772. [Google Scholar] [CrossRef]
- Wolin, K.Y.; Yan, Y.; Colditz, G.A. Physical activity and risk of colon adenoma: A meta-analysis. Br. J. Cancer 2011, 104, 882–885. [Google Scholar] [CrossRef]
- McClellan, J.L.; Steiner, J.L.; Day, S.D.; Enos, R.T.; Davis, M.J.; Singh, U.P.; Murphy, E.A. Exercise effects on polyp burden and immune markers in the Apc (Min+) mouse model of intestinal tumorigenesis. Int. J. Oncol. 2014, 45, 861–868. [Google Scholar] [CrossRef]
- Bryce, C. Association of 25-OH Vitamin D Status with Findings on Screening Colonoscopy. Mil. Med. 2018, 183, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. A critical review of Vitamin D and Cancer: A report of the IARC Working Group. Dermatoendocrinol 2009, 1, 25–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahendra, A.; Karishma, B.K.C.; Sharma, T.; Bansal, N.; Bansal, R.; Gupta, S. Vitamin D and gastrointestinal cancer. J. Lab. Physicians 2018, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Mayorga, G.; Larriba, M.; Crespo, P.; Muñoz, A. Mechanisms of action of vitamin D in colon cancer. J. Steroid Biochem. 2018, 185, 1–6. [Google Scholar] [CrossRef]
- El-Shami, K.; Oeffinger, K.C.; Erb, N.L.; Willis, A.; Bretsch, J.K.; Pratt-Chapman, M.L.; Cannady, R.S.; Wong, S.L.; Rose, J.; Barbour, A.L.; et al. American Cancer Society colorectal cancer survivorship care guidelines. CA Cancer J. Clin. 2015, 65, 428–455. [Google Scholar] [CrossRef]
- Division of Cancer Prevention. Post Polypectomy Nutrition Clinic. Available online: https://www.mcgill.ca/cancerprev/clinical/post-polypectomy-nutrition-clinic (accessed on 3 June 2019).
| Variables | n | % |
|---|---|---|
| Age (years) | ||
| 35–45 | 5 | 17 |
| 46–55 | 6 | 20 |
| 56–65 | 13 | 43 |
| 66–75 | 5 | 17 |
| 76–85 | 1 | 3 |
| Income (Saudi Riyal /month) | ||
| <5000 | 9 | 30 |
| 5000–10,000 | 8 | 27 |
| 10,000–15,000 | 6 | 20 |
| >15,000 | 7 | 23 |
| Education | ||
| Uneducated | 2 | 7 |
| Primary | 7 | 23 |
| Secondary | 3 | 10 |
| High school | 6 | 20 |
| College/diploma | 11 | 37 |
| Higher education | 1 | 3 |
| Anthropometric | Median | IQR (Q1–Q3) |
| Height (cm) | 164 | 154–169 |
| Weight (kg) | 70 | 63–73 |
| BMI (kg/m2) | 26 | 24–28 |
| BMI category | n | % |
| Normal weight (18.5–24.9 kg/m2) | 10 | 33 |
| Overweight (25.0–29.9 kg/m2) | 16 | 53 |
| Obese (>30.0 kg/m2) | 4 | 17 |
| Median | IQR (Q1–Q3) | % of Energy Intake | |
|---|---|---|---|
| Energy (kcal/day) | 1600 | 1384–2222 | |
| Carbohydrate (g/day) | 211 | 166–305 | 53 |
| Fat (g/day) | 65 | 49–80 | 36 |
| Protein (g/day) | 67 | 54–83 | 17 |
| Fiber (g/day) | 13 | 10–21 | |
| Cholesterol (mg/day) | 169 | 136–237 | |
| SFA (g/day) * | 24 | 17–26 | |
| MUFA (g/day) * | 21 | 17–27 | |
| PUFA (g/day) * | 12 | 10–22 |
| Food Group * | Food Items (Daily) | Overall (n = 30) | ||
|---|---|---|---|---|
| Median | Q1 | Q3 | ||
| Bread, Cereal, Rice, and Pasta group (recommended: 6–11 servings daily) | Cornflakes | 0.0 | 0.0 | 0.0 |
| Rice, kabsa, pasta, and saleeg | 0.7 | 0.4 | 1.4 | |
| White bread (e.g., white Arabic, white toast, and French toast) | 0.9 | 0.3 | 1.4 | |
| Brown bread | 0.2 | 0.0 | 1.0 | |
| Savories (e.g., pizza, pies, chips) | 0.3 | 0.1 | 0.4 | |
| Total | 2.5 | 1.9 | 4.0 | |
| Fruit group (recommended: 2–4 servings daily) | Fruit (e.g., apples, bananas, grapes, melons, and oranges) | 1.8 | 0.9 | 3.5 |
| Dried fruits | 0.1 | 0.0 | 0.2 | |
| Dates (dried and fresh) | 0.2 | 0.1 | 1.0 | |
| Fresh juices | 0.2 | 0.1 | 0.2 | |
| Total | 3.0 | 1.5 | 4.9 | |
| Vegetable group (recommended: 3–5 servings daily) | Salad vegetables (e.g., cucumbers, tomatoes, lettuce, and peppers) | 1.7 | 1.1 | 3.8 |
| Other vegetables (e.g., cabbage, cauliflower, aubergines, okra, onions, and spinach) | 1.5 | 0.8 | 2.0 | |
| Cooked, boiled, and fried potatoes | 0.2 | 0.2 | 0.3 | |
| Total | 4.0 | 2.6 | 5.6 | |
| Protein food group (recommended: 2–3 servings daily) | Peas, beans, and lentils | 0.3 | 0.1 | 0.6 |
| Nuts and seeds (e.g., peanuts, melon seeds, and pistachio nuts) | 0.4 | 0.1 | 0.6 | |
| Red meat (beef, lamb, brain, kidneys, and liver) | 0.3 | 0.2 | 0.4 | |
| Poultry (chicken) | 0.2 | 0.2 | 1.0 | |
| Meat products (e.g., shawarmas and burgers) | 0.1 | 0.0 | 0.2 | |
| Fish and fish products | 0.2 | 0.1 | 0.3 | |
| Egg and egg dishes | 0.4 | 0.2 | 0.4 | |
| Total | 2.1 | 1.6 | 2.6 | |
| Dairy products group (recommended: 2–3 servings daily) | Milk | 0.2 | 0.1 | 1.0 |
| Cheeses | 0.8 | 0.2 | 1.8 | |
| Yoghurts | 0.8 | 0.2 | 1.1 | |
| Ice cream | 0.1 | 0.0 | 0.1 | |
| Total | 2.4 | 1.2 | 3.1 | |
| Fats and sweets group (recommended: sparingly) | Fats (e.g., butter, olive oil, and vegetable oil) | 2.0 | 1.2 | 2.4 |
| Syrups, preserves, and sweeteners | 0.6 | 0.2 | 1.2 | |
| Total | 2.5 | 1.8 | 3.2 | |
| Miscellaneous | Black and green teas and coffees | 2.0 | 1.0 | 3.9 |
| Other beverages (e.g., juices and soda drinks) | 0.4 | 0.3 | 1.1 | |
| Sauces | 0.2 | 0.0 | 0.3 | |
| Pickles and spices | 1.3 | 0.7 | 2.0 | |
| Ginger, mint, parsley, and coriander | 1.2 | 0.3 | 2.0 | |
| Level (Score) | Overall (n = 30) |
|---|---|
| n (%) | |
| Very active (>40) | 0 (0) |
| Active (30–39) | 2 (7) |
| Moderate activity (20–29) | 3 (10) |
| Non-active (<20) | 25 (83) |
| Biomarkers | Median | IQR |
|---|---|---|
| Serum 25-hydroxy vitamin D (nmol/L) | 47.2 (n = 24/30) | 29.0–76.3 |
| Serum folate (nmol/L) | 23.1 (n = 24/30) | 17.2–31.5 |
| C-reactive protein (mg/dL) | ||
| Normal (n = 8/21) | <3.0 | - |
| Raised CRP (n = 13/21) | 11.8 | 7.5–15.4 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhaldy, A.A. Hospital-Based Preliminary Observations of Dietary Intake and Physical Activity in Saudi Patients with Colorectal Polyps: A Call for Nutrition Care Integration after Polypectomy Procedure. Gastrointest. Disord. 2020, 2, 96-106. https://doi.org/10.3390/gidisord2020009
Alkhaldy AA. Hospital-Based Preliminary Observations of Dietary Intake and Physical Activity in Saudi Patients with Colorectal Polyps: A Call for Nutrition Care Integration after Polypectomy Procedure. Gastrointestinal Disorders. 2020; 2(2):96-106. https://doi.org/10.3390/gidisord2020009
Chicago/Turabian StyleAlkhaldy, Areej Ali. 2020. "Hospital-Based Preliminary Observations of Dietary Intake and Physical Activity in Saudi Patients with Colorectal Polyps: A Call for Nutrition Care Integration after Polypectomy Procedure" Gastrointestinal Disorders 2, no. 2: 96-106. https://doi.org/10.3390/gidisord2020009
APA StyleAlkhaldy, A. A. (2020). Hospital-Based Preliminary Observations of Dietary Intake and Physical Activity in Saudi Patients with Colorectal Polyps: A Call for Nutrition Care Integration after Polypectomy Procedure. Gastrointestinal Disorders, 2(2), 96-106. https://doi.org/10.3390/gidisord2020009