Abstract
Introduction: Helicobacter pylori (H. pylori) is a well-recognized risk factor for upper gastrointestinal bleeding (UGIB). The exposure to tissue plasminogen activator (tPA), anti-platelets, and anticoagulants increases the risk of UGIB in acute ischemic stroke (AIS) patients, the risk stratification of H. pylori infection is not known. In this retrospective cross-sectional study, we aimed to evaluate the relationship between H. pylori and GIB in patients hospitalized with AIS. Methods: In the nationwide data, hospitalization for AIS was identified by primary diagnosis using International Classification of Diseases, clinical modification (ICD-9-CM) codes. Subgroup of patients with GIB and H. pylori were identified in AIS cohort. A stepwise multivariable logistic regression model was fitted to evaluate the outcome of upper GIB and role of H. Pylori in UGIB. Results: Overall 4,224,924 AIS hospitalizations were identified, out of which 18,629 (0.44%) had UGIB and 3122 (0.07%) had H. pylori. The prevalence of H. pylori-induced UGIB among UGIB in AIS was 3.05%. The prevalence of UGIB was markedly elevated among the H. pylori infection group (18.23% vs. 0.43%; p < 0.0001) compared to the non-H. pylori group. In multivariable regression analysis, H. pylori was associated with markedly elevated odds of UGIB (aOR:27.75; 95%CI: 21.07–36.55; p < 0.0001). Conclusion: H. pylori infection had increased risk-adjusted occurrence of UGIB amongst the AIS hospitalized patients. H. pylori testing may improve risk stratification for UGIB and lower the health care cost burden in stroke hospitalization.
1. Introduction
Patients with acute ischemic stroke (AIS) have various complications, including an estimated 1–5% incidence of gastrointestinal bleeding (GIB) [1,2,3]. Multiple risk factors are associated with GIB post-stroke, including older age, presence of underlying pathology, past history of peptic ulcer, history of hemorrhage, stroke severity at admission, and increased exposure to tissue plasminogen activator (tPA), antiplatelets, and anticoagulants [2,3]. The most common cause of upper GIB (UGIB) is peptic ulcer followed by less common causes such as esophagitis, erosive gastritis, duodenitis, Mallory–Weiss syndrome, angiodysplasia, neoplasm and Dieulafoy’s lesion [4,5,6,7]. The most common causes of lower GIB are diverticulosis, hemorrhoids, angiodysplasia, inflammatory disease, and cancer in descending frequency [8,9]. According to an international, prospective study called the Reduction of Atherothrombosis for Continued Health (REACH) Registry, >68,000 patients across six continents with established atherothrombotic or three atherothrombotic risk factors, 70% of 25,668 outpatients from 1599 practices in US have been treated with aspirin, 13% were given aspirin and antiplatelets, 8% aspirin and oral anticoagulants, and half among those who were not on aspirin were on anticoagulants or antiplatelet drugs [4]. Aspirin and antiplatelets cause ulcers and erosion, which increases risk of GIB, while anticoagulants exacerbate bleeding from existing gastrointestinal lesions [10]. The impact of aspirin and anticoagulants on outcomes is not well-characterized [11,12,13,14,15]. Bleeding can lead to hemodynamic instability, and antithrombotic may need to be discontinued, leading to a prothrombotic state [16]. GIB has led to an increased hospital length of stay and poorer neurological prognosis, consequently having a significant impact on morbidity and mortality [3].
If potential risk factors for GIB in stroke can be identified, these could be used to reduce risk. One of the major risk factors for UGIB is peptic ulcer disease (PUD). Helicobacter pylori (H. pylori) and non-steroidal anti-inflammatory drug (NSAID) usage are two of the most common risk factors of PUD [17,18,19,20,21]. H. pylori is a gram-negative, spiral-shaped microaerophilic bacterium, colonizing the gastric mucosa that has been associated seroepidemiologically with atherosclerosis [22]. H. pylori may be a lifelong bacterial infection. Proton pump inhibitors and H2 antagonists reduce the risk of GIB and can be used in stroke patients who are at greater risk; hence, identifying risk factors for GIB could play a vital role in the management of stroke patients [22]. However, data regarding GIB in AIS are limited.
In this retrospective cross-sectional study, we aimed to ascertain the burden of GIB in patients hospitalized for AIS with serological evidence of H. pylori infection and to assess the risk of GIB with chronic use of aspirin and NSAIDs, and treatment with aspirin, antiplatelets, anticoagulants and tPA. We used the nationally representative Nationwide Inpatient Sample (NIS) from 2003 to 2014.
2. Materials and Methods
We had obtained the data from the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project (HCUP) NIS files between January 2003 and December 2014. The NIS is a deidentified, the publicly available, largest inpatient care database in the USA and contains discharge-level data provided by 46 states that participate in the HCUP. This administrative dataset contains data of approximate a 20% stratified sample of all US hospitals which representing more than 95% of the national population. Discharge weight are provided to calculate the national estimate. For each hospitalization, an individual entry is available with one principle diagnosis and each hospitalization has up to 24 secondary diagnosis and 15 procedural diagnosis. Detailed information on NIS is available at http://www.hcup-us.ahrq.gov/db/nation/nis/nisdde.jsp.
2.1. Study Population
We used the 9th revision of the International Classification of Diseases, clinical modification codes (ICD-9-CM) to identify adult patients admitted with a primary diagnosis of AIS (ICD-9-CM codes 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91). These codes have been previously validated and are 35% sensitive, 99% specific, 96% positive predictive value (PPVs), and 79% negative predictive value for the diagnosis of ischemic stroke [23]. Similarly, Patients with UGIB were identified using ICD-9-CM codes 530.xx-535.xx (PPVs: 66% for 532.xx, 61% for 531.xx, 534xx, and 1% for 533.xx) [24] and H. pylori using ICD-9-CM code 041.86 (PPVs: 100%) [25]. We used ICD-9-CM codes to identify independent predictors (covariates), including the comorbidities of hypertension, diabetes mellitus, hypercholesterolemia, atrial fibrillation, use of anticoagulant and antiplatelet medications, chronic use of NSAIDs and aspirin, smoking (current/past), and use of IV tPA. Supplementary Table S1 lists all ICD-9-CM codes that were used for this study.
Patients were divided into UGIB subgroups stratified by H. pylori status. Age < 18 years and admissions with missing data for age, sex, and race were excluded.
2.2. Patient and Hospital Characteristics
Patient characteristics of interest were sex, age, race, insurance status and concomitant diagnoses as defined above. Race was defined by white (referent), African American, Hispanic, Asian or Pacific Islander, and Native American. Insurance status was defined by Medicare (referent), Medicaid, Private Insurance, and Other/Self-pay/No charge. Severity of co-morbid conditions were defined by using Deyo’s modification of the Charlson co-morbidity index (Supplementary Table S2). NIS data covered thirty-one facilities to be teaching hospitals if they have an American Medical Association-approved residency program, and are a member of the Council of Teaching Hospitals.
2.3. Outcomes
Our primary outcome of interest was UGIB with H. pylori, and secondary outcomes were death during hospitalization, All Patients Refined Diagnosis Related Groups (APRDRG) Risk of Mortality, APRDRG Severity estimate, discharge disposition, length of stay (LoS), and cost of hospitalization.
2.4. Statistical Analysis
We used SAS (version 9.4) for the all statistical analyses. From NIS data, weighted values of patient-level observations were obtained to produce a nationally representative estimate of hospitalized patients from the entire US population. We had considered p-values of < 0.05 as significant. We used chi-square test to evaluate the differences between categorical variables and paired student’s t-test to analyse the differences between continuous variables including age, length of stay and cost of hospitalization. Hierarchical mixed-effects survey logistic regression models with weighted analysis were used for the categorical dependent variables, including UGIB and H. Pylori infection, in order to estimate odds ratio (OR) and 95% confidence interval for the association between UGIB and H. Pylori.
Three-level hierarchical models (demographics and patient-level factors nested within hospital-level factors) were created as random effects within the model for the primary outcome. In the multivariate analysis, we included demographics (age, gender, race), patient-level hospitalization variables (admission day, primary payer, admission type, Median Household Income Category), hospital-level variables (hospital region, teaching versus nonteaching hospital, hospital bed size), comorbidities (mentioned in Table 1), concurrent conditions including hypertension, diabetes mellitus, hypercholesterolemia, atrial fibrillation, obesity, hemorrhagic conversion, smoking status, drug abuse, alcohol abuse, medication use (anticoagulant and antiplatelet medication, chronic use of NSAIDs and aspirin), use of IV tPA during the same hospitalization or in a different institution within the 24 h prior to admission to the facility, and Charlson’s co-morbidity index (CCI).

Table 1.
Characteristics of H. pylori in upper GI bleeding among acute ischemic stroke population.
We investigated the variation in UGIB due to H. pylori by creating three separate stepwise hierarchical survey logistic regression models with weights to account for sampling strategy:
Model 1: Model with H. pylori.
Model 2: Model 1 + basic demographic-adjusted model including age, race, and sex with patient-level variables: admission day, primary payer and urgent/emergent admission, comorbidities, CCI, concurrent conditions like hypertension, diabetes mellitus, hypercholesterolemia, atrial fibrillation, obesity, hemorrhagic conversion, smoking status, drug abuse, alcohol abuse, medication use (anticoagulant and antiplatelet medication, chronic use of NSAIDs and aspirin), and use of IV tPA during the same hospitalization or in a different institution within the 24 hours prior to admission to the facility.
Model 3: Model 2 + hospital-associated characteristics including hospital region, teaching status, and bed size.
For each model, c-index was calculated to validate the accuracy of the regressions. We used two-sided tests with considering p < 0.05 statistically significant.
2.5. Informed Consent
The data has been taken from Nationwide Inpatient Sample, which is a deidentified database from “Health Care Utilization Project (HCUP)” sponsored by the Agency for Healthcare Research and Quality, so informed consent or IRB approval was not needed for the study. The relevant ethical oversight and HCUP Data Use Agreement (HCUP-4Q28K90CU) were obtained for the data utilized in this study.
3. Results
3.1. Disease Hospitalizations
There were 4,224,924 hospitalizations due to AIS from 2003–2014 after excluding patients with age <18 years and admissions with missing data for age, gender, and race (Figure 1). Out of 4,224,924 hospitalizations, 18,629 (0.44%) had UGIB. Out of these 18,629 hospitalizations with AIS who had UGIB, 569 (3.05%) had concurrent H. pylori infection.
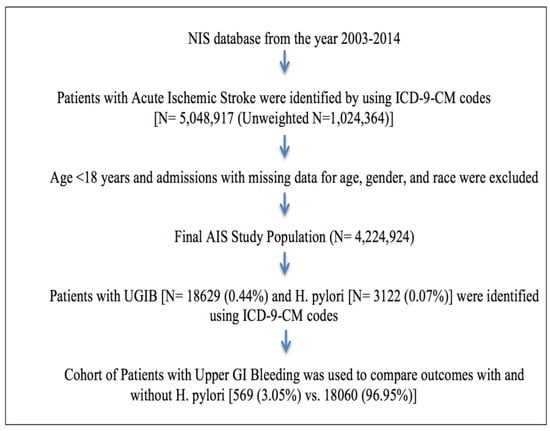
Figure 1.
Flowchart detailing cohort selection and analysis modeling.
3.2. Prevalence Trends
We analyzed trends of UGIB and H. pylori in AIS hospitalizations. As shown in Figure 2, trends of UGIB in AIS hospitalizations and H. pylori infections in AIS hospitalizations were slightly declining from years 2003 to 2014. (UGIB: 0.61% in 2003 to 0.35% in 2014 and H. pylori: 0.12% in 2003 to 0.06% in 2014; P-Trend < 0.0001). However, the trend of H. pylori infection in UGIB drastically decreased from 3.75% in 2003 to 1.98% in 2014, being highest 4.23 in 2004; p-Trend < 0.0001.
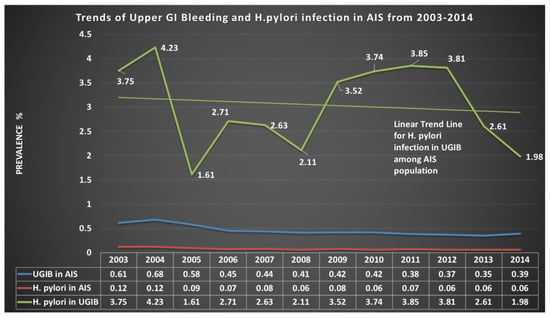
Figure 2.
Prevalence trend of H. pylori infection.
3.3. Demographics, Patient and Hospital Characteristics, and Comorbidities
AIS hospitalizations with UGIB with concomitant H. pylori infection (compared to no infection) were more likely to be male (64.30% vs. 54.52%, p < 0.0001), African American (29.95% vs. 18.78%, p < 0.0001) and Hispanic (14.93% vs. 7.98%, p < 0.0001). Co-morbidities such as diabetes (38.57% vs. 33.45%, p = 0.0114) and hypertension (80.36% vs. 72.96%, p < 0.0001), peripheral vascular disease, and solid tumor without metastasis were more common in patients with H. pylori than those without H. pylori infection. Hospitalizations with H. pylori were also associated with a higher percentage of Deyo’s Charlson Co-morbidity Index (CCI) 3, 4 and ≥5. AIS with UGIB hospitalizations in large, urban teaching hospitals and in the West region were more likely to have H. pylori infection (Table 1).
3.4. The Primary and Secondary Outcomes
Table 2 shows outcomes of H. pylori infections in UGIB patients among AIS hospitalizations. UGIB was the primary outcome. Prevalence of H. pylori among UGIB was 18.23% vs. 0.43%, p < 0.0001) without H. pylori. Secondary outcomes were all-cause in-hospital mortality, discharge disposition, loss of function and risk of death. Though prevalence of all-cause in hospital mortality, major/extreme likelihood of death on discharge, and discharge outcomes were not higher among UGIB with H. pylori, the prevalence of major loss of function was higher (65.91% vs. 52.62%, p < 0.0001).

Table 2.
Univariate analysis of outcomes of H. pylori infection.
3.5. Length of Stay and Cost of Hospitalization
Mean length of stay (10.2 days vs. 12.8 days, p = 0.0008) and total cost of hospitalization were lower ($68,945 vs. $94,812, p = 0.0005) among those with H. pylori infection (Table 2).
3.6. Regression Model Derivation
The overall unadjusted odds for UGIB in H. pylori infections among AIS (model 1) were 52.33 (95%CI: 42.45–64.50, p < 0.0001), while after adjusting for basic demographic with patient-level variables, comorbidities, CCI, concurrent conditions in model 2, the adjusted odds for UGIB in H. pylori infection were 27.64 (95%CI: 20.99–27.40, p < 0.0001) compared to without H. pylori infection. Model 3 suggested, H. pylori was linked with UGIB with odds of 27.75 (95%CI: 21.07–36.55, p < 0.0001). (Table 3).

Table 3.
Multivariate logistic regression analysis for the outcome of upper GI bleeding.
Table 3 also lists multivariate analysis of predictors of UGIB in AIS hospitalizations with H. pylori infections. Age group year 50–64 (compared to 18–34), male (compared to female) and African American (compare to White) race were significant predictors of UGIB with an adjusted OR of 1.50 (95%CI: 1.00–2.25, p = 0.0496), 1.39 (95%CI: 1.29–1.49, p < 0.0001), and 1.12 (95%CI: 1.01–1.23, p = 0.0248) respectively. Co-morbidities like diabetes (adjusted OR: 1.65, 95%CI: 1.02–2.65, p = 0.0409), obesity (aOR: 1.48, 95%CI: 1.04–2.11, p = 0.029), hypertension (aOR: 1.46, 95%CI: 1.24–1.71, p < 0.0001), coagulopathy (aOR: 1.85, 95%CI: 1.61–2.11, p < 0.0001), solid tumor without metastasis (aOR: 1.28, 95%CI: 1.04–1.56, p = 0.01888) and other neurological disorders (aOR: 3.31, 95%CI: 2.65–4.14, p < 0.0001) were significant predictors of UGIB with H. pylori infection amongst AIS hospitalizations. Also, more burden of comorbidities, CCI ≥ 5 (aOR: 4.85, 95%CI: 4.24–5.55, p < 0.0001) was associated with UGIB. Hospitals with more beds, urban hospital (teaching and nonteaching), and Western hospitals were associated with higher adjusted odds for UGIB.
Concurrent conditions/secondary diagnosis like atrial fibrillation (aOR: 1.12, 95%CI: 1.03–1.22, p = 0.0107), hemorrhagic conversion (aOR: 1.34, 95%CI: 1.1–1.64, p = 0.0045), alcohol abuse/dependence (aOR: 1.47, 95%CI: 1.26–1.72, p < 0.0001), and chronic use of NSAIDs (aOR: 3.19, 95%CI: 1.64–6.16, p = 0.0006) were also significant predictors of UGIB. Concurrent conditions like chronic (current) use of aspirin (aOR: 0.63, 95%CI: 0.54-0.74, p < 0.0001), current use of anti-platelet medicines (aOR: 0.66, 95%CI: 0.49–0.90, p = 0.0077), use of anti-coagulant medicines (aOR: 0.66, 95%CI: 0.55–0.78, p < 0.0001) had lower odds of UGIB.
We did not find that recombinant tissue plasminogen activator (t-PA) was associated with UGIB in patients with H. pylori (p = 0.2974).
3.7. Accuracy of the Model:
The c-index was 0.515, 0.705, 0.706 for unadjusted model 1, adjusted model 2 and adjusted model 3, respectively. Both adjusted model 2 and 3 have c-index > 0.7, which indicates a good model fit.
4. Discussion
Upper gastrointestinal bleeding (UGIB) is a common complication following an acute ischemic stroke, with an incidence ranging from 1–5% [1,2,3]. It is known that H. pylori serves as a positive risk factor for peptic ulcer disease (PUD), which could ultimately lead to UGIB [17,18,19,20,21]. Our results suggest that H. pylori was significantly associated with UGIB (adjusted odds ratio: 27.75), possibly due to the relationship between H. pylori and PUD. The study also significantly found that hospitalizations with UGIB with concomitant H. pylori infection were more likely to be male, African American and Hispanic.
H. pylori increases the risk of PUD through a number of virulence factors which influence its colonization and severity. In particular, cytotoxin-associated gene A (cagA), encodes a type IV secretion apparatus, which is used to inject CagA, which is thought to interact with multiple host proteins, into the host cell cytoplasm to cause inflammation and increased risk of ulcers. Similarly, all H. Pylori strains contain vacuolating toxin A (VacA), which produce vacA genes. The strains of H. pylori harboring the s1m1 allele and i1 allele are thought to also play a substantial role in the development of ulcers, due to their high toxicity [26]. Additionally, H. pylori gamma-glutamyl transpeptidase (GGT) also functions to increase peptic ulcer development through increasing the secretion of IL-8 and hydrogen peroxide in epithelial cells [26]. These mechanisms contribute to the ability of H. pylori to contribute to the formation of PUD, however, the percentage of ulcers not associated with H. pylori is rising due to increased use of NSAIDs.
PUD is significantly associated with the use of NSAIDs. In our study, we found that chronic use of NSAIDs was a significant predictor of UGIB. In a meta-analysis study done on the relationship between H. pylori infection and the use of NSAIDs in the pathogenesis of PUD, it was found that PUD was significantly more common in NSAID takers, irrespective of H. pylori infection and the use of NSAIDs increased the risk of ulcer bleeding in the presence of H. pylori infection [27]. However, previous studies have also demonstrated that H. pylori eradication is partially protective against ulcer development in NSAID users [28,29]. This demonstrates that although NSAID usage and H. pylori infection could be independently associated with the development of PUD, there is also some degree of overlap between them in the pathogenesis of PUD. Our study also found that chronic use of aspirin had lower odds of UGIB. However, this finding contradicts previous studies which found that aspirin doubles the risk of bleeding, even at doses as low as 75 mg daily [30]. In our study major risk factors for the upper GI events were alcohol consumption, drug, hypertension, which in presence of emotional stress could also contribute to ulcer formation by increasing secretion of gastric acid and damaging the mucosal barrier [31].
In one study, it was found that H. pylori is treated relatively poorly in inpatients compared to outpatients and that inpatients received therapy only 60% of the time when compared to outpatients, who received therapy 92% of the time [32]. This finding could also be attributed to relatively poor treatment of inpatients with H. pylori infection. The mean length of stay and cost of hospitalization could have been lower due to patients being discharged early, which could be due to the lack of appropriate care in hospitals.
Our study also showed utilization of IV-tPA had no significant role in UGIB among patients with or without H. pylori infection.
The main strength of this study is the number of hospitalizations from NIS database. The nationwide data is having inpatient records of approximately 9–12 million patients each year, which makes our study results more reliable. With the ICD-9-CM codes, we utilized have high PPV of stroke, H. pylori, and UGIB [23,24,25]. Thirumurthi et al. utilized serology and urea breath test to identify H. pylori infection and to compare PPV of ICD-9-CM code for in-patient and outpatient cohort [25]. The sensitivity and specificity of urea breath test are approximately 88–95% and 95–100%, respectively [33] and sensitivity and specificity of serologic assays found an overall of 85% and 79%, respectively to differentiate an active vs. past infection [34]. However, this study has several limitations. This is a retrospective cross-sectional study based on patients hospitalized for AIS. Due to the nature of this study, selection biases could exist. Sensitivity, specificity and PPV for other confounders and comorbidities could not be measured accurately in the NIS database. Additionally, data from clinical registries or administrative databases were obtained retrospectively or prospectively by chart abstractions based on the discharge diagnosis codes, billing codes etc. and hence are susceptible to coding errors.
5. Conclusions
These results indicate that the H. pylori infection is highly associated with UGIB amongst the patients with AIS. Believed risk factors like utilization of aspirin, anticoagulants, antiplatelets, and IV tPA have no role in increasing the risk of UGIB. Early identification and treatment of H. pylori infection amongst AIS patients improve the outcomes and risk stratification for GIB. These results emphasize the need for a hospital-based randomized study to evaluate the benefit of H. pylori infection identification and eradication in stroke patients and to track them to see the risk of GIB in future.
Supplementary Materials
The following are available online at https://www.mdpi.com/2624-5647/1/3/29/s1. Table S1: ICD-9-CM codes used in this analysis; Table S2: Deyo’s modification of Charlson’s co-morbidity index (CCI).
Author Contributions
Conceptualization, U.K.P.; methodology, U.K.P., M.S.D.; software, U.K.P.; validation, M.S.D., V.J., A.L. (Abhishek Lunagariya); formal analysis, U.K.P.; investigation, U.K.P.; resources, M.S.D.; data curation, M.D.; writing—original draft preparation, U.K.P., A.L. (Anusha Lekshminarayanan), M.D., N.P.; writing—review and editing, M.S.D., A.L. (Abhishek Lunagariya), V.J.; visualization, U.K.P.; supervision, M.S.D.; project administration, U.K.P.; funding acquisition, none.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- O’Donnell, M.J.; Karal, M.K.; Fang, J.; Saposnik, G.; Eikelboom, J.W.; Oczkowski, W.; Silva, J.; Gould, L.; D’Uva, C.; Silver, F.L. Gastrointestinal bleeding after acute ischemic stroke. Neurology 2008, 71, 650–655. [Google Scholar] [CrossRef]
- Hsu, H.L.; Lin, Y.H.; Huang, Y.C.; Weng, H.H.; Lee, M.; Huang, W.Y.; Lee, J.D. Gastrointestinal Hemorrhage after Acute Ischemic Stroke and Its Risk Factors in Asians. Eur. Neurol. 2009, 62, 212–218. [Google Scholar] [CrossRef]
- Chen, C.M.; Hsu, H.C.; Chuang, Y.W.; Chang, C.H.; Lin, C.H.; Hong, C.Z. Study on factors affecting the occurrence of upper gastrointestinal bleeding in elderly acute stroke patients undergoing rehabilitation. J. Nutr. Health Aging 2011, 15, 632–636. [Google Scholar] [CrossRef]
- Cannon, C.P.; Rhee, K.E.; Califf, R.M.; Boden, W.E.; Hirsch, A.T.; Alberts, M.J.; Cable, G.; Shao, M.; Ohman, E.M.; Steg, P.G.; et al. Current Use of Aspirin and Antithrombotic Agents in the United States Among Outpatients with Atherothrombotic Disease (from the Reduction of Atherothrombosis for Continued Health [REACH] Registry). Am. J. Cardiol. 2010, 105, 445–452. [Google Scholar] [CrossRef][Green Version]
- Van Leerdam, M.E. Epidemiology of acute upper gastrointestinal bleeding. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 209–224. [Google Scholar] [CrossRef]
- Hreinsson, J.P.; Kalaitzakis, E.; Gudmundsson, S.; Björnsson, E.S. Upper gastrointestinal bleeding: Incidence, etiology and outcomes in a population-based setting. Scand. J. Gastroenterol. 2013, 48, 439–447. [Google Scholar] [CrossRef]
- Enestvedt, B.K.; Gralnek, I.M.; Mattek, N.; Lieberman, D.A.; Eisen, G. An evaluation of endoscopic indications and findings related to nonvariceal upper-GI hemorrhage in a large multicenter consortium. Gastrointest. Endosc. 2008, 67, 422–429. [Google Scholar] [CrossRef]
- Farrell, J.J.; Friedman, L.S. Review article: The management of lower gastrointestinal bleeding. Aliment. Pharmacol. Ther. 2005, 21, 1281–1298. [Google Scholar] [CrossRef]
- Ghassemi, K.A.; Jensen, D.M. Lower GI Bleeding: Epidemiology and Management. Curr. Gastroenterol. Rep. 2013, 15, 333. [Google Scholar] [CrossRef]
- Abraham, N.S.; Hartman, C.; Richardson, P.; Castillo, D.; Street, R.L.; Naik, A.D. Risk of Lower and Upper Gastrointestinal Bleeding, Transfusions, and Hospitalizations with Complex Antithrombotic Therapy in Elderly Patients. Circulation 2013, 128, 1869–1877. [Google Scholar] [CrossRef]
- Wolf, A.T.; Wasan, S.K.; Saltzman, J.R. Impact of Anticoagulation on Rebleeding Following Endoscopic Therapy for Nonvariceal Upper Gastrointestinal Hemorrhage. Am. J. Gastroenterol. 2007, 102, 290–296. [Google Scholar] [CrossRef]
- Ahsberg, K.; Höglund, P.; Staël von Holstein, C. Mortality from peptic ulcer bleeding: The impact of comorbidity and the use of drugs that promote bleeding. Aliment. Pharmacol. Ther. 2010, 32, 801–810. [Google Scholar] [CrossRef]
- Lanas, A.; Aabakken, L.; Fonseca, J.; Mungan, Z.A.; Papatheodoridis, G.V.; Piessevaux, H.; Cipolletta, L.; Nuevo, J.; Tafalla, M. Clinical predictors of poor outcomes among patients with nonvariceal upper gastrointestinal bleeding in Europe. Aliment. Pharmacol. Ther. 2011, 33, 1225–1233. [Google Scholar] [CrossRef][Green Version]
- Mose, H.; Larsen, M.; Riis, A.; Johnsen, S.P.; Thomsen, R.W.; Sorensen, H.T. Thirty-Day Mortality After Peptic Ulcer Bleeding in Hospitalized Patients Receiving Low-Dose Aspirin at Time of Admission. Am. J. Geriatr. Pharmacother. 2006, 4, 244–250. [Google Scholar] [CrossRef]
- Ortiz, V.; Ortuño, J.; Rodríguez-Soler, M.; Iborra, M.; Garrigues, V.; Ponce, J. Outcome of non-variceal acute upper gastrointestinal bleeding in patients with antithrombotic therapy. Digestion 2009, 80, 89–94. [Google Scholar] [CrossRef]
- Eikelboom, J.W.; Mehta, S.R.; Anand, S.S.; Xie, C.; Fox, K.A.; Yusuf, S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006, 114, 774–782. [Google Scholar] [CrossRef]
- Lau, J.Y.; Sung, J.; Hill, C.; Henderson, C.; Howden, C.W.; Metz, D.C. Systematic review of the epidemiology of complicated peptic ulcer disease: Incidence, recurrence, risk factors and mortality. Digestion 2011, 84, 102–113. [Google Scholar] [CrossRef]
- Sostres, C.; Lanas, A. Epidemiology and demographics of upper gastrointestinal bleeding: Prevalence, incidence, and mortality. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 567–581. [Google Scholar] [CrossRef]
- Lee, I.; Cryer, B. Epidemiology and role of nonsteroidal antiinflammatory drugs in causing gastrointestinal bleeding. Gastrointest. Endosc. Clin. N. Am. 2011, 21, 597–612. [Google Scholar] [CrossRef]
- Garcia Rodriguez, L.A.; Barreales Tolosa, L. Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology 2007, 132, 498–506. [Google Scholar] [CrossRef]
- Lanza, F.L.; Chan, F.K.; Quigley, E.M. Guidelines for prevention of NSAID-related ulcer complications. Am. J. Gastroenterol. 2009, 104, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Vijayvergiya, R.; Vadivelu, R. Role of Helicobacter pylori infection in pathogenesis of atherosclerosis. World J. Cardiol. 2015, 7, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Birman-Deych, E.; Waterman, A.D.; Yan, Y.; Nilasena, D.S.; Radford, M.J.; Gage, B.F. Accuracy of ICD-9-CM Codes for Identifying Cardiovascular and Stroke Risk Factors. Med. Care 2005, 43, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.E.; Gurwitz, J.H.; Chan, K.A.; Donahue, J.G.; Beck, A.; Boles, M.; Buist, D.S.; Goodman, M.; LaCroix, A.Z.; Levin, T.R.; et al. Validation of diagnoses of peptic ulcers and bleeding from administrative databases: A multi-health maintenance organization study. J. Clin. Epidemiol. 2002, 55, 310–313. [Google Scholar] [CrossRef]
- Thirumurthi, S.; Desilva, R.; Castillo, D.L.; Richardson, P.; Abraham, N.S. Identification of Helicobacter pylori infected patients, using administrative data. Aliment. Pharmacol. Ther. 2008, 28, 1309–1316. [Google Scholar] [CrossRef]
- Testerman, T.L.; Morris, J. Beyond the stomach: An updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J. Gastroenterol. 2014, 20, 12781–12808. [Google Scholar] [CrossRef]
- Huang, J.Q.; Sridhar, S.; Hunt, R.H. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: A meta-analysis. Lancet 2002, 359, 14–22. [Google Scholar] [CrossRef]
- Musumba, C.; Jorgensen, A.; Sutton, L.; Van Eker, D.; Moorcroft, J.; Hopkins, M.; Pritchard, D.M.; Pirmohamed, M. The relative contribution of NSAIDs and Helicobacter pylori to the aetiology of endoscopically-diagnosed peptic ulcer disease: Observations from a tertiary referral hospital in the UK between 2005 and 2010. Aliment. Pharmacol. Ther. 2012, 36, 48–56. [Google Scholar] [CrossRef]
- Tang, C.L.; Ye, F.; Liu, W.; Pan, X.L.; Qian, J.; Zhang, G.X. Eradication of Helicobacter pylori infection reduces the incidence of peptic ulcer disease in patients using nonsteroidal anti-inflammatory drugs: A meta-analysis. Helicobacter 2012, 17, 286–296. [Google Scholar] [CrossRef]
- Chan, F.K.; Chung, S.C.; Suen, B.Y.; Lee, Y.T.; Leung, W.K.; Leung, V.K.; Wu, J.C.; Lau, J.Y.; Hui, Y.; Lai, M.S.; et al. Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low-dose aspirin or naproxen. N. Engl. J. Med. 2001, 344, 967–973. [Google Scholar] [CrossRef]
- Cryer, B.; Mahaffey, K.W. Gastrointestinal ulcers, Role of aspirin, and Clinical outcomes: Pathobiology, Diagnosis, and Treatment. Journal of Multidisciplinary Healthcare. J. Multidiscip. Healthc. 2014, 7, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Nazareno, J.; Driman, D.K.; Adams, P. Is Helicobacter pylori being treated appropriately? A study of inpatients and outpatients in a tertiary care centre. Can. J. Gastroenterol. Hepatol. 2007, 21, 285–288. [Google Scholar] [CrossRef][Green Version]
- Howden, C.W.; Hunt, R.H. Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am. J. Gastroenterol. 1998, 93, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Loy, C.T.; Irwig, L.M.; Katelaris, P.H.; Talley, N.J. Do commercial serological kits for Helicobacter pylori infection differ in accuracy? A meta-analysis. Am. J. Gastroenterol. 1996, 91, 1138–1144. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).