Histopathology of Barrett’s Esophagus and Early-Stage Esophageal Adenocarcinoma: An Updated Review
Abstract
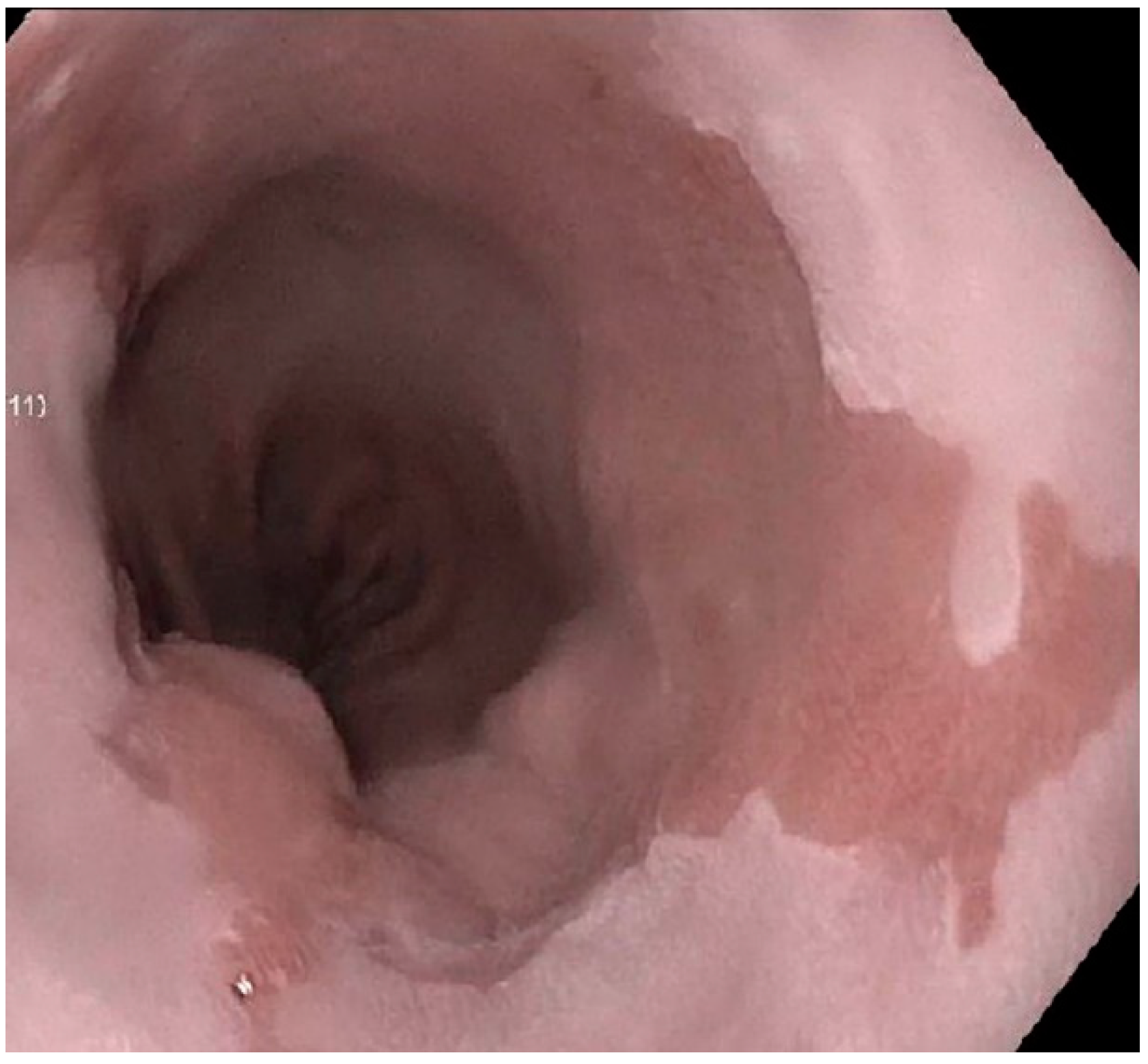
:1. Introduction
2. Histopathology of BE
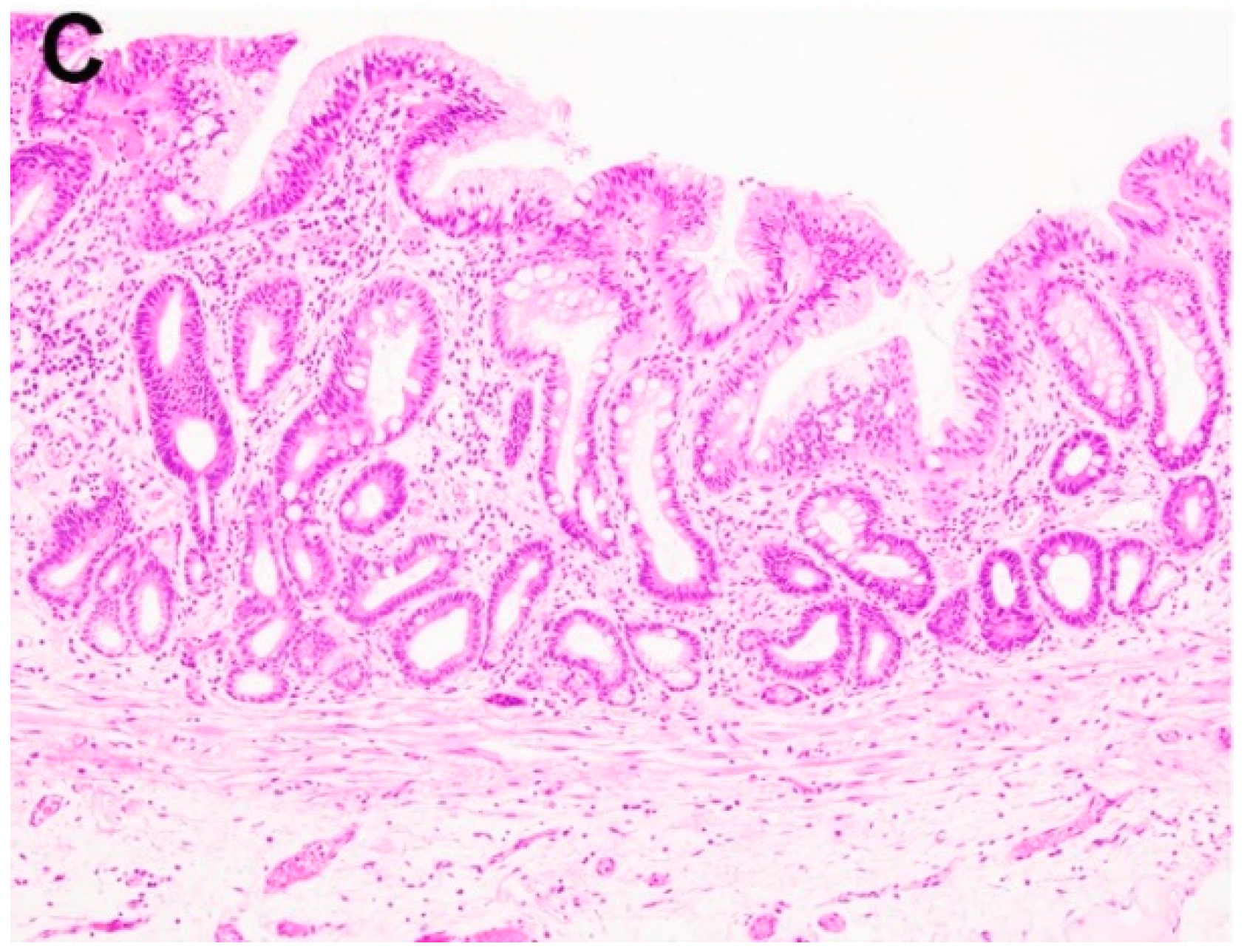
2.1. Negative for Dysplasia (ND)
2.2. Indefinite for Dysplasia (IND)
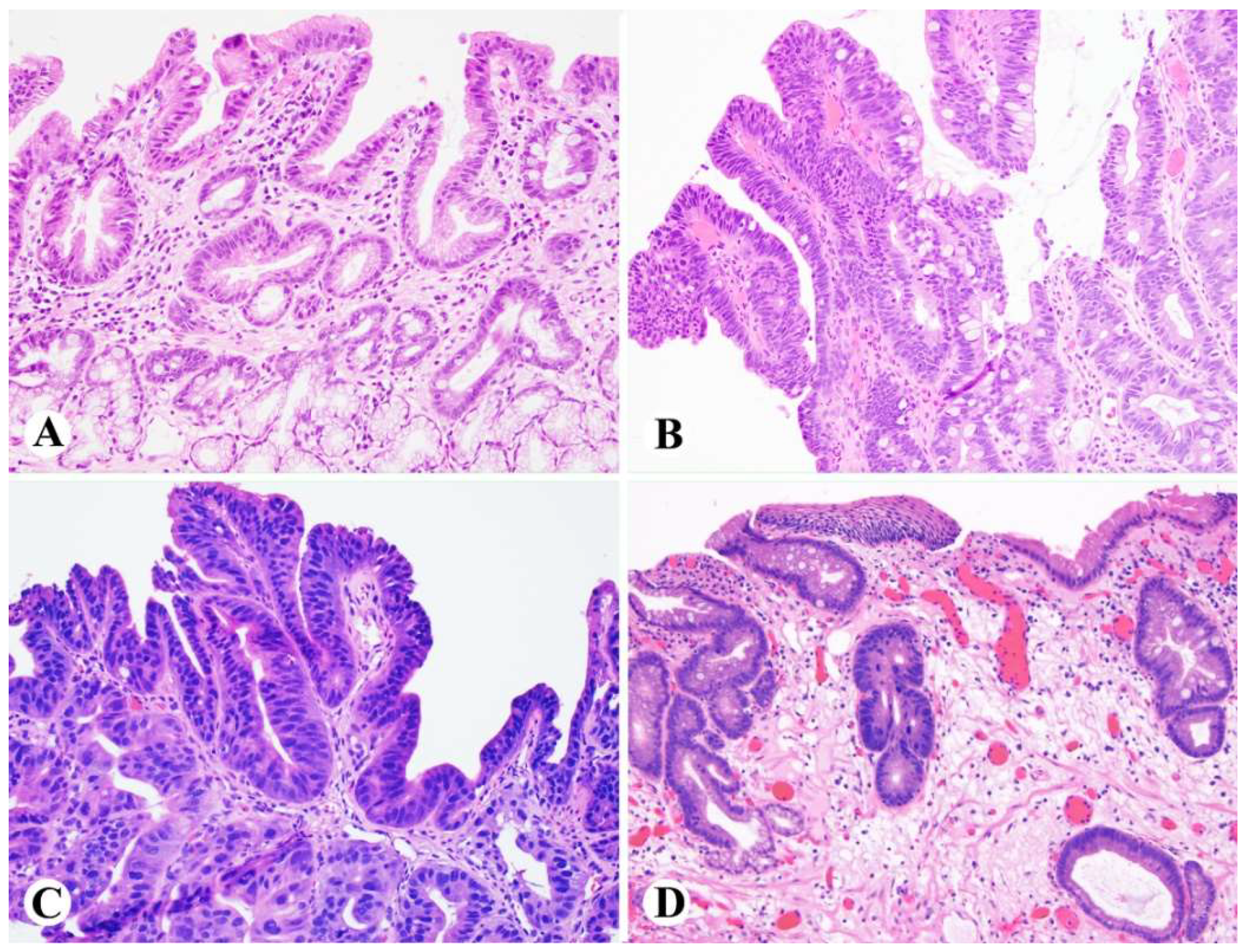
2.3. Low-Grade Dysplasia (LGD)
2.4. High-Grade Dysplasia (HGD)
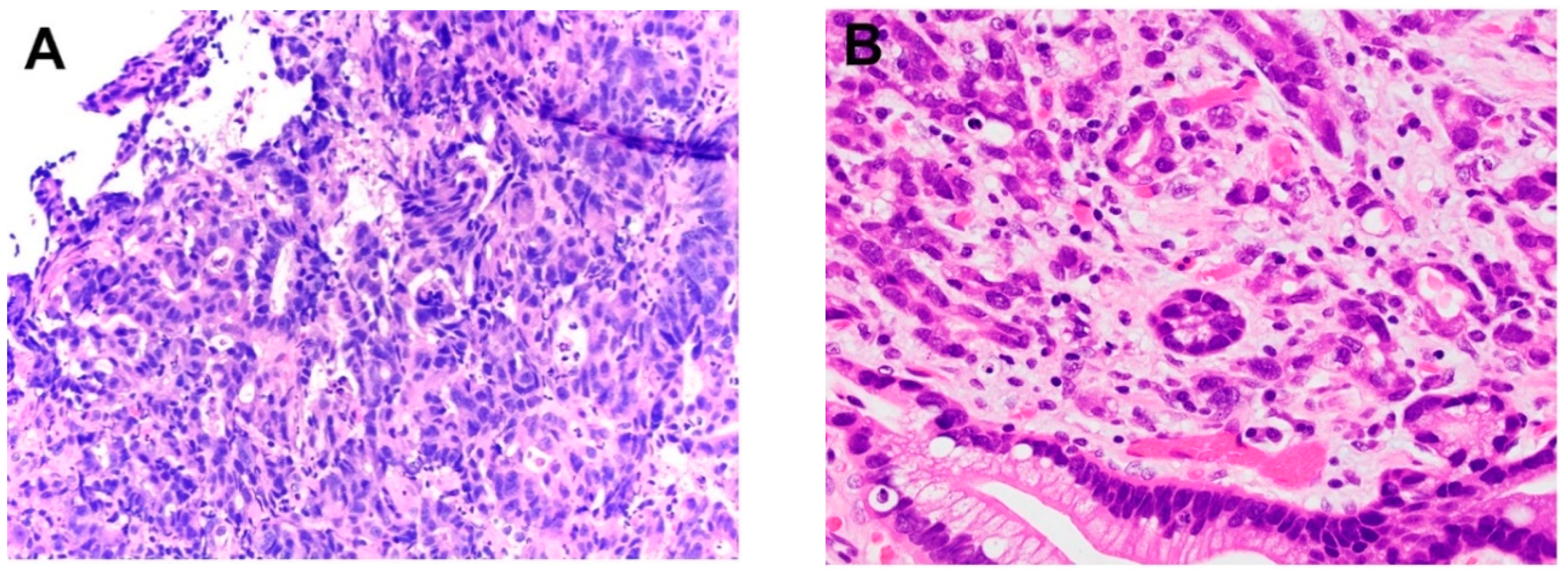
3. Histopathology of Intramucosal Carcinoma (IMC)
4. Histopathology of Submucosal Adenocarcinoma (SAC)
5. Ancillary Biomarkers for BE, Dysplasia, and Early Stage EAC
6. Genetic and Molecular Signature of BE and Early Stage EAC
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y. Epidemiology of esophageal cancer. World J. Gastroenterol. 2013, 19, 5598–5606. [Google Scholar] [CrossRef] [PubMed]
- Thrift, A.P.; Whiteman, D.C. The incidence of esophageal adenocarcinoma continues to rise: Analysis of period and birth cohort effects on recent trends. Ann Oncol. 2012, 23, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Bytzer, P.; Christensen, P.B.; Damkier, P.; Vinding, K.; Seersholm, N. Adenocarcinoma of the esophagus and Barrett’s esophagus: A population-based study. Am. J. Gastroenterol. 1999, 94, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Kuipers, E.J.; Spaander, M.C. Natural History of Barrett’s Esophagus. Dig. Dis. Sci. 2018, 63, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Graham, T.A.; Jansen, M. Insights into the Pathophysiology of Esophageal Adenocarcinoma. Gastroenterology 2018, 154, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H. From reflux esophagitis to Barrett’s esophagus and esophageal adenocarcinoma. World J. Gastroenterol. 2015, 21, 5210–5219. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef] [PubMed]
- Erőss, B.; Farkas, N.; Vincze, Á.; Tinusz, B.; Szapáry, L.; Garami, A.; Balaskó, M.; Sarlós, P.; Czopf, L.; Alizadeh, H.; et al. Helicobacter pylori infection reduces the risk of Barrett’s esophagus: A meta-analysis and systematic review. Helicobacter 2018, 23, e12504. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.; Moayyedi, P.; Corley, D.A.; DeCaestecker, J.; Falck-Ytter, Y.; Falk, G.; Vakil, N.; Sanders, S.; Vieth, M.; Inadomi, J.; et al. BOB CAT: A Large-Scale Review and Delphi Consensus for Management of Barrett’s Esophagus with No Dysplasia, Indefinite for, or Low-Grade Dysplasia. Am. J. Gastroenterol. 2015, 110, 662–682. [Google Scholar] [CrossRef] [PubMed]
- Naini, B.V.; Chak, A.; Ali, M.A.; Odze, R.D. Barrett’s oesophagus diagnostic criteria: Endoscopy and histology. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Wallner, B.; Sylvan, A.; Stenling, R.; Janunger, K.G. The esophageal Z-line appearance correlates to the prevalence of intestinal metaplasia. Scand. J. Gastroenterol. 2000, 35, 17–22. [Google Scholar] [PubMed]
- Shaheen, N.J.; Falk, G.W.; Iyer, P.G.; Gerson, L.B.; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am. J. Gastroenterol. 2016, 111, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.D.; Chaves, P. Low risk of adenocarcinoma and high-grade dysplasia in patients with non-dysplastic Barrett’s esophagus: Results from a cohort from a country with low esophageal adenocarcinoma incidence. United Eur. Gastroenterol. J. 2016, 4, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Riddell, R.H.; Odze, R.D. Definition of Barrett’s esophagus: Time for a rethink—Is intestinal metaplasia dead? Am. J. Gastroenterol. 2009, 104, 2588–2594. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Ertan, A.; Ergun, G.; Verm, R.; Bridges, M.; Woods, K.; Meriano, F.; Schmulen, A.C.; Colman, R.; Johnson, C.; et al. Goblet cell mimickers in esophageal biopsies are not associated with an increased risk for dysplasia. Arch. Pathol. Lab. Med. 2007, 131, 571–575. [Google Scholar] [PubMed]
- Hvid-Jensen, F.; Pedersen, L.; Drewes, A.M.; Sørensen, H.T.; Funch-Jensen, P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N. Engl. J. Med. 2011, 365, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.B.; Falk, G.W.; Richter, J.E. The incidence of adenocarcinoma and dysplasia in Barrett’s esophagus: Report on the Cleveland Clinic Barrett’s Esophagus Registry. Am. J. Gastroenterol. 1999, 94, 2037–2042. [Google Scholar] [PubMed]
- Drewitz, D.J.; Sampliner, R.E.; Garewal, H.S. The incidence of adenocarcinoma in Barrett’s esophagus: A prospective study of 170 patients followed 4.8 years. Am. J. Gastroenterol. 1997, 92, 212–215. [Google Scholar] [PubMed]
- Sinh, P.; Anaparthy, R.; Young, P.E.; Gaddam, S.; Thota, P.; Balasubramanian, G.; Singh, M.; Higbee, A.D.; Wani, S.; Gupta, N.; et al. Clinical outcomes in patients with a diagnosis of “indefinite for dysplasia” in Barrett’s esophagus: A multicenter cohort study. Endoscopy 2015, 47, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Kestens, C.; Leenders, M.; Offerhaus, G.J.; van Baal, J.W.; Siersema, P.D. Risk of neoplastic progression in Barrett’s esophagus diagnosed as indefinite for dysplasia: A nationwide cohort study. Endoscopy 2015, 47, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Shroff, S.; Feldman, M.; DeMarshall, M.; Price, C.; Tierney, A.; Falk, G.W. Risk of malignant progression in Barrett’s esophagus indefinite for dysplasia. Dis. Esophagus 2017, 30, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Salomao, M.A.; Lam-Himlin, D.; Pai, R.K. Substantial Interobserver Agreement in the Diagnosis of Dysplasia in Barrett Esophagus Upon Review of a Patient’s Entire Set of Biopsies. Am. J. Surg. Pathol. 2018, 42, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Vennalaganti, P.; Kanakadandi, V.; Goldblum, J.R.; Mathur, S.C.; Patil, D.T.; Offerhaus, G.J.; Meijer, S.L.; Vieth, M.; Odze, R.D.; Shreyas, S.; Parasa, S.; Gupta, N.; Repici, A.; Bansal, A.; Mohammad, T.; Sharma, P. Discordance Among Pathologists in the United States and Europe in Diagnosis of Low-Grade Dysplasia for Patients with Barrett’s Esophagus. Gastroenterology 2017, 152, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Coleman, H.G.; Yousef, F.; Johnston, B.T.; McManus, D.T.; Gavin, A.T.; Murray, L.J. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J. Natl. Cancer Inst. 2011, 103, 1049–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spechler, S.J. Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA 2013, 310, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Puli, S.; El-Serag, H.B.; Bansal, A.; Wani, S.; Sharma, P. Incidence of esophageal adenocarcinoma in patients with Barrett’s esophagus and high-grade dysplasia: A meta-analysis. Gastrointest. Endosc. 2008, 67, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Lomo, L.C.; Blount, P.L.; Sanchez, C.A.; Li, X.; Galipeau, P.C.; Cowan, D.S.; Ayub, K.; Rabinovitch, P.S.; Reid, B.J.; Odze, R.D. Crypt dysplasia with surface maturation: A clinical, pathologic, and molecular study of a Barrett’s esophagus cohort. Am. J. Surg. Pathol. 2006, 30, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Coco, D.P.; Goldblum, J.R.; Hornick, J.L.; Lauwers, G.Y.; Montgomery, E.; Srivastava, A.; Wang, H.; Odze, R.D. Interobserver variability in the diagnosis of crypt dysplasia in Barrett esophagus. Am. J. Surg. Pathol. 2011, 35, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Appelman, H.D.; Greenson, J.K.; Ramsburgh, S.R.; Orringer, M.B.; Chang, A.C.; McKenna, B.J. A histologically defined subset of high-grade dysplasia in Barrett mucosa is predictive of associated carcinoma. Am. J. Clin. Pathol. 2009, 132, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Downs-Kelly, E.; Mendelin, J.E.; Bennett, A.E.; Castilla, E.; Henricks, W.H.; Schoenfield, L.; Skacel, M.; Yerian, L.; Rice, T.W.; Rybicki, L.A.; et al. Poor interobserver agreement in the distinction of high-grade dysplasia and adenocarcinoma in pretreatment Barrett’s esophagus biopsies. Am. J. Gastroenterol. 2008, 103, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Patil, D.T.; Goldblum, J.R.; Rybicki, L.; Plesec, T.P.; Mendelin, J.E.; Bennett, A.E.; Castilla, E.A.; Henricks, W.H.; Schoenfield, L.; Skacel, M.; et al. Prediction of adenocarcinoma in esophagectomy specimens based upon analysis of preresection biopsies of Barrett esophagus with at least high-grade dysplasia: A comparison of 2 systems. Am. J. Surg. Pathol. 2012, 36, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.C.; Krasinskas, A.M.; Correa, A.M.; Hofstetter, W.L.; Ajani, J.A.; Swisher, S.G.; Wu, T.T. Duplication of the muscularis mucosae in Barrett esophagus: An underrecognized feature and its implication for staging of adenocarcinoma. Am. J. Surg. Pathol. 2007, 31, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Estrella, J.S.; Hofstetter, W.L.; Correa, A.M.; Swisher, S.G.; Ajani, J.A.; Lee, J.H.; Bhutani, M.S.; Abraham, S.C.; Rashid, A.; Maru, D.M. Duplicated muscularis mucosae invasion has similar risk of lymph node metastasis and recurrence-free survival as intramucosal esophageal adenocarcinoma. Am. J. Surg. Pathol. 2011, 35, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Rice, T.W.; Liu, X.; Goldblum, J.R.; Williams, S.J.; Rybicki, L.A.; Murthy, S.C.; Mason, D.P.; Raymond, D.P.; Blackstone, E.H. Intramucosal esophageal adenocarcinoma: primum non nocere. J. Thorac. Cardiovasc. Surg. 2013, 145, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, M.; Koppert, L.B.; Buskens, C.J.; Tilanus, H.W.; ten Kate, F.J.; Bergman, J.J.; Siersema, P.D.; van Dekken, H.; van Lanschot, J.J. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005, 446, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Boys, J.A.; Worrell, S.G.; Chandrasoma, P.; Vallone, J.G.; Maru, D.M.; Zhang, L.; Blackmon, S.H.; Dickinson, K.J.; Dunst, C.M.; Hofstetter, W.L.; et al. Can the Risk of Lymph Node Metastases Be Gauged in Endoscopically Resected Submucosal Esophageal Adenocarcinomas? A Multi-Center Study. J. Gastrointest. Surg. 2016, 20, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Manner, H.; Wetzka, J.; May, A.; Pauthner, M.; Pech, O.; Fisseler-Eckhoff, A.; Stolte, M.; Vieth, M.; Lorenz, D.; Ell, C. Early-stage adenocarcinoma of the esophagus with mid to deep submucosal invasion (pT1b sm2–3): The frequency of lymph-node metastasis depends on macroscopic and histological risk patterns. Dis. Esophagus 2017, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Buskens, C.J.; Westerterp, M.; Lagarde, S.M.; Bergman, J.J.; ten Kate, F.J.; van Lanschot, J.J. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest. Endosc. 2004, 60, 703–710. [Google Scholar] [CrossRef]
- Bollschweiler, E.; Baldus, S.E.; Schröder, W.; Prenzel, K.; Gutschow, C.; Schneider, P.M.; Hölscher, A.H. High rate of lymph-node metastasis in submucosal esophageal squamous-cell carcinomas and adenocarcinomas. Endoscopy 2006, 38, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Sepesi, B.; Watson, T.J.; Zhou, D.; Polomsky, M.; Litle, V.R.; Jones, C.E.; Raymond, D.P.; Hu, R.; Qiu, X.; Peters, J.H. Are endoscopic therapies appropriate for superficial submucosal esophageal adenocarcinoma? An analysis of esophagectomy specimens. J. Am. Coll. Surg. 2010, 210, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, A.H.; Bollschweiler, E.; Schröder, W.; Metzger, R.; Gutschow, C.; Drebber, U. Prognostic impact of upper, middle, and lower third mucosal or submucosal infiltration in early esophageal cancer. Ann. Surg. 2011, 254, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Ancona, E.; Rampado, S.; Cassaro, M.; Battaglia, G.; Ruol, A.; Castoro, C.; Portale, G.; Cavallin, F.; Rugge, M. Prediction of lymph node status in superficial esophageal carcinoma. Ann. Surg. Oncol. 2008, 15, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, D.; Origer, J.; Pauthner, M.; Graupe, F.; Fisseler-Eckhoff, A.; Stolte, M.; Pech, O.; Ell, C. Prognostic risk factors of early esophageal adenocarcinomas. Ann. Surg. 2014, 259, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Leers, J.M.; DeMeester, S.R.; Oezcelik, A.; Klipfel, N.; Ayazi, S.; Abate, E.; Zehetner, J.; Lipham, J.C.; Chan, L.; Hagen, J.A.; et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann. Surg. 2011, 253, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.P.; Jones, M.; Brown, I.; Gotley, D.C.; Martin, I.; Thomas, J.; Clouston, A.; Smithers, B.M. Risk stratification for early esophageal adenocarcinoma: analysis of lymphatic spread and prognostic factors. Ann. Surg. Oncol. 2010, 17, 2494–2502. [Google Scholar] [CrossRef] [PubMed]
- Zemler, B.; May, A.; Ell, C.; Stolte, M. Early Barrett’s carcinoma: The depth of infiltration of the tumour correlates with the degree of differentiation, the incidence of lymphatic vessel and venous invasion. Virchows Arch. 2010, 456, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Badreddine, R.J.; Prasad, G.A.; Lewis, J.T.; Lutzke, L.S.; Borkenhagen, L.S.; Dunagan, K.T.; Wang, K.K. Depth of submucosal invasion does not predict lymph node metastasis and survival of patients with esophageal carcinoma. Clin. Gastroenterol. Hepatol. 2010, 8, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hofstetter, W.L.; Rashid, A.; Swisher, S.G.; Correa, A.M.; Ajani, J.A.; Hamilton, S.R.; Wu, T.T. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am. J. Surg. Pathol. 2005, 29, 1079–1085. [Google Scholar] [PubMed]
- Alvarez Herrero, L.; Pouw, R.E.; van Vilsteren, F.G.; ten Kate, F.J.; Visser, M.; van Berge Henegouwen, M.I.; Weusten, B.L.; Bergman, J.J. Risk of lymph node metastasis associated with deeper invasion by early adenocarcinoma of the esophagus and cardia: Study based on endoscopic resection specimens. Endoscopy 2010, 42, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, R.; Oyama, T.; Abe, S.; Takahashi, H.; Ono, H.; Fujisaki, J.; Kaise, M.; Goda, K.; Kawada, K.; Koike, T.; et al. Risk of metastasis in adenocarcinoma of the esophagus: A multicenter retrospective study in a Japanese population. J. Gastroenterol. 2017, 52, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Landau, M.S.; Hastings, S.M.; Foxwell, T.J.; Luketich, J.D.; Nason, K.S.; Davison, J.M. Tumor budding is associated with an increased risk of lymph node metastasis and poor prognosis in superficial esophagealadenocarcinoma. Mod. Pathol. 2014, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.; Sillah, K.; Griffiths, E.A.; Swindell, R.; West, C.M.; Page, R.D.; Welch, I.M.; Pritchard, S.A. Tumour budding and a low host inflammatory response are associated with a poor prognosis in oesophageal and gastro-oesphageal junction cancers. Histopathology 2010, 56, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Thies, S.; Guldener, L.; Slotta-Huspenina, J.; Zlobec, I.; Koelzer, V.H.; Lugli, A.; Kröll, D.; Seiler, C.A.; Feith, M.; Langer, R. Impact of peritumoral and intratumoral budding in esophageal adenocarcinomas. Hum. Pathol. 2016, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.B.; Schaeffer, D.F. Tumor budding as a standardized parameter in gastrointestinal carcinomas: More than just the colon. Mod. Pathol. 2018, 31, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Koelzer, V.H.; Langer, R.; Zlobec, I.; Lugli, A. Tumor Budding in Upper Gastrointestinal Carcinoma. Front. Oncol. 2014, 4, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolte, M.; Kirtil, T.; Oellig, F.; Vogel, C.; Mueller, H.; May, A.; Ell, C.; Wittenberg, R. The pattern of invasion of early carcinomas in Barrett’s esophagus is dependent on the depth of infiltration. Pathol. Res. Pract. 2010, 206, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Glickman, J.N.; Wang, H.; Das, K.M.; Goyal, R.K.; Spechler, S.J.; Antonioli, D.; Odze, R.D. Phenotype of Barrett’s esophagus and intestinal metaplasia of the distal esophagus and gastroesophageal junction: An immunohistochemical study of cytokeratins 7 and 20, Das-1 and 45 MI. Am. J. Surg. Pathol. 2001, 25, 87–94. [Google Scholar] [CrossRef] [PubMed]
- McIntire, M.G.; Souc, G.; Vaughan, T.L.; Shahsafaei, A.; Odze, R.D. MUC2 is a highly specific marker of goblet cell metaplasia in the distal esophagus and gastroesophageal junction. Am. J. Surg. Pathol. 2011, 35, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Ahmed, S.; Clark, P. Immunohistochemical assessment for Cdx2 expression in the Barrett metaplasia-dysplasia-adenocarcinoma sequence. J. Clin. Pathol. 2011, 64, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Appelman, H.; Goldsmith, J.D.; Davison, J.M.; Hart, J.; Krasinskas, A.M. The Use of Ancillary Stains in the Diagnosis of Barrett Esophagus and Barrett Esophagus-associated Dysplasia: Recommendations from the Rodger C. Haggitt Gastrointestinal Pathology Society. Am. J. Surg. Pathol. 2017, 41, e8–e21. [Google Scholar] [CrossRef] [PubMed]
- Janmaat, V.T.; van Olphen, S.H.; Biermann, K.E.; Looijenga, L.H.J.; Bruno, M.B.; Spaander, M.C.W. Use of immunohistochemical biomarkers as independent predictor of neoplastic progression in Barrett’s oesophagus surveillance: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0186305. [Google Scholar] [CrossRef] [PubMed]
- Stachler, M.D.; Camarda, N.D.; Deitrick, C.; Kim, A.; Agoston, A.T.; Odze, R.D.; Hornick, J.L.; Nag, A.; Thorner, A.R.; Ducar, M.; Noffsinger, A.; et al. Detection of Mutations in Barrett’s Esophagus Before Progression to High-Grade Dysplasia or Adenocarcinoma. Gastroenterology 2018, 155, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Lebovitz, R.M.; Lechago, L.V.; Lechago, J. p53 protein accumulation in Barrett’s metaplasia, dysplasia, and carcinoma: a follow-up study. Gastroenterology 1993, 105, 1637–1642. [Google Scholar] [CrossRef]
- Younes, M.; Brown, K.; Lauwers, G.Y.; Ergun, G.; Meriano, F.; Schmulen, A.C.; Barroso, A.; Ertan, A. p53 protein accumulation predicts malignant progression in Barrett’s metaplasia: A prospective study of 275 patients. Histopathology 2017, 71, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Dorer, R.; Odze, R.D. AMACR immunostaining is useful in detecting dysplastic epithelium in Barrett’s esophagus, ulcerative colitis, and Crohn’s disease. Am. J. Surg. Pathol. 2006, 30, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, F.; Biermann, K.; Steyerberg, E.W.; Verheij, J.; Kalisvaart, M.; Looijenga, L.H.; Stoop, H.A.; Walter, L.; Kuipers, E.J.; Spaander, M.C.; et al. Value of α-methylacyl-CoA racemase immunochemistry for predicting neoplastic progression in Barrett’s oesophagus. Histopathology 2013, 63, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Horvath, B.; Singh, P.; Xie, H.; Thota, P.N.; Sun, X.; Liu, X. Expression of p53 predicts risk of prevalent and incident advanced neoplasia in patients with Barrett’s esophagus and epithelial changes indefinite for dysplasia. Gastroenterol. Rep. 2016, 4, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhou, Z.; Peters, J.H.; Khoury, T.; Zhai, Q.; Wei, Q.; Truong, C.D.; Song, S.W.; Tan, D. Expression of insulin-like growth factor II mRNA-binding protein 3 in human esophageal adenocarcinoma and its precursor lesions. Arch. Pathol Lab. Med. 2011, 135, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Vohra, P.; Chu, P.G.; Woda, B.; Rock, K.L.; Jiang, Z. An oncofetal protein IMP3: A new molecular marker for the detection of esophageal adenocarcinoma and high-grade dysplasia. Am. J. Surg. Pathol. 2009, 33, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Gadara, M.R.; Gonzalez, M.; Cartun, R.W.; Ligato, S. IMP3 Immunoreactivity is More Sensitive Than AMACR in Detecting Dysplastic Epithelium and Early Adenocarcinoma in Barrett Esophagus. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Koopman, T.; Smits, M.M.; Louwen, M.; Hage, M.; Boot, H.; Imholz, A.L. HER2 positivity in gastric and esophageal adenocarcinoma: Clinicopathological analysis and comparison. J. Cancer Res. Clin. Oncol. 2015, 141, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Bartley, A.N.; Washington, M.K.; Ventura, C.B.; Ismaila, N.; Colasacco, C.; Benson III, A.B.; Carrato, A.; Gulley, M.L.; Jain, D.; Kakar, S.; et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma. Arch. Pathol. Lab. Med. 2016, 140, 1345–1363. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.T.; Galipeau, P.C.; Sanchez, C.A.; Emond, M.J.; Reid, B.J. Determination of the frequency of loss of heterozygosity in esophageal adenocarcinoma by cell sorting, whole genome amplification and microsatellite polymorphisms. Oncogene 1996, 12, 1873–1878. [Google Scholar] [PubMed]
- Wu, T.T.; Watanabe, T.; Heitmiller, R.; Zahurak, M.; Forastiere, A.A.; Hamilton, S.R. Genetic alterations in Barrett esophagus and adenocarcinomas of the esophagus and esophagogastric junction region. Am. J. Pathol. 1998, 153, 287–294. [Google Scholar] [CrossRef]
- Choi, W.T.; Tsai, J.H.; Rabinovitch, P.S.; Small, T.; Huang, D.; Mattis, A.N.; Kakar, S. Diagnosis and risk stratification of Barrett’s dysplasia by flow cytometric DNA analysis of paraffin-embedded tissue. Gut 2018, 67, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Secrier, M.; Li, X.; de Silva, N.; Eldridge, M.D.; Contino, G.; Bornschein, J.; MacRae, S.; Grehan, N.; O’Donovan, M.; Miremadi, A.; et al. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat. Genet. 2016, 48, 1131–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galipeau, P.C.; Prevo, L.J.; Sanchez, C.A.; Longton, G.M.; Reid, B.J. Clonal expansion and loss of heterozygosity at chromosomes 9p and 17p in premalignant esophageal (Barrett’s) tissue. J. Natl. Cancer Inst. 1999, 91, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Stachler, M.D.; Taylor-Weiner, A.; Peng, S.; McKenna, A.; Agoston, A.T.; Odze, R.D.; Davison, J.M.; Nason, K.S.; Loda, M.; Leshchiner, I.; et al. Paired exome analysis of Barrett’s esophagus and adenocarcinoma. Nat. Genet. 2015, 47, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Orloff, M.; Peterson, C.; He, X.; Ganapathi, S.; Heald, B.; Yang, Y.R.; Bebek, G.; Romigh, T.; Song, J.H.; Wu, W.; et al. Germline mutations in MSR1, ASCC1, and CTHRC1 in patients with Barrett esophagus and esophageal adenocarcinoma. JAMA 2011, 306, 410–419. [Google Scholar] [CrossRef] [PubMed]

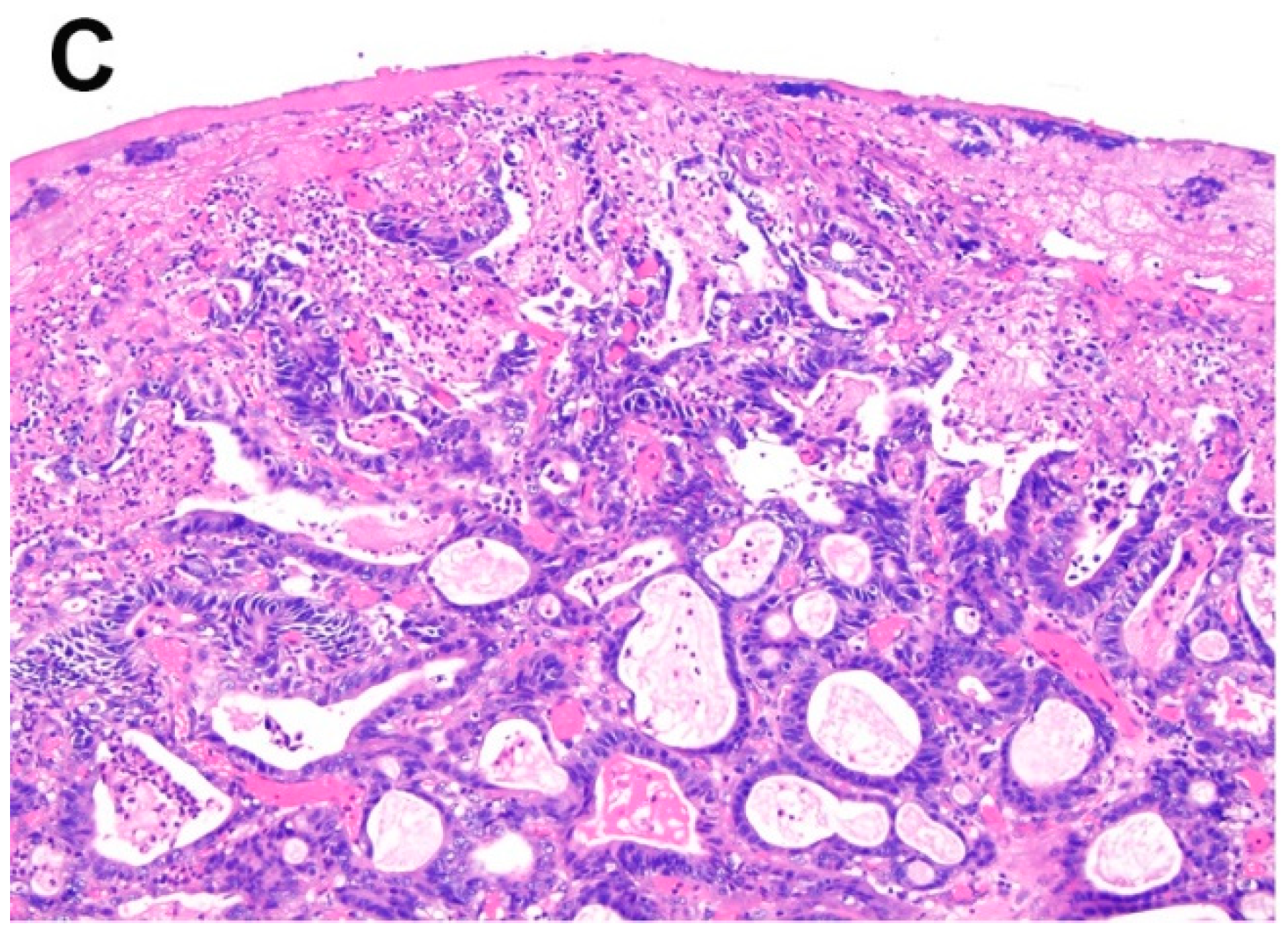
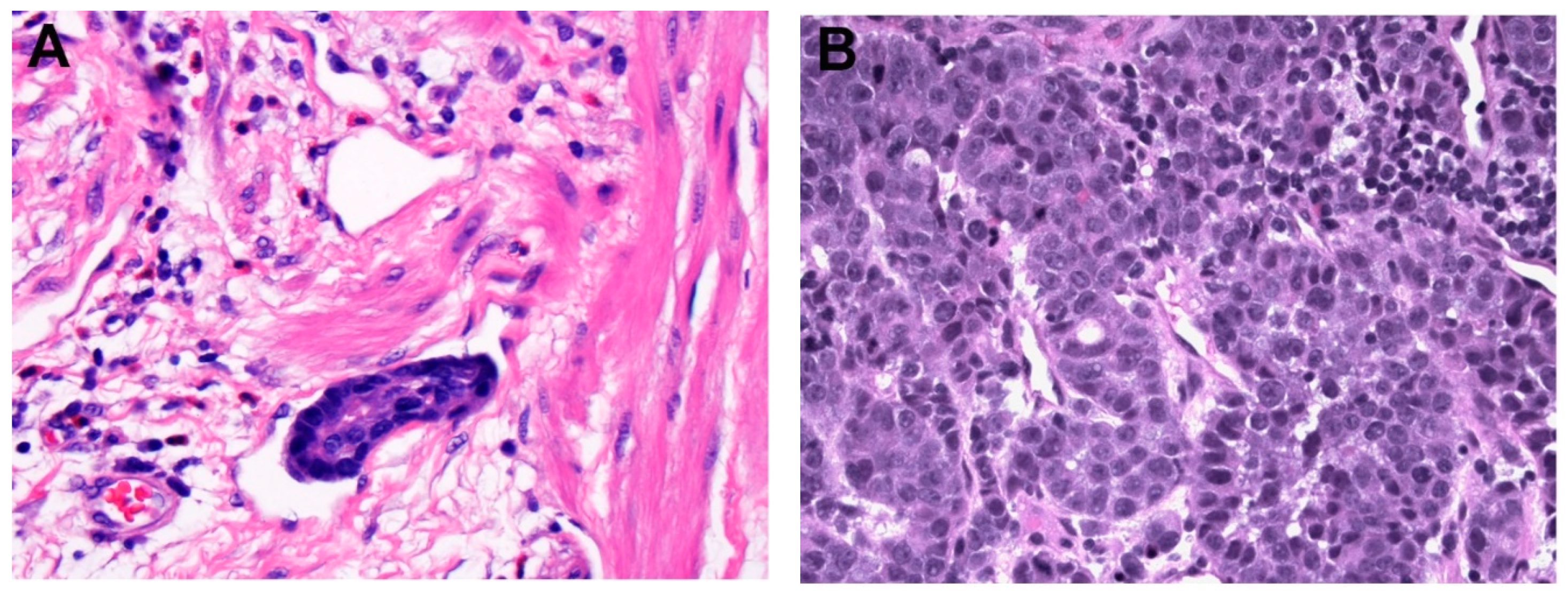
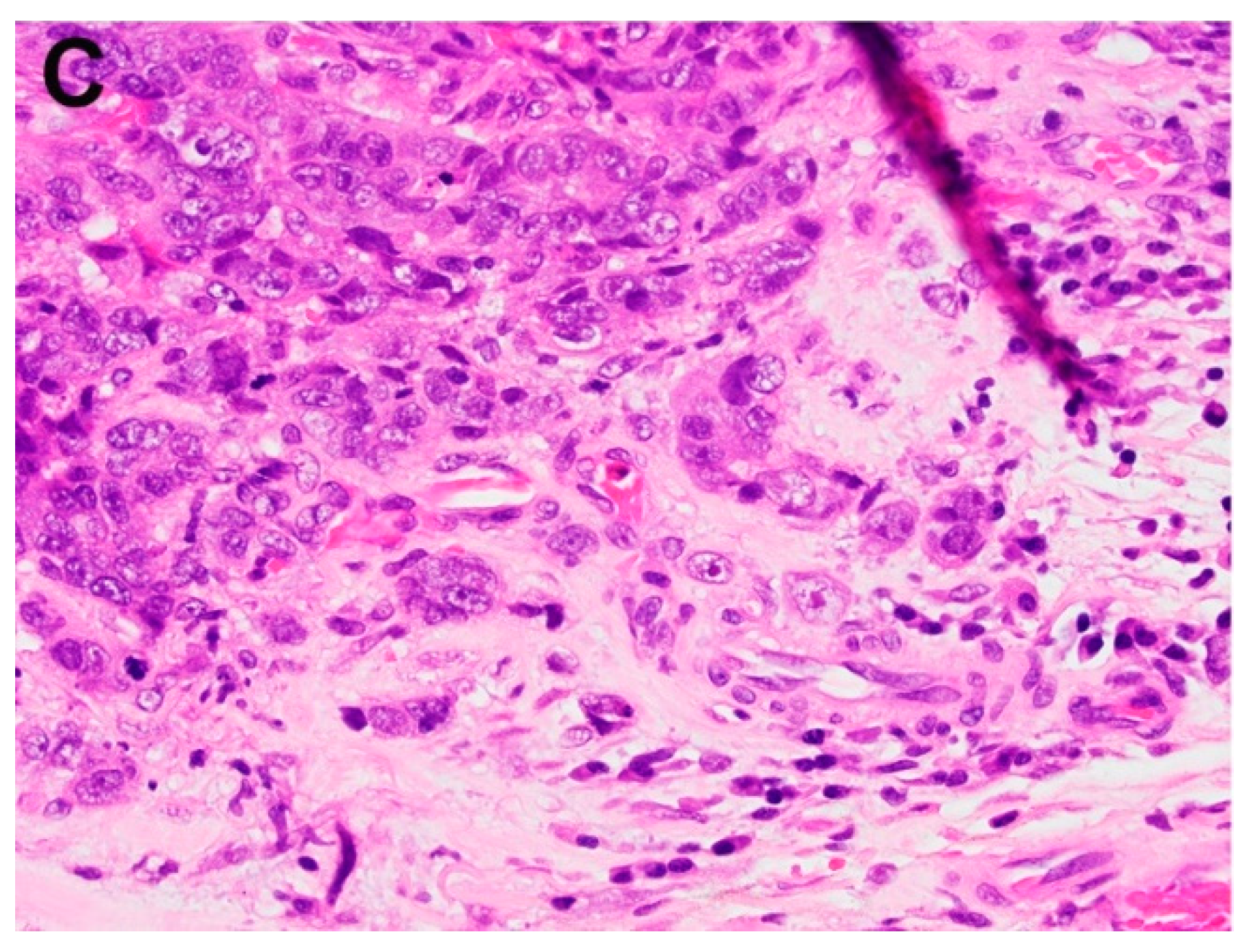
| Reference | Procedure | Subgroup | n | LN + | L + | V + | Recurrence | Metastasis | Mortality | HR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|
| Buskens et al., 2004 [35] | esophagectomy | sm1 | 16 | 0 (0%) | 0 (0%) | |||||
| sm2 | 13 | 3 (23.1%) | 4 (30.8%) | |||||||
| sm3 | 13 | 9 (69.2%) | 12 (92.3%) | |||||||
| Bollschweiler et al., 2006 [36] | esophagectomy | sm1 | 9 | 2 (22.2%) | ||||||
| sm2 | 4 | 0 (0%) | ||||||||
| sm3 | 9 | 7 (77.8%) | ||||||||
| Sepesi et al., 2010 [37] | esophagectomy | sm1 | 14 | 3 (21.4%) | 25% | |||||
| sm2 | 11 | 4 (36.4%) | 60% | |||||||
| sm3 | 4 | 2 (50.0%) | 100% | |||||||
| Westerterp et al., 2005 [32] | esophagectomy | sm1 | 25 | 0 (0%) | 0 (0%) | 4 (16%) | ||||
| sm2 | 23 | 6 (26.1%) | 4 (17.4%) | 11 (47.8%) | ||||||
| sm3 | 18 | 12 (66.7%) | 4 (22.2%) | 11 (61.1%) | ||||||
| Hölscher et al., 2011 [38] | esophagectomy | sm1 | 23 | 2 (8.6%) | ||||||
| sm2 | 15 | 2 (13.3%) | ||||||||
| sm3 | 28 | 12 (42.9%) | ||||||||
| Ancona et al., 2008 [39] | esophagectomy | sm1 | 36 | 3 (8.3%) | ||||||
| sm2 | 7 | 2 (28.6%) | ||||||||
| sm3 | 28 | 15 (53.6%) | ||||||||
| Lorenz et al., 2014 [40] | endoscopic resection and esophagectomy | sm1 | 37 | 3 (8.1%) | 12 (32.4%) | 3 (8.1%) | 3 (8.1%) | |||
| sm2 | 33 | 9 (27.3%) | 12 (36.4%) | 1 (3.0%) | 4 (12.1%) | |||||
| sm3 | 56 | 14 (25.0%) | 26 (46.4%) | 5 (8.9%) | 12 (21.4%) | |||||
| Leers et al., 2011 [41] | esophagectomy | sm1 | 19 | 4 (21.0%) | 1 | |||||
| sm2 | 9 | 1 (11.1%) | 1.07 (0.08–9.78) | |||||||
| sm3 | 23 | 6 (26.1%) | 0.75 (0.12–4.77) | |||||||
| Barbour et al., 2010 [42] | esophagectomy | sm1 | 12 | 2 (16.7%) | ||||||
| sm2 | 17 | 2 (11.8%) | ||||||||
| sm3 | 20 | 4 (20.0%) | ||||||||
| Summary of LN + Rate, [32,35,36,37,38,39,40,41,42] | sm1 | 191 | 19 (9.9%) | |||||||
| sm2 | 132 | 29 (22.0%) | ||||||||
| sm3 | 199 | 81 (40.7%) | ||||||||
| Zemler et al., 2010 [43] | endoscopic resection | sm1 | 61 | 15 (24.6%) | 2 (3.3%) | |||||
| sm2 | 19 | 8 (42.1%) | 0 (0%) | |||||||
| sm3 | 29 | 12 (41.4%) | 4 (13.8%) | |||||||
| Badreddine et al., 2010 [44] | esophagectomy | sm1 | 31 | 4 (12.9%) | 10 (32%) | 3 (9.6%) | 10 (32%) | |||
| sm2-3 | 49 | 10 (20%) | 17 (35%) | 4 (8.2%) | 21 (43%) | |||||
| Manner et al., 2017 [34] | endoscopic resection and esophagectomy | sm2 low risk | 12 | 0 (0%) | 0 (0%) | |||||
| sm2 high risk | 11 | 6 (54.5%) | 0 (0%) | |||||||
| sm3 low risk | 7 | 0 (0%) | 0 (0%) | |||||||
| sm3 high risk | 32 | 17 (53.1%) | 3 (9.4%) | |||||||
| Liu et al., 2005 [45] | esophagectomy | Superficial (<50%) | 12 | 1 (8.3%) | 3 (25.0%) | 3 (0.8–11.5) | ||||
| Deep (>50%) | 25 | 9 (36.0%) | 12 (48.0%) | 2.2 (0.7–7.1) | ||||||
| Alvarez Herrero et al., 2010 [46] | endoscopic resection | <500 μm | 12 | 0 (0%) | 0 (0%) | |||||
| >500 μm | 13 | 0 (0%) | 3 (23.1%) | |||||||
| Ishihara et al., 2017 [47] | endoscopic resection and esophagectomy | <500 μm | 59 | 6.80% | ||||||
| >500 μm | 195 | 22.9–50% |
| Reference | Stage | Staining | Methods | Cut-off | n | G3 | LN + | L + | V + | PNI | Tumor ≥ 2 cm | Survival (Month) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Landau et al., 2014 [48] | pT1 | H&E | TBF: a 20X microscopic field | TBF (0–2) | 151 | 6% | 11% | 40% | ||||
| (0.785 mm2) with 5 or more TB | TBF (≥ 3) | 59 | 39% | 41% | 75% | |||||||
| Brown et al., 2010 [49] | pT1-pT4 | H&E | Count the total number of TB at | TB < 5 | 31 ± 5.55 | |||||||
| a 20X microscopic field | TB ≥ 5 | 15 ± 1.14 | ||||||||||
| Thies et al., 2016 [50] | pT1-pT4 | Pan-CK | PTB: TB count in 10 HPFs along | LG PTB < 130 TB | 100 | 32% | 42% | 37% | 8% | 13% | ||
| the invasion front | HG PTB ≥ 130 TB | 99 | 63% | 66% | 71% | 23% | 50% | |||||
| ITB: TB count in 10 HPF within | LG ITB < 80 TB | 100 | 29% | 44% | 38% | 8% | 11% | |||||
| the main tumor body | HG ITB ≥ 80 TB | 100 | 66% | 63% | 69% | 23% | 52% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, F.; Hernandez Gonzalo, D.; Lai, J.; Liu, X. Histopathology of Barrett’s Esophagus and Early-Stage Esophageal Adenocarcinoma: An Updated Review. Gastrointest. Disord. 2019, 1, 147-163. https://doi.org/10.3390/gidisord1010011
Yin F, Hernandez Gonzalo D, Lai J, Liu X. Histopathology of Barrett’s Esophagus and Early-Stage Esophageal Adenocarcinoma: An Updated Review. Gastrointestinal Disorders. 2019; 1(1):147-163. https://doi.org/10.3390/gidisord1010011
Chicago/Turabian StyleYin, Feng, David Hernandez Gonzalo, Jinping Lai, and Xiuli Liu. 2019. "Histopathology of Barrett’s Esophagus and Early-Stage Esophageal Adenocarcinoma: An Updated Review" Gastrointestinal Disorders 1, no. 1: 147-163. https://doi.org/10.3390/gidisord1010011
APA StyleYin, F., Hernandez Gonzalo, D., Lai, J., & Liu, X. (2019). Histopathology of Barrett’s Esophagus and Early-Stage Esophageal Adenocarcinoma: An Updated Review. Gastrointestinal Disorders, 1(1), 147-163. https://doi.org/10.3390/gidisord1010011




