Corrosion Behavior of Electrochemical and Thermal Treated Titanium into Artificial Saliva: Effect of pH and Fluoride Concentration
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Samples
2.2. Electrochemical Oxidation
2.3. Structural and Morphological Analyses
2.4. Electrochemical Measurements
3. Experimental Results
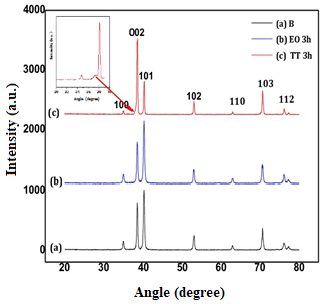
3.1. Surface Analysis
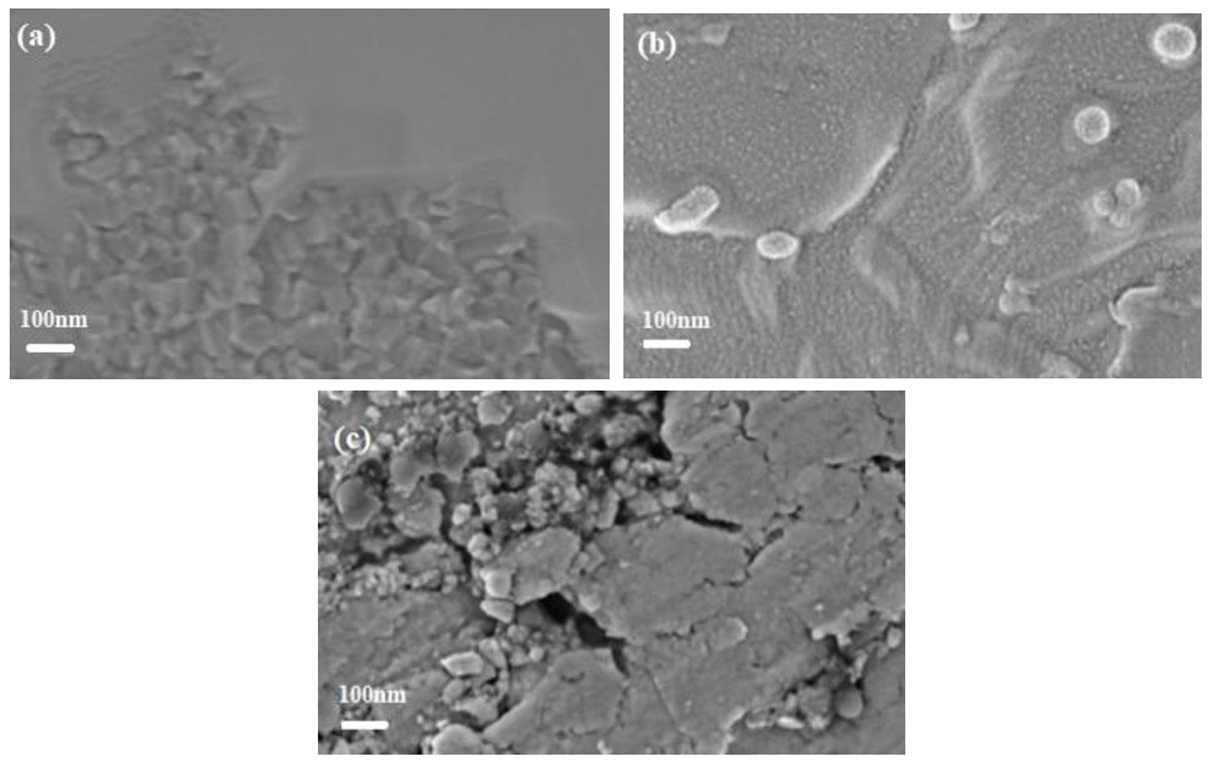
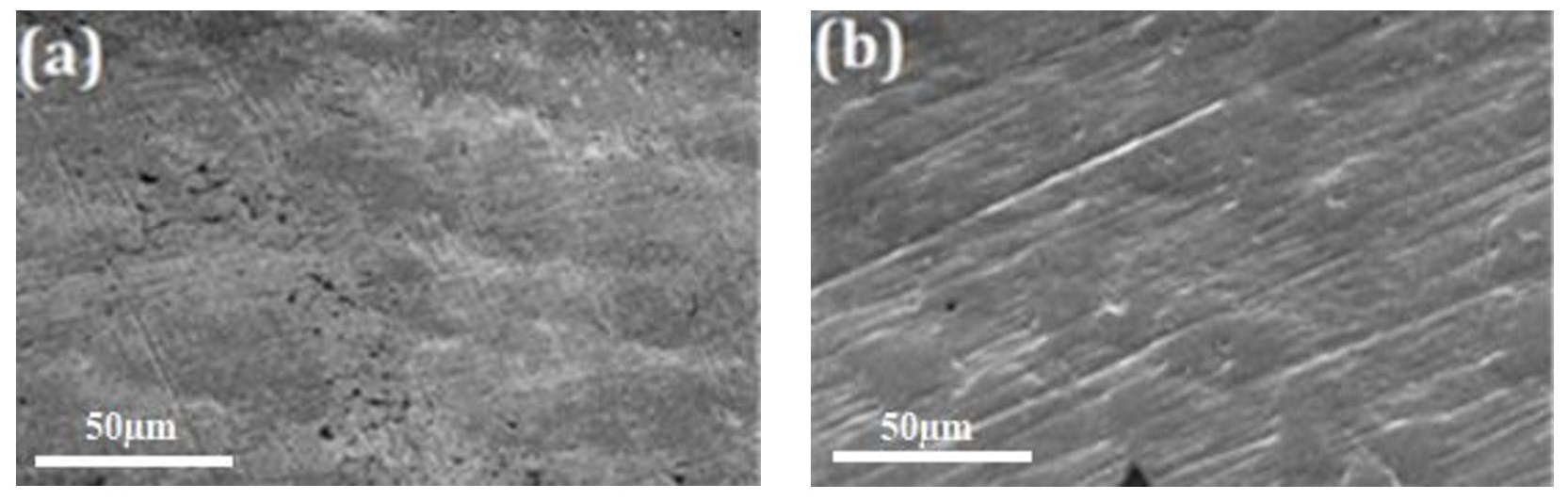
3.2. Morphological Analysis
3.3. Electrochemical Measurements
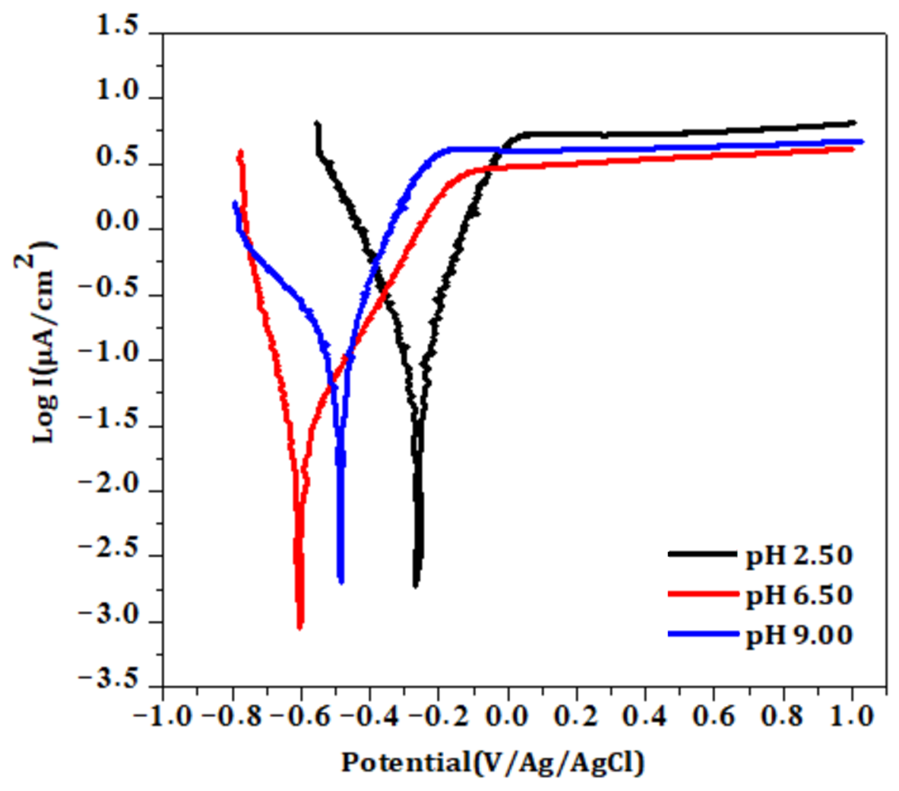
3.3.1. Effect of pH on B
- Potentiodynamic polarization curves
- b.
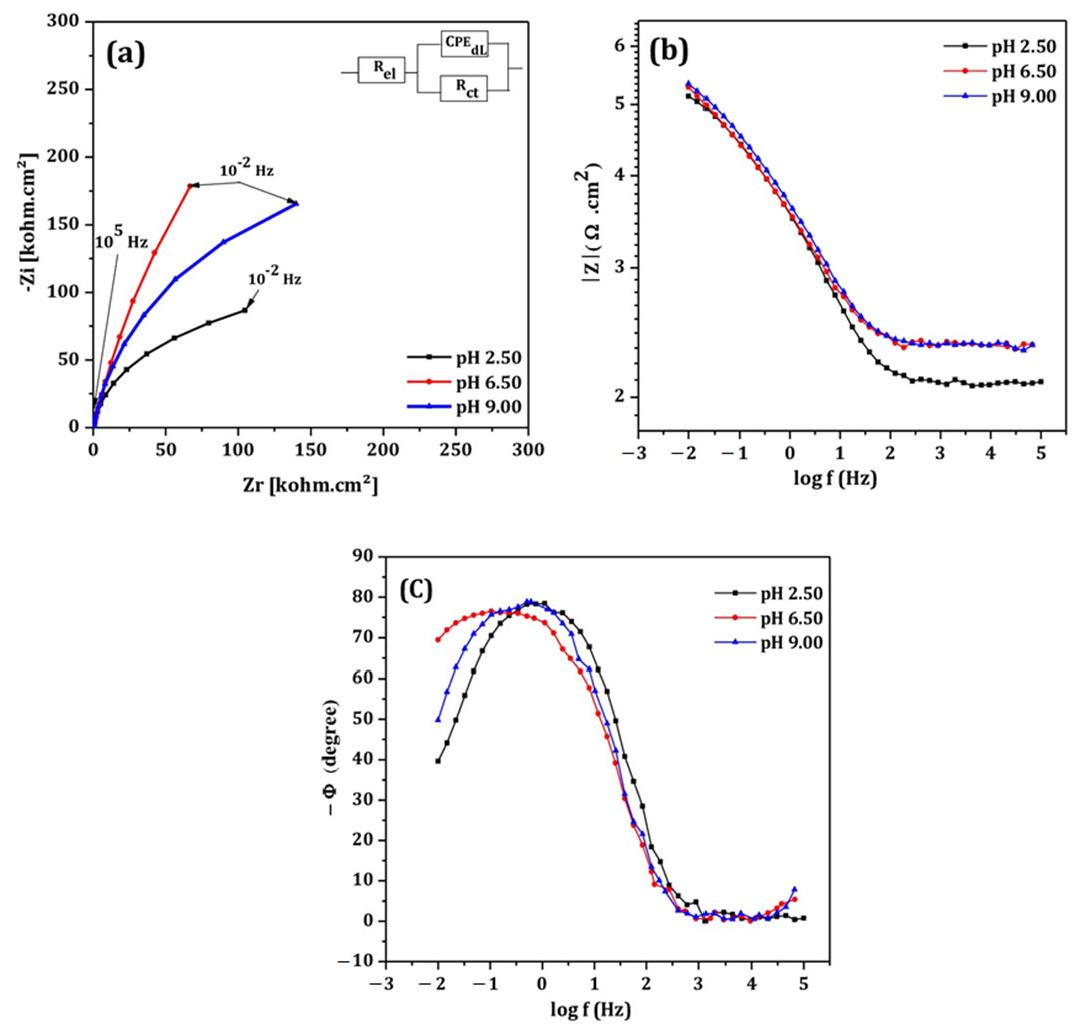
- Electrochemical impedance spectroscopy measurements
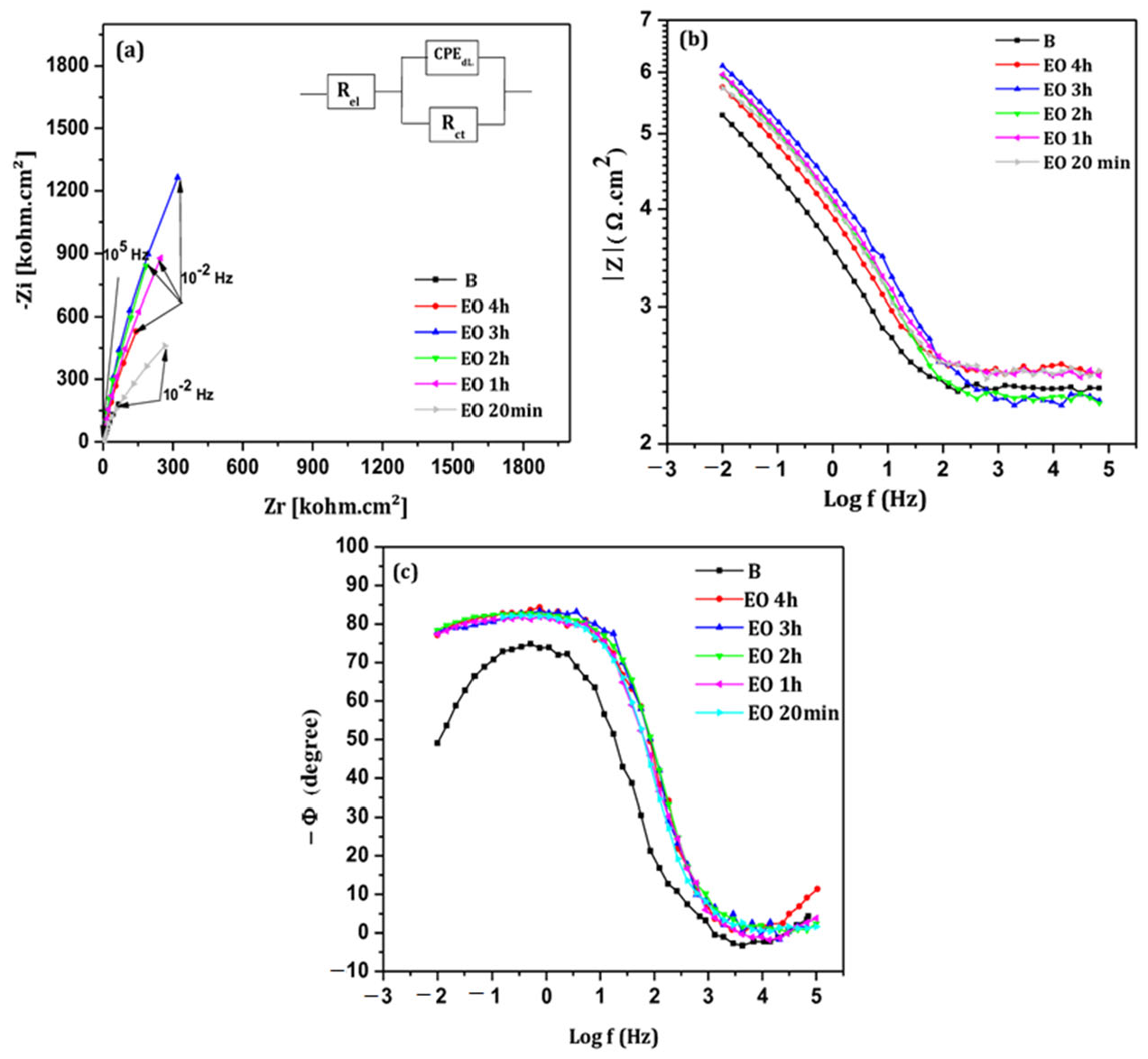
3.3.2. Effect of Surface Treatments
- Potentiodynamic polarization curves
- b.
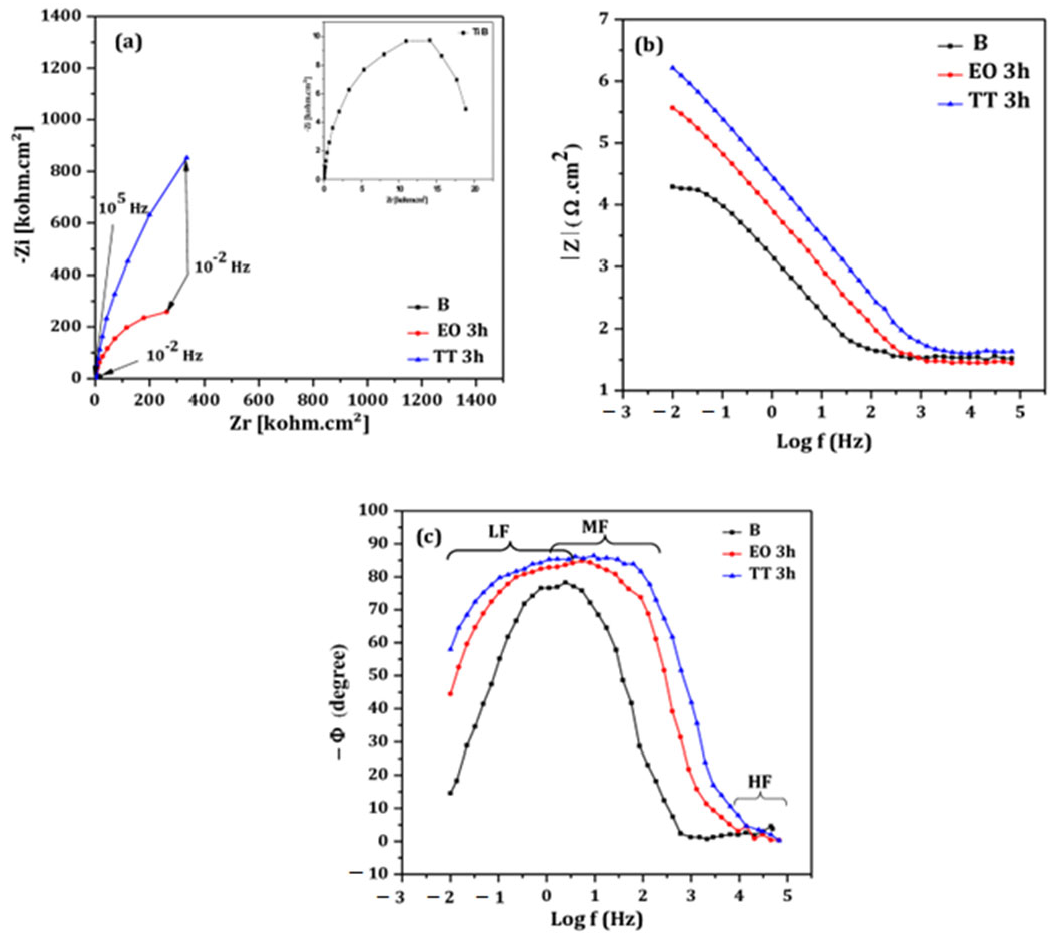
- EIS measurement
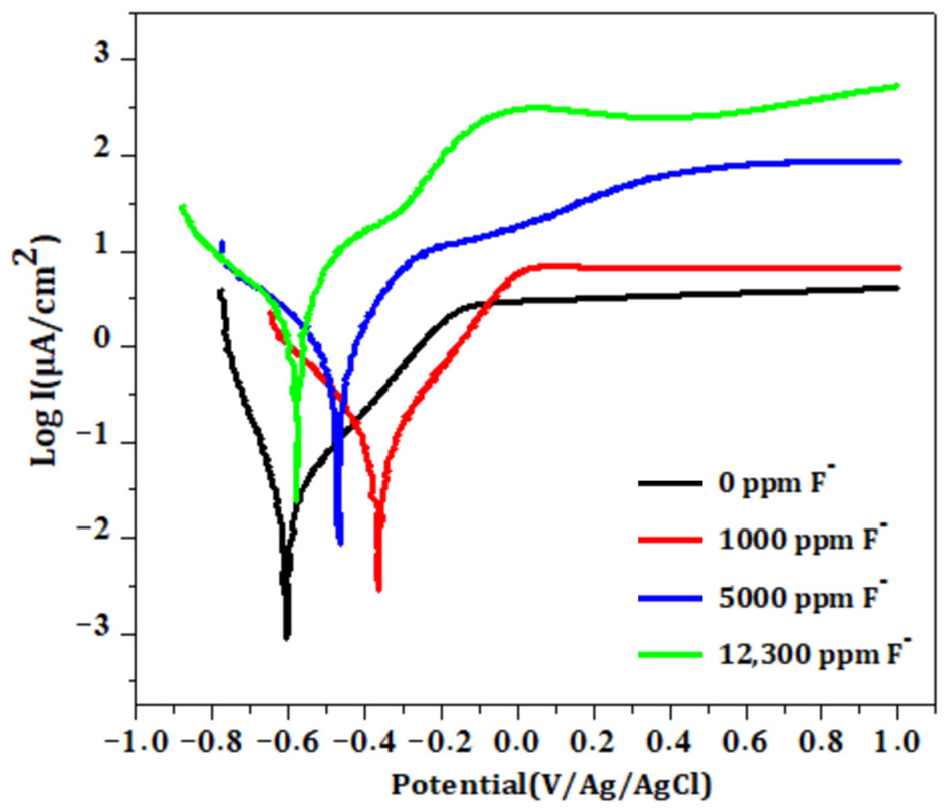
3.3.3. Effect of Fluoride Ions on the Bare Titanium Surface (B)
- Potentiodynamic polarization curves
- b.
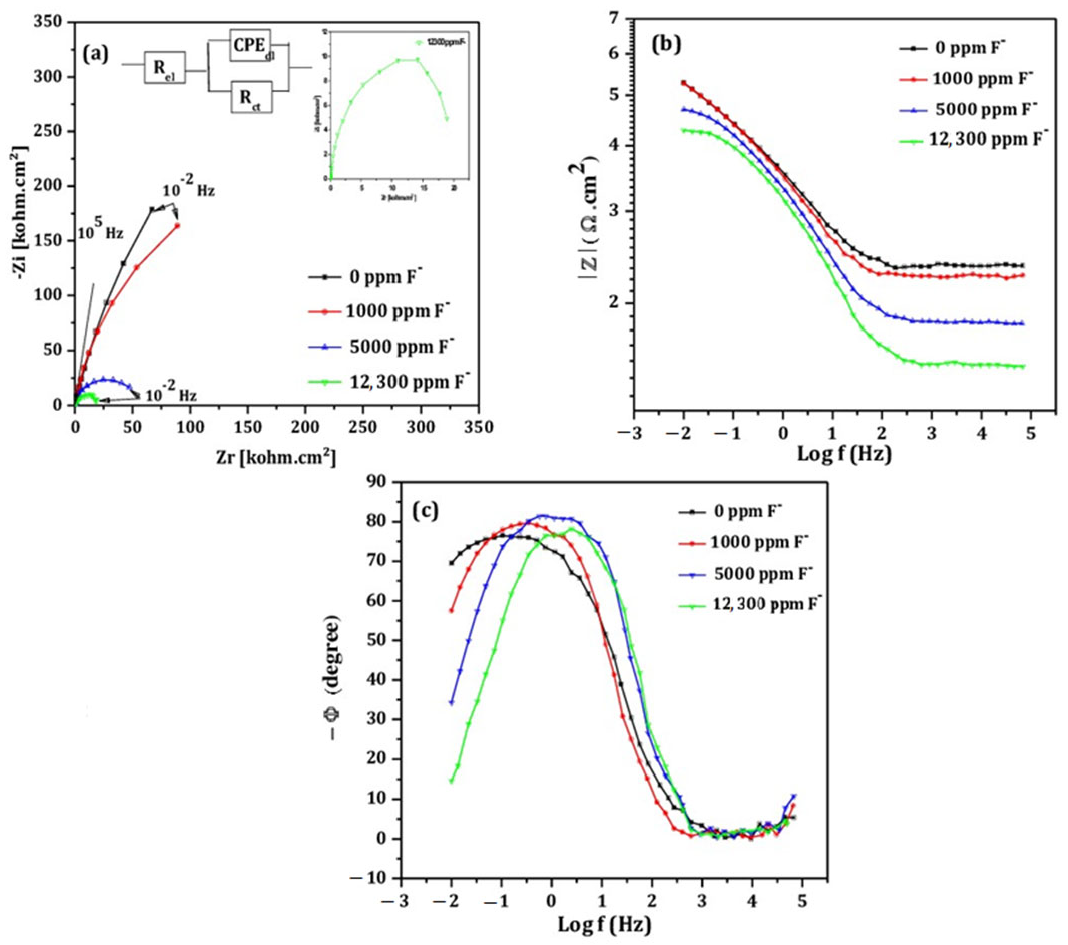
- EIS measurement
3.3.4. Corrosion Behavior of EO and TT Samples in 12,300 ppm Fluoride-Containing Saliva
- Potentiodynamic polarization curves
- b.
- EIS measurement
4. Conclusions
- The study demonstrated that corrosion rates increase at both low and high pH levels, underscoring the importance of maintaining near-neutral pH in oral environments to preserve the integrity of titanium implants.
- Treatment durations from 20 min to 4 h were tested, with 3 h identified as optimal for both electrochemical and thermal oxidation. During this time, TiO2 layers effectively passivated the titanium surface in neutral saliva (pH 6.5). However, EO-treated films showed nanoscale defects like pores and cracks that may reduce long-term protection.
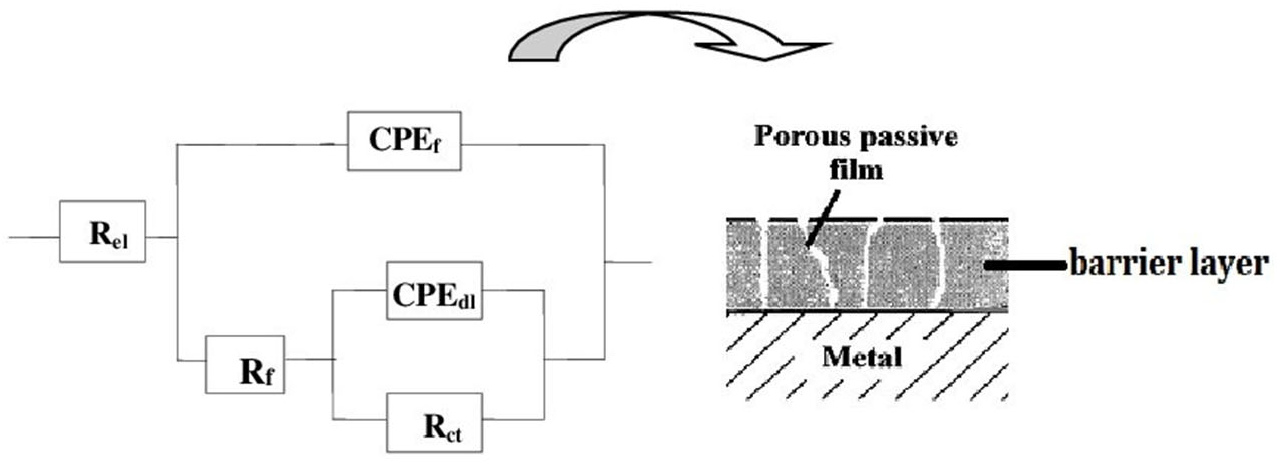
- Electrochemical tests performed at fluoride concentrations of 1000, 1500 and 12,300 ppm revealed a strong correlation between fluoride contact and corrosion severity. Higher fluoride levels led to increase corrosion current density and decrease polarization resistance, indicating significant degradation of the passive layer. EIS results at 12,300 ppm F− suggested the formation of a dual layer structure composed of an inner barrier layer and an outer porous layer, which likely accounts for the extensive pitting observed by SEM.
- Both EO and TT surface modifications significantly enhanced the corrosion resistance of titanium in artificial saliva, even under aggressive fluoride concentrations. Notably, the TT treated samples provided superior performance, attributed to the formation of a more compact and defect-free layer compared to EO.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rojo, L.; Gharibi, B.; McLister, R.; Meenan, B.J.; Deb, S. Self-assembled monolayers of alendronate on Ti6Al4V alloy surfaces enhance osteogenesis in mesenchymal stem cells. Sci. Rep. 2016, 6, 30548. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.K.; Lapovok, R.; Estrin, Y.S.; Rundell, S.; Wang, J.Y.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials 2010, 31, 3674–3683. [Google Scholar] [CrossRef] [PubMed]
- Chelariu, R.; Bolat, G.; Izquierdo, J.; Mareci, D.; Gordin, D.M.; Gloriant, T.; Souto, R.M. Metastable beta Ti-Nb-Mo alloys with improved corrosion resistance in saline solution. Electrochim. Acta 2014, 137, 280–289. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Huo, W.; Zhang, W.; Zhao, Y.; Zhang, Y. Electrochemical corrosion characteristics and biocompatibility of nanostructured titanium for implants. Appl. Surf. Sci. 2018, 434, 63–72. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, Y.J.; Park, C.H.; Lee, D.H.; Ko, Y.G.; Jang, J.H.; Lee, C.S. Enhanced osteoblast response to an equal channel angular pressing-processed pure titanium substrate with microrough surface topography. Acta Biomater. 2009, 5, 3272–3280. [Google Scholar] [CrossRef]
- Robin, A.; Meirelis, J.P. Influence of fluoride concentration and pH on corrosion behavior of Ti-6Al-4V and Ti-23Ta alloys in artificial saliva. Mater. Corros. 2007, 58, 173–180. [Google Scholar] [CrossRef]
- Marino, C.E.B.; Mascaro, L.H. EIS characterization of a Ti-dental implant in artificial saliva media: Dissolution process of the oxide barrier. J. Electroanal. Chem. 2004, 568, 115–120. [Google Scholar] [CrossRef]
- Schiff, N.; Grosgogeat, B.; Lissac, M.; Dalard, F. Influence of fluoride content and pH on the corrosion resistance of titanium and its alloys. Biomaterials 2002, 23, 1995–2002. [Google Scholar] [CrossRef]
- Mazare, A.; Totea, G.; Burnei, C.; Schmuki, P.; Demetrescu, I.; Ionita, D. Corrosion, antibacterial activity and haemocompatibility of TiO2 nanotubes as a function of their annealing temperature. Corros. Sci. 2016, 103, 215–222. [Google Scholar] [CrossRef]
- Li, J.; Li, S.J.; Hao, Y.L.; Huang, H.H.; Bai, Y.; Hao, Y.Q.; Guo, Z.; Xue, J.Q.; Yang, R. Electrochemical and surface analyses of nanostructured Ti-24Nb-4Zr-8Sn alloys in simulated body solution. Acta Biomater. 2014, 10, 2866–2875. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Marino, C.E.B.; Biaggio, S.R.; Rocha-Filho, R.C.; Bocchi, N. Voltammetric stability of anodic films on the Ti6Al4V alloy in chloride medium. Electrochim. Acta 2006, 51, 6580–6583. [Google Scholar] [CrossRef]
- Alves, V.A.; Reis, R.Q.; Santos, I.C.B.; Souza, D.G.; Gonçalves, T.F.; Pereira-da-Silva, M.A.; Rossi, A.; da Silva, L.A. In situ impedance spectroscopy study of the electrochemical corrosion of Ti and Ti–6Al–4V in simulated body fluid at 25 °C and 37 °C. Corros. Sci. 2009, 51, 2473–2482. [Google Scholar] [CrossRef]
- Mutlu, I. Synthesis and characterization of Ti−Co alloy foam for biomedical applications. Trans. Nonferrous Met. Soc. China. 2016, 26, 126–137. [Google Scholar] [CrossRef]
- Valentim, A.R.B.; Mathew, T.M.; Assunção, W.G.; Yuan, J.C.C.; Wimmer, M.; Sukotjo, C. Stability of cp-Ti and Ti-6Al-4V alloy for dental implants as a function of saliva pH- an electrochemical study. Clin. Oral Implants Res. 2012, 23, 1055–1062. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Barbosa, L.S.; Ariza, E.A.; Henriques, M.; Teughels, W.; Ponthiaux, P.; Celis, J.P.; Rocha, L.A. How do titanium and Ti6Al4V corrode in fluoridated medium as found in the oral cavity? An in vitro study. Mater. Sci. Eng. 2015, 47, 384–393. [Google Scholar] [CrossRef]
- Nakagawa, M.; Matsuya, S.; Shiraishi, T.; Ohta, M. Effect of fluoride concentration and pH on corrosion behavior of titanium for dental use. J. Dent. Res. 1999, 78, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Janužis, G.; Milvydaitė, G.; Miškinytė, M.; Latakas, D.; Griškonis, G. Fluoride Induced Corrosion Impact on Peri-Imlantitis: A Systemic Review. Ann. Dent. Spec. 2023, 11, 87–92. [Google Scholar] [CrossRef]
- Huang, H.H. Effect of fluoride and albumin concentration on the corrosion behavior of Ti-6Al-4V alloy. Biomaterials 2003, 24, 275–282. [Google Scholar] [CrossRef]
- Laurent, F.; Grosgogeat, B.; Reclaru, L.; Dalard, F.; Lissac, M. Comparison of corrosion behaviour in presence of oral bacteria. Biomaterials 2001, 22, 2273–2282. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Henriques, M.; Oliveira, R.; Teughels, W.; Celis, J.P.; Rocha, L.A. Do oral biofilms influence the wear and corrosion behavior of titanium? Biofouling 2010, 26, 471–478. [Google Scholar] [CrossRef]
- Murrell, S.; Marshall, T.; Moynihan, P.; Qian, F.; Wefel, J. Comparison of in vitro erosion potentials between beverages available in the United Kingdom and the United States. J. Dent. 2010, 38, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Assis, S.L.; Wolynec, S.; Costa, I. Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim. Acta 2006, 51, 1815–1819. [Google Scholar] [CrossRef]
- Souza, M.E.; Lima, L.; Lima, C.R.P.; Zavaglia, C.A.C.; Freire, C.M.A. Effects of pH on the electrochemical behaviour of titanium alloys for implant applications. J. Mater. Sci Mater. Med. 2009, 20, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Q.; Zhang, S.M.; Qiu, J. Surface analysis and corrosion behavior of pure titanium under fluoride exposure. J. Prosthet. Dent. 2020, 124, 239.e1–239.e8. [Google Scholar] [CrossRef]
- Souza, J.C.; Apaza-Bedoya, K.; Benfatti, C.A.; Silva, F.S.; Henriques, B. A comprehensive review on the corrosion pathways of titanium dental implants and their biological adverse effects. J. Met. 2020, 10, 1272. [Google Scholar] [CrossRef]
- Lindholm-Sethson, B.; Ardlin, B.I. Effects of pH and fluoride concentration on the corrosion of titanium. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. 2008, 86, 149–159. [Google Scholar] [CrossRef]
- Çomaklı, O.; Yazıcı, M.; Yetim, T.; Yetim, A.F.; Çelik, A. The effect of calcination temperatures on structural and electrochemical properties of TiO2 film deposited on commercial pure titanium. Surf. Coat. Technol. 2016, 285, 298–303. [Google Scholar] [CrossRef]
- Jamesh, M.; Kumar, S.; Narayanan, T.S.N.S.; Chu, P.K. Effect of thermal oxidation on the corrosion resistance of Ti6Al4V alloy in hydrochloric and nitric acid medium: Effect of thermal oxidation on the corrosion resistance of Ti6Al4V alloy. Mater. Corros. 2013, 64, 902–907. [Google Scholar] [CrossRef]
- Jayaraj, J.; Ranjith, P.M.; Ningshen, S.; Ramanathan, S. Studies on Corrosion of Titanium and Air-Oxidized Titanium in Fluorinated Nitric Acid. Trans. Indian Inst. Met. 2019, 72, 1917–1926. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Curto, B.D.; Pedeferri, M. Anodic oxidation of titanium: From technical aspects to biomedical applications. J. Appl. Biomater. Biomech. 2011, 9, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Doulache, M.; Trari, M.; Benchettara, A. The oxidation of titanium thin films in phosphoric medium. Prot. Met. Phys. Chem. Surf. 2014, 50, 200–208. [Google Scholar] [CrossRef]
- Minhas, B.; Dino, S.; Zuo, Y.; Qian, H.; Zhao, X. Improvement of Corrosion Resistance of TiO2 Layers in Strong Acidic Solutions by Anodizing and Thermal Oxidation Treatment. J. Mater. 2021, 14, 1188. [Google Scholar] [CrossRef]
- Pour, H.K.; Ansari, H.; Tehrani, A. In vitro effect of anodization of titanium abutments on their tensile bond strength to implant-supported lithium disilicate all-ceramic crowns. J. Dent. Res. 2023, 20, 99. [Google Scholar] [CrossRef]
- Ningrum, E.O.; Khoiroh, I.; Nastiti, H.I.; Affan, R.A.; Karisma, A.D.; Agustiani, E.; Widiyanto, S. Surface coating effect on corrosion resistance of titanium alloy bone implants by anodizing method. Int. J. Technol. 2023, 14, 749–760. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Mendez-Ramirez, C.T.; Carrera-Ramirez, M.G.; Baltazar-Zamora, M.A.; Santiago-Hurtado, G.; Almeraya-Calderon, F. Corrosion of anodized titanium alloys. Coatings 2024, 14, 809. [Google Scholar] [CrossRef]
- Guo, T.; Scimeca, J.C.; Ivanovski, S.; Verron, E.; Gulati, K. Enhanced Corrosion Resistance and Local Therapy from Nano-Engineered Titanium Dental Implants. Pharmaceutics 2023, 15, 315. [Google Scholar] [CrossRef]
- Djendel, A.; Ait Ahmed, N.; Knauth, P.; Eyraud, M. Improved corrosion and adhesion properties of titanium alloy for endoprostheses applications using a two-step anodization method. Surf. Coat. Technol. 2023, 461, 129437. [Google Scholar] [CrossRef]
- Mohammed, A.A. Evaluation of the corrosion resistance and biology properties for titanium surface coated with various composite coatings. Appl. Phys. A 2025, 131, 372. [Google Scholar] [CrossRef]
- Lu, W.Q.; Liu, Y.J.; Wu, X.; Liu, X.C.; Wang, J.C. Corrosion and passivation behavior of Ti-6Al-4V surfaces treated with high-energy pulsed laser: A comparative study of cast and 3D-printed specimens in a NaCl solution. Surf. Coat. Technol. 2023, 470, 129849. [Google Scholar] [CrossRef]
- Wang, X.; Qin, P.; Chen, L.Y.; Sun, H.; Zhang, L.C. Corrosion behavior and mechanisms of the heat-treated Ti5Cu produced by laser powder bed fusion. Corros. Sci. 2023, 221, 111336. [Google Scholar] [CrossRef]
- Lu, W.; Liu, Y.; Wu, X.; Liu, X.; Wang, J. Corrosion behavior and microstructural effects on passivation film mechanisms in forged Ti-5Al-5Mo-5V-1Cr-1Fe titanium alloy under laser surface remelting. Corros. Sci. 2024, 241, 112542. [Google Scholar] [CrossRef]
- Rossi, S.; Volgare, L.; Perrin-Pellegrino, C.; Chassigneux, C.; Dousset, E.; Eyraud, M. Dual Electrochemical Treatments to Improve Properties of Ti6Al4V Alloy. J. Mater. 2020, 13, 2479. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Mingo, B.; Mohedano, M.; Pardo, A.; Merino, M.C. In vitro corrosion performance of PEO coated Ti and Ti6Al4V used for dental and orthopaedic implants. Surf. Coat. Technol. 2016, 307, 1255–1264. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A. Electrochemical polarization: I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 56, 104. [Google Scholar] [CrossRef]
- Gaul, E. Coloring titanium and related metals by electrochemical oxidation. J. Chem. Educ. 1993, 70, 176–178. [Google Scholar] [CrossRef]
- Velten, D.; Biehl, V.; Aubertin, F.; Valeske, B.; Possart, W.; Breme, J. Preparation of TiO2 layers on cp-Ti and Ti6Al4V by thermal and anodic oxidation and by sol-gel coating techniques and their characterization. J. Biomed. Mater. Res. 2002, 59, 18–28. [Google Scholar] [CrossRef]
- Ting, C.C.; Chen, S.Y.; Liu, D.M. Preferential growth of thin rutile TiO2 films upon thermal oxidation of sputtered Ti films. Thin Solid Films 2002, 402, 290–295. [Google Scholar] [CrossRef]
- Helbert, V.S.; Nazarov, A.; Vucko, F.; Larché, N.; Thierry, D. Effect of Cathodic Polarisation Switch-Off on the Passivity and Stability to Crevice Corrosion of AISI 304L Stainless Steel. Materials 2021, 14, 2921. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhong, X.; Walton, J.; Thompson, G.E. Anodic Film Growth of Titanium Oxide Using the 3-Electrode Electrochemical Technique: Effects of Oxygen Evolution and Morphological Characterizations. J. Electrochem. Soc. 2016, 163, 75–82. [Google Scholar] [CrossRef]
- Cheung, K.H.; Pabbruwe, M.B.; Chen, W.F.; Koshy, P.; Sorrell, C.C. Thermodynamic and microstructural analyses of photocatalytic TiO2 from the anodization of biomedical-grade Ti6Al4V in phosphoric acid or sulfuric acid. Ceram. Int. 2021, 47, 1609–1624. [Google Scholar] [CrossRef]
- Lin, F.H.; Hsu, Y.S.; Lin, S.H.; Chen, T.M. The growth of hydroxyapatite on alkaline treated Ti−6Al−4V soaking in higher temperature with concentrated Ca2+/HPO42− simulated body fluid. Mater. Chem. Phys. 2004, 87, 24–30. [Google Scholar] [CrossRef]
- Fojt, J.; Joska, L.; Málek, J. Corrosion behaviour of porous Ti−39Nb alloy for biomedical applications. Corros. Sci. 2013, 71, 78–83. [Google Scholar] [CrossRef]
- Mareci, D.; Ungureanu, G.; Aelenei, D.M.; Mirza-Rosca, J.C. Electrochemical characteristics of titanium based biomaterials in artificial saliva. Mater. Corros 2007, 58, 848–856. [Google Scholar] [CrossRef]
- González, J.E.G.; Mirza-Rosca, J.C. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. J. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- Qu, Q.; He, Y.; Li, L.; Yang, M.; Lai, B.; Chen, Y. Effect of fluorine ion on the corrosion of Ti-6Al-4V alloy in artificial saliva. Int. J. Electrochem. Sci. 2015, 10, 7453–7464. [Google Scholar] [CrossRef]
- Janužis, G.; Milvydaitė, G.; Miškinytė, M.; Latakas, D.; Griškonis, G. A Systematic Review on the Effects of Fluoride-Induced Corrosion in Peri-Implantitis. Turk. J. Dent. Hyg. 2023, 3, 61–67. [Google Scholar] [CrossRef]
- Wang, L.; Wang, M.; Zhong, M.; Li, X.; Cui, Z. Degradation of thermal oxide film on pure titanium in an acidic environment containing fluoride. Npj Mater. Degrad. 2022, 6, 65. [Google Scholar] [CrossRef]




| wt% | Ti | O |
|---|---|---|
| B | 92 ± 1 | 8 ± 2 |
| TT | 74 ± 1 | 26 ± 2 |
| EO | 74 ± 1 | 26 ± 2 |
| pH | Ecorr (mV/ Ag/AgCl) | Icorr × 10−9 (A·cm−2) | Average Tafel Slope (mV dec−1) | RP × 106 (Ω·cm2) | Ipass × 10−6 (A·cm−2) | |
|---|---|---|---|---|---|---|
| βa | βc | |||||
| 2.50 | −266 ± 13 | 91 ± 4 | 141 ± 7 | 162 ± 8 | 0.36 ± 0.04 | 6.30 ± 0.3 |
| 6.50 | −613 ± 30 | 23 ± 1 | 219 ± 11 | 94 ± 5 | 1.24 ± 0.12 | 4.00 ± 0.2 |
| 9.00 | −481 ± 24 | 81 ± 4 | 115 ± 5 | 222 ± 11 | 0.40 ± 0.04 | 4.50 ± 0.2 |
| pH | Rel (Ω·cm2) | CPEdl × 10−6 (Ω−1·cm−2·sn) | n | Rct (kΩ·cm2) | Chi-Squared |
|---|---|---|---|---|---|
| 2.50 | 211 | 78 | 0.91 | 300 | 0.002 |
| 6.50 | 228 | 57 | 0.86 | 1390 | 0.002 |
| 9.00 | 228 | 43 | 0.90 | 420 | 0.003 |
| Ecorr (mV/Ag/AgCl) | Icorr × 10−9 (A·cm−2) | Average Tafel Slope (mV dec−1) | RP × 106 (Ω·cm2) | Ipass × 10−6 (A·cm−2) | ||
|---|---|---|---|---|---|---|
| βa | βc | |||||
| B | −613 ± 30 | 23 ± 1 | 219 ± 11 | 94 ± 5 | 1.24 ± 0.12 | 4.00 ± 0.2 |
| EO 4 h | −549 ± 27 | 12 ± 0.6 | 400 ± 20 | 87 ± 4 | 2.58 ± 0.26 | 1.68 ± 0.09 |
| EO 3 h | −144 ± 7 | 6 ± 0.3 | 399 ± 20 | 177 ± 9 | 8.87 ± 0.89 | 0.32 ± 0.02 |
| EO 2 h | −376 ± 19 | 6 ± 0.3 | 313 ± 16 | 107 ± 5 | 5.77 ± 0.58 | 0.44 ± 0.02 |
| EO 1 h | −293 ± 15 | 7 ± 0.4 | 308 ± 15 | 152 ± 8 | 6.31 ± 0.63 | 0.52 ± 0.03 |
| EO 20 min | −238 ± 12 | 18 ± 0.9 | 251 ± 13 | 128 ± 6 | 2.04 ± 0.20 | 1.58 ± 0.08 |
| Ecorr (mV/Ag/AgCl) | Icorr × 10−9 (A·cm−2) | Average Tafel Slope (mV dec−1) | RP × 106 (Ω·cm2) | Ipass × 10−6 (A·cm−2) | ||
|---|---|---|---|---|---|---|
| βa | βc | |||||
| B | −613 ± 30 | 23 ± 1 | 219 ± 11 | 94 ± 5 | 1.24 ± 0.12 | 4.00 ± 0.2 |
| TT 4 h | −331 ± 17 | 10 ± 0.5 | 195 ± 10 | 124 ± 6 | 3.29 ± 0.33 | 0.16 ± 0.01 |
| TT 3 h | −194 ± 10 | 7 ± 0.4 | 335 ± 17 | 105 ± 5 | 4.96 ± 0.50 | 0.15 ± 0.01 |
| TT 2 h | −286 ± 14 | 10 ± 5 | 416 ± 21 | 142 ± 7 | 4.59 ± 0.46 | 0.19 ± 0.01 |
| TT 1 h | −267 ± 13 | 12 ± 6 | 433 ± 22 | 139 ± 7 | 3.80 ± 0.38 | 0.26 ± 0.01 |
| TT 20 min | −218 ± 11 | 17 ± 1 | 304 ± 15 | 85 ± 4 | 1.69 ± 0.17 | 0.56 ± 0.03 |
| Electrochemical Oxidation | |||||
|---|---|---|---|---|---|
| Rel (Ω·cm2) | CPEdl × 10−6 (Ω−1·cm−2·sn) | n | Rct (kΩ·cm2) | Chi-Squared | |
| B | 228 | 57 | 0.86 | 1390 | 0.002 |
| EO 4 h | 213 | 15 | 0.87 | 3460 | 0.002 |
| EO 3 h | 197 | 10 | 0.92 | 11,210 | 0.004 |
| EO 2 h | 229 | 15 | 0.94 | 7300 | 0.006 |
| EO 1 h | 291 | 14 | 0.94 | 3800 | 0.003 |
| EO 20 min | 297 | 20 | 0.90 | 1490 | 0.002 |
| Thermal Oxidation | |||||
|---|---|---|---|---|---|
| Rel (Ω·cm2) | CPEdl × 10−6 (Ω−1·cm−2·sn) | n | Rct (kΩ·cm2) | Chi-Squared | |
| B | 228 | 57 | 0.86 | 1390 | 0.002 |
| TT 4 h | 297 | 9 | 0.93 | 4880 | 0.006 |
| TT 3 h | 226 | 8 | 0.92 | 6325 | 0.008 |
| TT 2 h | 252 | 9 | 0.94 | 4700 | 0.002 |
| TT 1 h | 218 | 7 | 0.94 | 3220 | 0.002 |
| TT 20 min | 296 | 9 | 0.92 | 1491 | 0.002 |
| Ecorr (mV/Ag/AgCl) | Icorr × 10−9 (A·cm−2) | Average Tafel Slope (mV dec−1) | RP × 103 (Ω·cm2) | Ipass × 10−6 (A·cm−2) | ||
|---|---|---|---|---|---|---|
| βa | βc | |||||
| 0 ppm F− | −613 ± 30 | 23 ± 1 | 219 ± 11 | 94 ± 5 | 1242 ± 124 | 4 ± 0.2 |
| 1000 ppm F− | −354 ± 17 | 90 ± 5 | 186 ± 9 | 221 ± 11 | 488 ± 48 | 7 ± 0.4 |
| 5000 ppm F− | −470 ± 24 | 770 ± 36 | 177 ± 9 | 277 ± 14 | 61 ± 6 | 87 ± 4 |
| 12,300 ppm F− | −579 ± 29 | 1765 ± 88 | 143 ± 7 | 278 ± 14 | 23 ± 2 | 589 ± 29 |
| Rel (Ω·cm2) | CPEf × 10−6 (Ω−1·cm−2·sn) | n1 | Rf (Ω·cm2) | CPEdl × 10−6 (Ω−1·cm−2·sn) | n2 | Rct (kΩ·cm2) | Chi-Squared | |
|---|---|---|---|---|---|---|---|---|
| 0 ppm | 228 | - | - | - | 57 | 0.86 | 1390 | 0.002 |
| 1000 ppm | 180 | - | - | - | 58 | 0.91 | 524 | 0.002 |
| 5000 ppm | 67 | - | - | - | 62 | 0.92 | 150 | 0.004 |
| 12,300 ppm | 37 | 150 | 0.88 | 33 | 1 | 0.30 | 21 | 0.002 |
| Ecorr (mV/Ag/AgCl) | Icorr × 10−9 (A·cm−2) | Average Tafel Slope (mV dec−1) | RP × 103 (Ω·cm2) | Ipass × 10−6 (A·cm−2) | ||
|---|---|---|---|---|---|---|
| βa | βc | |||||
| B | −579 ± 29 | 1765 ± 88 | 143 ± 7 | 278 ± 14 | 23 ± 2 | 589 ± 29 |
| EO 3 h | −465 ± 24 | 53 ± 3 | 169 ± 8 | 130 ± 7 | 602 ± 60 | 68 ± 3 |
| TT 3 h | −206 ± 10 | 9 ± 0.5 | 258 ± 13 | 100 ± 5 | 3481 ± 348 | 0.25 ± 0.01 |
| Rel (Ω·cm2) | CPEf × 10−6 (Ω−1·cm−2·sn) | n1 | Rf (kΩ·cm2) | CPEdl × 10−6 (Ω−1·cm−2·sn) | n2 | Rct (kΩ·cm2) | Chi-Squared | |
|---|---|---|---|---|---|---|---|---|
| B | 37 | 150 | 0.88 | 0.033 | 1.35 | 0.30 | 21 | 0.002 |
| EO 3 h | 29 | 21 | 0.94 | 25 | 0.71 | 0.60 | 533 | 0.006 |
| TT 3 h | 42 | 7 | 0.97 | 1610 | 28,150 | 0.93 | 1583 | 0.006 |
| wt% | Ti | O |
|---|---|---|
| TT | 44 | 56 |
| EO | 79 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakaa, F.; Ferkhi, M.; Khaled, A.; Amira, S.; Eyraud, M. Corrosion Behavior of Electrochemical and Thermal Treated Titanium into Artificial Saliva: Effect of pH and Fluoride Concentration. Corros. Mater. Degrad. 2025, 6, 52. https://doi.org/10.3390/cmd6040052
Kakaa F, Ferkhi M, Khaled A, Amira S, Eyraud M. Corrosion Behavior of Electrochemical and Thermal Treated Titanium into Artificial Saliva: Effect of pH and Fluoride Concentration. Corrosion and Materials Degradation. 2025; 6(4):52. https://doi.org/10.3390/cmd6040052
Chicago/Turabian StyleKakaa, Faiza, Mosbah Ferkhi, Ammar Khaled, Sabah Amira, and Marielle Eyraud. 2025. "Corrosion Behavior of Electrochemical and Thermal Treated Titanium into Artificial Saliva: Effect of pH and Fluoride Concentration" Corrosion and Materials Degradation 6, no. 4: 52. https://doi.org/10.3390/cmd6040052
APA StyleKakaa, F., Ferkhi, M., Khaled, A., Amira, S., & Eyraud, M. (2025). Corrosion Behavior of Electrochemical and Thermal Treated Titanium into Artificial Saliva: Effect of pH and Fluoride Concentration. Corrosion and Materials Degradation, 6(4), 52. https://doi.org/10.3390/cmd6040052






